Abstract
Little is known about the sylvatic transmission cycle of Trypanosoma cruzi in the Gran Chaco ecoregion. We conducted surveys to identify the main sylvatic hosts of T. cruzi, parasite discrete typing units and vector species involved in Pampa del Indio, a rural area in the humid Argentinean Chaco. A total of 44 mammals from 14 species was captured and examined for infection by xenodiagnosis and polymerase chain reaction amplification of the hyper-variable region of kinetoplast DNA minicircles of T. cruzi (kDNA-PCR). Ten (22.7%) mammals were positive by xenodiagnosis or kDNA-PCR. Four of 11 (36%) Didelphis albiventris (white-eared opossums) and six of nine (67%) Dasypus novemcinctus (nine-banded armadillos) were positive by xenodiagnosis and or kDNA-PCR. Rodents, other armadillo species, felids, crab-eating raccoons, hares and rabbits were not infected. Positive animals were highly infectious to the bugs that fed upon them as determined by xenodiagnosis. All positive opossums were infected with T. cruzi I and all positive nine-banded armadillos with T. cruzi III. Extensive searches in sylvatic habitats using 718 Noireau trap-nights only yielded Triatoma sordida whereas no bug was collected in 26 light-trap nights. Four armadillos or opossums fitted with a spool-and-line device were successfully tracked to their refuges; only one Panstrongylus geniculatus was found in an armadillo burrow. No sylvatic triatomine was infected with T. cruzi by microscopical examination or kDNA-PCR. Our results indicate that two independent sylvatic transmission cycles of T. cruzi occur in the humid Chaco. The putative vectors of both cycles need to be identified conclusively.
Keywords: Trypanosoma cruzi, Didelphis albiventris, Dasypus novemcinctus, reservoir, molecular epidemiology, discrete typing unit, vector
The protozoan Trypanosoma cruzi, the etiologic agent of Chagas disease, has been detected in more than 180 mammalian species from 7 orders and 25 families in the Americas (Barretto 1979; World Health Organization 2002; Noireau et al., 2009). The main hosts of T. cruzi are marsupials, edentates, rodents and carnivores in sylvatic habitats, and humans, dogs, cats and rodents in domestic or peridomestic transmission cycles. Originally a vector-borne zoonosis affecting sylvatic mammals, current estimates suggest that between 10 and 17 million people may be infected with T. cruzi (World Health Organization 2002; Schofield et al., 2006). An increasing number of food-borne outbreaks of human T. cruzi infection originating from different sylvatic sources has been recorded during recent decades (Alarcón de Noya et al., 2010).
Trypanosoma cruzi has recently been classified into six discrete typing units (DTUs) ranging from T. cruzi I (TcI) to T. cruzi VI (TcVI) (Zingales et al., 2009, 2012). DTUs were initially defined as "sets of stocks that are genetically more related to each other than to any other stock and that are identifiable by common genetic, molecular or immunological markers" (Tibayrenc, 1998). TcI has the widest distribution range from USA to northern Argentina and Chile; TcIII has been detected from western Venezuela down to the southern cone countries, and TcIV occurs north of the Amazon basin and in the USA, though there are some reports south of the Amazon (Barnabé et al., 2000; Yeo et al., 2005; Noireau et al., 2009; Llewellyn et al., 2009). TcIII and TcIV have usually been isolated from sylvatic mammals, but there are a few reports in humans from Colombia, Bolivia and Venezuela (Ramírez et al., 2010). Several DTUs occur mainly (TcII) or are confined to (TcV and TcVI) the southern cone countries where they frequently infect humans and dogs (Montamat et al., 1992; Brenière et al., 2002; Diosque et al., 2003; Burgos et al., 2007; Cardinal et al., 2008). Distinct transmission cycles may occur in parallel in the same forest fragment (Miles, 1979; Lisboa et al., 2004). The impressive genetic diversity of T. cruzi and heterogeneity in transmission cycles support the need for new detailed studies at defined spatial scales for better understanding of transmission dynamics and parasite evolution (Miles et al., 2003).
