Abstract
Lymphatic dysfunction in lymphedema results in chronic accumulation of interstitial fluid and life-long tissue swelling. In the absence of restored lymphatic drainage via adequate lymphangiogenesis, the interstitial environment can remodel in ways that decrease the elevated interstitial stress. Presently, relatively little is known about the glycosaminoglycans (GAGs) that become upregulated in the interstitium during lymphedema. We employed a mouse tail model of acute lymphedema that reproduces important features of the chronic human condition to establish a relationship between hyaluronan (HA) and sulfated GAG concentration with tissue swelling. We found that HA was upregulated by tissue injury at day 5 and became upregulated again by skin swelling (HA content increasing by 27% relative to controls at days 15 and 20). Surprisingly, the second phase of HA expression was associated with the declining phase of the tail skin swelling (tail diameter significantly decreasing by 17% from day 10 peak to day 20), demonstrating that HA is upregulated by tissue swelling and may help to counteract the edema in the mouse tail. This finding was confirmed by intradermal injection of an HA degrading enzyme (hyaluronidase) to the swollen tail, which was found to worsen the tail swelling. Sulfated GAGs, including chondroitin sulfate (CS), were not regulated by tissue swelling. The results demonstrate that HA, but not sulfated GAGs, is upregulated in the interstitium by acute tissue swelling. We speculate that HA expression during lymphedema may be part of a natural adaptive mechanism of the interstitial environment to reduce capillary filtration and increase interstitial fluid outflow following lymphatic obstruction and fluid accumulation.
Introduction
Lymphatic dysfunction in lymphedema results in chronic accumulation of interstitial fluid and life-long tissue swelling. In the absence of restored lymphatic drainage via adequate lymphangiogenesis, the interstitial environment can remodel in ways that decrease the elevated interstitial stress. Presently, relatively little is known about the particular interstitial molecules that become predominant in the skin to reduce tissue swelling. Collagen and lipid deposition, for example, have been shown to increase in swollen skin.1,2 In addition to collagens, which strengthen the tensile properties of skin to counteract the swelling force, glycosaminoglycans (GAGs) increase tissue hydrostatic pressure by stabilizing water (ie, restricting water mobility) due to their high negative charge density.
GAGs are long unbranched sugar polymers with repeating disaccharides present in the extracellular matrix of skin. In the interstitium, the polyanionic GAGs stabilize water due to the increased osmotic pressure obtained from attracted cations, such as Ca2+ and Na+. There are two main types of GAGs, nonsulfated GAGs (mainly hyaluronan) and sulfated GAGs (chondroitin sulfate, dermatan sulfate, keratan sulfate, and heparan sulfate).3,4 Hyaluronan (HA) and chondroitin sulfate (CS) are the main GAGs found in skin, with HA representing approximately 75% of all GAGs present in the dermis.5 Numerous sulfated patterns exist among these broad categories of sulfated GAGs6 and variations in sulfation patterns may be related to GAG function in both normal and diseased states.7 Each additional sulfate adds one negative charge to every disaccharide in the GAG. Because the negative charge density on the GAG chain is directly proportional to its water attractiveness and resistivity to fluid flow, the sulfation of the GAGs and the different degree of sulfation may be an important parameter in lymphedema. Hyaluronan has a mobile form and can also bind to other GAGs in the form of proteoglycans,8 thereby immobilizing hydrophilic molecules within the interstitium. It has previously been shown that HA content is increased in sites of injury.9 High levels of HA have been found in infarcted cardiac tissue exhibiting edema,10 acute alveolitis,11,12 and edema resulting from organ transplantation.13
Although GAG concentration has been reported to increase in the edematous human arm,14 the types of GAGs involved and their discreet roles have not been clarified. Thus, it is unknown how GAGs are acting in secondary lymphedema. An upregulation of interstitial GAGs may exacerbate the swelling by increasing the swelling pressure (via water binding). However, the increased interstitial fluid pressure from the GAG upregulation may simultaneously oppose capillary filtration and increase fluid outflow. We hypothesized that the content of HA and sulfated GAGs may become altered in acute lymphedema of the mouse tail, providing us an opportunity to gain insight into the roles of GAGs in the chronic human form of lymphedema. We employed an existing mouse tail model of experimental lymphedema that reproduces important features of the human condition to establish a relationship between HA and sGAG concentration with tissue swelling.
Materials and Methods
Experimental lymphedema
Tail skin lymphedema was created in Balb/c mice by excising a 2-mm circular band of dermis (which contains the lymphatic capillary network) 2 cm from the base of the tail, leaving the underlying bone, muscle, tendons, and major blood vessels intact, as was performed previously.15–20 Tail swelling was determined by measuring the peak tail diameter using ImageJ software from digital images of the tail distal to the wound site. Two images of the tail at a 90° rotation from each other were captured for each mouse tail with a DP71 color camera mounted to a stereo microscope, and the two peak tail diameters were averaged together. Animal protocols were approved by the Animal Care and Use Committee of Michigan Technological University.
In order to clarify the regulation of glycosaminoglycans (GAGs) by lymphedema, mice were euthanized at appropriate endpoints and a small portion of the tail containing the wound region was cryosectioned for histological analysis, while the remaining length of the tail skin (away from the wound site) was digested and ground to quantify sulfated GAG and hyaluronan concentration in the swollen skin.
