Abstract
Purpose of review
The autologous antigen-presenting cell immunotherapy, sipuleucel-T, was the first and remains the only US Food and Drug Administration-approved immunotherapy for prostate cancer. In this article, we will summarize recent clinical data on several additional immune-directed strategies, some of which have now entered phase 3 trials.
Recent findings
Multiple studies are now testing sipuleucel-T in different disease settings and/or in combination with conventional and novel hormonal therapies. In addition, a poxviral-based vaccine has shown promise in early-phase clinical studies and has now entered phase 3 testing in men with metastatic prostate cancer. Next, a DNA vaccine has been evaluated in men with biochemically recurrent prostate cancer and has shown early signs of clinical efficacy. Finally, several studies are evaluating the role of immune checkpoint blockade using ipilimumab in pivotal phase 3 trials in prostate cancer patients with advanced disease, as well as in earlier phase studies in combination with androgen ablation.
Summary
The abundance of new treatment options for men with advanced prostate cancer will challenge the role of immunotherapy in these patients. Future progress may rely on optimal combination and sequencing of various immunotherapies with androgen-directed approaches as well as with other standard prostate cancer therapies, an effort which is now just beginning.
Keywords: androgen ablation, immune checkpoints, immunotherapy, prostate cancer, vaccine
INTRODUCTION
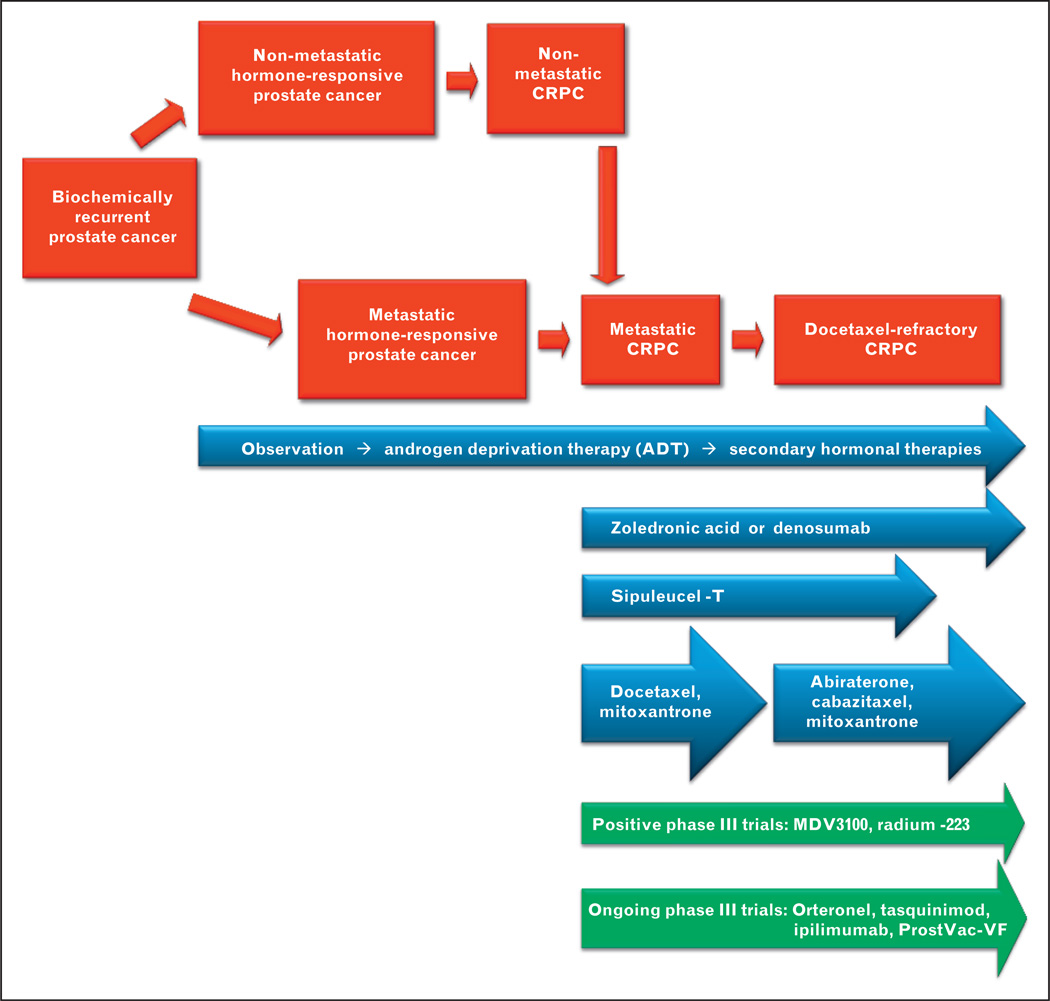
The past few years have witnessed an explosion of new treatment options for men with advanced prostate cancer [1]. Although depleting or blocking the action of androgens has been the mainstay of therapy for patients with recurrent prostate cancer, additional treatment approaches are urgently needed because these androgen-directed therapies are not curative and the disease invariably progresses to a state known as castration-resistant prostate cancer (CRPC). In 2005 docetaxel emerged on the scene and was the first agent to improve survival in men with metastatic CRPC [2]. After a 5-year hiatus the field of prostate cancer therapeutics received a significant boost in 2010 with the US Food and Drug Administration (FDA) approval of sipuleucel-T [3▪▪] a personalized antigen-presenting cell-based immunotherapy product indicated for the treatment of asymptomatic or minimally symptomatic metastatic CRPC. Since that pivotal point four additional drugs have demonstrated a survival advantage in men with metastatic CRPC: the chemotherapy agent cabazitaxel (FDA-approved in men with docetaxel-refractory CRPC) [4] the androgen synthesis inhibitor abiraterone (also FDA-approved in docetaxel-pretreated patients) [5] the radiopharmaceutical agent radium-223 (FDA-approval pending) [6], and the androgen-signaling inhibitor MDV3100 (FDA-approval pending) [7]. Multiple additional agents are now in phase 3 development for men with advanced prostate cancer and are likely to further expand the treatment arsenal (Fig. 1).