The Gran Chaco—a 1.3 million km2 plain mainly extending over Argentina (62%), Paraguay (25%) and Bolivia (12%)—is the most biodiverse ecoregion after the Amazon (The Nature Conservancy et al., 2005). A marked East-West gradient in annual rainfall created two subsections (humid in the east and dry in the west) home to 44 complex units of ecological systems. The Gran Chaco is hyperendemic for Chagas disease and other neglected infectious diseases (Gürtler, 2009; Gürtler et al., 2007a). In this region, T. cruzi infects various species of marsupials (e.g. Didelphis albiventris, white-eared opossums), armadillos (especially Dasypus novemcinctus, nine-banded armadillos), rodents (Calomys musculinus and C. laucha) and carnivores including skunks, ferrets, foxes and coatis (Carcavallo and Martínez, 1968; Barretto, 1979; Wisnivesky-Colli et al., 1992; Yeo et al., 2005; Ceballos et al., 2006). In the Argentinean and Paraguayan Chaco, TcI and TcIII mainly occurred in opossums and nine-banded armadillos, respectively (Montamat et al., 1992; Luca d’Oro et al., 1993; Brisse et al., 2000; Diosque et al., 2003; Yeo et al., 2005; Ceballos et al., 2006; Cardinal et al., 2008), whereas TcV and TcVI were more prevalent in domestic environments in Triatoma infestans, humans, dogs and cats. In the Bolivian Chaco there is also evidence of the occurrence of TcIII (Llewellyn et al., 2009). The most conspicuous potential vectors of T. cruzi in the Gran Chaco are T. infestans, Triatoma sordida, Triatoma guasayana and Triatoma eratyrusiformis (Carcavallo and Martínez, 1968; Carcavallo et al., 1998).
Large-scale changes in land use and habitat fragmentation throughout Latin America may impact on extant sylvatic transmission cycles of T. cruzi in different ways (Ceballos et al., 2006; Vaz et al., 2007). In the Gran Chaco, deforestation and rapid expansion of intensified agriculture have caused large degradation and fragmentation of natural forests and significant biodiversity losses (Bucher and Huszar, 1999). An assessment of the impacts of ongoing environmental changes requires more information on the structure of transmission cycles in distinct biological communities over time. As part of a multi-site project on the eco-epidemiology and control of Chagas disease in the Gran Chaco including sister sites in Bolivia and Paraguay, the current study sought to identify the main sylvatic hosts of T. cruzi in the humid Argentinean Chaco, circulating parasite DTUs, and vector species involved.
Materials and methods
Study area
Field work was conducted in a well-defined rural area (450 km2) in the municipality of Pampa del Indio (25° 55’S 56° 58’W), Province of Chaco, Argentina. The study area belongs to the eastern Chaco of the low Paraguay River (The Nature Conservancy et al., 2005) and has been described elsewhere (Gurevitz et al., 2011). In common agreement with the provincial Chagas disease control program, Pampa del Indio was selected for this study because it had high indices of infestation and the last treatment with residual insecticides by official vector control programs had been in 1997. Before all houses in the study area were sprayed with pyrethroid insecticides in November 2007, the prevalence of house infestation (as determined by pooling results from several methods: timed manual collections with a dislodging agents, knockdown during insecticide spraying, and householders’ bug collections) with T. infestans (46%) and infection with T. cruzi in domestic bugs (22%), dogs (26%) and cats (29%) were indicative of active domestic transmission (Gurevitz et al., 2011; Cardinal et al.,, unpublished results). Mean monthly temperatures range from 15°C in winter to 28°C in summer, and mean annual rainfall (954 mm) mainly occurs in summer and fall. Rural villagers mostly have a subsistence economy based on raising goats or poultry, growing various crops and cotton, and exploiting forest resources. The landscape includes a mosaic of crop or cotton fields and native dry forest with various degrees of degradation.
Mammal and triatomine capture
Mammals were sampled in sylvatic habitats around six rural villages (Santa Rita, Santos Lugares, El Salvaje, La Loma, Campo Los Toros and Los Ciervos) in August 2008. Medium-sized mammals (opossums, armadillos, felids and rabbits) were live-captured with National traps baited with beef or chicken scraps soaked in fish sauce deployed every 50 m along transects lines. Rodents were caught with Sherman traps baited with peanut butter, corn and barley seeds; they usually were deployed in association with a National trap. National-like, home-made traps were used by experienced local hunters who also caught mammals manually. The research team also caught two Procyon cancrivorus using tranquilizing darts (Telazol®, Tiletamine & Zolazepam, Fort Dodge). Traps were deployed in degraded woods, grasslands and in fields cultivated or covered by stubble, and their location georeferenced (Garmin Legend C). Traps were checked every morning and re-baited when needed. The total catch effort was 1,599 trap-nights with National or home-made traps, and 440 trap-nights with Sherman traps.
