Crb fulfills a protective role during light exposure by limiting oxidative damage resulting from Rac1–NADPH oxidase complex activity.
Abstract
Drosophila melanogaster Crumbs (Crb) and its mammalian orthologues (CRB1–3) share evolutionarily conserved but poorly defined roles in regulating epithelial polarity and, in photoreceptor cells, morphogenesis and stability. Elucidating the molecular mechanisms of Crb function is vital, as mutations in the human CRB1 gene cause retinal dystrophies. Here, we report that Crb restricts Rac1–NADPH oxidase-dependent superoxide production in epithelia and photoreceptor cells. Reduction of superoxide levels rescued epithelial defects in crb mutant embryos, demonstrating that limitation of superoxide production is a crucial function of Crb and that NADPH oxidase and superoxide contribute to the molecular network regulating epithelial tissue organization. We further show that reduction of Rac1 or NADPH oxidase activity or quenching of reactive oxygen species prevented degeneration of Crb-deficient retinas. Thus, Crb fulfills a protective role during light exposure by limiting oxidative damage resulting from Rac1–NADPH oxidase complex activity. Collectively, our results elucidate an important mechanism by which Crb functions in epithelial organization and the prevention of retinal degeneration.
Introduction
The physiology of epithelia relies on the polarized architecture of individual epithelial cells along the apical-basal axis (Tepass et al., 2001). Functional interactions between the apical protein Crumbs (Crb) and lateral polarity modules is essential for formation and size regulation of membrane domains in Drosophila melanogaster epithelial cells, and hence for controlling embryonic tissue morphogenesis (Bilder et al., 2003; Tanentzapf and Tepass, 2003; Laprise et al., 2006; Laprise et al., 2009; Laprise et al., 2010; Laprise and Tepass, 2011). For instance, Crb maintains epithelial integrity by repressing Rac1 and phosphoinositide 3-kinase (PI3K), which act in a positive feedback loop and participate in lateral membrane stability (Laprise et al., 2002; Gassama-Diagne et al., 2006; Jeanes et al., 2009; Chartier et al., 2011). In return, Rac1 and PI3K signaling limit Crb levels at the apical membrane and Crb-dependent apical membrane growth (Pirraglia et al., 2010; Chartier et al., 2011). Thus, mutually antagonistic interactions between Crb and the PI3K/Rac1 module are instrumental for epithelial tissue organization.
GTP-loaded Rac1 is also required for NADPH oxidase activity (Hordijk, 2006). Therefore, active Rac1 contributes to the production of superoxide by this enzymatic complex (Sundaresan et al., 1996; Park et al., 2004; Hordijk, 2006). Although superoxide and other reactive oxygen species (ROS) are crucial mediators of many physiological responses to environmental cues (Forman et al., 2002), cellular ROS levels need to be tightly regulated. Uncontrolled ROS production is cytotoxic and known to contribute to retinal pathologies (Leslie, 2006; Komeima et al., 2007; Lambeth, 2007; Kang et al., 2009; Usui et al., 2009).
Crb is essential for photoreceptor cell morphogenesis (Izaddoost et al., 2002; Pellikka et al., 2002), and lack of Crb is associated with light-induced retinal degeneration (Johnson et al., 2002). Similarly, mutations in the human CRB1 gene, which encodes a Crb orthologue, cause degenerative retinal diseases such as retinitis pigmentosa and Leber congenital amaurosis (den Hollander et al., 1999; Gosens et al., 2008; Bulgakova and Knust, 2009). Therefore, it is of special clinical interest to elucidate the molecular mechanisms acting downstream of CRB1, for which Drosophila Crb represents a good model (den Hollander et al., 2001; Bulgakova and Knust, 2009). Here, we show that Crb limits Rac1–NADPH oxidase-dependent superoxide production to maintain epithelial organization and to prevent retinal degeneration.
Results and discussion
Crb controls ROS production in epithelia
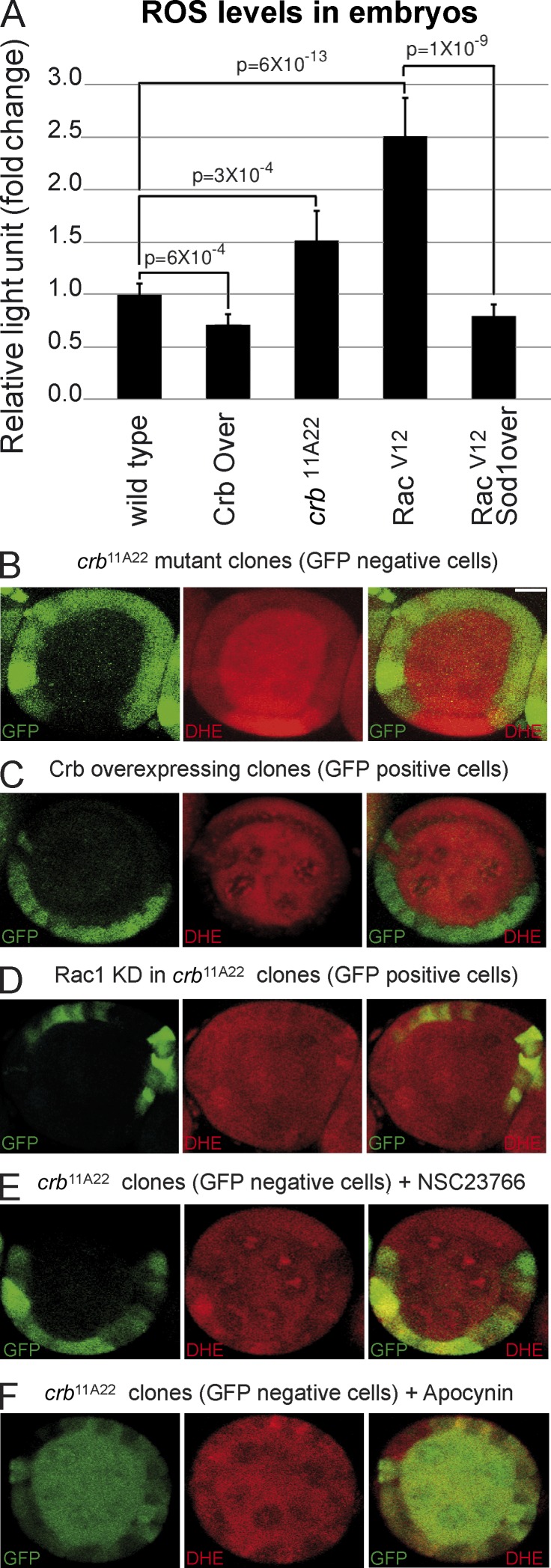