Immunofluorescence and immunohistochemistry
The swollen tail skin near the wound site was snap frozen, cryosectioned at 10 microns thickness, fixed in 4% formaldehyde, and immunolabeled for hyaluronan (HA) or chondroitin sulfate (CS). For HA detection, cross-sections were labeled for HA using biotinylated hyaluronan binding protein (HABP, 5 μg/ml, from Sigma Aldrich). Color was added to the biotin conjugate with the VectaStain ABC kit (Vector Labs) and cell nuclei were counterstained with Gil's hematoxylin (Sigma). For CS detection, cross-sections were immunolabeled with an anti-chondroitin sulfate antibody (Sigma) and then with a fluorescence conjugated secondary antibody. Cell nuclei were counter-stained with DAPI (Vector). Images were captured with the 10X objective lens of an Olympus brightfield/ fluorescence microscope affixed with a DP70 camera. Images were then stitched together using Adobe Photoshop software.
Hyaluronan quantification
A Hyaluronan Enzyme-Linked Immunosorbent Assay Kit (HA-ELISA, from Echelon) was used to quantify HA in the mouse tail skin. Briefly, harvested swollen and control tissue was suspended in 0.5 M NaCl overnight and further processed by homogenizing. Each sample was syringe filtered through 0.22 micron pore-sized filters. Supernatants were further processed by following the manufacturer's instructions. Values calculated from the standard curve were divided by the dry weight for each sample and normalized to the control values.
Sulfated glycosaminoglycan (sGAG) quantification
The Blyscan Assay (Blyscan Assay from Biocolor life science assays) was employed to detect all sulfated forms of GAGs, including chondroitin, dermatan, keratan, and heparan sulfates. Briefly, harvested swollen and control tissue was suspended in a papain extraction reagent to release the GAGs from the tissue, as described in the Blyscan product manual. Tissue was incubated overnight at 65°C with occasional vortexing and grinding of the tissue. Following centrifugation to remove the particulates, the supernatant was run in duplicate with the Blyscan Assay kit by following the manufacturer's instructions. Values calculated from the standard curve were divided by the dry weight of the tissue of each sample and normalized to control values.
Exogenous hyaluronidase enzymatic treatment
Twenty-six additional mice were used to evaluate the effects of exogenous hyaluronidase (HAse) treatment on the tail swelling. Hyaluronidase is an enzyme that degrades the GAG hyaluronan. Following surgery, mice were treated with hyaluronidase or PBS (control) via injections. Injections were made into the tail skin distal to the surgical wound every 3 days from day 12 to day 18 post surgery, for a total of three injections per mouse. The enzyme concentrations employed were similar to a previously published study.21 For the injections, hyaluronidase (Type IV-S, H3884, Sigma Aldrich) was diluted to a concentration of 56 U (low dose), 112 U (medium dose), and 225 U (high dose), in a volume of 2.5 μl sterile PBS. Control mice were injected with the same 2.5 μl volume of sterile PBS without enzyme.
Statistical methods
At least five animals were used for each data point. Data are presented as means with standard deviations. P values were calculated by ANOVA with JMP statistical software. Results were reported as significant with p values less than or equal to 0.05, and as highly significant with p values less than or equal to 0.01.
Results
Sustained acute lymphedema in the mouse tail
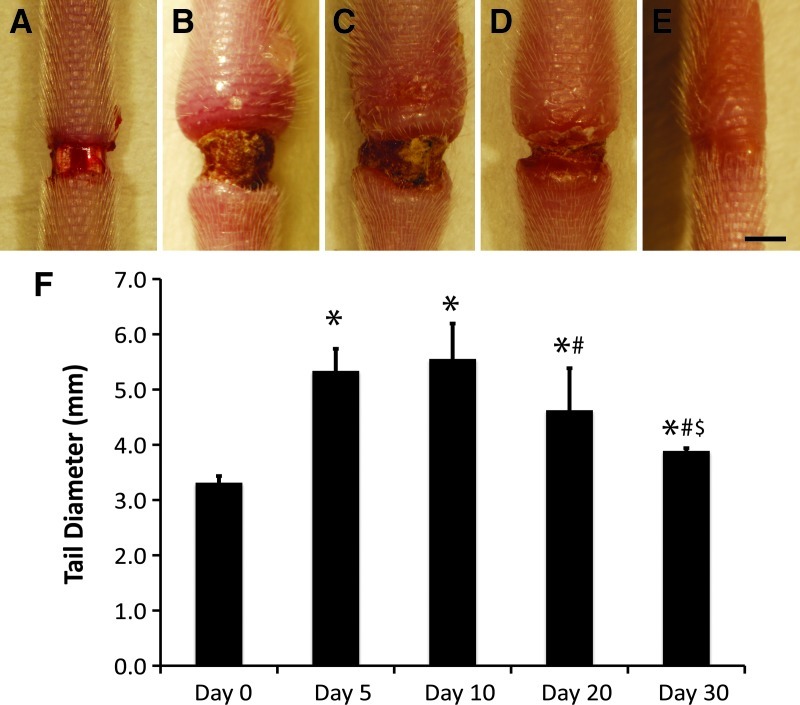
Acute lymphedema was sustained over 30 days by excision of an annulus of skin near the base of the tail, which obstructed the outflow of accumulating fluid in the mouse tail (Fig. 1A–E). Similar to previous studies using this approach, the tails were significantly swollen by day 5, with increased swelling resolution apparent between days 20 and 30 (Fig. 1F, p<0.05 by ANOVA for all days relative to day 0, at day 20 relative to day 10, and at day 30 relative to days 20 and 10, n=5). The sustained tissue swelling produced in the tail provides a rich environment of interstitial adaptations where it is possible to investigate the extracellular matrix (ECM) remodeling associated with lymphedema. By comparison between normal, swollen, and restored ECM, we are able to identify interstitial molecules that may be adaptive during lymphedema and determine whether these molecules play a role in either exacerbating or opposing fluid accumulation.