Biosafety and animal processing procedures were performed according to protocols approved by the Ethical Committee Dr. Carlos Barclay (Buenos Aires). Transit permits were also obtained from the provincial government. The captured animals were transported to the field laboratory to assess their clinical status before and after anesthesia. Parenteral anesthesia was performed with tiletamine clorhydrate and zolacepan clorhydrate (Zelazol®, Fort Dodge) or ketamine clorhydrate (Vetaset®, Fort Dodge) combined with xylacine (Ronpun®, Bayer) in doses appropriate to species and weight (Carpenter et al., 2001). Inhalatory anesthesia with Isofluorane® was used in rodents. Pregnant females were not anesthetized and were kept in quiet environments during procedures. Didelphis opossums and armadillos were assigned to age classes based on tooth eruption and wear (Schweigmann et al., 1999, Genoways and Timm, 2003). All animals were sexed and measured from snout to base of tail and tail. Animals were marked with numbered metal tags (National Band & Tag co.) and/or tattooed depending on the species, and then released at the capture site once they recovered fully from anesthesia.
Searches for sylvatic foci of triatomine bugs with mouse-baited sticky traps (Noireau traps) were conducted by three team members during three weeks in February–March 2008 (using 354 trap-nights in 12 sampling areas) and 2009 (using 364 trap-nights in 11 sampling areas). Baited sticky traps were deployed during late afternoons in hollow tree trunks (live or dead), fallen logs, and in the entrance of eight burrows; all sites were georeferenced and identified with a numbered piece of cloth as described elsewhere (Ceballos et al., 2009). High tree holes were accessed by means of a telescopic ladder. Temperature and relative humidity were measured with a data logger (Hobo). Traps were left overnight and recovered early in the following morning. The trapped triatomine bugs were carefully removed and stored in labeled tubes with folded filter paper. Light traps (a 1.25 × 2 m frame holding a white cloth illuminated with a black light tube) were set up on appropriate evenings for triatomine flight (i.e., with wind <10 km/h, >20°C and no rain) from 20:45 (sunset) to 23:00 hs (Vazquez-Prokopec et al., 2004). Wind speed was recorded with a hand-held electronic anemometer and temperature recorded with an automated electronic sensor during light-trapping efforts. Light traps were deployed far from houses in wood openings; they were operated in pairs in six areas in 2008 and in triplets or pairs in five areas in 2009 (12 and 14 light trap-nights, respectively). During light-trapping nights temperatures were 20.7–30.4°C and wind speed averaged 0.19 m/sec (range, 0 to 4.42 m/sec).
To locate any associated triatomine bug in opossum or armadillo burrows and tree holes, five armadillos from three species and one Didelphis opossum were fitted with a spool-and-line tracking device (weight, 70 g; range, 1,500–2,000 m) at the site of capture (Miles et al., 1981), released there in the evening and tracked during the next two days. Animals could not be tracked more than two days because the spool-and-line device was frequently dropped off when the animals entered some holes or the line was used up (one opossum). Burrows or tree holes where the tracked animals sought shelter were marked with a colored tape and georeferenced for subsequent searches of triatomine bugs when the tracking was completed. A vacuum cleaner fitted with a flexible hose was used to aspirate all materials and rubble within the nest or burrow. Because this procedure was considered ineffective, the burrow was dug up and all the material collected and screened for triatomine bugs.
All triatomines collected were identified to species and counted by stage as described by Canale et al. (2000). Given that the taxonomic identification of small nymphs based on morphological characters is difficult, first- or second-instar nymphs were individually placed in labeled plastic vials, artificially fed with uninfected rabbit blood and kept alive until molting.
Parasitologic methods
Blood samples were drawn by venipuncture from all specimens. An aliquot was diluted 1:1 in guanidine buffer for PCR and the remainder was centrifuged during 15 min. at 3,000 rpm for serum collection. Each animal was examined by xenodiagnosis with 5 (rodents), 10 (small armadillos and cavids) or 20 (other medium-sized mammals) uninfected fourth-instar nymphs of T. infestans contained in wooden boxes applied on the host during 25 min and checked for its degree of blood engorgement (Gürtler et al., 2007b). Pools of feces from five xenodiagnosis bugs that had fed on a given specimen were examined microscopically (Zeiss) at 400× magnification 30 and 60 days post-exposure. Bugs from each T. cruzi-positive pool were re-examined individually to assess the individual host’s infectiousness to the vector (i.e., number of infected bugs fed on a given individual divided by the total number of insects examined for infection at least once, excluding bugs that died prior to the first examination). The numbers of exuviae and of dead bugs in each box were recorded as an index of xenodiagnosis quality. Of 629 bugs used in xenodiagnosis, 94.1% were alive on first examination and 15.1% of those alive at first inspection molted within the 60-day observation period.