FIGURE 1.
Current and emerging treatment options for men with advanced prostate cancer. Red panels show a series of prostate cancer clinical states, beginning with early recurrent disease and progressing to advanced chemotherapy-refractory prostate cancer. Blue panels show current Food and Drug Administration-approved treatment options for men with recurrent or advanced prostate cancer. Green panels show selected emerging treatment options on the horizon. CRPC, castration-resistant prostate cancer.
Amidst this panoply of effective agents, the role of prostate cancer immunotherapy will be challenged by the fact that all the other available treatment modalities produce measurable tumor response in addition to improving overall survival (OS). Thus, the maximal impact of immune-directed approaches may be realized only when they are combined or sequenced with other standard therapies for prostate cancer. This review will highlight selected immunotherapy approaches for the treatment of advanced prostate cancer, describing how they are being combined with other conventional therapies, with a focus on androgen-directed therapies. Indeed, the concept of combination immunotherapy and hormonal therapy is supported by ample preclinical and clinical data [8,9].
SIPULEUCEL-T
The approval of sipuleucel-T, an autologous PAP (prostatic acid phosphatase) directed cell-based immunotherapy manufactured using patients’ own antigen-presenting cells, represented a landmark in the treatment of metastatic cancers because it was the first therapeutic vaccine to show a survival advantage in any tumor type [10]. In the pivotal 512-patient phase 3 study leading to FDA-approval, men with minimally/asymptomatic metastatic CRPC who received sipuleucel-T demonstrated superior survival than patients receiving placebo (25.8 versus 21.7 months; hazard ratio 0.78; P = 0.03), but there was no difference in other measurable endpoints such as prostate-specific antigen (PSA) responses or radiographic tumor responses [3▪▪]. Whereas docetaxel was the only other life-prolonging therapy for men with CRPC at the time of its approval, sipuleucel-T now competes with four additional treatment modalities that have all been shown to improve survival: cabazitaxel, abiraterone, radium-223, and MDV3100. This change in the therapeutic landscape, together with an increased awareness that immunotherapy will likely prove most effective in a minimal-disease setting [11▪] and/or when combined with other standard therapies [12], has motivated the design of several clinical trials evaluating sipuleucel-T in novel contexts (Table 1).
Table 1.
