Abstract
The presence of viable but nonculturable bacteria in human clean-catch and mouse bladder-isolated urine specimens was investigated. Viable but nonculturable bacteria are alive but do not give rise to visible growth under nonselective growth conditions. Urine specimens obtained from human female volunteers with or without an active urinary tract infection were found to contain, on average, significantly more viable than culturable forms of bacteria. Additional support for the presence of viable but nonculturable cells in urine specimens considered sterile was obtained from examination of urine specimens obtained directly from the bladder of healthy mice. Because the viability assay used to study the viable but nonculturable condition is by necessity growth independent, and hence indirect, the accuracy of this assay that scores cells with intact cell membranes as being viable was studied. Greater than 95% of Escherichia coli cells exposed to lethal doses of UV irradiation were found to lose their membrane integrity within a day, a time frame similar to that used to examine urine specimens. These data suggest that viable but nonculturable cells can occur within regions of the urinary tract previously considered sterile.
Urinary tract infections (UTIs) affect as many as 50% of women at least once during their lifetime (29, 32), and 25% of those who acquire a UTI will have another infection within the following 6 months (17). A UTI occurs when the urinary tract is infected with microorganisms, and uropathogenic Escherichia coli accounts for greater than 80% of all UTI cases (4, 30). One method of diagnosing a UTI is by culturing urine specimens; a threshold of 100,000 CFU/ml in clean-catch urine specimens is considered to indicate a UTI (4, 28). This threshold is not an absolute indicator, as both asymptomatic bacteriuria and patients with UTI symptoms having no culturable urine bacteria occur (29, 32).
Urine within the urinary tract is generally considered sterile (14). This conclusion is based upon a lack of culturable cells present in urine specimens obtained via clean-catch and catheterization methods. The presence of viable bacteria in the urine specimens of healthy patients would impact on hypotheses to explain recurrent UTIs as well as diagnostic procedures. Most recurrent UTIs result from reinfection; however, a higher percentage than would be expected by chance are caused by the index strain (6, 18, 26, 27, 41). The physical location and physiological status of index strain cells that remain after successful antibiotic therapy are unknown. Observations with a mouse model indicate that uropathogenic E. coli cells can remain in the urinary tract following antibiotic therapy (33, 39, 44). It has been proposed that the bladder epithelium can act as a persistent reservoir for “quiescent” uropathogenic E. coli bacteria, which, when reentering an active replicative state, can lead to an infection (42, 43). We hypothesized that recurrent infections could be caused by index strain cells escaping disease treatment and subsequent detection by entering the quiescent-like viable but nonculturable condition in vivo (39).
The viable but nonculturable state is defined as one in which cells are viable yet do not undergo sufficient division to give rise to visible growth on nonselective growth medium (9, 10, 11). The viable but nonculturable condition has been reported to occur in many gram-negative bacteria, including human-pathogenic E. coli (19). Cells are induced to enter the viable but nonculturable state by one or a combination of environmental stresses, such as starvation, temperature shift, and exposure to a heavy metal (1, 19). Assays used to document the viability of viable but nonculturable cells must be growth independent. The most common microscope-based assays measure cell metabolic activity, such as through observation of chemical reduction of redox dyes (40) or the presence of an intact cell membrane (22). What distinguishes the viable but nonculturable state from similar bacterial cell responses such as cell stress, cell starvation, and cell wounding are that viable but nonculturable cells can remain in this condition for long periods of time and only viable but nonculturable cells do not give rise to visible growth when placed under nonselective growth conditions. By proteome analysis, the viable but nonculturable state has recently been shown to be mechanistically distinct from the starvation response (23). The ability of viable but nonculturable cells to regain the ability to grow, termed resuscitation, has been documented; for resuscitation of E. coli alone, see references 13, 21, 31, and 34.
The aim of this study was to characterize the physiological status of bacteria found in clean-catch urine specimens obtained from human females and bladder-isolated urine specimens from mice as a first step in examining the role of the viable but nonculturable state in persistent UTI infections.
MATERIALS AND METHODS
Human urine specimens.
