Abstract
It is known that patients with mastocytosis have an increased risk of anaphylaxis. This also appears to be the case with patients with evidence of a clonal mast cell disorder resulting in the monoclonal mast cell activation syndrome (MMAS) who do not express the full mastocytosis phenotype. Most patients with mastocytosis are recognized by their characteristic skin lesions. An increased level of baseline serum mast cell tryptase is also an indicator for a possible clonal mast cell disorder including mastocytosis. Other markers for mast cell clonality and for mastocytosis include abnormal immunostaining of mast cells with CD25 and CD2, clustering of mast cells in tissues, abnormal mast cell morphology, and the presence of a mutation in the proto-oncogene c-kit encoding for the mast cell growth receptor KIT. As recognition depends on an understanding of mastocytosis, and this disease should be considered in patients with recurrent anaphylaxis, we describe the features of mast cell clonality, MMAS and mastocytosis; and review recent findings.
Introduction
Mast cells express high affinity IgE Fc receptors (FcεRI) on their surface, contain cytoplasmic granules which are major sources of histamine and other inflammatory mediators, and are activated to release and generate these mediators by IgE-dependent and non-IgE-dependent mechanisms [1]. Disturbances either in the release of mast cell mediators or in mast cell proliferation are associated with clonal mast cell disorders including monoclonal mast cell activation syndrome (MMAS) and mastocytosis respectively, which are in turn associated with some cases of anaphylaxis [2]. Molecular mechanisms have been identified which may link increased releasability of mast cell mediators and conditions leading to increased mast cell numbers [3]. Patients with mastocytosis have an increased risk to develop anaphylaxis [4, 5] and those with anaphylaxis may suffer from unrecognized mastocytosis or may display incomplete features of the disease [6–8].
Description
Mastocytosis is a disorder characterized by increased numbers of mast cells in the skin, bone marrow, gastrointestinal tract, liver, spleen, and lymph nodes [9, 10]. The prevalence is unknown; the incidence has been roughly estimated to be three to seven new patients per million per year [9]. Most cases are sporadic with only a limited number (50–100) of cases with mastocytosis reported to pass from generation to generation [11]. Mastocytosis presents at any age, although most cases occur during the first 2 years of life (childhood-onset) or after puberty (adult-onset) [9]. Mastocytosis in childhood often is self-limited and involves only the skin; whereas the course in patients with adult-onset disease is normally chronic and includes systemic involvement.
Pathogenesis
The most important survival and growth factor for mast cells is the KIT ligand stem cell factor (SCF) [12]. The hypothesis of early studies, that SCF might be elevated in skin lesions associated with mastocytosis [13], however, was not confirmed by later studies on SCF levels in skin and blood, at least for adult patients [14].
Rather, it is now thought that an associated and early event in the evolution of mastocytosis is the occurrence of an activating mutation in c-kit, the gene for KIT [10, 15]. KIT is a transmembrane tyrosine kinase growth receptor expressed on mast cells. Ligation of KIT by SCF promotes dimerization of the receptor and subsequent intrinsic tyrosine kinase activation. The resulting phosphorylated tyrosine residue serves as a docking site for intracellular signaling pathways leading to mast cell proliferation and activation.
In 1995, Nagata et al. identified a point mutation consisting of a substitution of valine for aspartic acid in the catalytic domain of c-kit (D816V) in the peripheral blood of patients with mastocytosis and predominately myelodysplastic features [16]. Subsequently, the same mutation was identified in adult patients with different forms of mastocytosis in tissues where mast cells are abundant, such as bone marrow, skin and spleen [17]. It is now believed that more than 90% of adults with mastocytosis have the D816V mutation, if bone marrow mononuclear cells are examined [17]. In a subset of patients, primarily those with more severe disease, the clone expands sufficiently to be detected in peripheral blood [16].
Thus, mastocytosis appears connected with the presence of activated KIT, at least in adult patients. The huge variance of symptomatology and disease severity among patients with mastocytosis, however, appears to depend on secondary or coexisting factors [2]. For example, a gain of function polymorphism in the gene for the IL-4 receptor alpha chain (Q576R) has been reported to be associated with less extensive mast cell involvement [18]. As early addition of IL-4 to human mast cell cultures decreases mast cell number by down-regulating KIT expression, the hypothesis is that the polymorphism in the IL-4 receptor results in increased IL-4-induced signaling, limiting the mast cell proliferation by KIT.
In children, the D816V c-kit mutation, and other less common mutations in c-kit, such as V560G, D816Y, D816F, D816H, E839K, R815K, D820G, V533D, V559A, del419, K509I, F522C, and A533D have been detected occasionally in biopsies of lesional skin or bone marrow, mainly in those with more severe forms of mastocytosis [2, 15]. However, overall c-kit mutations are untypical in children with infant-onset maculopapular cutaneous mastocytosis [2]. Thus, in many children, mastocytosis appears to have a different basis from that in most adults.