Feces from microscope-positive xenodiagnosis bugs were cultured in biphasic medium (brain-heart-infusion and nutrient agar mixed with defibrinated rabbit blood) as described by Lauricella et al. (2005). Parasite cultures were cryopreserved and DNA extracted after boiling samples for 15 min (Marcet et al., 2006). Blood samples mixed with guanidine-EDTA buffer were boiled for 15 min. and DNA was extracted with DNeasy Blood & Tissue Kit following manufacturers’ instructions (QIAGEN Sciences, Maryland, USA).
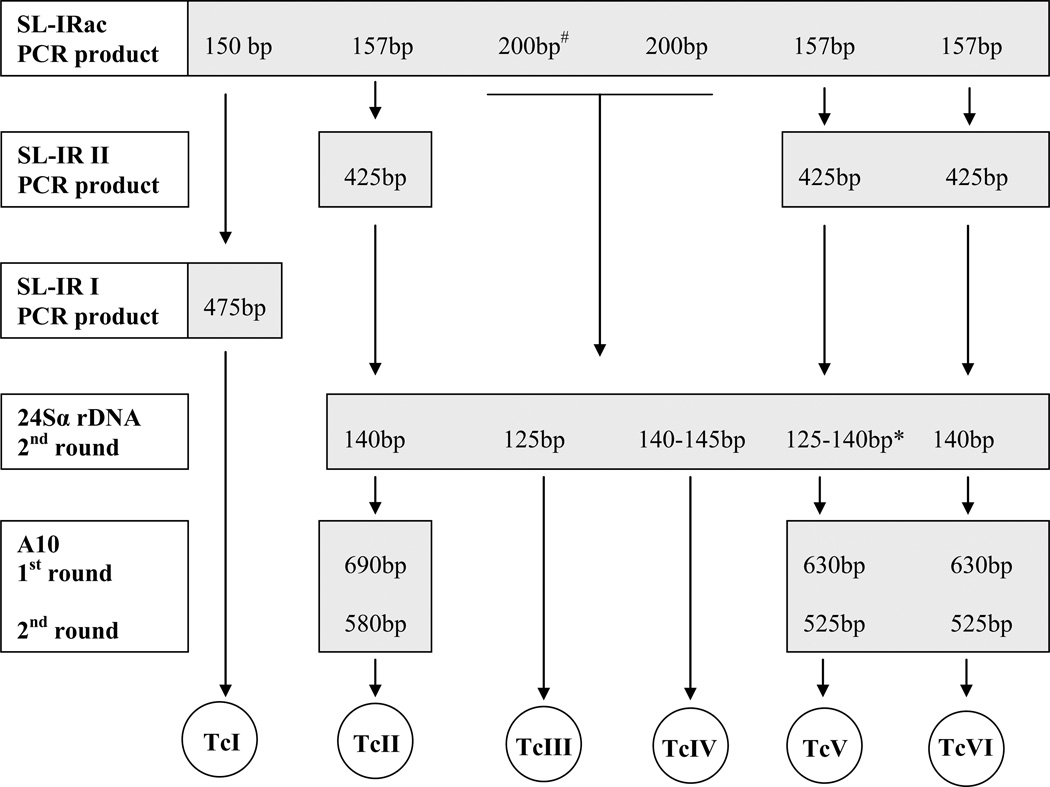
Third- to fifth-instars and adult triatomine bugs were microscopically examined for T. cruzi infection at 400× and by PCR targeted to 330 base pair (bp) fragment of the minicircles of the kinetoplastid genome (kDNA-PCR). Infections with T. cruzi were revealed by kDNA-PCR using primers and cycling conditions previously described (Schijman et al., 2003; Burgos et al., 2005). Each PCR run included 100 fg of T. cruzi DNA as positive control and sterile distilled water as a negative control. Aliquots of 12 µl of PCR products were visualized under UV light after electrophoresis in 2% agarose gels containing Gel-red® (Biotium). T. cruzi DTUs were identified in culture samples of each infected animal by PCR-based strategies targeted to the intergenic region of spliced-leader genes (SL-IRac, SL-IRII, SL-IRI) and 24s alpha ribosomal DNA genomic markers with the incorporation of Taq platinum (Invitrogen, USA) as described by Burgos et al. (2007) (Figure 1). TCC/TC1 and TCC/TC2 primers were used at the start of genotyping procedures to identify the DTU infecting one of the xenodiagnosis-positive D. albiventris (Brisse et al., 2001).