FIG. 1.
Acute lymphedema appearance and resolution over 30 days. Acute lymphedema was induced in the mouse tail skin over a period of 30 days by a 2-mm wide surgical excision of the skin that was left unprotected. Injury sites and swollen tails are shown at days 0 (A), 5 (B), 10 (C), 20 (D), and 30 (E). Scale bar (E, bottom, right)=2 mm. The evolution of tail swelling over the 30-day period is graphically depicted (F). Tail diameters are normalized to the average initial (preswelling) diameter of the tails, n=5 mice per group. *Statistical significance relative to control; #Statistical significance relative to day 10; $Statistical significance relative to day 20.
Hyaluronan is increased in lymphedema
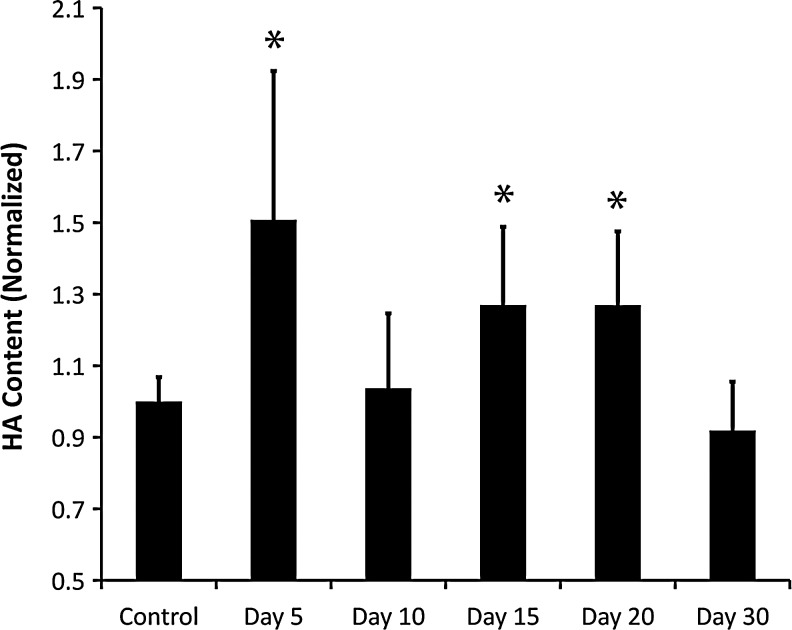
In order to compare hyaluronan (HA) concentration between normal, lymph edematous, and restored tissue, we employed an HA-specific ELISA to quantify the HA in the skin. Similar approaches have been used successfully to identify changes in GAG concentration,22,23 but have not been employed in edematous skin. This quantification approach revealed a highly significant increase of HA at post-surgical days 5, 15, and 20 relative to the nonoperated control skin (Fig. 2; p values of 0.0001, 0.0092, and 0.0093, respectively, by ANOVA, n=6). These results show that HA peaked at day 5 post surgery and returned to physiological levels by day 10, when swelling had peaked. A second increase was evident at days 15 and 20, which had returned to physiological levels by day 30. HA content at day 10 was found to be significantly less than at days 15 and 20 (Fig. 2; p values of 0.01 and 0.008, respectively). The first peak at day 5 and decline by day 10 is consistent with HA's known role in wound healing,24 although the first peak in HA expression at day 5 may have also contributed to the swelling. The second HA increase occurs during tissue swelling and is associated temporaly with a decreased tissue swelling. The second phase, which occurs after the injury-induced HA increase has subsided, demonstrates that HA expression becomes increased in lymphedema, possibly due to biosynthesis from cells stimulated by low interstitial flow or accummulated as a consequence of reduced lymphatic drainage. HA's association with the swelling resolution trend rather than peak swelling (day 10) is strongly suggestive that HA may be important for reducing the swelling in acute lymphedema.
FIG. 2.
Hyaloronan is increased in the tail skin during lymphedema. Hyaloronan was quantified from mouse tail skin over the 30-day period of acute lymphedema. Values are normalized to the unoperated control HA concentration. *Statistical significance relative to control; n=6 mice per group.
