Introduction
Among the various somatic mutations in the epidermal growth factor receptor (EGFR) found in non-small-cell lung cancer (NSCLC), the most common include inframe deletions of exon 19 (~45%) and the exon 21 L858R (~40%). Clinical trials have ascertained that the oral EGFR tyrosine kinase inhibitors (TKIs), gefitinib and erlotinib, lead to superior response rates (RRs) and progression-free survivals (PFSs) than standard chemotherapies in these NSCLCs (1;2). The reported RRs to gefitinib/erlotinib exceed 70%, with median PFSs of ~9-12months and overall survival times beyond 20-24months (1). Other less prevalent EGFR mutations, such as the exon 18 G719X (~3%) and the exon 21 L861Q (~2%) display RRs that exceed 50% and prolonged PFS in gefitinib/erlotinib-treated patients (3). EGFR exon 20 insertion mutations (~5%) are associated with preclinical and clinical resistance to gefitinib/erlotinib (4).
Other less common EGFR mutations have not been completely characterized. This is the case of the exon 18 deletion/insertion mutation delE709_T710insD. Herein, we report the response to erlotinib of an EGFR delE709_T710insD mutated NSCLC, and provide a review of the literature on the pattern of response to EGFR TKIs of this mutation type.
Case report, methods and results
Case report
An 88-year-old white Caucasian female with a never smoking history presented with stage IV NSCLC (adenocarcinoma) with multiple pulmonary nodules, an osteolytic rib lesion, and metastatic lymph nodes. Her ECOG performance status was 0. The tumor, obtained from a transbronchial left lower lobe biopsy, did not contain an ALK translocation by FISH. Tumor-derived DNA was genotyped and found to have wild-type KRAS and the EGFR delE709_T710insD exon 18 mutation (using dideoxynucleotide sequencing).
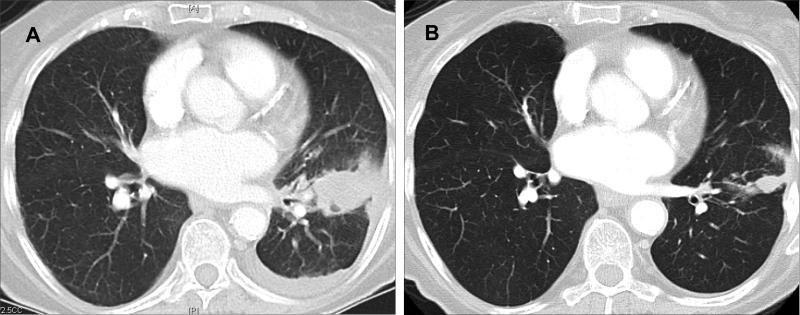
The patient was started on erlotinib 150mg/day but was only able to tolerate this dose for 3 weeks. Due to intolerable rash, gastro-intestinal symptoms and anorexia the dose was reduced to erlotinib 75mg/day. At the latter dose, the patient continued to have a characteristic EGFR TKI-induced rash that was tolerable. Imaging studies following initiation of erlotinib demonstrated significant improvement of the patient's tumor lesions (Figure). Measurement of target lesions indicated that the best response was a reduction of 47% in the sum of the largest diameter of the target tumors’ dimensions, which classifies as a partial response (PR) using RECIST. The last imaging study was obtained at the 4-month mark of therapy and the clinical response was maintained for the 6 months of follow-up. However, the patient decided to discontinue erlotinib at the 6-month mark of therapy. Further follow-up for clinical and radiographic progression was censored at that time point.
Figure.
Computed tomography images of the thorax of an adenocarcinoma of the lung harboring the EGFR delE709_T710insD mutation before (A) and after (B) erlotinib.