Fig.1.
Decision key for discriminating T. cruzi DTUs following Burgos et al (2007)
# An additional 150 bp band may occasionally appear.
* In samples that amplified 125+140 bp 24Sα-rDNA fragments, mixed populations by TcV+TcII/TcVI cannot be excluded.
For sequence analysis of SL-IR amplicons, the 200 bp amplicons obtained in SL-IRac-PCRs were purified using a QIAquick PCR purification kit (QIAgen®) and sequenced (Macrogen, Korea). Electropherograms of forward and reverse sequences were edited using the program Chromas Version 1.45. A consensus of forward and reverse sequences (contig) was created. SL-IR sequences were aligned using the ClustalW algorithm in MEGA 5 (Tamura et al., 2011) and adjusted visually. SL-IR sequences from TcIII and TcIV reference strains were obtained from the GenBank database: M5631 (AY367126), CANIII (AY367123) (Sturm et al., 2003), M6241 (AF050522) and MT4167 (AF050523) (Fernandes et al., 1998).
Results
A total of 44 mammals from 14 species was captured (Table 1). Of these, 23 mammals were captured with National or similar home-made traps (1.4 per 100 trap-nights); 13 rodents were caught with Sherman traps (3 per 100 traps-nights), and 8 mammals were caught manually or with darts. D. albiventris opossums (mean weight, 739.1 g; mean body length, 31 cm) belonged to age classes IV (5) or V (5) and were approximately 6 and 9 months of age, respectively. All D. novemcinctus armadillos were adults >1 year of age (mean weight, 3,431.3 g; mean body length, 41 cm).
Table 1.
Prevalence of infection with T. cruzi determined by xenodiagnosis and kDNA-PCR in sylvatic mammals captured in Pampa del Indio (Chaco), August 2008.
| Host | No. captured and examined |
No. (%) positive by | |||
|---|---|---|---|---|---|
| Scientific name | Common name | Xenodiagnosis | kDNA-PCR | Total | |
| Didelphis albiventris | White-eared opossum | 11 | 3 (27.3) | 4 (36.4) | 4 (36.4) |
| Dasypus novemcinctus | Nine-banded armadillo | 9 | 6 (66.7) | 5 (55.6) | 6 (66.7) |
| Chaetophractus villosus | Big hairy armadillo | 1 | 0 | 0 | 0 |
| Chaetophractus vellerosus | Screaming hairy armadillo | 2 | 0 | 0 | 0 |
| Tolypeutes matacus | Three-banded armadillo | 2 | 0 | 0 | 0 |
| Oncifelis geoffroyi | Geoffroy's cat | 2 | 0 | 0 | 0 |
| Procyon cancrivorus | Crab-eating raccoon | 2 | 0 | 0 | 0 |
| Sylvilagus brasiliensis | Brazilian rabbit | 1 | 0 | 0 | 0 |
| Galea musteloides | Common yellow-toothed cavy | 1 | 0 | 0 | 0 |
| Oligoryzomys cf. chacoensis | Chacoan colilargo | 6 | 0 | 0 | 0 |
| Oligoryzomys cf. flavescens | Flavescent colilargo | 4 | 0 | 0 | 0 |
| Calomys ”callosus“ | Large vesper mouse | 1 | 0 | 0 | 0 |
| Akodon toba | Chaco grass mouse | 1 | 0 | 0 | 0 |
| Graomys centralis | Central leaf-eared mouse | 1 | 0 | 0 | 0 |
| Total | 44 | 9 (20.5) | 9 (20.5) | 10 (22.7) | |
One xenodiagnosis-positive D. novemcinctus and one Oligoryzomys cf. flavescens were not examined by kDNA-PCR due to insufficient blood samples.