Selected ongoing prostate cancer immunotherapy trials
| Agent | Description | Phase 2 studies | Phase 3 studies |
|---|---|---|---|
| Sipuleucel-T | Autologous PAP-directed cellular immunotherapy | Prior to radical prostatectomy in the neoadjuvant setting (NCT00715104) Sequencing with standard androgen ablation in men with PSA-recurrent prostate cancer (NCT01431391) Combination with abiraterone in men with metastatic CRPC (NCT01487863) |
N/A |
| ProstVac-VF | PSA-encoding poxviral vaccine | Combined with flutamide, versus flutamide alone (NCT00450463) Sequencing with docetaxel, versus docetaxel alone (NCT01145508) |
Randomized trial in men with chemo-naive metastatic CRPC: used alone, or together with GM-CSF, compared with placebo (NCT01322490) |
| pTVG-HP | PAP-encoding DNA vaccine | Priming doses followed by personalized versus fixed booster regimen (NCT00849121) Vaccination and GM-CSF versus GM-CSF alone (NCT01341652) |
N/A |
| Ipilimumab | Fully human anti-CTLA-4 monoclonal antibody | Combination with androgen ablation in men with hormone-naive metastatic prostate cancer (NCT01377389) Combination with androgen ablation in men with metastatic CRPC (NCT01498978) |
Randomized trial of ipilimumab versus placebo (in combination with bone XRT) in docetaxelrefractory metastatic CRPC (NCT00861614) Randomized trial of ipilimumab versus placebo in chemo-naive metastatic CRPC (NCT01057810) |
| 177Lu-J591 | Radiolabeled anti-PSMA monoclonal antibody | Combination with ketoconazole in nonmetastatic CRPC (NCT00859781) Combination with docetaxel in metastatic CRPC (NCT00916123) |
N/A |
CRPC, castration-resistant prostate cancer; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; GM-CSF, granulocyte-macrophage colony-stimulating factor; PAP, prostatic acid phosphatase; PSA, prostate-specific antigen; PSMA, prostate specific membrane antigen; XRT, radiation therapy.
The earliest setting in which prostate cancer immunotherapy could be tested is prior to primary therapy (surgery or radiation) for localized disease. To this end, sipuleucel-T has been evaluated in the neoadjuvant space in a phase 2 study enrolling 40 men who are scheduled to undergo subsequent radical prostatectomy (NCT00715104). In this study, individuals were treated with three infusions of sipuleucel-T before radical prostatectomy, with the primary endpoint being analysis of antitumor immune responses in resected prostate glands compared with that in core biopsy specimens collected prior to sipuleucel-T administration. In addition, the potential for augmentation of antitumor immune responses by combining immunotherapy with androgen deprivation therapy in the presurgical setting is an attractive concept. Indeed, key neoadjuvant studies support this combinatorial approach, showing that (even when used alone) androgen ablation induces dense immunological infiltrates into the prostate gland [13,14].
The next setting in which an immunotherapy could be investigated is the biochemical-recurrence state, characterized by PSA elevation after primary prostate cancer therapy [15]. This disease state represents an ideal setting for immune-based approaches because disease burden is at a minimum and tumor-induced tolerance is expected to be low. Because such patients are often treated with androgen deprivation therapy (although this does not result in any cures), one attractive approach is to combine androgen ablation (given for a defined period of time) with immunotherapy in an effort to eradicate residual prostate cancer cells (or maintain them in a dormant state) in the absence of continued androgen ablation [9]. Ample preclinical data support this notion, demonstrating that androgen deprivation can potentiate antitumor immunity and augment vaccine efficacy [8,16]. However, the optimal sequencing of androgen ablation with immunotherapy is not yet clear (i.e., whether immunotherapy should be delivered before androgen ablation, as a priming maneuver; or after androgen ablation, as an immunological boost). To answer this question clinically, a 60-patient randomized phase 2 trial has been initiated (NCT01431391) in which a standard three-dose course of sipuleucel-T is being administered either 6 weeks before or 12 weeks after a 12-month course of androgen deprivation therapy in men with biochemically recurrent prostate cancer at high risk of metastatic progression. The primary objective of this study is to evaluate PAP-specific effector T-cell and B-cell responses, in an attempt to establish which sequence results in the more robust antitumor immune response. This study will also compare PSA progression and metastasis-free survival in the two treatment arms [17].