Urine specimens from females infected with a UTI were collected from patients of Concord Urgent Care (Concord, N.C.), Eastland Urgent Care (Charlotte, N.C.), and Brocker Health Center (University of North Carolina-Charlotte), as well as from volunteers of the University of North Carolina-Charlotte campus. Participants provided a self-caught midstream voided specimen after cleansing with a towelette and completed a questionnaire detailing their UTI history. Dipstick and microscopic assays were performed on each sample within 24 h of sample collection. For UTI patients, urine specimens were collected at the initial clinic visit, prior to initiation of antibiotic therapy. Non-UTI urine specimens included in this study were obtained from uninfected females who had not engaged in vaginal intercourse within the previous 72 h. When not examined directly after collection, urine specimens were stored at 5°C.
The Institutional Review Board Committees at the University of North Carolina at Charlotte and the Carolinas HealthCare System approved this study as described in Institutional Review Board protocols 99-08-03 (University of North Carolina-Charlotte) and 08-99-26E (Carolinas HealthCare System).
Isolation of urine specimens from mouse bladders.
Four- to seven-week-old female BALB/c mice (Charles River Laboratories, Research Triangle Park, N.C.) were used in all studies. Mice housed in cages were allowed food pellets and water ad libitum and were exposed to 12-h alternating light and dark cycles.
Mice were anesthetized with 2.5% Avertin (0.015 ml/g of weight), and the fur was washed and soaked with iodine. The skin was then cut over the bladder and resected. The abdominal muscles were resected to expose the bladder. The bladder was then rinsed with 70% ethanol prior to inserting a 27-gauge 0.5-in. needle into the bladder and withdrawing the urine specimens into a 1-ml syringe. The mice were then euthanized by cervical dislocation prior to anesthesia recovery.
The Institutional Animal Care and Use Committee at the University of North Carolina at Charlotte approved all experiments involving animal use in this study as described in protocol 98/99-03.
Culturability assay.
For urine analysis, 50-μl samples of undiluted urine specimens and various dilutions were spot plated in triplicate onto two sets of nonselective Luria-Bertani (LB) and brain heart infusion (BHI) media. For urine specimens from five noninfected females, samples were also plated on sheep's blood agar (SBA). For mouse urine specimens, the volume was recorded and then, if needed, increased to 500 μl with 0.9% NaCl. Each plate set was then placed in a 5% CO2 incubator and an aerobic incubator at 37°C and scored at 24 h and again after either 48 or 72 h. No significant difference was observed between the two time points. The highest CFU counts obtained from the various growth conditions were used in data analysis. BHI with incubation in 5% CO2 consistently yielded the highest number of CFU from human urine specimens; for mouse bladder urine specimens, there was no significant difference in CFU counts among the different media.
As described in the text, for a subset of samples growth was examined under a wider range of conditions. Those conditions were growth on LB, BHI, and SBA (blood agar) medium, incubated under anaerobic, 5% CO2, and anaerobic conditions (with a GasPak bell jar system).
For UV-irradiated E. coli cells, various sample dilutions were spot plated in triplicate onto LB plates and incubated aerobically and scored as described above.
Bacterial identification from human urine specimens.
Random colonies from human UTI samples were morphologically characterized and inoculated into Enterotube II multimedia tubes (Becton Dickinson, Cockeysville, Md.) for the rapid identification of aerobic, facultative anaerobic gram-negative rods. E. coli was the predominant species in the urine specimens of all UTI patients.
Viability assay.
The BacLight LIVE/DEAD bacterial viability kit (Molecular Probes, Inc., Eugene, Oreg.) differentiates viable cells with an intact cell membrane (i.e., viable) from those with a compromised membrane (i.e., dead) based on the differential permeability of two fluorescing dyes. This assay was used as per the manufacturer's instructions. Briefly, 1 ml of each sample was incubated with the kit reagents (with 1 μl of reagent A and 3 μl of reagent B) in the dark for 30 to 60 min and then collected onto 0.22-μm-pore-size black polycarbonate filters (Osmonics Inc., Livermore, Calif.), washed with 5 ml of 0.9% NaCl, and examined with an Olympus BX60 epifluorescent microscope utilizing a HBO103W/2 Mercury burner. The number of both red (i.e., dead) and green (i.e., viable) rod-shaped cells per field was recorded. The concentration of cells that would give rise to an average of 1 cell per field of vision was 1 × 104 cells/ml.
UV irradiation.