Previous studies have revealed that activation of KIT markedly potentates FcεRI-mediated mast cell degranulation [19]. In a study in human mast cells, it was sought how these pathways were linked and how upstream signals produced by FcεRI and KIT were integrated to produce these downstream synergistic responses in mast cells [3]. It was shown that Linker of Activation of T cells 2 (LAT2) has a role both in antigen-mediated and KIT-enhanced degranulation. Using knock-out mice deficient in specific tyrosine kinases, it was demonstrated that FcεRI employs the tyrosine kinases Lyn and Syk for LAT2 phosphorylation, whereas KIT directly phosphorylates LAT2 [3]. There was evidence for a role of LAT2 in regulating PLCγ1-dependent calcium mobilization in mast cells. Further, phosphoinositide 3-kinase appeared to be a critical player in the amplification pathway utilized by KIT activation for the potentiation of antigen-mediated responses. These insights provide the molecular basis for the observation that in mastocytosis, where an activating mutation of KIT exists, elevated KIT signaling may further potentate mast cell activation.
Clinical features
Cutaneous involvement
Maculopapular cutaneous mastocytosis (MPCM) is the presenting feature in most children and the majority of adult patients with systemic mastocytosis [9]. This form has also been termed urticaria pigmentosa (UP). The classical lesion of MPCM is a hyperpigmented macula or slightly elevated papula. In adults, the mean diameter of the lesions is three millimeters [20]. Lesions occur in a symmetric and disseminated distribution; the trunk and thighs tend to have the highest density of lesions (Figure 1A). In some patients the lesions are few and not easily recognized (Figure 1B). When mechanically irritated, the lesions develop an edematous wheal. This reaction is referred to as Darier’s sign. In children, lesions of MPCM are larger (mean diameter of five millimeters), and tend to be more hyperpigmented. The trunk is the most affected site. In addition, MPCM may have other clinical presentations that are less typical [10, 21]. Thus, MPCM may be telangiectatic and lack pigment (telangiectasia macularis eruptiva perstans, [TMEP]), plaque-like (multiple mastocytomas) or nodular [10, 22].
Fig. 1. Typical maculopapular cutaneous mastocytosis (urticaria pigmentosa) in adults.
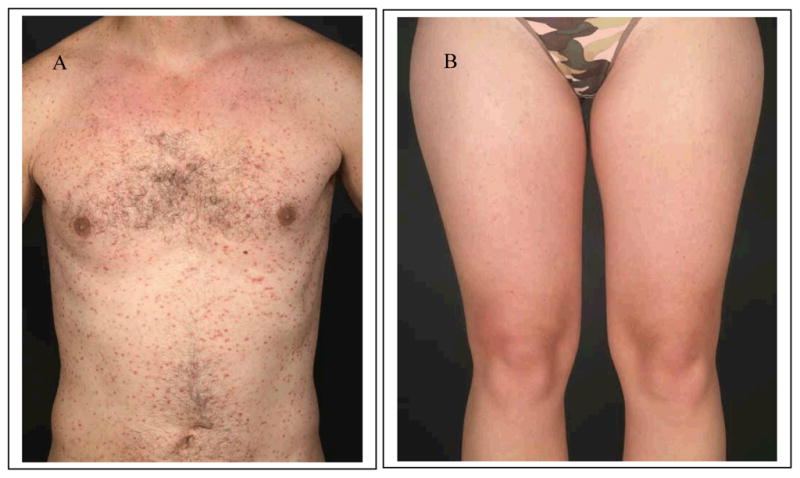
Typical maculopapular cutaneous mastocytosis (urticaria pigmentosa) is the most common form of cutaneous mastocytosis. In adults, lesions consist of numerous small red-brown macules and slightly elevated disseminated papules (A). In less obvious cases, however, lesions are few or less well recognizable (B) and may be overlooked.
Mastocytomas and diffuse cutaneous mastocytosis (DCM) are further manifestations of cutaneous mastocytosis (CM) [9]. Solitary mastocytomas are common in children. Most are present at birth or develop in infancy. These lesions are flat or mildly elevated, well demarcated, solitary yellowish red-brown plaques or nodules, typically 2–5 cm in diameter. DCM is a rare disorder characterized by diffuse mast cell infiltration of large areas of the skin that presents in infants in the first year of life. Severe edema and leathery indurations of the skin leads to accentuation of skin folds (pseudo-lichenified skin) and a peau-d’orange-like appearance. Systemic complications include hypotension and gastrointestinal hemorrhage. Infants and young children with considerable mast cell infiltration of the skin sometimes exhibit blister formation in the first three years of life. MPCM and other forms of CM have been classified in a consensus nomenclature (Table 1) [10].
Table 1.
Classification of cutaneous forms of mastocytosis (adapted from [10])
| Maculopapular cutaneous mastocytosis/Urticaria pigmentosa |
| Subvariants: Typical urticaria pigmentosa |
| Plaque-form |
| Nodular |
| Telangiectasia macularis eruptiva perstans (TMEP) |
| Diffuse cutaneous mastocytosis |
| Mastocytoma of skin |
WHO classification of systemic involvement
Whereas in children internal organ involvement (systemic mastocytosis, SM) is unusual, MPCM in adults is associated with SM in the majority of cases [10]. WHO criteria for SM consist of the major criterion of multifocal mast cell infiltrates in the bone marrow or other extracutaneous organ(s) and four minor criteria (Table 2)[21]: 25% or more of mast cells in non-cutaneous biopsy sections with spindle-shaped or abnormal morphology, or >25% of mast cells in bone marrow aspirate smears are immature or atypical; detection of a c-kit mutation at codon 816 in non-cutaneous organ(s); mast cells in extracutaneous organs co-expressing KIT with CD2 and/or CD25; and a baseline serum total tryptase persistently of >20 ng/mL. If at least one major and one minor, or at least three minor criteria for SM are fulfilled, the diagnosis is made. If the major criterion is absent and bone marrow mast cells are KIT+ and/or CD2+ and/or CD25+ in the presence of mediator related symptoms, the findings are consistent with a clonal mast cell disorder MMAS [10]. Criteria defining the mast cell burden, involvement of non-mast cell lineages and aggressiveness of disease subclassify SM in subvariants (Table 3) [21].