With the advent of novel hormonal therapies, such as abiraterone and MDV3100, another intriguing concept would be the combination of these agents with immunotherapy approaches. However, the optimal timing of these newer androgen-directed therapies with sipuleucel-T remains unknown. Aiming to shed light on this issue, a 60-patient randomized phase 2 study has been recently launched (NCT01487863); this trial will allocate men with metastatic CRPC to receive 6 months of abiraterone beginning concurrently with the first dose of sipuleucel-T, or 6 weeks after the last dose of sipuleucel-T. As mentioned above, this study’s primary objective is to compare the peripheral immune response between the two treatment arms, although it is certainly conceivable that larger trials employing clinical endpoints could follow. One potential caveat with this trial is that abiraterone treatment requires the concurrent administration of prednisone (10 mg daily), and the effects of corticosteroids on sipuleucel-T efficacy are unknown. In summary, the ongoing trials discussed above will help clarify both the potential utility of sipuleucel-T in early-stage disease as well as its optimal combination with conventional and novel hormonal therapies, although confirmatory trials with placebo-control groups and clinical endpoints will be required for label-expansion purposes.
PROSTVAC-VF
ProstVac-VF (Bavarian Nordic, Washington, DC, USA) is a PSA-targeted poxviral-based vaccine approach that has been developed through a series of iterative preclinical and clinical studies. The current version utilizes a heterologous prime–boost strategy (vaccinia prime, fowlpox boost), and also includes three costimulatory molecules serving to increase PSA-specific immune responses [18]. Similar to the experience with sipuleucel-T, a randomized phase 2 study demonstrated improved survival among men with metastatic CRPC who received ProstVac-VF compared with those receiving an empty-vector placebo (25.1 versus 16.6 months; hazard ratio 0.56; P = 0.006), whereas there was no impact on the primary endpoint of progression-free survival [19▪▪]. Following from these encouraging results, a multinational randomized phase 3 trial was recently launched (NCT01322490) in which 1200 men with chemotherapy-naive metastatic CRPC will be allocated to one of three treatment arms: ProstVac-VF given alone, ProstVac-VF and subcutaneous granulocyte-macrophage colony-stimulating factor (GM-CSF), or placebo. The primary endpoint of this pivotal trial is OS.
In addition, there has been considerable interest in combining ProstVac-VF with other standard prostate cancer therapies (Table 1). In an intriguing phase 2 study involving 42 men with nonmetastatic CRPC, patients were randomized to receive ProstVac-VF followed by nilutamide versus nilutamide followed by ProstVac-VF [20]. This study suggested an improved survival in men receiving ProstVac-VF before nilutamide rather than the opposite sequence (6.2 versus 3.7 years; P = 0.045). A similar phase 2 trial is currently open (NCT00450463) in which 64 patients with nonmetastatic CRPC are being randomized to ProstVac-VF and nilutamide versus nilutamide alone. The primary endpoint of this study is time-to-treatment-failure, with a secondary endpoint of time-to-metastasis.
Another ongoing study aims to test the hypothesis that an accelerated antitumor immune response may augment the effect of standard chemotherapy, a notion that is supported by robust preclinical data [21]. A randomized phase 2 trial (NCT01145508) is allocating 144 men with metastatic chemotherapy-untreated CRPC to ProstVac-VF followed by up to 12 cycles of docetaxel chemotherapy, or to docetaxel chemotherapy upfront, and will use OS as its primary endpoint. Although the concept that immunotherapy can facilitate a chemotherapy response has been established in preclinical experiments, the above trial is particularly important because well designed clinical studies proving this phenomenon are lacking.
DNA VACCINES
An alternative immune-directed strategy that differs from those discussed above involves the use of cell-free DNA plasmids encoding specific-target antigens [22]. Such DNA-based vaccines have the advantage of being relatively simple to manufacture and also allow rapid evaluation of a number of target antigens, but are generally unable to induce strong immune responses. One of the first studies employing a DNA vaccine was a phase 1 trial involving a PAP-encoding plasmid (known as pTVG-HP) that was administered to men with biochemically recurrent prostate cancer. This trial demonstrated the induction of PAP-specific cytolytic T-cell responses [23▪], and also suggested a slowing of PSA doubling time in a number of treated patients [24]. One intriguing finding of this study was the variable time course required to mount specific immune responses in different patients (sometimes taking several months to develop), suggesting the notion of a more personalized approach whereby some patients may require a longer treatment period (or more booster immunizations) than others.