The recent discovery that Crb represses Rac1 activation raises the intriguing possibility that Crb controls superoxide production by the NADPH oxidase complex, which requires active Rac1 to function in mammals (Hordijk, 2006). In support of this hypothesis, we found that expression of an activated form of Rac1 (Rac1V12) in Drosophila embryos increased ROS production (Fig. 1 A). This Rac1V12-induced ROS overproduction is suppressed by overexpression of Superoxide dismutase 1 (Sod1; Fig. 1 A), which suggests that superoxide is the primary ROS produced downstream of Rac1. Indeed, Sod1 catalyses the first step of an enzymatic chain reaction, ultimately leading to conversion of superoxide to water and dioxygen, thus contributing to elimination of this highly reactive oxygen radical (Johnson and Giulivi, 2005). Similarly, crb mutant embryos showed an increased ROS production when compared with their wild-type counterparts, whereas overexpression of Crb in fly embryos reduced the amount of ROS (Fig. 1 A). These data show that Crb is a negative regulator of ROS production in embryonic tissues. To further explore the role of Crb in regulating oxidation in epithelial cells, we used mosaic analysis in the Drosophila follicular epithelium loaded with dihydroethidium (DHE), which becomes fluorescent upon oxidation (Afanasev, 2009). This approach allows for the comparison of mutant and normal cells within the same epithelium. DHE fluorescence was increased in crb homozygous mutant cells compared with neighboring crb/+ and wild-type cells (Fig. 1 B). In contrast, Crb overexpression strongly suppressed DHE fluorescence (Fig. 1 C), showing that Crb limits ROS production, not only in embryos, but also in adult epithelia. Knockdown of Rac1 or inhibition of its activation with NSC23766 suppressed the overproduction of ROS normally observed in crb mutant cells (compare Fig. 1, D and E, with Fig. 1 B; Fig. S1 B). Similar results were obtained with apocynin, which inhibits NADPH oxidase activity (Fig. 1 F). Together, these data propose that Crb limits superoxide levels through repression of Rac1 and NADPH oxidase activity in epithelial tissues.
Figure 1.
Crb controls ROS production through the inhibition of Rac1 and NADPH oxidase in epithelial tissues. (A) Monitoring of ROS production using lucigenin assay in wild-type embryos, Crb overexpressing embryos (Crb Over.), crb11A22 mutant embryos (null allele), Rac1V12-expressing embryos, and in embryos expressing Rac1V12 and overexpressing Sod1 (Sod1 over.). Error bars represent the 95% confidence level. (B) crb homozygous mutant clones were produced in adult crb/+ female flies (mutant clones are GFP negative). Ovaries were then dissected, incubated in the presence of the ROS-sensing DHE probe, and mounted for confocal microscopy analysis. (C) Crb was clonally overexpressed in the follicular epithelium, and ROS production was assessed using DHE and confocal microscopy. Crb overexpressing clones are positively marked with GFP. (D) Determination of ROS levels using DHE in crb mutant clones in which Rac1 was knocked down. (E and F) After induction of crb homozygous mutant clones in crb/+ female flies (mutant clones are GFP-negative), ovaries were incubated in the presence of the Rac1 activation inhibitor NSC23766 (E) or the NADPH oxidase complex inhibitor apocynin (F). Then, ROS levels were determined using DHE and confocal microscopy. Bar, 10 µm.
Counteraction of superoxide production by Crb maintains epithelial integrity in vivo
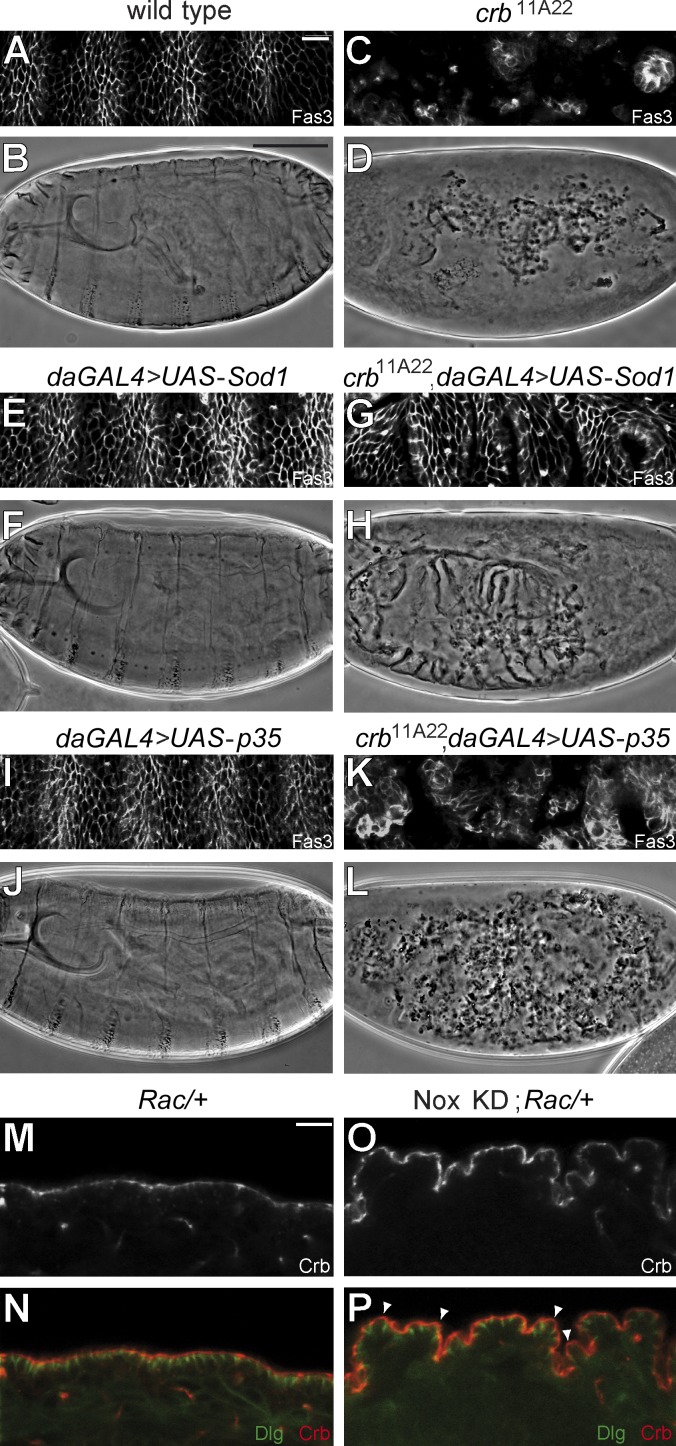
Deregulation of Rac1 signaling contributes to epithelial breakdown in the absence of Crb, as attenuation of Rac1 activity rescues the crb mutant phenotype (Chartier et al., 2011). To determine whether superoxide produced by NADPH oxidase is a Rac1 effector responsible for epithelial disorganization associated with Crb deficiency, we overexpressed Sod1 in crb mutant embryos (null allele crb11A22). Although absence of Crb is associated with epithelial collapse resulting in scattered cysts of epithelial cells and dispersed grains of cuticle (Fig. 2, C and D; Tepass et al., 1990), crb mutant embryos overexpressing Sod1 produced uninterrupted layers of well-organized epithelial tissues and continuous sheets of cuticle (Fig. 2, G and H). These data illustrate that superoxide overabundance in crb mutant embryos participates in epithelial tissue disintegration, and that limitation of superoxide production is a crucial function of Crb in maintaining epithelial tissue integrity in vivo.