To confirm the increase of HA in lymphedema, we labeled tail skin cross sections for HA to determine the HA intensity and distribution in the swollen skin (Fig. 3). HA was seen to be weakly present in normal skin (Fig. 3A), became strongly present and evenly distributed at day 5 (Fig. 3B), was weakly present again at day 10 (Fig. 3C), and was seen to be moderately present and unevenly distributed at day 20 (Fig. 3D). HA had returned to normal levels by day 30 (Fig. 3C). The strong increase and even distribution of HA at day 5 throughout the swollen tissue suggests that the initial swelling may produce an injury-related increase in the HA content. The HA present in the cross section at day 20 appears more strongly expressed in the dermis relative to the subcutaneous region of the skin. This HA distribution may have the effect of increasing fluid outflow through the subcutaneous region, which has a higher hydraulic conductivity than the dermis. These results parallel the ELISA quantification data and confirm that HA becomes increased in lymphedema of the mouse tail.
FIG. 3.
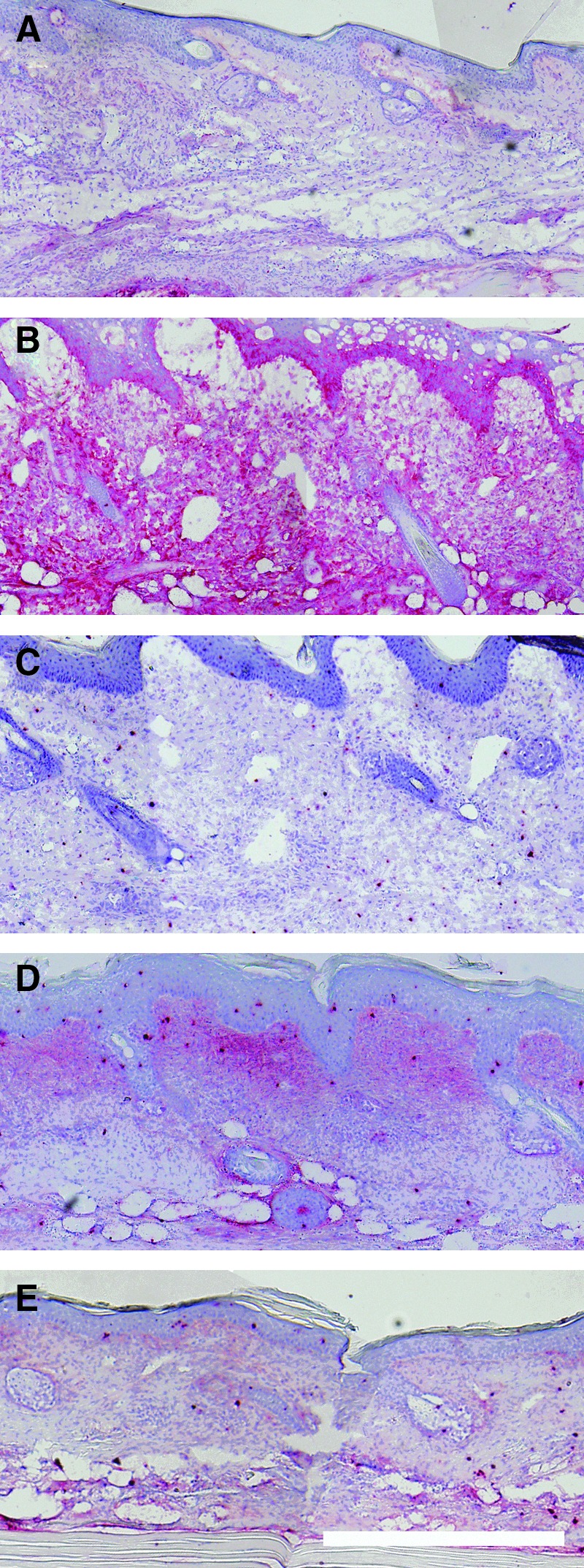
Hyaloronan is increased in swollen tail skin cross sections. Hyaloronan was detected in cross sections to reveal intensity and distribution. Images shown at days 0 (A), 5 (B), 10 (C), 20 (D), and 30 (E). Epidermis is at the top of each image. Scale bar (E, bottom, right)=0.5 mm.
Hyaluronidase treatment worsens the tail swelling
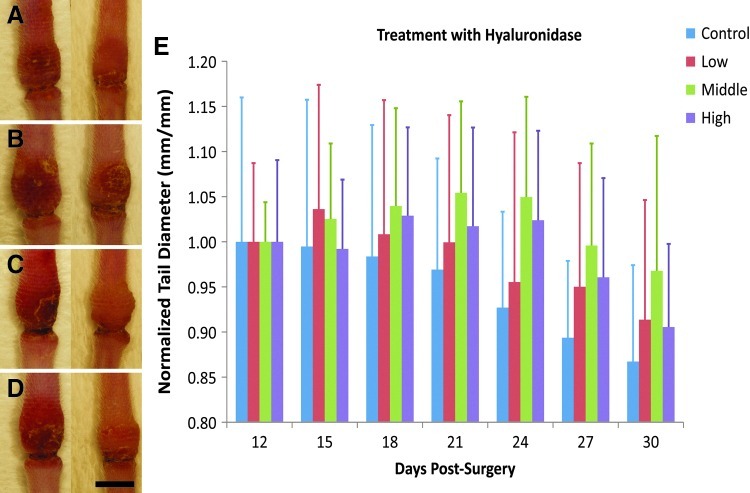
Because it appeared that an increased HA content was associated with a reduced tail swelling, we treated lymph edematous mouse tail skin with the HA degrading enzyme hyaluronidase (HAase) to determine if a reduction in the HA would have the opposite effect. To accomplish this, acute lymphedema was produced in the mouse tail as before, and 3 groups of mice received repeated injections of different concentrations of HAase, with a fourth group receiving PBS injections. To prevent interference with HA's physiological role in wound repair, injections were first initiated on day 12, after the decline of the first peak in HA expression and shortly preceding the second phase of increased HA expression (Fig. 2). The tail diameters were measured before each injection, shown in Figure 4. It was found that the mouse tails that received the medium and high HAase dose developed significantly increased tail swelling relative to the control tissue (p<0.05 for control vs. high dose and p<0.005 for control vs. medium dose, by repeated measures ANOVA). There were no differences found between the medium or high dose groups. Thus, HAase treatment worsened the tail swelling, consistent with an ameliorative role for HA in lymphedema.