An innovative phase 2 trial making use of a tailored approach is currently ongoing (NCT00849121) in men with nonmetastatic CRPC (Table 1). In this study, patients are randomized to either a predetermined vaccination schedule (six doses given every 2 weeks followed by 3 monthly boosts) or to a more adaptive vaccine regimen (in which the six dose run-in is followed by either biweekly, monthly, or 3 monthly boosts based on observed cellular immune responses). The co-primary endpoints of this trial are safety and immunogenicity, whereas secondary endpoints include PSA doubling time modulation and 1-year metastasis-free survival. Another interesting phase 2 trial enrolling men with biochemically recurrent prostate cancer compares the pTVG-HP vaccine and GM-CSF against GM-CSF used alone (NCT01341652), a reasonable comparator arm since prior studies have shown that GM-CSF in itself can induce PSA responses in a small proportion of patients [25]. Studies combining pTVG-HP with androgen ablation or other standard prostate cancer therapies have not yet been initiated.
IPILIMUMAB
Ipilimumab [26,27], a monoclonal antibody blocking the immune checkpoint molecule cytotoxic T-lymphocyte antigen-4 (CTLA-4), has received renewed attention following the recent studies of improved OS in patients with advanced melanoma [28]. In that disease, two pivotal phase 3 trials demonstrated an extension of survival using ipilimumab in patients with previously treated metastatic melanoma [29▪▪] as well as in those with previously untreated metastatic disease [30▪]. However, because CTLA-4 normally serves to attenuate autoimmune disease in the normal host, treatment with ipilimumab has been associated with a number of immune-related adverse events including grade 3 and grade 4 autoimmune breakthrough phenomena (in ~15% of patients). The frequency and severity of such immune-related toxicities has prompted the development of standardized treatment algorithms mandating rapid immunosuppressive interventions for suspected autoimmune events in an effort to limit the seriousness of such events.
Stimulated by the encouraging results in melanoma patients, ipilimumab has also been evaluated in men with prostate cancer in a number of phase 1 and 2 studies. From these trials, it has collectively emerged that CTLA-4 blockade has some activity in advanced prostate cancer as judged by PSA response rates of 10–20% and objective response rates of 5–10% [31,32]. To definitively assess the safety and efficacy of ipilimumab in men with metastatic CRPC, two randomized phase 3 trials are currently underway. In the first study (NCT00861614), 800 men with docetaxel-refractory CRPC are being randomized to low-dose bone-directed radiation therapy followed by ipilimumab (given every 3 weeks for four doses) or to placebo; the primary endpoint of this trial is OS. In the second study (NCT01057810), 600 men with chemotherapy-naive metastatic CRPC are being randomized to either ipilimumab or placebo; the primary endpoint of this trial is also OS. Although these studies may potentially lead to FDA-approval of ipilimumab for men with CRPC in the next several years, a role for ipilimumab at that time is far from clear in the setting of a rapidly evolving overall treatment landscape for this disease.
Meanwhile, additional trials are focused on using ipilimumab in men with early-stage disease and/or in combination with androgen deprivation or other therapies (Table 1). As highlighted above, the combination of immunotherapy and androgen ablation is supported by a strong scientific rationale [9]. To this end, one trial has combined ipilimumab with androgen ablation in the neoadjuvant setting. Preliminary data from this study suggest meaningful pathological responses in prostatectomy specimens from several patients [33], although a control arm of androgen ablation alone was not used in this trial. In addition, a recently launched phase 2 trial (NCT01377389) will test the combination of androgen deprivation and ipilimumab in 48 men with newly diagnosed hormone-naive metastatic prostate cancer, setting as its primary endpoint an undetectable PSA level after 7 months on study. Another phase 2 study (NCT01498978) will test the combination of ipilimumab and androgen suppression in 30 men with metastatic prostate cancer who have not achieved an undetectable PSA level after at least 6 months of androgen ablation; this trial aims to study whether the addition of ipilimumab in these patients can drive the PSA to undetectable levels.