Figure 2.
Repression of superoxide production by Crb is crucial for epithelial integrity. (A–L) Panels show a portion of the lateral epidermis of late-stage embryos immunostained for Fasciclin 3 (Fas3; A, C, E, G, I, and K) or a cuticle preparation (B, D, F, H, J, and L) for the following genotypes: wild-type (A and B), crb11A22 (null allele; C and D), daGAL4/UAS-Sod1 (overexpression of Sod1 in a wild-type background; E and F), crb11A22, daGAL4/crb11A22, UAS-Sod1 (overexpression of Sod1 in a crb11A22 mutant background; G and H), UAS-p35; daGAL4 (expression of BacA/p35 in a wild-type background; I and J), or UAS-p35; crb11A22/crb11A22, daGAL4 (expression of BacA/p35 in a crb11A22 mutant background; K and L). (M and N) Panels show Crb staining (M) or costaining of Crb and Dlg (N; red and green, respectively) in the ventral epidermis of a stage 16 Rac heterozygous mutant embryo (ptcGAL4/+; Rac1J11, Rac2Δ, MtlΔ/+). (O and P) Crb staining or staining of Crb (red) and Dlg (green) in the ventral epidermis of a stage 16 embryo knockdown for Nox and heterozygous for Rac (ptcGAL4/UAS-shRNA Nox; Rac1J11, Rac2Δ, MtlΔ/+). Bars: (A, C, E, G, I, and K) 10 µm; (B, D, F, H, J, and L) 100 µm; (M–P) 10 µm.
Under certain circumstances, superoxide and other ROS induce apoptosis (Ray et al., 2012), which is responsible for massive loss of epithelial cells in the absence of Crb (Tepass et al., 1990). Expression of the antiapoptotic BacA/p35 protein in crb mutant embryos increased the number of epithelial cell cysts and cuticle granules, but suppression of cell death had no impact on epithelial tissue organization (Fig. 2, K and L; Tanentzapf and Tepass, 2003). This implies that the rescue of the crb mutant phenotype resulting from overexpression of Sod1 is not simply the result of increased cell survival, which suggests a role for superoxide in epithelial polarity regulation. In accordance, reduction of superoxide production through the knockdown of the catalytic subunit of NADPH oxidase (Nox) in a Rac-sensitized background mimics loss of Rac1 activity and resulted in an irregular surface of the embryonic ectoderm caused by the elongated apical membrane of individual epithelial cells (Fig. 2, M, O, P, arrowheads; and Fig. S1 C). Thus, superoxide is an integral component of the molecular network regulating apical membrane size and epithelial tissue organization. Together, these data strongly argue that Rac1 acts as a member of the NADPH oxidase complex to sustain the antagonistic functional relationship with Crb, which is vital for a proper apical/basolateral ratio and epithelial cell architecture (Chartier et al., 2011). NADPH oxidase and superoxide likely contribute to the positive feedback loop linking Rac1 and PI3K, which supports lateral membrane stability and limits Crb-dependent apical membrane growth (Laprise et al., 2002; Gassama-Diagne et al., 2006; Jeanes et al., 2009; Chartier et al., 2011). Indeed, ROS are known inducers of PI3K signaling (Lee et al., 2002; Leslie et al., 2003; Kwon et al., 2004; Leslie, 2006), and such a redox-dependent positive feedback loop linking Rac1–NADPH oxidase and PI3K was recently shown to be important for polarization of migrating neutrophils (Kuiper et al., 2011). This suggests that this signaling module has a broad impact on cell polarity ranging from organization of the apical-basal axis to directional migration. Thus, our data describe a novel molecular function of Crb that is crucial for its role in organizing and maintaining epithelial tissue structure in vivo, namely the control of NADPH oxidase activity and superoxide production. Thereby, our results also identify superoxide as a critical regulator of epithelial tissue integrity.