FIG. 4.
Hyaluronidase (HAase) treatment worsens the tail swelling. Control tissue (A) was compared to mouse tails that received HAase in low (B), medium (C), and high doses (D). Images show tails at days 12 (left) and 24 (right), with distal direction at the top and proximal direction at the bottom of each image. Peak swelling diameters for each tail are graphically depicted in E. Scale bar (D, bottom, right)=5 mm, n=7 mice for the low and high dose groups, and n=6 mice for the control and medium dose groups.
Sulfated GAGs are not increased in lymphedema
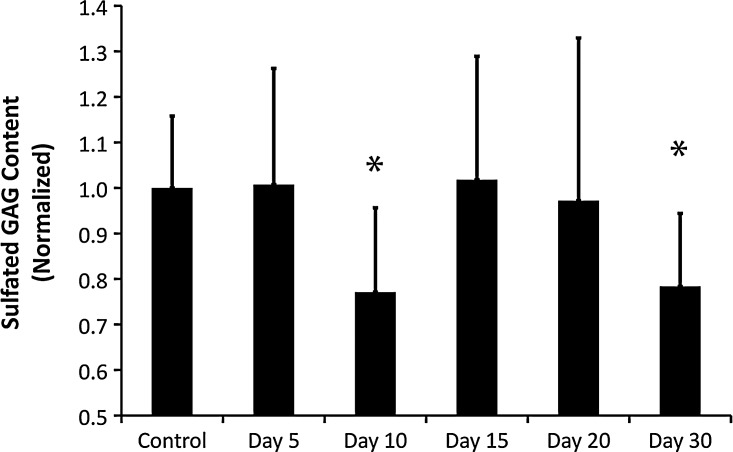
In order to compare sGAG concentration between normal, lymph edematous, and restored tissue, we quantified the sGAG concentration in the skin. Quantification of sGAG demonstrated that sGAG did not become increased in lymphedema. Indeed, a small yet significant reduction in sGAG concentration was seen at days 10 and 30 (Fig. 5, p values of 0.0243 and 0.0289, respectively, by ANOVA, n=6). However, normal sGAG levels were detected at all days of significant tissue swelling other than day 10, suggesting that tissue swelling was not the cause of the day 10 reduced sGAG. Interestingly, the reductions seen at days 10 and 30 were associated with a return of the HA content to normal levels (Fig. 2). Because several sGAGs are able to form interstitial aggregates with HA through hydrophobic associations on the polymer chains,25 it is possible that the HA and sGAG reductions at day 10 and 30 are related. It may be the case that sGAGs become enzymatically cleaved during wound repair when local cells release enzymes to degrade HA between days 5 and 10. These GAGs would then have an increased ability to diffuse through the tissue. The reduction of both HA and sGAG at day 30 may be due to a general washout effect as capillary filtration is restored to normal levels and soluble GAG is able to drain more effectively from the limb.
FIG. 5.
Sulfated GAG is not increased in the tail skin during lymphedema. CS was quantified from mouse tail skin over the 30-day period of acute lymphedema. Values are normalized to the unoperated control HA concentration. *Statistical significance relative to control; n=6 mice per group.
In addition to hyaluronan (which does not become sulfated), chondroitin sulfate (CS) is a dominant GAG in the skin. Therefore, we labeled skin cross sections for CS to determine the CS intensity and distribution in the swollen skin (Fig. 6). CS appeared evenly distributed throughout the skin at all time points. Unlike what was found for HA, CS did not appear to be regulated by tissue injury, swelling, or recovery. Although the sGAG assay showed a reduced content at days 10 and 30, a similar trend was not evident in the CS-labeled tissue sections. This is possibly due to the modest reduction of ∼20% that was detected by the quantitative method, which may not be visible with a less sensitive histological labeling approach. It is also possible that a decrease of a different sGAG (such as heparan or keratan sulfate) caused the decrease detected by the quantative method at days 10 and 30.
FIG. 6.