Table 2.
WHO Classification of mastocytosis (adapted from [10])
| Cutaneous mastocytosis (CM) |
| Indolent systemic mastocytosis (ISM) |
| Subvariants: Isolated bone marrow mastocytosis |
| Smouldering systemic mastocytosis |
| Systemic mastocytosis with an associated clonal hematologic non-mast-cell lineage disease (SM-AHNMD) |
| Aggressive systemic mastocytosis |
| Subvariant: Lymphadenopathic mastocytosis with eosinophilia |
| Mast cell leukemia |
| Mast cell sarcoma |
| Extracutaneous mastocytoma |
Table 3.
| Major criterion |
| Multifocal dense infiltrates of mast cells in bone marrow and/or other extracutaneous organs |
Minor criteria
|
One major and one minor; or three minor criteria are needed for the diagnosis of systemic mastocytosis
In children, CM is normally the only manifestation, and resolves in more than 50% of such cases during puberty [23]. Most adults fall into the indolent systemic mastocytosis (ISM) category [4, 17]. Although progression of skin involvement may occur, the disease tends to remain stable over many years and evolution of disease into more severe forms is uncommon [10]. Most adults with CM show focal or diffuse accumulations of mast cells in the bone marrow [9, 10]. Osteoporosis is not uncommon, and in occasional cases, osteopenia, sclerosis, cystic lesions and, in severe disease, pathologic fractures are reported [9, 10]. Patients with extensive disease may exhibit hepatomegaly, splenomegaly or lymphadenopathy caused by accumulation of mast cells in these organs. Anemia, thrombocytopenia and eosinophilia are less common.
Patients in the more aggressive categories are less likely to exhibit involvement of the skin and have a less favorable prognosis [10]. Those patients may have a definable hematological disorder such as a myelodysplastic syndrome, myeloproliferative disorder, acute leukemia, or a malignant lymphoma. In aggressive mastocytosis and mast cell leukemia, the clinical course is determined by the rapidity of the increase in mast cell numbers.
Mast cell mediator-induced symptoms
Some patients with mastocytosis report flushing, shortness of breath, palpitations, nausea, diarrhea, hypotension or even syncope [9, 24]. Lethargy and fatigue lasting several hours may follow. Gastrointestinal complaints are common in patients with SM [9, 24]. Abdominal pain is the most frequent symptom, followed by nausea, diarrhea, and vomiting. Diarrhea is not generally related to gastric hypersecretion and has been attributed to altered intestinal secretion, structural mucosal abnormalities, and hypermotility [25]. These symptoms are believed to result in part from an excess release of mast cell mediators.
Patients sometimes describe recurrent spontaneous episodes with a single symptom or a combination of symptoms, and where findings sometimes resemble anaphylaxis. The presence, frequency and severity of these symptoms cannot be predicted by the degree of organ involvement, although such symptoms are considerably more frequent with systemic disease [25]. Severe or protracted anaphylaxis may occur in patients with extensive disease, and even fatal reactions have been described [26]. Those episodes may be IgE-mediated, as following a bee sting in a sensitized individual; although anaphylaxis in a patient with MMAS or SM may occur in the absence of demonstrable allergic sensitivity.
Mastocytosis and anaphylaxis
Frequency of anaphylaxis in patients with mastocytosis
The cumulative prevalence of anaphylaxis in adults with the diagnosis of mastocytosis has been reported to be as high as 49%, and thus is considerably higher than expected in the general population [4]. In a another study, the frequency of anaphylaxis in adults with mastocytosis was reported to be 22% [5]. In children with CM, the prevalence was significantly lower and was reported to be 6% and 9%, respectively [4, 5]. One difference between these studies was the definition of anaphylaxis, for which there is yet no universal agreement. In addition to different patient populations, in the first study, anaphylaxis was more broadly defined according to World Allergy Organization criteria as a severe, life-threatening generalized or systemic hypersensitivity reaction [27]. In the second study, anaphylaxis was diagnosed only when more than one organ system symptoms were present or if there was a laryngeal edema [5], according to the recommendations of a consensus symposium [28]. In adults, those with SM had an increased risk for anaphylaxis as compared to patients with CM only [4]. In children, the risk to develop anaphylaxis was restricted to those with extensive skin involvement and high serum levels of tryptase [4].
Clinical features of anaphylaxis
The most frequent symptoms of anaphylaxis in patients with mastocytosis are decreased blood pressure and tachycardia Also observed are dizziness, dyspnea, flushing, nausea and diarrhoea [4]. Severe reactions are typical for patients with mastocytosis. In 55 patients with insect sting allergy and confirmed mastocytosis, 81% of patients experienced severe anaphylaxis with shock or cardiopulmonary arrest, whereas clinical reactions of this severity occurred in only 17% of 504 patients without evidence for mastocytosis and normal tryptase levels [29]. In another study in patients with mastocytosis, where the severity of anaphylaxis was rated, 60% reported severe symptoms and 43% experienced loss of consciousness [4]. In addition, there are reports of fatal anaphylactic reactions in patients with mastocytosis [26]. The risk for anaphylaxis appears not to be strictly associated with the mast cell load. For example, we care for patients with the smouldering variant of SM, which is characterized by a high mast cell load and highly elevated serum tryptase levels; and where we have yet to have a report of anaphylaxis.