Uropathogenic E. coli cells of the rifampin-resistant strain ES80 (21) were exposed to 312-nm-wavelength UV light from a radiation box at 1.34 mW of UV from a distance of 5 cm; 15 ml of exponentially growing ES80 cells at a concentration of 6 × 108 cells/ml was placed in an 85-mm-diameter sterile petri dish. The lid of the dish was removed, and the cells were exposed to the UV light for 25 min (35 mJ/cm2). Following exposure, the cells were kept at 5°C and monitored at the indicated times for culturability as described above and membrane integrity with the viability assay described above. Lysed cells were calculated as the difference in the concentration of visible cells (both green- and red-fluorescing cells) and the starting cell concentration.
Statistical analysis.
Because the data from human patients were not normally distributed, a nonparametric analysis of variance was used to determine if significant differences were present. Upon determining that results did not vary between parametric and nonparametric analyses, a parametric analysis of variance was used in which comparisons were made post hoc. Data were analyzed with a one-way analysis of variance or t test, and comparisons between three patient groups were made post hoc with Tukey's test (SAS).
The concentration of viable but nonculturable cells was determined by subtracting the concentration of CFU from the concentration of viable cells.
RESULTS
Clean-catch urine specimens from human females contain viable but nonculturable forms of bacteria.
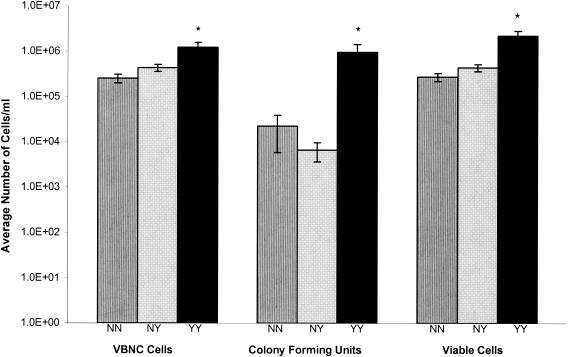
Viable but nonculturable cells are enumerated as the difference in the number of viable and culturable cells. To determine if viable but nonculturable cells were present in clean-catch human urine specimens, the concentrations of culturable and viable bacteria were determined in urine specimens obtained from 36 female volunteers. Of these 36 volunteers, 11 had an active UTI, 19 did not have an active UTI but had had at least one previous UTI, and 11 did not have and had not previously had a UTI. The average concentration of culturable bacteria present in the urine specimens of all patients was 2.7e5 CFU/ml (standard error = 1.4e5) and 8.7e5 viable cells/ml (standard error = 2.2e5). This result indicates that there are significant numbers of viable but nonculturable bacteria present in human urine specimens (an average of 6.0e5 viable but nonculturable cells/ml; standard error, 1.2e5).
Although the concentration of culturable and viable but nonculturable bacteria differed among the patient groups (Fig. 1), all contained viable but nonculturable cells. As expected, the average concentration of culturable cells in urine specimens was higher from UTI patients (9.8e5 CFU/ml) than from non-UTI patients (1.2e4 CFU/ml). The concentration of culturable cells observed in this study from the urine specimens of non-UTI patients is similar to what has previously been reported (15). Although all patient categories contained at least 2.5e5 viable but nonculturable cells/ml, UTI patients had significantly more viable but nonculturable cells than did non-UTI patients (Fig. 1). There was no significant difference in the culturable, viable, or viable but nonculturable cell concentrations between non-UTI patients with or without a previous UTI history (Fig. 1).
FIG. 1.
Concentration and physiological status of bacteria found in human urine specimens. Urine was collected, as described in the text, from women who had never had a UTI (NN column), who had no current UTI but had had at least one previous UTI (NY columns), and who had a current UTI (YY column). Error bars indicate the positive standard error of the mean. * indicates a significant difference between this and the other two participant categories.
Viable but nonculturable cells are found in mouse bladder urine specimens.
Although urine specimens were obtained as clean-catch samples, it is possible that some urine specimens contained bacterial contaminants from regions of the urinary tract not considered sterile (e.g., external skin or the distal region of the urethra). To obtain urine specimens known to be free of bacterial contamination as well as to extend the observations obtained with humans to a different species, laboratory mice were examined. Healthy BALB/c mice were anesthetized, and urine specimens were sterilely removed directly from the bladder as described in Materials and Methods. The average concentration of culturable cells present in urine specimens obtained from five mice was 1.5e2 (standard deviation, 2.2e2) CFU/ml. This concentration would be classified as a negative culture result with standard criteria (46). The average concentration of viable cells present in urine specimens obtained from six mice was 4.5e6 (standard deviation, 2.8e6) viable cells/ml; when reexamined after 24 h at 4°C, the concentration did not significantly change (4.8e6 viable cells/ml; standard deviation, 2.7e6). These data indicate that there were approximately 4.5e6 viable but nonculturable cells/ml in bladder urine specimens. As with human samples above, cells scored as viable might actually be recently killed bacterial cells whose membrane had not yet become compromised sufficiently to take up propidium iodide.