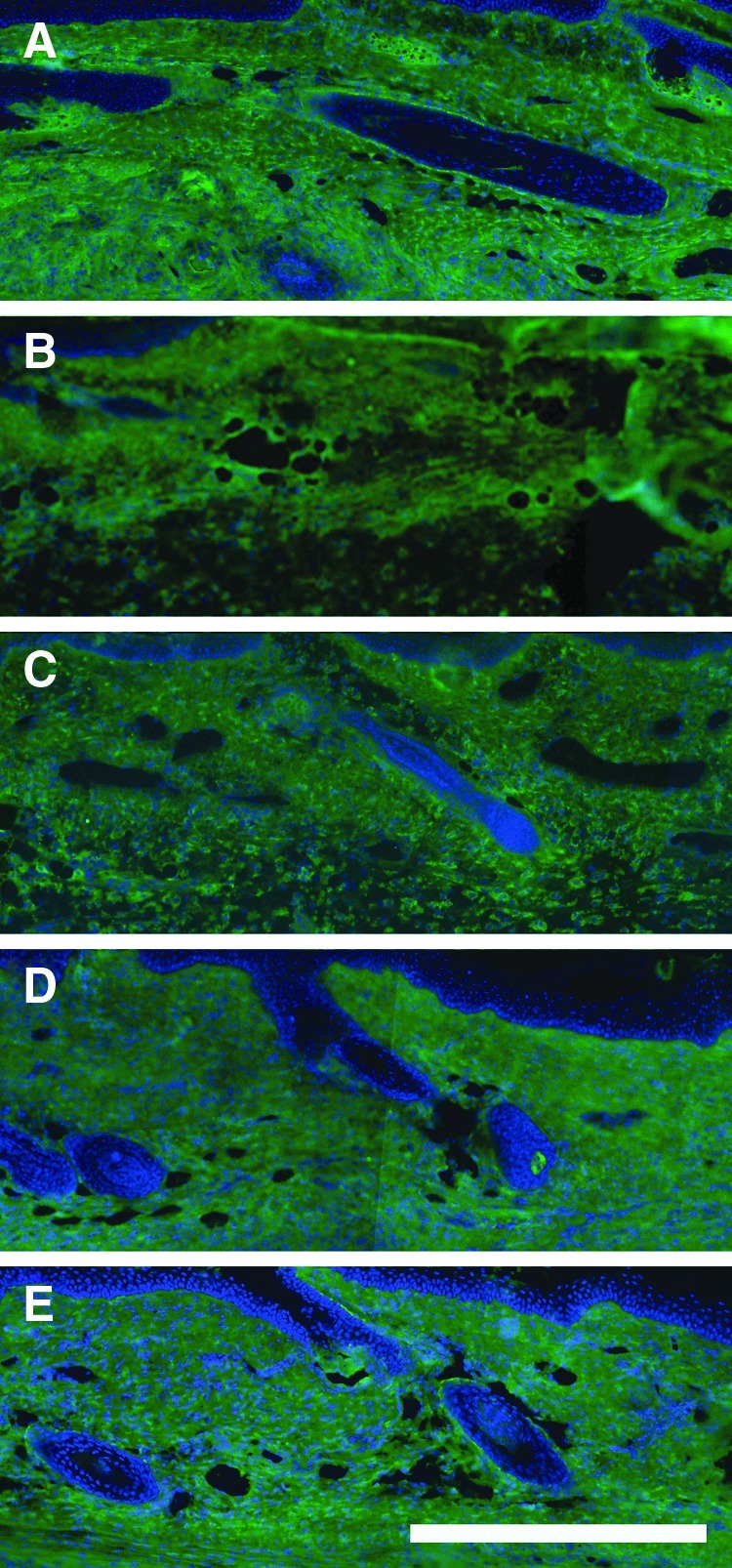
Chondroitin sulfate is not increased in swollen tail skin cross sections. CS was detected in cross sections to reveal intensity and distribution. Images shown at days 0 (A), 5 (B), 10 (C), 20 (D), and 30 (E). Epidermis is at the top of each image. Scale bar (E, bottom, right)=0.5 mm.
Conclusions
The interstitial environment retains intrinsic capacity to modify microvascular filtration and interstitial fluid dynamics during pathological states by adjusting the content of the extracellular matrix (ECM) proteins and glycosaminoglycans (GAGs). GAGs are long unbranched polysaccharides endowed with mechanical properties that affect interstitial flow and interstitial fluid pressure, properties that are central to the pathophysiology of lymphedema. GAGs form a major component of the ECM and strongly attract water due to their large number of negative charges and thereby act to resist interstitial flow 26 and increase swelling pressure.27 We recreated the interstitial environment of fluid accumulation and tissue swelling found in lymphedema by surgically disrupting the lymphatic capillaries and vessels to decrease fluid outflow from the mouse tail. Under these conditions, the mouse tail skin rapidly experiences sustained swelling with marked changes in the content of interstitial macromolecules, including upregulation of collagen matrix,1,2 followed by near complete swelling resolution. This allowed us to assess the role of GAG alterations that take place in the interstitial environment during tissue swelling. We used quantitative methods to correlate nonsulfated and sulfated GAG concentration with the degree of tissue swelling.