Elicitors of anaphylaxis
As in patients without SM, in patients with mastocytosis the most frequent reported elicitors of anaphylaxis are insect venoms, drugs and foods [4, 5]. Atopic diseases and the prevalence of allergy in patients with mastocytosis are similar to the prevalence in the general population [5]. Specific IgE antibodies were commonly found in patients with insect venom allergy, but not to drugs and foods [5, 7]. Foods were implicated in the onset of an anaphylactic episode by some patients with mastocytosis, but this was not confirmed following clinical evaluation. Specific IgE to relevant foods is seldom found [5]. There is insufficient evidence to conclude that histamine in foods can elicit systemic reactions in patients with mastocytosis. The hypothesis that patients with reduced levels of diaminooxidase (a histamine-degrading enzyme) may experience anaphylaxis following histamine intake is not supported by the observation that diaminooxidase levels were no different in patients with mastocytosis with and without anaphylaxis [4].
Some drugs reported to elicit anaphylaxis in patients with mastocytosis [4, 5], such as opiates (including morphine and codeine) and muscle relaxants, may in some instances, directly activate mast cells in some, but not all, patients. Severe anaphylactoid reactions as well as coagulopathy have been reported in a few patients with mastocytosis undergoing general anesthesia, or following upper gastrointestinal endoscopy [9]. Other pharmaceutical agents, such as aspirin (acetylsalicylic acid) and other NSAIDs and alcohol are described to elicit pseudo-allergic reactions in a subset of patients with mastocytosis and have been reported to be augmentation factors for IgE-mediated allergic reactions. Antibiotics (such as betalactams) and radiocontrast media are also associated with episodes of anaphylaxis in some patients with and without mastocytosis [4].
Hymenoptera venom is a prominent trigger of systemic reactions. Severe and fatal reactions have been described in patients with mastocytosis [9, 30, 31]. In few cases with UP and hymenoptera venom anaphylaxis, no sensitization could be detected by means of skin tests and determination of specific IgE antibodies [32]. However, larger series found evidence that these systemic reactions are normally IgE-mediated insect sting allergies [7, 33].
Physical factors, such as heat, mechanical stimulation and exercise, may sometimes lead to mast cell degranulation and whealing in the skin, but rarely provoke systemic anaphylaxis [4, 33]. Patients do report that these and other factors in combination (such as exercise, heat and alcohol) may elicit anaphylaxis in summation. In one study, 26% of anaphylactic reactions were reported to have developed after a combination of elicitors [4]. In other patients with mastocytosis, anaphylaxis remains idiopathic despite an extensive search for an allergic basis.
Diagnosis
Mastocytosis is recognized in most patients because of the presence of characteristic cutaneous lesions [10]. A positive Darier’s sign and/or histological examination of the skin using metachromatic stains, or by immunohistochemistry using antibodies to mast cell tryptase, helps confirm the diagnosis of cutaneous disease. Different forms of precursor and mature forms of mast cell tryptase have been described, namely α-, β-, γ-, and δ-tryptase [34]. The monoclonal antibodies prepared against tryptase used in the assay commercially available (Phadia, Uppsala, Sweden) recognize mature and precursor forms of α- and β-tryptases only, whereas the other forms are either not recognized or not present in human mast cells. This is clinically relevant, because α/β-tryptase precursors seem to be continuously secreted by human mast cells, their level in the serum probably providing a measure of systemic mast cell involvement, whereas mature tryptase, presumably β-tryptase, is stored in secretory granules and is released only during granule exocytosis, with levels thereby reflecting mast cell activation.
Thus, whereas biochemical demonstration of elevated serum levels of total (α/β-) mast cell tryptase (>20 ng/mL) raises the suspicion of SM, in CM, tryptase levels tend to be within the normal range. Particularly in adults with CM and elevated serum tryptase, bone marrow biopsy and aspiration should be considered for staging of disease and for exclusion of associated haematological abnormalities. The most sensitive method to support the diagnosis of SM in the bone marrow aspirate is to identify the co-expression of CD2 and/or CD25 in CD117 (KIT)-positive mast cells by flow cytometry or by immunohistochemical analysis of bone marrow biopsies [35, 36]. In addition, a c-kit mutational analysis should be performed [37]. The D816V point mutation is best detected in bone marrow tissues, where the number of malignant mast cells is high. The diagnosis of SM and determination of the subcategory is made according to WHO criteria [21]. In patients with SM, the size of the liver and spleen may be evaluated by ultrasound or CT. Bone density is determined by the DXA method because of the risk of osteoporosis.
If patients have experienced anaphylaxis, the identification of any possible elicitor is important to help avoid further episodes. With skin tests and specific IgE antibodies combined with history, a relevant allergy may be detected. Cellular tests monitoring basophil histamine release or basophil activation may be helpful in some patients who resist diagnosis by standard means [26, 31].