Rac1, NADPH oxidase, and ROS are responsible for light-induced degeneration of Crb-deficient photoreceptor cells
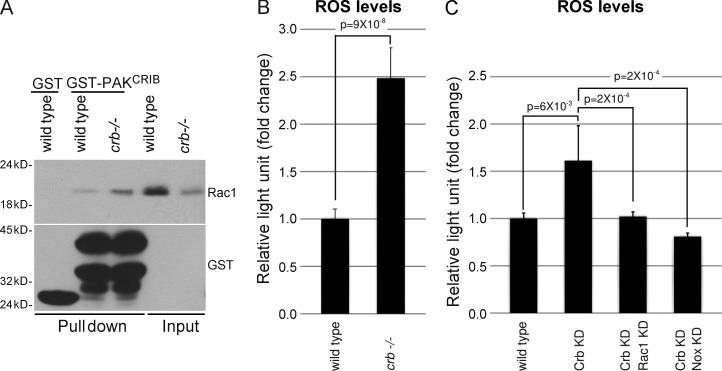
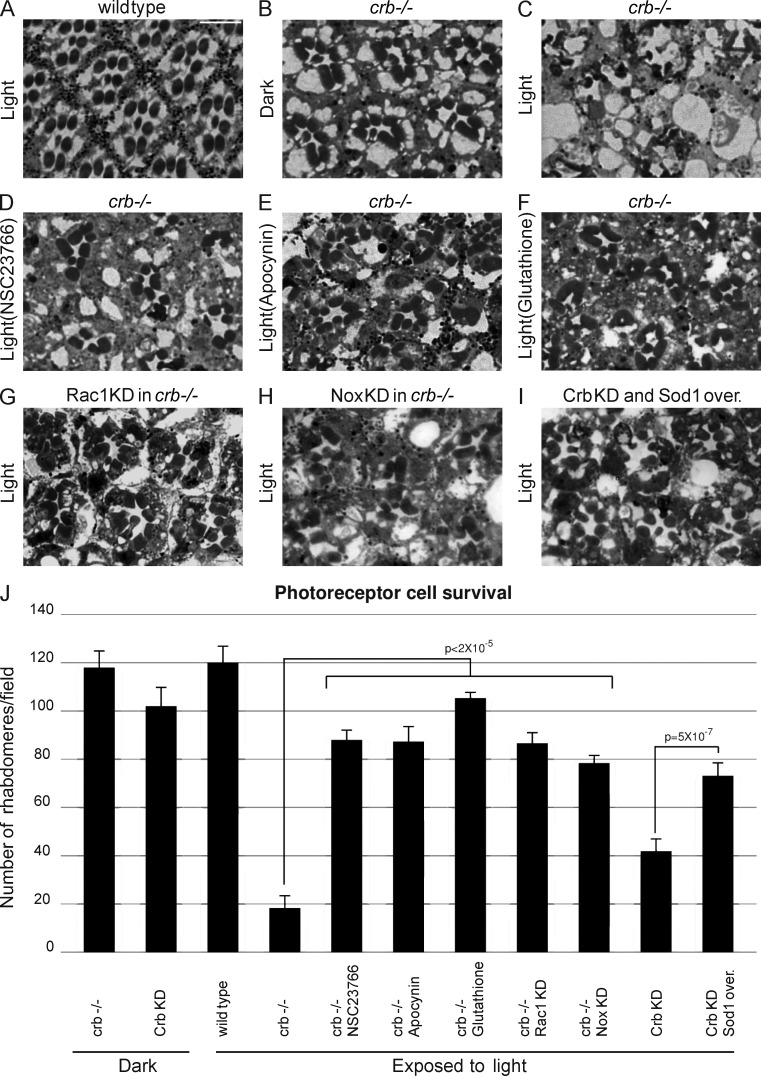
In addition to being involved in apical-basal polarity and the integrity of simple epithelia, Crb is required for survival of photoreceptor cells exposed to light (Johnson et al., 2002). Our data suggest that Crb may fulfill these two functions using common molecular mechanisms. Indeed, comparison of active Rac1 levels in wild-type heads and heads of flies carrying crb homozygous mutant retinas revealed an increased amount of GTP-bound Rac1 in Crb-deficient tissues, although Rac1 expression is consistently lower in crb mutant eyes (Fig. 3 A). Next, we found that crb mutant and Crb knockdown retinas showed an increased ROS production when compared with their wild-type counterparts (Fig. 3, B and C; and Fig. S2, A–E). This increase in ROS levels requires Rac1 and NADPH oxidase, as knockdown of either Rac1 or Nox suppressed ROS overabundance associated with the knockdown of Crb (Fig. 3 C). These data show that Crb is a negative regulator of Rac1–NADPH oxidase-dependent ROS production in photoreceptors, as we observed in epithelia (Fig. 1, A–C). Thus, control of this pathway seems to be a general function of Crb. This raises the intriguing possibility that Crb may counteract a Rac1–NADPH oxidase → superoxide pathway to limit oxidative stress and preserve cell viability after exposure to light. To explore this hypothesis, flies carrying crb mutant retinas were fed with NSC23766 or apocynin to inhibit Rac1 and NADPH oxidase, respectively, or with glutathione to quench cellular ROS. Notably, these molecules all blocked light-induced retinal degeneration in crb mutant eyes (compare Fig. 4, D–F, with Fig. 4 C). Quantification of photoreceptor cells revealed that improved cell survival is statistically significant (Fig. 4 J). In support of this drug-based approach, we observed that reduction of Rac1 or Nox levels using small hairpin RNAs (shRNAs), or lowering superoxide abundance by overexpression of Sod1, suppressed collapse of crb mutant or Crb knockdown retinas (compare Fig. 4, G and H, with Fig. 4 C; compare Fig. 4 I with Fig. S2 C; Fig. 4 J). Knockdown of the Rac1-related proteins Rac2 and Mtl had no impact on photoreceptor cell survival, which suggests a specific role for Rac1 in photoreceptor cell death associated with the loss of Crb (Fig. S2 F). Collectively, these data demonstrate that deregulation of the Rac1–NADPH oxidase pathway and the resulting superoxide overproduction are responsible for photoreceptor cell death in the absence of Crb.
Figure 3.
Crb represses Rac1 activation and ROS production in photoreceptor cells. (A) Heads from wild-type flies or flies carrying crb mutant retinas were homogenized. A portion of each homogenate was kept to monitor Rac1 expression levels (input), and GST-PAKCRIB was used to pull down active Rac1, which was detected by Western blotting. Native GST was used as a negative control. Western blotting controlled the amount of GST or GST-PAKCRIB used in each experiment. (B) Lucigenin assays were performed to compare ROS production in control and crb mutant retinas. (C) Comparison of ROS production in retinas after knockdown of Crb or concomitant knockdown of Crb and Rac1 or Crb and Nox. Error bars represent the 95% confidence level.
Figure 4.
Crb controls Rac1 and NADPH oxidase activity and ROS production to maintain retinal integrity. (A–I) Heads from flies maintained under constant illumination (light; A and C–I) or kept in total darkness (dark; B) were fixed, sectioned, and stained for light microscopy analysis. Panels show cross section of a wild-type retina (A), crb mutant (crb−/−) retinas (B and C), crb mutant retina of a fly fed with NSC23766 while submitted to light stress (D), crb mutant retina of a fly raised on a diet containing apocynin (E), crb mutant retina of a fly fed with glutathione (F), crb mutant retina knocked down for Rac1 (G), crb mutant retina in which Nox was knocked down (H), and a Crb knockdown retina in which Sod1 was overexpressed (Sod1 over.; I). Bar, 5 µm. (J) Rhabdomeres were counted on retinal cross sections to quantify the number of surviving photoreceptor cells after 7 d of constant illumination. Reduction of Rac1 activity using NSC23766 or knockdown (Rac1 KD) significantly increased the number of surviving photoreceptor cells in crb mutant retinas. Chemical inhibition of NADPH oxidase using apocynin or knockdown of Nox (Nox KD) had a similar effect. Finally, ROS quenching with glutathione or superoxide detoxification by Sod1 overexpression improved cell fitness in crb mutant or Crb knockdown retinas. Error bars represent the 95% confidence level.