An interesting question is whether GAG levels regulate edema clearance or whether edema (and fluid flow variations) regulate GAG levels. We found that hyaluronan (HA) but not sulfated GAGs became increased during the skin swelling. An increased interstitial HA content could occur from increased cellular synthesis or deficient lymphatic clearance. Thus, either biosynthesis or variations in lymphatic drainage due to lymphedema may account for the altered HA levels measured at days 5 versus 10 versus 20 (Fig. 2). The increased HA content was associated with a declining trend in the tissue swelling and may be causally related to edema clearance. We confirmed this possibility by treating swollen mouse tails with hyaluronidase (HAase), which degrades HA. Treatment with HAase worsened the tail skin swelling. Thus, HA levels may be regulated by tissue edema and may in turn regulate edema clearance. We speculate that the increased interstitial fluid pressure derived from the increased HA content may confer an opposition to interstitial fluid entry and/or an increased interstitial outflow during the resolution phase of acute lymphedema.
The concentration of GAGs has been reported to become significantly increased in the lymph fluid of humans with lymphedema.14 This work showed that the interstitial protein concentration in breast cancer-related lymphedema is inversely related to the swelling, which means counterintuitively that, when the protein content is reduced, the swelling is worse. The implications of this finding have been reviewed here.28 Second and largely ignored, the GAG concentration is increased while at the same time there is a net decrease in the interstitial osmotic pressure (due to the reduction in interstitial protein content).14,29,30 The effect of reduced interstitial osmotic pressure (from reduced protein content) and increased interstitial fluid pressure (from increased GAG content) is to increase pressure opposing capillary filtration while increasing fluid outflow across the obstruction, thus counteracting edema. Thus, GAG upregulation may be part of a natural adaptive mechanism of the interstitial environment to reduce filtration and increase outflow following lymphatic obstruction and fluid accumulation. This may lead to reduced swelling in acute lymphedema as the obstruction heals and outflow resistance subsides, but may contribute to chronic swelling in the human secondary lymphedema due to a sustained outflow resistance.
Acknowledgment
This work was funded by National Institutes of Health Grants R21-AR-053094, R21-HL-093568, and R15-HL-093705.
Author Disclosure Statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Rutkowski JM. Moya M. Johannes J. Goldman J. Swartz MA. Secondary lymphedema in the mouse tail: Lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res. 2006;72:161–171. doi: 10.1016/j.mvr.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rutkowski JM. Markhus CE. Gyenge CC. Alitalo K. Wiig H. Swartz MA. Dermal collagen and lipid deposition correlate with tissue swelling and hydraulic conductivity in murine primary lymphedema. Am J Pathol. 2010;176:1122–1129. doi: 10.2353/ajpath.2010.090733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson RL. Busch SJ. Cardin AD. Glycosaminoglycans: Molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991;71:481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi NS. Mancera RL. The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des. 2008;72:455–482. doi: 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 5.Akimoto S. Hayashi H. Ishikawa H. Disaccharide analysis of the skin glycosaminoglycans in systemic sclerosis. Br J Dermatol. 1992;126:29–34. doi: 10.1111/j.1365-2133.1992.tb08399.x. [DOI] [PubMed] [Google Scholar]
- 6.Bulow HE. Hobert O. The molecular diversity of glycosaminoglycans shapes animal development. Annu Rev Cell Dev Biol. 2006;22:375–407. doi: 10.1146/annurev.cellbio.22.010605.093433. [DOI] [PubMed] [Google Scholar]
- 7.Kuwaba K. Kobayashi M. Nomura Y. Irie S. Koyama Y. Elongated dermatan sulphate in post-inflammatory healing skin distributes among collagen fibrils separated by enlarged interfibrillar gaps. Biochem J. 2001;358:157–163. doi: 10.1042/0264-6021:3580157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasegawa K. Yoneda M. Kuwabara H. Miyaishi O. Itano N. Ohno A. Zako M. Isogai Z. Versican, a major hyaluronan-binding component in the dermis, loses its hyaluronan-binding ability in solar elastosis. J Invest Dermatol. 2007;127:1657–1663. doi: 10.1038/sj.jid.5700754. [DOI] [PubMed] [Google Scholar]
- 9.Riessen R. Wight TN. Pastore C. Henley C. Isner JM. Distribution of hyaluronan during extracellular matrix remodeling in human restenotic arteries and balloon-injured rat carotid arteries. Circulation. 1996;93:1141–1147. doi: 10.1161/01.cir.93.6.1141. [DOI] [PubMed] [Google Scholar]