Mastocytosis may present as anaphylaxis [9]. Sometimes the diagnosis of MPCM is made during the course of the evaluation of an anaphylactic episode. Thus in all patients with anaphylaxis, a skin examination should be performed to exclude CM [26]. Basal serum tryptase level should be determined. As serum tryptase levels may stay elevated for several hours after severe anaphylaxis, it is important to repeat the determination after some days. In cases with anaphylaxis and basal tryptase values >20 ng/ml, a bone marrow biopsy to exclude SM may be warranted. This examination may also be considered in patients with recurrent anaphylactic episodes even without clearly elevated tryptase levels. There have been cases with idiopathic anaphylaxis, in whom the diagnosis of SM was made by bone marrow biopsy[26, 32].
The activating D816V KIT mutation and other minor criteria for the diagnosis of mastocytosis have been reported in some patients with anaphylaxis who do not meet not the full diagnostic criteria for mastocytosis [8]. In a report on 12 patients with unexplained anaphylaxis, who did neither exhibit urticaria pigmentosa, nor the characteristic bone marrow biopsy finding of multifocal mast-cell aggregates for SM, five patients had evidence of 1 or more minor criteria for mastocytosis [6]. The D816V KIT mutation was found in all three patients in CD25-positive bone marrow cells where the analysis was performed. This report and others led a consensus conference to conclude that if the major criterion for the diagnosis of mastocytosis is absent and bone marrow mast cells are KIT+ and/or CD2+ and/or CD25+ in the presence of mediator related symptoms, the diagnosis should be MMAS [10].
In a later study, the incidence of a clonal mast cell disorder (either mastocytosis or MMAS) was assessed in 44 subjects with a serum baseline total tryptase level greater than 11.4 ng/ml who were among 379 patients with a prior systemic immediate hypersensitivity reaction to a hymenoptera sting. Resultant data indicated that the majority of these patients had an underlying clonal mast cell disorder, either SM or MMAS, by assessing the bone marrow for mast cell granulomas, spindle-shaped mast cells and mast cells expressing surface CD2 or CD25; and the serum for an elevated tryptase level [7]. These results demonstrate the presence of an aberrant mast-cell population carrying clonal markers in patients diagnosed with idiopathic or hymenoptera venom anaphylaxis. In patients with anaphylaxis and increased serum tryptase levels, a BM examination may thus be indicated for the diagnosis of a clonal mast cell disease.
Therapy
There is no cure for mastocytosis and treatment remains largely symptomatic [2, 9, 10]. All patients with mastocytosis should be informed about the disease, including prognosis and complications. Therapy for mastocytosis encompasses avoidance of trigger factors, targeting symptoms of mast cell mediator release and therapy of skin lesions. Cytoreductive forms of treatment are only indicated in patients with aggressive mastocytosis, or an associated hematological disorder, including mast cell leukemia.
Avoidance of trigger factors and treatment of anaphylaxis
Specific information on trigger factors and signs of mast cell degranulation, potentially leading to anaphylactic reactions, should to be provided. A list of trigger factors known to induce symptoms in patients is given in Table 4 [9]. Not all patients with mastocytosis have reactions provoked by all factors. For example, many patients with mastocytosis tolerate radiocontrast media, anesthesia, morphine, dextrometorphan, aspirin, and other analgesics. When in question, patients should undergo graded challenges to determine sensitivity.
Table 4.
Triggers for mast cell mediator release*
| Mechanical irritation of the skin (rubbing, scratching, friction) |
| Heat (e.g. hot shower), cold, sudden change of temperature |
| Physical exercise |
| Insect stings (e. g. bee and wasp stings) |
| Drugs (radiocontrast media, drugs used in general anaesthesia, codeine morphine, dextromethorphan, aspirin, and other analgesics) |
| Infection |
| Alcohol |
Note not all patients with mastocytosis have reactions provoked by all factors. For example, many patients with mastocytosis tolerate radiocontrast media, anaesthesia, morphine, dextrometorphan, aspirin, and other analgesics. When in question, patients should undergo graded challenges to determine sensitivity.
It has been stated that adults with mastocytosis as well as children with bullous lesions and with more severe involvement, and especially those with previous reactions, are at increased risk for anaphylaxis [4]. Thus, we recommend that patients at risk carry an emergency kit for self medication which includes epinephrine and, as warranted, an antihistamine and a corticosteroid [38].
Patients with mastocytosis also have to be considered at increased risk when administered iodinated contrast media, when undergoing general anesthesia, or during endoscopic procedures. Protocols aiming at ameliorating these reactions employ premedication with H1- and H2-antihistamines, sometimes corticosteroids depending upon the procedure, and availability of means of resuscitation [26]. In 22 children with mastocytosis, the frequency and adverse reactions to routine anesthesia was assessed [39]. In 29 anaesthetic procedures, including 24 cases with general anaesthesia, these procedures were tolerated with the exception of 2 cases with flushing and 4 cases with nausea and/or vomiting even without specific premedication. Thus, as long as the anaesthetist is informed about the patient and regular stabilizing medications are continued, at least in children, a premedication may not be routinely necessary before general anaesthesia.