It has been previously suggested that Rhodopsin, which initiates the phototransduction cascade, participates in light-induced degeneration of crb mutant retinas (Johnson et al., 2002; Pocha et al., 2011). Crb influences Rhodopsin transport through stabilization of Myosin V, which could thus act downstream of Crb to sustain photoreceptor cell survival (Pocha et al., 2011). Data from the literature suggest that the role of Rhodopsin in Crb-deficient photoreceptor cell death might be linked to the Rac1–NADPH oxidase pathway. Indeed, Rac1 operates downstream of Rhodopsin in Drosophila and mammals (Chang and Ready, 2000; Balasubramanian and Slepak, 2003). Consequently, Rac1 is activated by light (Balasubramanian and Slepak, 2003; Belmonte et al., 2006), which triggers relapse of crb mutant retinas (Johnson et al., 2002). Thus, it is plausible that Crb fulfills a protective role during light exposure by limiting Rac1–NADPH oxidase complex activity and oxidative damage downstream of light-activated Rhodopsin. Overall, we identified several key players promoting retinal degeneration in the absence of Crb, a finding with great potential for the clinical setting. Indeed, mutations in the human CRB1 gene cause diverse retinal dystrophies (den Hollander et al., 1999; Gosens et al., 2008; Bulgakova and Knust, 2009). Crb controls retinal stability and epithelial organization through the modulation of Rac1–NADPH oxidase activity (this study and Chartier et al., 2011). Human CRB1 can substitute for Drosophila Crb in vivo to promote epithelial integrity (den Hollander et al., 2001), strongly arguing that CRB1 also regulates Rac1–NADPH oxidase-dependent signaling. It is imperative to test this premise, as it might highlight several therapeutic approaches to treat patients with mutations in CRB1 gene. Prevention of oxidative stress with antioxidant molecules and/or strategies to counteract Rac1 and NADPH oxidase in retinas would be promising avenues. Of note, reduced Rac1 expression in rods has a beneficial effect on cell survival after intense light exposure without compromising retinal structure or function, and the NADPH oxidase inhibitor apocynin was already used in vivo to prevent death of photoreceptor cells (Williams and Griendling, 2007; Haruta et al., 2009; Usui et al., 2009).
In conclusion, we defined a signaling pathway inhibited by Crb supporting its function in epithelial organization and prevention of retinal degeneration. In epithelial cells, Crb-dependent inhibition of Rac1 limits NADPH oxidase and PI3K activation to preserve epithelial tissue integrity (this study and Chartier et al., 2011). In retinas, loss of the inhibitory action of Crb coupled to light, a known activator of Rac1 (Balasubramanian and Slepak, 2003; Belmonte et al., 2006), results in ROS accumulation and photoreceptor cell death. Thereby, our study contributes to illuminate the molecular mechanisms regulating epithelial polarity, a crucial aspect of epithelial tissue morphogenesis and physiology in most organs. Our results may also serve as a basis to establish therapeutic strategies to fight retinal dystrophies resulting from mutations in CRB1.
Materials and methods
Drosophila genetics and clonal analysis
Mutant allele and transgenic lines used in this study were: crb11A22, UAS-RacV12, UAS-crbwt2e, Rac1J11, Rac2Δ, MtlΔ, UAS-p35, UAS-Sod1, da-GAL4, enGAL4, GMR-GAL4, P{GD14463}v39177 (shRNA directed against crb), P{TRiP.JF02813}attP2 (shRNA directed against Rac1), and P{KK111991}VIE-260B (shRNA directed against Nox; all alleles and transgenic lines are described in Flybase). Using these lines, the following recombinant chromosomes were generated: (a) UAS-RacV12, UAS-Sod1; (b) GMR-GAL4, P{GD14463}v39177; (c) crb11A22, UAS-Sod1; and (d) FRT82B, crb11A22, P{TRiP.JF02813}attP2. Sod1 and BacA/p35 were expressed in embryos by crossing the corresponding upstream activating sequence (UAS) lines with da-GAL4 flies at 25°C. Sod1 was overexpressed in crb mutant embryos by crossing crb11A22, da-GAL4 flies to crb11A22, UAS-Sod1 flies at 25°C. Crb was stably knocked down in the retina of GMR-GAL4, P{GD14463}v39177 flies. These animals were crossed to the UAS-Sod1 strain to overexpress Sod1 in Crb-deficient retinas.
Mutant clones homozygous for crb were produced in the follicular epithelium using the Flippase recognition target (FRT)/Flippase (FLP) system in which the FLP recombinase was under the control of a heat shock promoter (hsFLP). Newly hatched w*, hsFLP/+; FRT82B, crb11A22/FRT82B, ubi-GFP, or hsFLP/+; Act5C(−FRT)GAL4.Switch, UAS-GFP/+; FRT82B, tub-Gal80/FRT82B, crb11A22, P{TRiP.JF02813}attP2 females were heat-shocked three times for 2 h at 37°C at 24-h intervals. 24 h after the third heat shock, ovaries were dissected and processed for determination of ROS levels (described in a following section). To generate Crb overexpressing clones, the same protocol was applied to flies of the following genotype: hsFLP, y1, w*/+; UAS-crbwt2e/UAS-GFP; Act5C(-FRT)GAL4.Switch/+.
Crb mutant whole eye clones were produced using the FRT/FLP system as described previously (Pellikka et al., 2002). The genotype of flies in which clones were induced was: y1, w*; eyeless-GAL4, UAS-FLP1/+; FRT82B, GMR-hid, l(3)CL-R[1]/FRT82B, crb11A22.
Knockdown of Rac1 or Nox in crb mutant photoreceptors was achieved by crossing yd2, w1118, eyFLP; GMR-GAL4/CyO; FRT82B, l(3)cl-R31/TM3 with P{KK111991}VIE-260B; FRT82B, crb11A22/TM3 or with FRT82B, crb11A22, P{TRiP.JF02813}attP2/TM6B.
Crb mutant embryos used for lucigenin assays were obtained from crb germ line clone females (P{ry[+t7.2]=hsFLP}1, y[1] w[1118]; FRT82B, P{ovoD1-18}3R/FRT82B, crb11A22) that were heat shocked three times for 2 h at 37°C as second and third instar larvae and crossed to crb/TM3 males.