Membrane of cells killed by UV breaks down within the time used to examine urine specimens.
Identifying nongrowing bacteria as viable but nonculturable requires a viability assay that differentiates dead cells from viable but nonculturable cells. The assay in this study uses cell membrane integrity as an indicator of cell viability. False-positives arise if a recently dead cell is examined before its membrane integrity has been compromised. To eliminate false-positives requires determining how long it takes a dead cell's membrane to degrade sufficiently to allow uptake of propidium iodide. This time was determined by exposing cells to lethal doses of UV, a method that should not directly affect the cell membrane (16, 45), and then following the change in viable cells over time.
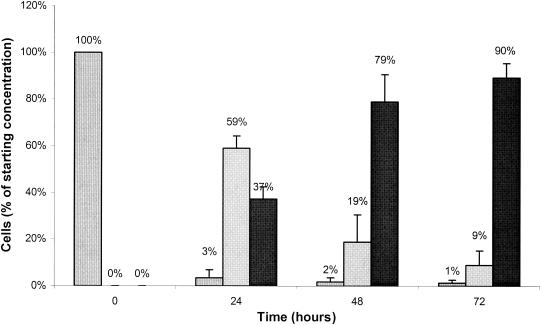
Exponentially growing uropathogenic E. coli cells at a starting concentration of 6 × 108 cells/ml were placed in a sterile petri dish and subjected to UV light exposure for 25 min at a wavelength of 312 nm and an intensity of 35 mJ/cm2 as described in Materials and Methods. Following exposure, the cells were kept at 5°C (to mimic urine specimen storage conditions) and monitored for membrane integrity with the live/dead BacLight bacterial viability kit. Membrane integrity was measured immediately following and at 24, 48, and 72 h following UV exposure. No culturable E. coli cells were detected by 24 h after UV exposure (Fig. 2). When examined with the viability assay, by 24 h after irradiation, 59% of the exposed cells fluoresced red, 37% had lysed, and 3% fluoresced green. By 48 h after exposure, 79% of the starting cells had lysed and 2% fluoresced green. By 72 h after exposure, 90% of the cells had lysed and 1% fluoresced green.
FIG. 2.
Time required to degrade the cell membrane of bacteria exposed to lethal doses of UV light. E. coli cells were examined after the indicated times following exposure to UV light for 25 min as described in the text. Cell concentration is given as a percentage of the starting concentration for live cells (striped columns), dead cells (gray columns), and lysed cells (black columns). Error bars represent the standard error of the mean.
To confirm that cells from urine specimens scored above as viable but nonculturable were actually viable, samples from eight human patients (four with and four without an active UTI) included in Fig. 1 were reexamined after storage for 2 days at 5°C. There was no significant change in the concentration of viable but nonculturable cells from day 0 to day 2, 3.54 × 105 ± 6.0 × 104 cells/ml and 5.21 × 105 ± 9.1 × 104 cells/ml, respectively. Similar results were observed with mouse urine specimens; after storage at 5°C for an additional 24 h, the viable cell concentration changed from 4.5 × 106 ± 2.5 × 106 to 4.8 × 106 ± 2.7 × 106. These results indicate that the LIVE/DEAD assay accurately measured the concentration of viable cells.
DISCUSSION
Urine located within the urinary tract, excluding the distal region of the urethra, is considered sterile in healthy individuals, as indicated by the absence of culturable bacterial cells. In this study, urine specimens were examined for the presence of viable but nonculturable bacteria in two animal species. The total number of culturable and viable cells was determined in human urine specimens obtained from female individuals with or without an active UTI. Although the absolute numbers of culturable and viable cells present in urine specimens varied among individuals, the average number of viable cells was significantly higher than the average number of culturable cells in all patient categories (Fig. 1). This is consistent with viable but nonculturable bacteria being present in the urine found in the urinary tract of humans. Women with an active UTI had a higher concentration of viable but nonculturable cells than did the subjects in the other categories. The average concentration of viable but nonculturable cells in women without an active UTI was not affected by the UTI history.