Diet should be modified only in cases where foods have been proven to elicit symptoms. Patients with mastocytosis and hymenoptera venom exposure are at risk for severe anaphylaxis. Thus, specific immunotherapy should be considered in patients with hymenoptera venom allergy and then administered under close supervision [31]. The majority of patients with mastocytosis reportedly tolerate immunotherapy without significant side effects and appear protected following this approach [33, 40]. However, there does appear to be some increased risk for adverse reactions during initiation of immunotherapy, as well as for therapy failures [31, 33]. An increased maintenance dose of insect venom has been reported to carry better success rates by sting provocation [41]. Also, in the light of two fatal cases of anaphylaxis after discontinuation of SIT in patients with mastocytosis [30], lifelong immunotherapy should be considered [26].
In rare cases, initiation of specific immunotherapy with insect venom leads to recurrent anaphylaxis, even with antihistamine premedication. In those cases, comedication with omalizumab (anti-IgE) has been reported to induce tolerance. In a case of recurrent anaphylaxis to induction of specific immunotherapy, the injection of 300 mg of omalizumab between four days and one hour reportedly led to tolerance [42]. This approach also appears worthy of consideration in patients with both idiopathic recurrent anaphylaxis and mastocytosis who do not respond to standard antimediator therapy, as has been described in two atopic patients with ISM [43]. Most patients with mastocytosis and idiopathic anaphylaxis, however, are sufficiently controlled by standard antimediator therapy with antihistamines with or without low-dose corticosteroids.
Therapy of mast cell mediator-induced symptoms
Recommendations for the treatment of mastocytosis have been published [10]. In brief, nonsedating H1-antihistamines are the agents of choice for pruritus and associated wheal and flare of skin lesions. For prophylaxis of recurrent episodes of anaphylaxis or persistent pruritus, antihistamines should be administered daily. Gastric acid hypersecretion, peptic ulcer disease, and reflux esophagitis may be managed with H2-receptor blockers and/or proton pump inhibitors. Cromolyn sodium may be helpful for gastrointestinal symptoms, particularly in children. Anticholinergics may be tried to control diarrhea. Systemic corticosteroids are used restrictedly in cases of malabsorption or ascites. Calcium, vitamin D and bisphosphonates are the drugs of choice for osteoporosis.
Therapy of skin lesions
Application of topical corticosteroids under occlusion, or UV therapy (PUVA, UVA1) has been reported to lead to dermatologic improvement in patients with CM [9]. Both are effective in reducing pruritus and urtication, but relapse typically occurs a few months after therapy is discontinued. The benefits of these forms of therapy have to be weighed against the potential for inducing side effects. Thus, topical corticosteroids should be considered primarily for the treatment of localized skin lesions, such as mastocytomas causing flushing or blistering. Topical treatment with the raft modulator miltefosine has been tried as a novel therapeutic option for mastocytosis [44]. However, twice daily application of miltefosine solution led to irritation rather than reduction of skin swelling and skin numbers, possibly due to the presence of potentially irritating alkanol propandiol as a vehicle.
Therapy of aggressive forms of mastocytosis
Patients with SM-AHNMD are managed using therapy appropriate for the associated hematological disorder [2, 10]. Chemotherapy has not been shown to produce remission or to effectively prolong survival in patients with mast cell leukemia; and is not indicated in the management of ISM, as it may lead to bone marrow suppression without improving the symptoms of mastocytosis. A partial response to interferon-α2b, often in combination with corticosteroids, has been reported in some patients with aggressive disease [2]. Cladribrine (2-cholorodeoxyadenosin) has been reported to reduce the mast cell burden in case reports and may be used in aggressive mastocytosis [2]. It should be considered; however, that cladribine can induce pancytopenia, and has an unknown potential oncogenicity. Splenectomy may be performed in the management of patients with aggressive mastocytosis and hypersplenism leading to portal hypertension.
Newer therapeutic approaches being investigated target neoplastic mast cells bearing CD25, or inhibit tumor necrosis factor (Thalidomid, Lenalidomid) [2]. Bone marrow transplantation is under investigation, although results to date have been disappointing or have to be reproduced [2].
The tyrosine kinase inhibitor imatinib is not generally indicated in patients with typical D816V mutations in KIT, as steric conformations of the receptor interfere with the action of the drug [45]. The drug has been reported to reduce mast cell load and symptoms in patients with mutations in c-kit at other sites [46]. Other tyrosine kinase inhibitors, such as dasatinib, PKC412 and AMN 107, inhibit KIT with mutations at codon 816. Data available indicate a somewhat limited effect of these drugs on aggressive mast cell disease and/or significant toxicity [2]. It has been proposed that a multiple drug approach using combination therapy with targeted agents that have different mechanisms of action be considered [2].
Acknowledgments
This work was in part supported by the Division of Intramural Research, NIAID/NIH.