Immunofluorescence
Fixation protocols for embryos and ovaries were based on methods described previously (Tepass et al., 1990; Tanentzapf et al., 2000). In brief, dechorionated embryos were fixed for 10 min in a 15% formaldehyde solution under a heptane phase. The aqueous phase was then replaced by methanol, and strong agitation was performed to remove the vitelline membrane. Devitellinized embryos were further incubated in methanol for 15 min. Ovaries were fixed for 20 min in 4% formaldehyde solution and incubated for 5 min in methanol. Anti–Fasciclin 3 (Fas3; 7G10) and anti-Dlg (4F3) were from Developmental Studies Hybridoma Bank, and were used at a dilution of 1:50. Anti-Crb was diluted 1:500 (Pellikka et al., 2002). Secondary antibodies were conjugated to Cy3 (Jackson ImmunoResearch Laboratories, Inc.) or Alexa Fluor 488 (1:400 dilution; Molecular Probes).
Cuticle preparation
Embryos were dechorionated in 2% sodium hypochlorite, mounted in Hoyer’s mounting media/lactic acid (50:50), and incubated at 85°C overnight.
Western blotting
Isolated fly heads or dechorionated embryos were homogenized in lysis buffer (1% Triton X-100, 150 mM NaCl, 1 mM EDTA, 40 mM Tris, pH 7.6, 0.1 mM phenylmethylsulfonyl fluoride, 10 µg/ml leupeptin, 1 µg/ml pepstatin, 10 µg/ml aprotinin, 0.1 mM orthovanadate, and 40 mM β-glycerophosphate) and processed for SDS-PAGE followed by electrotransfer on nitrocellulose membranes. Membranes were blocked in PBS containing 5% nonfat powdered milk and 0.05% Tween 20 for 1 h at room temperature. Incubation with primary antibodies was performed overnight at 4°C (diluted in blocking solution), whereas secondary antibodies were incubated for 1 h at room temperature. Primary antibodies used were: rat anti-Crb (1:3,000; Pellikka et al., 2002), mouse anti-Rac1 (clone 102; 1:1,000; BD), rabbit anti-Akt (1:1,000; Cell Signaling Technology), mouse anti-Actin (1:10,000; EMD Millipore), and rabbit anti-GST (1:10,000; provided by J.-Y. Masson, Laval University, Quebec City, Canada). HRP-conjugated secondary antibodies were from GE Healthcare and used at a 1:1,000 dilution.
Determination of Rac1 activation levels
Fly heads were homogenized in ice-cold lysis buffer (25 mM Hepes, pH 7.5, 1% Nonidet P-40, 10 mM MgCl2, 100 mM NaCl, 5% glycerol, 0.1 mM sodium orthovanadate, 0.1 mM phenylmethylsulfonyl fluoride, 5 mM sodium fluoride, 10 µg/ml aprotinin, and 10 µg/ml leupeptin; Picard et al., 2009). 50 µg of head lysates were kept to measure the total amount of Rac1 in each sample. Then, active Rac1 was pulled down from 1 mg of the same samples with glutathione-Sepharose beads coupled to 20 µg of the Cdc42/Rac interactive binding (CRIB) domain of p21-activated kinase (PAK) fused to GST (45 min, 4°C). GST loaded on Sepharose beads was used as a negative control. Beads were then washed twice with ice-cold wash buffer (25 mM Hepes, pH 7.5, 40 mM NaCl, 30 mM MgCl2, 0.5% Nonidet P-40, and 1 mM DTT). Active Rac1 levels or the total amount of Rac1 present in head extracts was analyzed by Western blotting.
Determination of ROS levels
Determination of NADPH oxidase activity.
Embryos or fly heads were crushed with a glass homogenizer in suspension buffer (250 mM Hepes, pH 7.6, 120 mM NaCl, 5.9 mM KCl, 1.2 mM MgSO4, 1.75 mM MgCl2, 11 mM glucose, and 0.5 mM EDTA) and further lysed with a constant cell disruptor (Constant Systems Ltd.) at 27,000 psi. Homogenates were cleared by centrifugation at 3,500 g for 5 min. After addition of NADPH and dark-adapted lucigenin (final concentration of 100 µM and 50 µM, respectively), NADPH oxidase activity was monitored in supernatants with a luminometer (Glomax 20/20; Promega). Recorded signals were normalized to protein amount in each sample (determined with a bicinchoninic acid protein assay; Thermo Fisher Scientific). Histogram bars represent mean values. Error bars represent 95% confidence interval. A Wilcoxon rank test was used to assess the statistical significance of experiments.
DHE fluorescence.
Ovaries were dissected and loaded for 5 min with 20 µM DHE dissolved in Shields and Sang M3 insect (M3) medium (Invitrogen) supplemented with 2% FBS, 2.5% fly extract, and 0.0125 IU/ml insulin (referred to as complete M3 medium). Ovaries were then washed three times with complete M3 medium and mounted in Vectashield (Vector Laboratories) for confocal microscopy observation. Where indicated, ovaries were treated for 15 min with 200 µM NSC23766 or 500 µM apocynin before incubation with DHE. These inhibitors were maintained for the remaining steps of the protocol. At least 22 clones were observed for each condition.
Light-stress experiments
Newly hatched flies were exposed to constant illumination (800 lux) or kept in total darkness at 25°C for 7 d. Drosophila eyes were then fixed (Pellikka et al., 2002), embedded in Spurr’s resin, sectioned, and stained (0.025% toluidine blue, 0.1% methylene blue, and 45% methanol). Where indicated, flies were fed with a 5% sucrose solution (absorbed on Whatman paper) or a 5% sucrose solution supplemented with 220 µM glutathione, 10 mM Apocynin, or 500 µM NSC23766 (chemicals were given to flies 3 d before light stress and supplied fresh daily during the entire experiment). Drosophila eyes were then processed as described for light microscopy.
Quantification of photoreceptor cell survival.
Pictures of semithin sections of fly retina taken with the 100× lens (from at least three independent flies) were used for rhabdomere quantification. A Student’s t test was performed to assess the statistical significance.
Primers
Primers used were: actin 5C (forward, 5′-AGCCGCTCCATCAGCCAGC-3′; reverse, 5′-TGTCGACAACCAGAGCAGCAACT-3′); Nox (forward, 5′-GCATCGGTACTGGAAAGCTC-3′; reverse, 5′-GACAGCAGATTGACGAACCA-3′).