- 10.Waldenstrom A. Martinussen HJ. Gerdin B. Hallgren R. Accumulation of hyaluronan and tissue edema in experimental myocardial infarction. J Clin Invest. 1991;88:1622–1628. doi: 10.1172/JCI115475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson K. Eklund A. Malmberg P. Bjermer L. Lundgren R. Belin L. Hyaluronic acid (hyaluronan) in BAL fluid distinguishes farmers with allergic alveolitis from farmers with asymptomatic alveolitis. Chest. 1992;101:109–114. doi: 10.1378/chest.101.1.109. [DOI] [PubMed] [Google Scholar]
- 12.Nettelbladt O. Tengblad A. Hallgren R. Lung accumulation of hyaluronan parallels pulmonary edema in experimental alveolitis. Am J Physiol. 1989;257:L379–384. doi: 10.1152/ajplung.1989.257.6.L379. [DOI] [PubMed] [Google Scholar]
- 13.Johnsson C. Hallgren R. Elvin A. Gerdin B. Tufveson G. Hyaluronidase ameliorates rejection-induced edema. Transpl Int. 1999;12:235–243. doi: 10.1007/s001470050216. [DOI] [PubMed] [Google Scholar]
- 14.Bates DO. Levick JR. Mortimer PS. Change in macromolecular composition of interstitial fluid from swollen arms after breast cancer treatment, and its implications. Clin Sci (Lond) 1993;85:737–746. doi: 10.1042/cs0850737. [DOI] [PubMed] [Google Scholar]
- 15.Goldman J. Conley KA. Raehl A. Bondy DM. Pytowski B. Swartz MA. Rutkowksi JM. Jaroch DB. Ongstad EL. Regulation of lymphatic capillary regeneration by interstitial flow in skin. Am J Physiol Heart Circ Physiol. 2007;292:H2176–H2183. doi: 10.1152/ajpheart.01011.2006. [DOI] [PubMed] [Google Scholar]
- 16.Goldman J. Rutkowski JM. Shields JD. Pasquier MC. Cui Y. Schmokel HG. Willey S. Hicklin DJ. Pytowski B. Swartz MA. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J. 2007;21:1003–1012. doi: 10.1096/fj.06-6656com. [DOI] [PubMed] [Google Scholar]
- 17.Ongstad EL. Bouta EM. Roberts JE. Uzarski JS. Gibbs SE. Sabel MS. Cimmino VM. Roberts MA. Goldman J. Lymphangiogenesis-independent resolution of experimental edema. Am J Physiol Heart Circ Physiol. 2010;299:H46–54. doi: 10.1152/ajpheart.00008.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uzarski J. Drelles MB. Gibbs SE. Ongstad EL. Goral JC. McKeown KK. Raehl AM. Roberts MA. Pytowski B. Smith MR, et al. The resolution of lymphedema by interstitial flow in the mouse tail skin. Am J Physiol Heart Circ Physiol. 2008;29:H1326–1334. doi: 10.1152/ajpheart.00900.2007. [DOI] [PubMed] [Google Scholar]
- 19.Boardman KC. Swartz MA. Interstitial flow as a guide for lymphangiogenesis. Circ Res. 2003;92:801–808. doi: 10.1161/01.RES.0000065621.69843.49. [DOI] [PubMed] [Google Scholar]
- 20.Goldman J. Le TX. Skobe M. Swartz MA. Overexpression of VEGF-C causes transient lymphatic hyperplasia but not increased lymphangiogenesis in regenerating skin. Circ Res. 2005;96:1193–1199. doi: 10.1161/01.RES.0000168918.27576.78. [DOI] [PubMed] [Google Scholar]
- 21.Mennuni C. Calvaruso F. Zampaglione I. Rizzuto G. Rinaudo D. Dammassa E. Ciliberto G. Fattori E. La Monica N. Hyaluronidase increases electrogene transfer efficiency in skeletal muscle. Hum Gene Ther. 2002;13:355–365. doi: 10.1089/10430340252792495. [DOI] [PubMed] [Google Scholar]
- 22.Riley GP. Harrall RL. Constant CR. Chard MD. Cawston TE. Hazleman BL. Glycosaminoglycans of human rotator cuff tendons: Changes with age and in chronic rotator cuff tendinitis. Ann Rheumatic Dis. 1994;53:367–376. doi: 10.1136/ard.53.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rugheimer L. Johnsson C. Maric C. Hansell P. Hormonal regulation of renomedullary hyaluronan. Acta Physiol. 2008;193:191–198. doi: 10.1111/j.1748-1716.2007.01795.x. [DOI] [PubMed] [Google Scholar]
- 24.Tammi R. Pasonen-Seppanen S. Kolehmainen E. Tammi M. Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J Invest Dermatol. 2005;124:898–905. doi: 10.1111/j.0022-202X.2005.23697.x. [DOI] [PubMed] [Google Scholar]
- 25.Sabaratnam S. Coleman PJ. Badrick E. Mason RM. Levick JR. Interactive effect of chondroitin sulphate C and hyaluronan on fluid movement across rabbit synovium. J Physiol. 2002;540:271–284. doi: 10.1113/jphysiol.2001.013468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levick J. Flow through interstitium and other fibrous matrices. Quart Rev Exp Physiol. 1987;72:409–438. doi: 10.1113/expphysiol.1987.sp003085. [DOI] [PubMed] [Google Scholar]
- 27.Maroudas AI. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature. 1976;260:808–809. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]
- 28.Bates DO. An interstitial hypothesis for breast cancer related lymphoedema. Pathophysiology. 2010;17:289–294. doi: 10.1016/j.pathophys.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bates DO. Levick JR. Mortimer PS. Starling pressures in the human arm and their alteration in postmastectomy oedema. J Physiol. 1994;477:355–363. doi: 10.1113/jphysiol.1994.sp020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bates DO. Levick JR. Mortimer PS. Subcutaneous interstitial fluid pressure and arm volume in lymphoedema. Int J Microcirc Clin Exp. 1992;11:359–373. [PubMed] [Google Scholar]