The composite prevalence of T. cruzi infection (as determined by xenodiagnosis or PCR) over all mammals examined was 22.7% (95% confidence interval, CI, 10.3–35.1%) (Table 1). Three (27.3%) D. albiventris opossums (all in age class IV) and 6 (66.7%) D. novemcinctus armadillos were xenodiagnosis-positive. Parasite isolation through culture was successful in all xenodiagnosis-positive individuals. Blood samples from xenodiagnosis-positive animals displayed the 330 bp band indicative of T. cruzi infection by kDNA-PCR. Only one xenodiagnosis-negative opossum was positive by kDNA-PCR. The combined prevalence of T. cruzi infection was 36.4% (95% CI, 7.9–64.8%) in D. albiventris and 66.7% (95% CI, 35.9–97.5%) in D. novemcinctus. Among animals positive by xenodiagnosis or kDNA-PCR, on average D. albiventris infected 46.1% of T. infestans nymphs (95% CI, 34.8–57.3%) whereas D. novemcinctus infected a substantially larger (84.5%) frequency of nymphs (95% CI, 77.9–91.1%). Host species with non-infected individuals belonged to Cricetidae, Dasypodidae, Felidae, Procyonidae, and Leporidae.
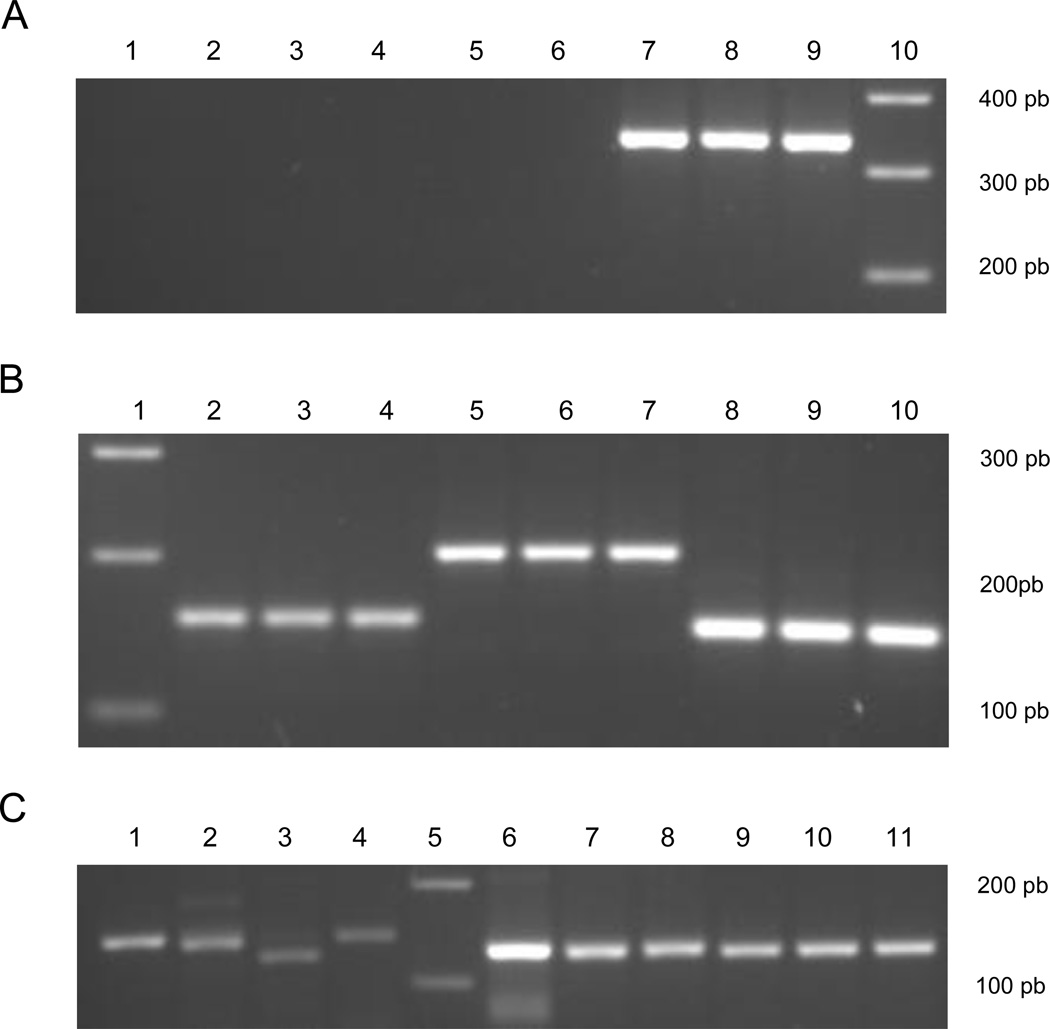
Fig. 2a illustrates the band patterns of the PCR products obtained when using TCC/TC1 and TCC/TC2 primers with DNA from T. cruzi infecting a D. albiventris opossum DNA. Absence of amplification of the 300 bp and the presence of 350 bp fragments of the intergenic region of the mini-exon genes (Brisse et al., 2001) indicated TcI. Another two D. albiventris opossums examined with the protocol developed by Burgos et al. (2007) presented the 150 bp band for the SL-IRac leader sequence (primers UTCC/TCac) (Fig. 2b) and the 475 bp band for the SL-IRI leader sequence (primers UTCC/TC2), indicative of TcI. All isolates from D. novemcinctus armadillos displayed the 200 bp band for the SL-IRac leader sequence (primers UTCC/TCac) and the 125 bp band for the 24sα ribosomal DNA-HnPCR sequence (primers D76/D71), indicative of TcIII (Fig. 2c). The DTU of the kDNA-PCR-positive sample of the xenodiagnosis-negative opossum could not be identified.