Although midstream-catch urine specimens were obtained from human participants, it is possible that not all samples were collected free of contamination. To minimize the chance for external contamination, urine specimens that was obtained directly from the surgically resected bladder of mice were examined. Three of five mice had no detectable CFU, and the average CFU concentration was 150 cells/ml. By contrast, the average concentration of viable cells was greater than 7e6 cells/ml. Greater than 99.9% of cells present in urine specimens were viable but nonculturable. Hence, viable but nonculturable forms of bacteria are present in the urinary tract of both humans and mice.
There are at least two hypotheses to explain these results. First, the viable bacteria are viable but nonculturable. Second, bacteria scored as viable were actually dead; this would occur if cells fluorescing green were dead yet retained an intact cell membrane. This scenario can arise if dead cells are examined before the integrity of their membrane has become compromised.
To evaluate this second alternative hypothesis requires a determination of the time required for membrane breakdown sufficient to allow uptake of propidium iodide in cells killed via a method that has minimal impact on cell membrane integrity. Because UV light does not directly damage the cell membrane (16, 45), uropathogenic E. coli cells were exposed to lethal doses of UV irradiation and then stored in the same manner as urine specimens. When cells were examined 24 h after exposure, 97% of the cells had been killed. Killed cells can be separated into those that have not lysed (i.e., red fluorescing) and those that have lysed (i.e., lack sufficient cell membrane integrity to be recognized as a cell; the concentration of lysed cells is estimated by subtractive analysis). That 37% of the cells had lysed after 24 h suggests that membrane integrity was compromised well before 24 h.
Although the urine specimens in this study were examined within 24 h, following standard clinical practices (7), eight samples from human subjects were reexamined 48 h after collection, and bladder-collected mouse urine specimens were reexamined 24 h after collection. There was no significant change in the concentration of viable but nonculturable cells in the human samples or of viable cells in the mouse samples during this time period. These data indicate that the green-fluorescing cells in the initial sample analysis were in fact viable.
Further examination of cells 48 and 72 h after UV irradiation reveals only a slight additional decrease or no significant change in the concentration of viable cells. Not surprisingly, the percentage of the starting cells that lysed during this time increased to 90%. It is possible that the few cells remaining viable 24 h after UV exposure reflect those cells that escaped UV killing by becoming viable but nonculturable. It has previously been reported that UV irradiation can induce E. coli to become viable but nonculturable (19, 37). Although those studies used slightly different UV conditions, a similar viable but nonculturable response may have occurred here. If the 3% of cells remaining viable 24 h after UV irradiation were in fact viable but nonculturable, it can be concluded that the membranes of all dead cells become compromised within 24 h. This interpretation further supports the validity of this viability assay when used on samples examined at least 24 h after collection or treatment.
Enumerating the viable but nonculturable cell concentration requires an accurate determination of both CFU and viable cell concentrations. Although common pathogens in the urinary tract (e.g., uropathogenic E. coli) can grow under all of the conditions used in this study, it is possible that strict anaerobes or microbes that cannot grow on LB or BHI are also present. To address this concern, urine specimens from five non-UTI females having had a previous UTI were grown on LB, BHI, and SBA under aerobic, 5% CO2, and anaerobic conditions. A comparison of CFU counts indicated that growth on SBA resulted in the same number of colonies as did BHI and more than did LB. Furthermore, the highest colony counts were obtained from BHI and SBA when grown in 5% CO2. To minimize underestimating CFU concentrations, results from whichever growth condition yielded the highest colony counts were used in data analysis. While it is possible that some bacterial species present in the urinary tract would not be counted with this process, it is doubtful that these cells would be present at a level that would change this study's conclusions.
Although both pathogenic and nonpathogenic bacterial cells were examined in this study, these results impact on hypotheses concerning the etiology of persistent UTIs (and perhaps interstitial cystitis as well [12]), and attempts to explain the urinary tract symptoms in patients without significant bacteriuria. There is increasing evidence that recurrent infections caused by the index strain arise from a reservoir of bacteria that are residing in the urinary tract (33, 39, 42, 44). We address the physiological status of those bacteria found in urine specimens.