Microscope image acquisition
Confocal images were acquired with a confocal microscope system (FV1000; Olympus) and the Fluoview 3.0 acquisition software. GFP expressing or Cy3- and Alexa Fluor 488–labeled specimens were mounted in Vectashield (Vector Laboratories) and imaged at room temperature using a 40× UPLS Apochromat lens (NA 0.90). Cuticle preparations were mounted in Hoyer’s mounting media/lactic acid (50:50), and pictures were acquired with a camera (CoolSNAP fx; Photometrics) and acquisition software (MetaVue 7.7.7; Molecular Devices) coupled to a microscope (Eclipse 600; Nikon; 20× Plan fluor lens; NA 0.50). Retinal sections were mounted in permount and imaged using the same system as for cuticle preparations, except that a 100× Plan fluor lens was used (NA 1.20). Some images were uniformly processed in Photoshop (Adobe) using the brightness/contrast and unsharp mask tools.
Online supplemental material
Fig. S1 shows that the expression of Rac1V12 is not affected by concomitant overexpression of Sod1 in embryos, and shows validation of Rac1 and Nox knockdown. Fig. S2 shows that Crb knockdown is efficient and that knockdown of Rac2 or Mtl had no impact on survival of Crb-knockdown photoreceptor cells. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201203083/DC1.
Supplementary Material
Acknowledgments
We are grateful to Y. Coulombe for technical support and to E. Paquet for statistical analysis. We thank U. Tepass, A. Wodarz, E. Knust, N. Lamarche-Vanne, J.-Y. Masson, the Bloomington Drosophila Stock Center, the Developmental Studies Hybridoma Bank, and the Vienna Drosophila RNAi Center for reagents. Sectioning of retinas was performed at Centre de recherche Université Laval Robert-Giffard EM facility.
This work was supported by an operating grant from the Canadian Institute of Health Research (CIHR) to P. Laprise (200903MOP-199572-CP-CFBA), who is a CIHR new investigator (177372). F. Chartier was supported by a postdoctoral fellowship from CIHR.
Footnotes
Abbreviations used in this paper:
- Crb
- Crumbs
- DHE
- dihydroethidium
- FLP
- Flippase
- FRT
- Flippase recognition target
- PAK
- p21-activated kinase
- PI3K
- phosphoinositide 3-kinase
- ROS
- reactive oxygen species
- shRNA
- small hairpin RNA
- Sod1
- Superoxide dismutase 1
- UAS
- upstream activating sequence
References
- Afanasev I. 2009. Detection of superoxide in cells, tissues and whole organisms. Front Biosci (Elite Ed). 1:153–160 [DOI] [PubMed] [Google Scholar]
- Balasubramanian N., Slepak V.Z. 2003. Light-mediated activation of Rac-1 in photoreceptor outer segments. Curr. Biol. 13:1306–1310 10.1016/S0960-9822(03)00511-6 [DOI] [PubMed] [Google Scholar]
- Belmonte M.A., Santos M.F., Kihara A.H., Yan C.Y., Hamassaki D.E. 2006. Light-Induced photoreceptor degeneration in the mouse involves activation of the small GTPase Rac1. Invest. Ophthalmol. Vis. Sci. 47:1193–1200 10.1167/iovs.05-0446 [DOI] [PubMed] [Google Scholar]
- Bilder D., Schober M., Perrimon N. 2003. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat. Cell Biol. 5:53–58 10.1038/ncb897 [DOI] [PubMed] [Google Scholar]
- Bulgakova N.A., Knust E. 2009. The Crumbs complex: from epithelial-cell polarity to retinal degeneration. J. Cell Sci. 122:2587–2596 10.1242/jcs.023648 [DOI] [PubMed] [Google Scholar]
- Chang H.Y., Ready D.F. 2000. Rescue of photoreceptor degeneration in rhodopsin-null Drosophila mutants by activated Rac1. Science. 290:1978–1980 10.1126/science.290.5498.1978 [DOI] [PubMed] [Google Scholar]
- Chartier F.J., Hardy E.J., Laprise P. 2011. Crumbs controls epithelial integrity by inhibiting Rac1 and PI3K. J. Cell Sci. 124:3393–3398 10.1242/jcs.092601 [DOI] [PubMed] [Google Scholar]
- den Hollander A.I., ten Brink J.B., de Kok Y.J., van Soest S., van den Born L.I., van Driel M.A., van de Pol D.J., Payne A.M., Bhattacharya S.S., Kellner U., et al. 1999. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat. Genet. 23:217–221 10.1038/13848 [DOI] [PubMed] [Google Scholar]
- den Hollander A.I., Johnson K., de Kok Y.J., Klebes A., Brunner H.G., Knust E., Cremers F.P. 2001. CRB1 has a cytoplasmic domain that is functionally conserved between human and Drosophila. Hum. Mol. Genet. 10:2767–2773 10.1093/hmg/10.24.2767 [DOI] [PubMed] [Google Scholar]
- Forman H.J., Torres M., Fukuto J. 2002. Redox signaling. Mol. Cell. Biochem. 234–235:49–62 10.1023/A:1015913229650 [DOI] [PubMed] [Google Scholar]
- Gassama-Diagne A., Yu W., ter Beest M., Martin-Belmonte F., Kierbel A., Engel J., Mostov K. 2006. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat. Cell Biol. 8:963–970 10.1038/ncb1461 [DOI] [PubMed] [Google Scholar]
- Gosens I., den Hollander A.I., Cremers F.P., Roepman R. 2008. Composition and function of the Crumbs protein complex in the mammalian retina. Exp. Eye Res. 86:713–726 10.1016/j.exer.2008.02.005 [DOI] [PubMed] [Google Scholar]
- Haruta M., Bush R.A., Kjellstrom S., Vijayasarathy C., Zeng Y., Le Y.Z., Sieving P.A. 2009. Depleting Rac1 in mouse rod photoreceptors protects them from photo-oxidative stress without affecting their structure or function. Proc. Natl. Acad. Sci. USA. 106:9397–9402 10.1073/pnas.0808940106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordijk P.L. 2006. Regulation of NADPH oxidases: the role of Rac proteins. Circ. Res. 98:453–462 10.1161/01.RES.0000204727.46710.5e [DOI] [PubMed] [Google Scholar]
- Izaddoost S., Nam S.C., Bhat M.A., Bellen H.J., Choi K.W. 2002. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature. 416:178–183 10.1038/nature720 [DOI] [PubMed] [Google Scholar]
- Jeanes A., Smutny M., Leerberg J.M., Yap A.S. 2009. Phosphatidylinositol 3′-kinase signalling supports cell height in established epithelial monolayers. J. Mol. Histol. 40:395–405 10.1007/s10735-010-9253-y [DOI] [PubMed] [Google Scholar]
- Johnson F., Giulivi C. 2005. Superoxide dismutases and their impact upon human health. Mol. Aspects Med. 26:340–352 10.1016/j.mam.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Johnson K., Grawe F., Grzeschik N., Knust E. 2002. Drosophila crumbs is required to inhibit light-induced photoreceptor degeneration. Curr. Biol. 12:1675–1680 10.1016/S0960-9822(02)01180-6 [DOI] [PubMed] [Google Scholar]