Frequency of EGFR delE709_T710insX among EGFR mutated NSCLCs
We next evaluated the frequency of EGFR delE709_T710insX mutations in the Wellcome Trust Sanger Institute COSMIC online database of EGFR mutations in lung cancer as of March 13th 2012 (http://www.sanger.ac.uk/perl/genetics/CGP/cosmic?action=bycancer&coords=AA%3AAA&start=1&end=1211&ln=EGFR&sn=lung&display=Apply). EGFR delE709_T710insD was only identified in 5/9539 (0.05%) EGFR mutated NSCLCs and delE709_T710insX (insA/insG/insD) in 7/9539 (0.07%) EGFR mutated NSCLCs.
Response of EGFR delE709_T710insD to EGFR TKIs
Two additional cases of patients whose tumors harbored EGFR delE709_T710insD and that received gefitinib have been reported (3;5). The calculated disease control rate to EGFR TKIs for the EGFR delE709_T710insD cohort was 66% (2/3 cases), and in our current report and another patient the PFS exceed 4 months. One of the cases was reported twice with divergent responses; in one publication as a PR (5) and in another as stable disease to gefitinib 250 mg/day (3); and in both the PFS was reported as 5 months. We assume this case had significant tumor regression but only met criteria for an unconfirmed PR by RECIST. The other case had progressive disease as best response to gefitinib 250 mg/day with a PFS of 0.9 months (3). These data indicate that the majority of NSCLCs with EGFR E709_T710delETinsD had tumor regression upon exposure to EGFR TKIs.
Discussion
EGFR delE709_T710insD exon 18 mutations account for less than 0.1% of previously reported EGFR mutations in NSCLC. Our case and the 2 additional cases reported in the literature provide evidence that EGFR delE709_T710insD may lead to enhanced sensitivity to reversible EGFR TKIs. Confirmatory in vitro studies will be needed to confirm this assertion. The clinical observation that patients with tumors with this mutation achieved radiographic tumor regression may be indicative that other patients with EGFR delE709_T710insD-bearing tumors can benefit from gefitinib and/or erlotinib at their usual clinical doses. In summary, EGFR delE709_T710insD is a rare but potentially EGFR TKI responsive mutation in NSCLC. The case presented here will be added to the Vanderbilt's DNA-mutation inventory to refine and enhance cancer treatment (DIRECT) database (http://www.mycancergenome.org/direct.php), with the goal of enhancing the ability of oncologists to select therapies for patients with uncommon EGFR mutated NSCLCs (6).
Funding/Grant Support/Acknowledgments
This work was supported in part by fellowships from the American Society of Clinical Oncology Conquer Cancer Foundation (DBC), an American Cancer Society grant RSG 11-186 (DBC), and National Institutes of Health grants CA090578 (DBC, SK). The funding agencies provided financial research support and were not involved in the writing of this manuscript.
Footnotes
Conflict of interest: DBC received consulting fees from Pfizer, Roche and AstraZeneca. No other conflict of interest is stated.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaughan EM, Costa DB. Genotype-driven therapies for non-small cell lung cancer: focus on EGFR, KRAS and ALK gene abnormalities. Ther Adv Med Oncol. 2011;3(3):113–125. doi: 10.1177/1758834010397569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 3.Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY, Yang PC. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17(11):3812–3821. doi: 10.1158/1078-0432.CCR-10-3408. [DOI] [PubMed] [Google Scholar]
- 4.Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012;13(1):e23–e31. doi: 10.1016/S1470-2045(11)70129-2. [DOI] [PubMed] [Google Scholar]
- 5.Wu SG, Chang YL, Lin JW, Wu CT, Chen HY, Tsai MF, et al. Including total EGFR staining in scoring improves EGFR mutations detection by mutation-specific antibodies and EGFR TKIs response prediction. PLoS One. 2011;6(8):e23303. doi: 10.1371/journal.pone.0023303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yatabe Y, Pao W, Jett JR. Encouragement to submit data of clinical response to EGFR-TKIs in patients with uncommon EGFR mutations. J Thorac Oncol. 2012;7(5):775–776. doi: 10.1097/JTO.0b013e318251980b. [DOI] [PubMed] [Google Scholar]