One reservoir that is the source of viable but nonculturable cells in urine specimens could be the previously described quiescent intracellular cells found within epithelial cells lining the urinary tract (33, 39, 44). It has recently been reported that following uropathogenic E. coli infection, large intracellular bacterial colonies form “pod” structures in the epithelial cells in the mouse bladder (2). Upon death of the epithelial cell, these bacteria can be released. Although at least some of these cells are culturable, the physiological status of all of the cells contained in a pod is unknown. As this model can satisfactorily describe the progression of a uropathogenic E. coli infection, it does not explain the source of viable but nonculturable cells in patients with no known previous UTI.
Regardless of how viable but nonculturable cells enter the urinary tract, that they may preferentially end up in the urine specimens could be explained if viable but nonculturable cells have a reduced binding affinity for epithelial cells. Although this has not been examined for uropathogenic E. coli, viable but nonculturable Enterococcus faecalis cells were found to retain the ability to adhere to epithelial cells, but the efficiency of attachment is reduced by at least 50% (38).
A direct comparison of our results with studies that have examined urine specimens from healthy individuals for bacteria via microscopy is hindered by differing methods of data reporting and the lack of standardized evaluation criteria. Most studies do not report an average cell concentration, but instead report the percentage of individuals having a positive and negative result. And microscopy results have been scored as positive if there has been greater than 0 (24, 25, 35), 1 or more (3, 36, 47), 2 or more (5, 8, 46), and 5 or more (20) bacterial cells per oil immersion field. These differing criteria can significantly impact the reported percentage of positive samples. However, both the lack of a standard for microscopy criteria and the use of a different staining protocol seem insufficient to explain the high cell counts reported in this study. That over a 2-year period six investigators with multiple staining kits obtained similar results minimizes technical error to explain the results obtained here.
This study presents evidence that viable but nonculturable cells are a significant part of the normal microbial flora found in the urinary tract of both mice and humans. The role of viable but nonculturable cells in persistent UTI infections is currently under investigation.
Acknowledgments
This work was supported in part by funds provided by the Foundation for the Carolinas (to T.R.S.), the University of North Carolina at Charlotte, and Sigma Xi Grant-In-Aid of Research awards (to B.R. and H.H.). We also acknowledge the Graduate School of the University of North Carolina for support of publication costs.
Footnotes
The first six authors are listed alphabetically.
REFERENCES
- 1.Alexander, E., D. Pham, and T. R. Steck. 1999. The viable but nonculturable condition is induced by copper in Agrobacterium tumefaciens and Rhizobium leguminosarum. Appl. Environ. Microbiol. 65:3754-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed]
- 3.Appelbaum, P. C., and C. C. Olmstead. 1982. Evaluation of Gram-stain screen and Micro-ID methods for direct identification of Enterobacteriaceae from urine. Med. Microbiol. Immunol. 170:173-184. [DOI] [PubMed] [Google Scholar]
- 4.Barnett, B. J., and D. S. Stephens. 1997. Urinary tract infection: an overview, Am. J. Med. Sci. 314:245-249. [DOI] [PubMed] [Google Scholar]
- 5.Baron, E. J., M. B. Tyburski, R. Almon, and M. Berman. 1988. Visual and clinical analysis of Bac-T-Screen urine screen results. J. Clin. Microbiol. 26:2382-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brauner, A., S. H. Jacobson, and I. Kuhn. 1992. Urinary Escherichia coli causing recurrent infections: a prospective follow-up of biochemical phenotypes. Clin. Nephrol. 38:318-323. [PubMed] [Google Scholar]
- 7.Campbell, P. C. 1998. In P. C. Walsh, A. Retik, E. D. Vaughan, and A. J. Wein (ed.), Campbell's urology, 7th ed. W. B. Saunders Co., Philadelphia, Pa.
- 8.Cardoso, C. L., C. B. Muraro, V. L. D. Siqueira, and M. Guilhermetti. 1998. Simplified technique for detection of significant bacteriuria by microscopic examination of urine. J. Clin. Microbiol. 36:820-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colwell, R. R., and D. J. Grimes. 2000. Semantics and strategies, p. 1-6. In R. R. Colwell and D. J. Grimes (ed.), Nonculturable microorganisms in the environment. American Society for Microbiology, Washington, D.C.
- 10.Colwell, R. R. 2000. Viable but nonculturable bacteria: a survival strategy. J. Infect. Chemother. 6:121-125. [DOI] [PubMed] [Google Scholar]
- 11.Colwell, R. R. 2000. Bacterial death revisited, p. 325-342. In R. R. Colwell and K. J. Grimes (ed.), Nonculturable microorganisms in the environment. American Society for Microbiology, Washington, D.C.