PROSTATE-SPECIFIC MEMBRANE ANTIGEN-TARGETED MONOCLONAL ANTIBODIES
An alternative approach to the use of active immunotherapies (as discussed thus far) is the use of passive immunotherapy (i.e., the administration of specific antitumor antibodies) [34]. The concept of anticancer antibody treatment is exemplified by agents such as trastuzumab for Her2/neu-positive breast cancer, as well as bevacizumab for multiple metastatic cancers. In a similar fashion, a high-affinity humanized monoclonal antibody against prostate-specific membrane antigen (PSMA), known as J591, is being developed for prostate cancer patients [35▪]. Initial studies with this agent showed excellent tumor targeting, but a relatively low rate of PSA responses and objective tumor responses. As a result, subsequent development of J591 has focused on radiolabeled constructs, especially those conjugated to 177Lutetium [36]. This radioimmunotherapy approach has shown early signs of success (manifested by PSA reductions in several men), while also allowing simultaneous treatment as well as imaging of these patients. There are currently several ongoing clinical trials investigating 177Lu-J591 in patients with advanced prostate cancer (Table 1), either as monotherapy or combined with other modalities. One phase 2 study combining 177Lu-J591 with ketoconazole (an adrenal androgen inhibitor) is of particular interest. In this trial, 140 men with nonmetastatic CRPC are being randomized to ketoconazole and 177Lu-J591 versus ketoconazole and placebo (NCT00859781); the primary endpoint of this trial is metastasis-free survival at 18 months.
CONCLUSION
With the FDA-approval of sipuleucel-T in 2010, the promise of immunotherapy is beginning to be realized but since that time the treatment landscape for men with advanced prostate cancer has changed considerably, and is likely to expand further in the next few years. For this reason, one of the challenges of the near future will be to determine the role that immunotherapy will play amidst a large selection of additional effective therapies ranging from chemotherapies to novel androgen-directed approaches to radiopharmaceutical drugs to small-molecule targeted therapies. Because many of these treatment modalities will be capable of producing measurable clinical benefits in addition to extending survival, they may become the preferred treatment options over immunotherapy agents. One way in which immunotherapies may remain relevant in the new treatment paradigm is in combination with other forms of prostate cancer therapy. Specifically, the integration of immunotherapies with androgen-directed therapies appears to hold much promise, although our understanding of the optimal timing and sequencing of each immunotherapy with conventional and novel hormonal approaches remains largely unknown. In addition, the high rate of PSA and tumor responses observed using some of the new hormonal agents (e.g., abiraterone, MDV3100) might make an additive effect of immunotherapy difficult to detect. Moreover, the need to administer potentially immunosuppressive doses of prednisone with many of the standard prostate cancer therapies (e.g., docetaxel, cabazitaxel, abiraterone) will further complicate the integration of immunotherapy with these agents. Despite these potential challenges, it is likely that immunotherapy will have an important role in the treatment of prostate cancer moving forward, but the field must now focus its efforts on defining optimal combination regimens that will result in long-term immune-mediated responses and even cures for patients with this disease.
KEY POINTS.
Prostate cancer is an immune-responsive malignancy as evidenced by a survival benefit achieved using the autologous cellular immunotherapy product, sipuleucel-T, in men with asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer.
Several additional immunotherapy agents (including a poxviral-based vaccine, a plasmid DNA vaccine, an immune checkpoint blocking antibody, and a radiolabeled targeted monoclonal antibody) are currently being investigated in phase 2 and 3 trials.
As new hormonal therapies are available, an understanding of the optimal combination and sequencing of various immunotherapies with standard and novel androgen-directed approaches will be paramount in moving the field forward.
Future progress in immunotherapy for prostate cancer will likely focus on earlier disease settings (such as the biochemically recurrent state), as well as on rational combinations of immune-directed agents with conventional therapies for prostate cancer.
Acknowledgements
NCI R01 CA12715; 1P50CA58236-15; the Patrick C. Walsh Fund; the OneInSix Foundation; the Prostate Cancer Foundation.
Footnotes
Conflicts of interest
E.S.A. has served as a consultant/advisor for Sanofi-Aventis. C.G.D. has served as a consultant/advisor for Amplimmune Inc, Bristol-Myers Squibb, and Dendreon Inc. C.G.D. also has stock ownership in Amplimmune Inc.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 350).
- 1.Antonarakis ES, Eisenberger MA. Expanding treatment options for metastatic prostate cancer. N Engl J Med. 2011;364:2055–2058. doi: 10.1056/NEJMe1102758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 3. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. This is the pivotal randomized phase III trial leading to the FDA-approval of sipuleucel-T for men with asymptomatic or minimally symptomatic metastatic CRPC.