References
- 1.Metz M, Brockow K, Metcalfe DD, Galli SJ. Mast cells, basophils and mastocytosis. In: Rich, Fleisher, Shearer, et al., editors. Clinical Immunology – Principles and Practice, Edition. London, Edinburgh, New York: Mosby International Ltd; 2008. pp. 22.21–22.16. [Google Scholar]
- 2.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–956. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tkaczyk C, Horejsi V, Iwaki S, et al. NTAL phosphorylation is a pivotal link between the signaling cascades leading to human mast cell degranulation following Kit activation and Fc epsilon RI aggregation. Blood. 2004;104:207–214. doi: 10.1182/blood-2003-08-2769. [DOI] [PubMed] [Google Scholar]
- 4.Brockow K, Jofer C, Behrendt H, Ring J. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63:226–232. doi: 10.1111/j.1398-9995.2007.01569.x. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez de Olano D, de la Hoz Caballer B, Nunez Lopez R, et al. Prevalence of allergy and anaphylactic symptoms in 210 adult and pediatric patients with mastocytosis in Spain: a study of the Spanish network on mastocytosis (REMA) Clin Exp Allergy. 2007;37:1547–1555. doi: 10.1111/j.1365-2222.2007.02804.x. [DOI] [PubMed] [Google Scholar]
- 6.Akin C, Scott LM, Kocabas CN, et al. Demonstration of an aberrant mast-cell population with clonal markers in a subset of patients with “idiopathic” anaphylaxis. Blood. 2007;110:2331–2333. doi: 10.1182/blood-2006-06-028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonadonna P, Perbellini O, Passalacqua G, et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels. J Allergy Clin Immunol. 2009 doi: 10.1016/j.jaci.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Sonneck K, Florian S, Mullauer L, et al. Diagnostic and subdiagnostic accumulation of mast cells in the bone marrow of patients with anaphylaxis: Monoclonal mast cell activation syndrome. Int Arch Allergy Immunol. 2007;142:158–164. doi: 10.1159/000096442. [DOI] [PubMed] [Google Scholar]
- 9.Brockow K. Urticaria pigmentosa. Immunol Allergy Clin North Am. 2004;24:287–316. doi: 10.1016/j.iac.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Valent P, Akin C, Escribano L, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37:435–453. doi: 10.1111/j.1365-2362.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- 11.Brockow K, Metcalfe DD. Mastocytosis. Curr Opin Allergy Clin Immunol. 2001;1:449–454. doi: 10.1097/01.all.0000011059.59188.08. [DOI] [PubMed] [Google Scholar]
- 12.Akin C, Metcalfe DD. The biology of Kit in disease and the application of pharmacogenetics. J Allergy Clin Immunol. 2004;114:13–19. doi: 10.1016/j.jaci.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 13.Longley BJ, Jr, Morganroth GS, Tyrrell L, et al. Altered metabolism of mast-cell growth factor (c-kit ligand) in cutaneous mastocytosis. N Engl J Med. 1993;328:1302–1307. doi: 10.1056/NEJM199305063281803. [DOI] [PubMed] [Google Scholar]
- 14.Brockow K, Akin C, Huber M, et al. Levels of mast-cell growth factors in plasma and in suction skin blister fluid in adults with mastocytosis: correlation with dermal mast- cell numbers and mast-cell tryptase. J Allergy Clin Immunol. 2002;109:82–88. doi: 10.1067/mai.2002.120524. [DOI] [PubMed] [Google Scholar]
- 15.Orfao A, Garcia-Montero AC, Sanchez L, Escribano L. Recent advances in the understanding of mastocytosis: the role of KIT mutations. Br J Haematol. 2007;138:12–30. doi: 10.1111/j.1365-2141.2007.06619.x. [DOI] [PubMed] [Google Scholar]
- 16.Nagata H, Worobec AS, Oh CK, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A. 1995;92:10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Montero AC, Jara-Acevedo M, Teodosio C, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108:2366–2372. doi: 10.1182/blood-2006-04-015545. [DOI] [PubMed] [Google Scholar]
- 18.Daley T, Metcalfe DD, Akin C. Association of the Q576R polymorphism in the interleukin-4 receptor alpha chain with indolent mastocytosis limited to the skin. Blood. 2001;98:880–882. doi: 10.1182/blood.v98.3.880. [DOI] [PubMed] [Google Scholar]
- 19.Gilfillan AM, Peavy RD, Metcalfe DD. Amplification mechanisms for the enhancement of antigen-mediated mast cell activation. Immunol Res. 2009 doi: 10.1007/s12026-008-8046-9. in press. [DOI] [PMC free article] [PubMed]
- 20.Brockow K, Akin C, Huber M, Metcalfe D. Assessment of the extent of cutaneous involvement in children and adults with mastocytosis: relationship to symptomatology, tryptase levels, and bone marrow pathology. J Am Acad Dermatol. 2003;48:508–516. doi: 10.1067/mjd.2003.98. [DOI] [PubMed] [Google Scholar]
- 21.Valent P, Horny H, Li C, et al. Mastocytosis. In: Jaffe E, Harris N, Stein H, Vardiman J, editors. World Health Organization classification of tumors. Pathology and genetics of tumors of hematopoietic and lymphoid tissue, Edition. Lyon: IARC Press; 2001. pp. 293–302. [Google Scholar]
- 22.Simpson JK, Brockow K, Turner ML, et al. Generalized erythematous macules and plaques associated with flushing, repeated syncope, and refractory anemia. J Am Acad Dermatol. 2002;46:588–590. doi: 10.1067/mjd.2002.120446. [DOI] [PubMed] [Google Scholar]