- 12.Dominque, G. J., G. M. Ghoniem, K. L. Bost, C. Fermin, and G. H. Liset. 1995. Dormant microbes in interstitial cystitis. J. Urol. 153:1321-1326. [PubMed] [Google Scholar]
- 13.Dukan, S., Y. Levi, and D. Touati. 1988. Recovery of culturability of an HOCl-stressed population of Escherichia coli after incubation in phosphate buffer: resuscitation or regrowth? Appl. Environ. Microbiol. 63:4204-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dukes, C. E. 1939. Urine examination and clinical interpretation, p. 28-88. Oxford Medical Publications, New York, N.Y.
- 15.Eschenback, D. A., K. L. Patton, T. M. Hooton, A. S. Meier, A. Stapleton, J. Aura, and K. Agnew. 2001. Effects of vaginal intercourse with and without condom on vaginal flora and vaginal epithelium. J. Infect. Dis. 183:913-918. [DOI] [PubMed] [Google Scholar]
- 16.Fiksdal, L., and I. Tryland. 1999. Effect of UV light irradiation, starvation and heat on Escherichia coli β-D-galactosidase activity and other potential viability parameters. J. Appl. Microbiol. 87:62-71. [DOI] [PubMed] [Google Scholar]
- 17.Foxman, B. 1990. Recurring urinary tract infections: incidence and risk factors. J. Public Health 880:331-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foxman, B., B. Gillespie, J. Koopman, L. Zhang, K. Palin, P. Tallman, J. V. Marsh, S. Spear, J. D. Sobel, M. J. Marty, and C. F. Marrs. 2000. Risk factors for second urinary tract infection among college women. Am. J. Epidemiol. 151:1194-1205. [DOI] [PubMed] [Google Scholar]
- 19.Gauthier, M. J. 2000. Environmental parameters associated with the viable but nonculturable state, p. 87-112. In R. R. Colwell and D. J. Grimes (ed.), Nonculturable microorganisms in the environment. American Society for Microbiology, Washington, D.C.
- 20.Greenberg, N. D., J. Stamler, J. Zackler, and S. L. Andelman. 1965. Detection of urinary tract infections in pregnant women. Public Health Rep. 80:805-811. [PMC free article] [PubMed] [Google Scholar]
- 21.Grey, B., and T. R. Steck. 2001. Concentrations of copper thought to be toxic to Escherichia coli can induce the viable but nonculturable condition. Appl. Environ. Microbiol. 67:5325-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haugland, R. P. 1996. Handbook of fluorescent probes and research chemicals. Molecular Probes, Inc., Eugene, Oreg.
- 23.Heim, S., M. D. M. Lleo, B. Bonato, C. A. Guzman, and P. Canepari. 2002. The viable but nonculturable state and starvation are different stress responses of Enterococcus faecalis, as determined by proteome analysis. J. Bacteriol. 184:6739-6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinze, P. A., L. D. Thrupp, and C. R. Anselmo. 1979. A rapid (4-6 hour) urine-culture system for direct identification and direct antimicrobial susceptibility testing. Am. J. Clin. Pathol. 71:177-183. [DOI] [PubMed] [Google Scholar]
- 25.Hoeprich, P. D. 1960. Culture of the urine. J. Lab. Clin. Med. 56:899-907. [PubMed] [Google Scholar]
- 26.Ikaheimo, R. A., A. Siitonen, T. Heiskanen, U. Karkkainen, P. Kuosmanen, P. Lipponen, and P. H. Makela. 1996. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin. Infect. Dis. 22:91-99. [DOI] [PubMed] [Google Scholar]
- 27.Karkkainen, U. M., R. Ikaheimo, M. L. Katila, and A. Siitonen. 2000. Recurrence of urinary tract infections in adult patients with community-acquired pyelonephritis caused by E. coli: a 1-year follow-up. Scand. J. Infect. Dis. 32:495-499. [DOI] [PubMed] [Google Scholar]
- 28.Kass, E. H. 1957. Bacteriuria and the diagnosis of infections of the urinary tract. AMA Arch. Intern. Med. 100:709-714. [DOI] [PubMed] [Google Scholar]
- 29.Kunin, C. M., L. VanArsdale White, and T. H. Hua. 1993. A reassessment of the importance of “low-count” bacteriuria in young women with acute urinary symptoms. Ann. Intern. Med. 119:454-460. [DOI] [PubMed] [Google Scholar]
- 30.Kunin, C. M. 1996. Urinary tract infections and pyelonephritis, p. 602-605. In J. C. Bennett and F. Plum (ed.), Cecil textbook of medicine, 20th ed. W. B. Saunders, Philadelphia, Pa.