Fig. 2.
Identification of T. cruzi DTUs by PCR strategies.
A) Amplification of the intergenic region of the mini-exon genes with primers TCC/TC2, lane (1) reference stock Tu 18 (TcII); lane (2) reference stock M5631 (TcIII); lane (3) reference stock Can III (TcIV); lane (4) reference stock PAH 263 (TcV); lane (5) reference stock CL-Brener (TcVI); lane (6) D. novemcinctus; lane (7) reference stock CA-1 K98 (TcI); lane (8–9) D. albiventris; lane (10) 100 bp ladder. B) Amplification of the intergenic region of the mini-exon genes with primers UTCC/TCac, lane (1) 100 bp ladder; lane (2) reference stock Tu 18 (TcII); lane (3) reference stock PAH 263 (TcV); lane (4) reference stock CL-Brener (TcVI); lane (5) reference stock Can III (TcIV); lane (6) reference stock M5631 (TcIII); lane (7) D. novemcinctus; lane (8) reference stock CA-1 K98 (TcI); lane (9–10) D. albiventris.
C) Amplification of the D7 ribosomal DNA 24S-alpha domain with primers D71/D76, lane (1) reference stock Tu 18 (TcII); lane (2) reference stock Can III (TcIV); lane (3) reference stock PAH 263 (TcV); lane (4) reference stock CL-Brenner (TcVI); lane (5) 100 bp ladder; lane (6) reference stock CA-1 K98 (TcI); lane (7–8) D. albiventris; lane (9) reference stock M5631 (TcIII); lane (10) D. novemcinctus.
Sequence analysis of SL-IR amplicons shows that five T. cruzi isolates infecting Dasypus novemcinctus (Dn 1, Dn 27, Dn 33, Dn 43 and Dn 44) were more closely related to TcIII than to TcIV, with 98.5% and 82.1% sequence identity with Tc III and Tc IV consensus sequences, respectively (Fig. 3). GenBank accession numbers are Dn 1 JX154668, Dn_27 JX154669, Dn_33 JX154670, Dn_43 JX154671, and Dn_44 JX154672.
Fig. 3.
Sequence analysis of spliced-leader intergenic region (SL-IR) amplicons from DTUs Tc III and Tc IV. Alignment of a 134 bp region within the SL-IR of five T. cruzi isolates infecting Dasypus novemcinctus (Dn) and reference strains Tc III and Tc IV: M5631 (AY367126), M6241 (AF050522), MT4167 (AF050523) and CANIII (AY367123). Boxed areas correspond to the nucleotide polymorphisms found among Tc III and Tc IV sequences. Conserved sites (.); gaps (−).
Intensive searches of sylvatic foci of triatomine bugs with Noireau traps yielded a total of 22 triatomine nymphs in 17 (4.8% of 354) positive traps in summer 2008 (including 10 fourth- or fifth-instar nymphs of T. sordida, and 12 first- or second-instar nymphs of Triatoma sp.). Similarly, a total of 25 triatomine nymphs was collected in 20 (5.5% of 364) positive Noireau traps in summer 2009 (18 third- to fifth-instar nymphs of T. sordida and 4 first- or second-instars of Triatoma sp). The frequency of Noireau traps positive for triatomine bugs did not differ significantly between years (χ2 = 0.176, 1 df, P = 0.67). No T. infestans was collected during both years. None of the apparently active armadillo burrows was positive for triatomine bugs. Bugs were collected from live trees (38.3%), fallen tree trunks (38.3%) and standing dry trees (23.4%). Nearly all of the bugs were unfed or little fed, and all were negative for T. cruzi by microscopical observation and kDNA-PCR. No triatomine bug was collected with light traps deployed in sylvatic habitats during both years.