- 4.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 5.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nilsson S, Franzén L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–594. doi: 10.1016/S1470-2045(07)70147-X. [DOI] [PubMed] [Google Scholar]
- 7.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drake CG, Doody AD, Mihalyo MA, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci. 2007;12:4957–4971. doi: 10.2741/2441. [DOI] [PubMed] [Google Scholar]
- 10.May KF, Jr, Gulley JL, Drake CG, et al. Prostate cancer immunotherapy. Clin Cancer Res. 2011;17:5233–5238. doi: 10.1158/1078-0432.CCR-10-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a Poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–674. doi: 10.1007/s00262-009-0782-8. This is an elegant demonstration that prostate cancer immunotherapy appears to be more effective in men with less aggressive disease or lower disease burden.
- 12.Antonarakis ES, Drake CG. Current status of immunological therapies for prostate cancer. Curr Opin Urol. 2010;20:241–246. doi: 10.1097/MOU.0b013e3283381793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gannon PO, Poisson AO, Delvoye N, et al. Characterization of the intraprostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009;348:9–17. doi: 10.1016/j.jim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Antonarakis ES, Feng Z, Trock BJ, et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int. 2012;109:32–39. doi: 10.1111/j.1464-410X.2011.10422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh YT, Gray A, Higgins SA, et al. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate. 2009;69:571–584. doi: 10.1002/pros.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonarakis ES, Kibel AS, Lin DW, et al. Design of an open-label randomized phase II trial examining the effect of sequencing of sipuleucel-T and androgen deprivation therapy on immune markers in prostate cancer patients with a rising PSA after primary therapy [abstract] J Clin Oncol. 2011;29:189. [Google Scholar]
- 18.Madan RA, Arlen PM, Mohebtash M, et al. ProstVac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009;18:1001–1011. doi: 10.1517/13543780902997928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. This is an important trial demonstrating a potential survival advantage with ProstVac-VF in men with metastatic CRPC, although survival was not the primary endpoint of this study.
- 20.Madan RA, Gulley JL, Schlom J, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14:4526–4531. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zitvogel L, Apetoh L, Ghiringhelli F, et al. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alam S, McNeel DG. DNA vaccines for the treatment of prostate cancer. Expert Rev Vaccines. 2010;9:731–745. doi: 10.1586/erv.10.64. [DOI] [PubMed] [Google Scholar]
- 23. Becker JT, Olson BM, Johnson LE, et al. DNA vaccine encoding prostatic acid phosphatase (PAP) elicits long-term T-cell responses in patients with recurrent prostate cancer. J Immunother. 2010;33:639–647. doi: 10.1097/CJI.0b013e3181dda23e. This is an interesting study investigating the immunological responses to treatment using the DNA-based pTVG-HP vaccine in men with biochemically recurrent prostate cancer.
- 24.McNeel DG, Dunphy EJ, Davies JG, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase (PAP) in patients with stage D0 prostate cancer. J Clin Oncol. 2009;27:425–430. doi: 10.1200/JCO.2008.19.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Small EJ, Reese DM, Um B, et al. Therapy of advanced prostate cancer with granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 1999;5:1738–1744. [PubMed] [Google Scholar]
- 26.Hoos A, Ibrahim R, Korman A, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37:533–546. doi: 10.1053/j.seminoncol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer. Preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37:430–439. doi: 10.1053/j.seminoncol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17:6958–6962. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. This is the pivotal randomized phase III trial leading to the FDA-approval of ipilimumab for patients with previously treated metastatic melanoma.
- 30. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. This is a randomized phase III trial showing a survival advantage in patients with metastatic melanoma receiving ipilumab combined with dacarbazine as first-line therapy.
- 31.Beer TM, Slovin SF, Higano CS, et al. Phase I trial of ipilimumab alone and in combination with radiotherapy in patients with metastatic castration-resistant prostate cancer [abstract] J Clin Oncol. 2008;26:5004. [Google Scholar]
- 32.Fong L, Kwek SS, O’Brien S, et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granberg C, Thompson RH, Quevedo JF, et al. Down-staging of locally-advanced prostate cancer with anti-CTLA-4 monoclonal antibody prior to radical prostatectomy [abstract] J Clin Oncol. 2009;27:16103. [Google Scholar]
- 34.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tagawa ST, Beltran H, Vallabhajosula S, et al. Antiprostate-specific membrane antigen-based radioimmunotherapy for prostate cancer. Cancer. 2010;116:1075–1083. doi: 10.1002/cncr.24795. This is an excellent review summarizing anti-PSMA 177Lu-J591 radioimmunotherapy for prostate cancer.
- 36.Bander NH, Milowsky MI, Nanus DM, et al. Phase I trial of 177Lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23:4591–4601. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]