- 31.Mizunoe, Y., S. N. Wai, A. Takade, and S. Yoshida. 1999. Restoration of culturability of starvation-stressed and low-temperature-stressed Escherichia coli O157 cells by using H2O2-degrading compounds. Arch. Microbiol. 172:63-67. [DOI] [PubMed] [Google Scholar]
- 32.Morgan, M. G., and H. McKenzie. 1993. Controversies in the laboratory diagnosis of community-acquired urinary tract infection, Eur. J. Clin. Microbiol. Infect. Dis. 12:491-504. [DOI] [PubMed] [Google Scholar]
- 33.Mulvey, M. A., J. D. Schilling, and S. J. Hultgren. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69:4572-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohtomo, R., and M. Saito. 2001. Increase in the culturable cell number of Escherichia coli during recovery from saline stress: possible implication for resuscitation from the VBNC state. Microb. Ecol. 42:208-214. [DOI] [PubMed] [Google Scholar]
- 35.Parker, R. H., N. M. Nord, G. F. Croft, and P. D. Hoeprich. 1996. Reliability of a commercial triphenyltetrazolium chloride reduction test for detecting significant bacteriuria. Am. J. Med. Sci. 251:260-265. [DOI] [PubMed] [Google Scholar]
- 36.Pezzlo, M. T., G. L. Tan, E. M. Peterson, and L. M. de la Maza. 1982. Screening of urine cultures by three automated systems. J. Clin. Microbiol. 15:468-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pommepuy, M., M. Butin, A. Derrien, M. Gourmelon, R. R. Colwell, and M. Cormier. 1996. Retention of enteropathogenicity by viable but nonculturable Escherichia coli exposed to seawater and sunlight. Appl. Environ. Microbiol. 62:4621-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pruzzo, C., R. Tarsi, M. del Mar Lleo, C. Signoretto, M. Zampini, R. R. Colwell, and P. Canepari. 2002. In vitro adhesion to human cells by viable but nonculturable Enterococcus faecalis. Curr. Microbiol. 45:105-110. [DOI] [PubMed] [Google Scholar]
- 39.Rivers, B., and T. R. Steck. 2001. Viable but nonculturable uropathogenic bacteria are present in the mouse urinary tract following urinary tract infection and antibiotic therapy. Urol. Res. 29:60-66. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez, G. G., D. Phipps, K. Ishiguro, and H. F. Ridgeway. 1992. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl. Environ. Microbiol. 58:1801-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo, T. A., A. Stapleton, S. Wenderoth, T. M. Hooton, and W. E. Stamm. 1995. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J. Infect. Dis. 172:440-445. [DOI] [PubMed] [Google Scholar]
- 42.Schilling, J. D., and S. J. Hultgren. 2002. Recent advances into the pathogenesis of recurrent urinary tract infections: the bladder as a reservoir for uropathogenic Escherichia coli. Int. J. Antimicrob. Agents 19:457-460. [DOI] [PubMed] [Google Scholar]
- 43.Schilling, J. D., M. A. Mulvey, and S. J. Hultgren. 2001. Dynamic interactions between host and pathogen during acute urinary tract infections. Urology 57(Suppl. 6A):56-61. [DOI] [PubMed] [Google Scholar]
- 44.Schilling, J. D., R. G. Lorenze, and S. J. Hultgren. 2002. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect. Immun. 70:7042-7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villarino, A., O. M. M. Bouvet, B. Regnault, S. Martin-Delautre, and P. A. D. Grimont. 2000. Cellular activities in ultra-violet killed Escherichia coli. Int. J. Food Microbiol. 55:245-247. [DOI] [PubMed] [Google Scholar]
- 46.Washington, J. A., C. M. White, M. Laganiere, and L. H. Smith. 1981. Detection of significant bacteriuria by microscopic examination of urine specimens. Lab. Med. 12:294-296. [Google Scholar]
- 47.Wellstood, S. A. 1986. Direction identification and susceptibility testing by the AutoMicrobic system of gram-negative bacilli from urine specimens. J. Clin. Microbiol. 23:1068-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]