- 23.Caplan RM. The natural course of urticaria pigmentosa. Arch Dermatol. 1963;87:146–157. doi: 10.1001/archderm.1963.01590140008002. [DOI] [PubMed] [Google Scholar]
- 24.Horan RF, Austen KF. Systemic mastocytosis: retrospective review of a decade’s clinical experience at the Brigham and Women’s Hospital. J Invest Dermatol. 1991;96:5S–13S. [PubMed] [Google Scholar]
- 25.Travis WD, Li CY, Bergstralh EJ, et al. Systemic mast cell disease. Analysis of 58 cases and literature review [published erratum appears in Medicine (Baltimore) 1990 Jan;69(1):34] Medicine (Baltimore) 1988;67:345–368. [PubMed] [Google Scholar]
- 26.Brockow K, Ring J, Przybilla B, Ruëff F. Klinik und Therapie der Anaphylaxie bei Mastozytose. Allergo Journal. 2008;17:556–562. [Google Scholar]
- 27.Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 28.Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 29.Ludolph-Hauser D, Rueff F, Fries C, et al. Constitutively raised serum concentrations of mast-cell tryptase and severe anaphylactic reactions to Hymenoptera stings. Lancet. 2001;357:361–362. doi: 10.1016/S0140-6736(00)03647-3. [DOI] [PubMed] [Google Scholar]
- 30.Oude Elberink JN, de Monchy JG, Kors JW, et al. Fatal anaphylaxis after a yellow jacket sting, despite venom immunotherapy, in two patients with mastocytosis. J Allergy Clin Immunol. 1997;99:153–154. doi: 10.1016/s0091-6749(97)70314-2. [DOI] [PubMed] [Google Scholar]
- 31.Rueff F, Placzek M, Przybilla B. Mastocytosis and Hymenoptera venom allergy. Curr Opin Allergy Clin Immunol. 2006;6:284–288. doi: 10.1097/01.all.0000235903.10548.63. [DOI] [PubMed] [Google Scholar]
- 32.Florian S, Krauth MT, Simonitsch-Klupp I, et al. Indolent systemic mastocytosis with elevated serum tryptase, absence of skin lesions, and recurrent severe anaphylactoid episodes. Int Arch Allergy Immunol. 2005;136:273–280. doi: 10.1159/000083954. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez de Olano D, Alvarez-Twose I, Esteban-Lopez MI, et al. Safety and effectiveness of immunotherapy in patients with indolent systemic mastocytosis presenting with Hymenoptera venom anaphylaxis. J Allergy Clin Immunol. 2008;121:519–526. doi: 10.1016/j.jaci.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz LB. Diagnostic Value of Tryptase in Anaphylaxis and Mastocytosis. Immunol Allergy Clin N Am. 2006:451–463. doi: 10.1016/j.iac.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Escribano L, Orfao A, Diaz-Agustin B, et al. Indolent systemic mast cell disease in adults: immunophenotypic characterization of bone marrow mast cells and its diagnostic implications. Blood. 1998;91:2731–2736. [PubMed] [Google Scholar]
- 36.Horny HP, Valent P. Histopathological and immunohistochemical aspects of mastocytosis. Int Arch Allergy Immunol. 2002;127:115–117. doi: 10.1159/000048180. [DOI] [PubMed] [Google Scholar]
- 37.Sotlar K, Escribano L, Landt O, et al. One-step detection of c-kit point mutations using peptide nucleic acid-mediated polymerase chain reaction clamping and hybridization probes. Am J Pathol. 2003;162:737–746. doi: 10.1016/S0002-9440(10)63870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ring J, Brockow K, Duda D, et al. Akuttherapie anaphylaktischer Reaktionen. Allergo Journal. 2007;16:420–434. [Google Scholar]
- 39.Carter MC, Uzzaman A, Scott LM, et al. Pediatric mastocytosis: routine anesthetic management for a complex disease. Anesth Analg. 2008;107:422–427. doi: 10.1213/ane.0b013e31817e6d7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonadonna P, Zanotti R, Caruso B, et al. Allergen specific immunotherapy is safe and effective in patients with systemic mastocytosis and Hymenoptera allergy. J Allergy Clin Immunol. 2008;121:256–257. doi: 10.1016/j.jaci.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Rueff F, Wenderoth A, Przybilla B. Patients still reacting to a sting challenge while receiving conventional Hymenoptera venom immunotherapy are protected by increased venom doses. J Allergy Clin Immunol. 2001;108:1027–1032. doi: 10.1067/mai.2001.119154. [DOI] [PubMed] [Google Scholar]
- 42.Kontou-Fili K. High omalizumab dose controls recurrent reactions to venom immunotherapy in indolent systemic mastocytosis. Allergy. 2008;63:376–378. doi: 10.1111/j.1398-9995.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- 43.Carter MC, Robyn JA, Bressler PB, et al. Omalizumab for the treatment of unprovoked anaphylaxis in patients with systemic mastocytosis. J Allergy Clin Immunol. 2007;119:1550–1551. doi: 10.1016/j.jaci.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 44.Hartmann K, Siebenhaar F, Belloni B, et al. Topical treatment with the raft modulator miltefosine – a novel therapeutic option for mastocytosis? 2008 doi: 10.1111/j.1365-2133.2009.09434.x. submitted. [DOI] [PubMed] [Google Scholar]
- 45.Akin C, Brockow K, D’Ambrosio C, et al. Effects of tyrosine kinase inhibitor STI571 on human mast cells bearing wild-type or mutated c-kit. Exp Hematol. 2003;31:686–692. doi: 10.1016/s0301-472x(03)00112-7. [DOI] [PubMed] [Google Scholar]
- 46.Akin C, Fumo G, Yavuz AS, et al. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004;103:3222–3225. doi: 10.1182/blood-2003-11-3816. [DOI] [PubMed] [Google Scholar]