- Kang K.H., Lemke G., Kim J.W. 2009. The PI3K-PTEN tug-of-war, oxidative stress and retinal degeneration. Trends Mol. Med. 15:191–198 10.1016/j.molmed.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeima K., Rogers B.S., Campochiaro P.A. 2007. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J. Cell. Physiol. 213:809–815 10.1002/jcp.21152 [DOI] [PubMed] [Google Scholar]
- Kuiper J.W., Sun C., Magalhães M.A., Glogauer M. 2011. Rac regulates PtdInsP3 signaling and the chemotactic compass through a redox-mediated feedback loop. Blood. 118:6164–6171 10.1182/blood-2010-09-310383 [DOI] [PubMed] [Google Scholar]
- Kwon J., Lee S.R., Yang K.S., Ahn Y., Kim Y.J., Stadtman E.R., Rhee S.G. 2004. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. USA. 101:16419–16424 10.1073/pnas.0407396101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth J.D. 2007. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic. Biol. Med. 43:332–347 10.1016/j.freeradbiomed.2007.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprise P., Tepass U. 2011. Novel insights into epithelial polarity proteins in Drosophila. Trends Cell Biol. 21:401–408 10.1016/j.tcb.2011.03.005 [DOI] [PubMed] [Google Scholar]
- Laprise P., Chailler P., Houde M., Beaulieu J.F., Boucher M.J., Rivard N. 2002. Phosphatidylinositol 3-kinase controls human intestinal epithelial cell differentiation by promoting adherens junction assembly and p38 MAPK activation. J. Biol. Chem. 277:8226–8234 10.1074/jbc.M110235200 [DOI] [PubMed] [Google Scholar]
- Laprise P., Beronja S., Silva-Gagliardi N.F., Pellikka M., Jensen A.M., McGlade C.J., Tepass U. 2006. The FERM protein Yurt is a negative regulatory component of the Crumbs complex that controls epithelial polarity and apical membrane size. Dev. Cell. 11:363–374 10.1016/j.devcel.2006.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprise P., Lau K.M., Harris K.P., Silva-Gagliardi N.F., Paul S.M., Beronja S., Beitel G.J., McGlade C.J., Tepass U. 2009. Yurt, Coracle, Neurexin IV and the Na(+),K(+)-ATPase form a novel group of epithelial polarity proteins. Nature. 459:1141–1145 10.1038/nature08067 [DOI] [PubMed] [Google Scholar]
- Laprise P., Paul S.M., Boulanger J., Robbins R.M., Beitel G.J., Tepass U. 2010. Epithelial polarity proteins regulate Drosophila tracheal tube size in parallel to the luminal matrix pathway. Curr. Biol. 20:55–61 10.1016/j.cub.2009.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.R., Yang K.S., Kwon J., Lee C., Jeong W., Rhee S.G. 2002. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 277:20336–20342 10.1074/jbc.M111899200 [DOI] [PubMed] [Google Scholar]
- Leslie N.R. 2006. The redox regulation of PI 3-kinase-dependent signaling. Antioxid. Redox Signal. 8:1765–1774 10.1089/ars.2006.8.1765 [DOI] [PubMed] [Google Scholar]
- Leslie N.R., Bennett D., Lindsay Y.E., Stewart H., Gray A., Downes C.P. 2003. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 22:5501–5510 10.1093/emboj/cdg513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.S., Lee S.H., Park D., Lee J.S., Ryu S.H., Lee W.J., Rhee S.G., Bae Y.S. 2004. Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol. Cell. Biol. 24:4384–4394 10.1128/MCB.24.10.4384-4394.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellikka M., Tanentzapf G., Pinto M., Smith C., McGlade C.J., Ready D.F., Tepass U. 2002. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 416:143–149 10.1038/nature721 [DOI] [PubMed] [Google Scholar]
- Picard M., Petrie R.J., Antoine-Bertrand J., Saint-Cyr-Proulx E., Villemure J.F., Lamarche-Vane N. 2009. Spatial and temporal activation of the small GTPases RhoA and Rac1 by the netrin-1 receptor UNC5a during neurite outgrowth. Cell. Signal. 21:1961–1973 10.1016/j.cellsig.2009.09.004 [DOI] [PubMed] [Google Scholar]
- Pirraglia C., Walters J., Myat M.M. 2010. Pak1 control of E-cadherin endocytosis regulates salivary gland lumen size and shape. Development. 137:4177–4189 10.1242/dev.048827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocha S.M., Shevchenko A., Knust E. 2011. Crumbs regulates rhodopsin transport by interacting with and stabilizing myosin V. J. Cell Biol. 195:827–838 10.1083/jcb.201105144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P.D., Huang B.W., Tsuji Y. 2012. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24:981–990 10.1016/j.cellsig.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan M., Yu Z.X., Ferrans V.J., Sulciner D.J., Gutkind J.S., Irani K., Goldschmidt-Clermont P.J., Finkel T. 1996. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem. J. 318:379–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G., Tepass U. 2003. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat. Cell Biol. 5:46–52 10.1038/ncb896 [DOI] [PubMed] [Google Scholar]
- Tanentzapf G., Smith C., McGlade J., Tepass U. 2000. Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J. Cell Biol. 151:891–904 10.1083/jcb.151.4.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U., Theres C., Knust E. 1990. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 61:787–799 10.1016/0092-8674(90)90189-L [DOI] [PubMed] [Google Scholar]
- Tepass U., Tanentzapf G., Ward R., Fehon R. 2001. Epithelial cell polarity and cell junctions in Drosophila. Annu. Rev. Genet. 35:747–784 10.1146/annurev.genet.35.102401.091415 [DOI] [PubMed] [Google Scholar]
- Usui S., Oveson B.C., Lee S.Y., Jo Y.J., Yoshida T., Miki A., Miki K., Iwase T., Lu L., Campochiaro P.A. 2009. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J. Neurochem. 110:1028–1037 10.1111/j.1471-4159.2009.06195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H.C., Griendling K.K. 2007. NADPH oxidase inhibitors: new antihypertensive agents? J. Cardiovasc. Pharmacol. 50:9–16 10.1097/FJC.0b013e318063e820 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.