Of seven animals fitted with the spool-and-line device, four (1 opossum, 1 six-banded E. sexcinctus and 2 D. novemcinctus armadillos) were successfully tracked to six refuges. Failed tracking attempts occurred when the spools did not fit in the burrows where the animals sought refuge and were dropped off (1 opossum, 1 C. vellerosus and 1 T. matacus). All devices were retrieved. Only one of the four burrows excavated (an armadillo burrow where an opossum sought refuge) contained a Panstrongylus geniculatus fourth-instar nymph recently fed, not infected with T. cruzi.
Discussion
Our study indicates that D. albiventris opossums and D. novemcinctus armadillos are probably the main sylvatic reservoir hosts of two independent transmission cycles of TcI and TcIII in the humid Argentinean Chaco. Both hosts were highly infectious to T. infestans – another feature of important reservoir hosts. No other sylvatic host of T. cruzi was detected, and there was a close agreement between xenodiagnosis and kDNA-PCR outcomes.
The finding of TcIII in nine-banded armadillos is the first such report from the Argentinean Chaco. TcIII infections were further confirmed by the analysis of sequence polymorphisms within the 200bp amplicons obtained in the SL-IRac PCR. D. novemcinctus has been found infected with TcIII throughout most of its range (Noireau et. al., 2009; Llewellyn et al., 2009). Previous findings of TcIII elsewhere in the dry Argentinean Chaco included one Conepatus chinga skunk, three domestic dogs and one adult peridomestic T. infestans (Ceballos et al., 2006; Cardinal et al., 2008). In the Paraguayan Chaco, TcIII was found in D. novemcinctus and Chaetophractus spp. armadillos, Monodelphis domestica (Yeo et al., 2005) and domestic dogs (Chapman et al., 1984). D. albiventris has been found infected with TcI throughout its range with very few exceptions (Diotaiuti et al., 1995; Barnabé et al., 2000; Diosque et al., 2003; Yeo et al., 2005; Ceballos et al., 2006; Luca D’Oro et al., 1993). Our findings further support the theory that arboreal transmission cycles including opossums are usually associated with TcI, whereas terrestrial transmission cycles with armadillos as reservoir hosts include TcIII (Yeo et al., 2005; Cardinal et al., 2008; Llewellyn et al., 2009), though this relationship may not be absolute (Marcili et al., 2009).
The observed prevalence of T. cruzi in D. novemcinctus armadillos (67%) exceeds previous findings in the Gran Chaco, although the number of animals we examined was smaller. The first reported finding of T. cruzi-infected nine-banded armadillos (16%) in the humid Argentinean Chaco was based on less sensitive and more laborious methods (Mazza et al., 1930; Romaña and Schürman, 1931). A more recent regional xenodiagnosis survey of various armadillo species (including D. novemcinctus) surprisingly found no T. cruzi infection among 81 specimens from the Argentinean Chaco (Martínez et al., 1983). In two sites located in the dry and humid Paraguayan Chaco, T. cruzi infections were recorded in D. novemcinctus (44.7%, TcIII and TcV), E. sexcinctus (17.4%, TcII, TcIII and TcV) and Chaetophractus spp. (3.6%, TcIII) (Yeo et al., 2005).
Dasypus novemcinctus has a wide range from northern Argentina and Uruguay to the southern USA, and its population size is apparently increasing despite being hunted for meat consumption (IUCN 2010.2, http://www.iucnredlist.org/apps/redlist/details/6290/0). The risks of human infection associated with skinning infected wild mammals or consuming undercooked armadillos or opossums are well known. Rural villagers from our study area and Paraguay (Yeo et al., 2005) frequently reported keeping armadillos in captivity at their homes for some time before eating them. This is a potential entry point of TcIII into the domestic cycle through domestic or peridomestic triatomine bugs feeding on them.
The occurrence of T. cruzi infection in Didelphis opossums aged < 9 months indicates an active sylvatic transmission cycle. D. albiventris opossums were as frequently infected with T. cruzi (36%) as they were 70 years earlier (25–45%) as determined by less sensitive diagnostic methods (Mazza and Schreiber, 1938). In the dry Chaco, 49.3% of D. albiventris harbored T. cruzi-like parasites in fresh blood preparations in the 1930s (Canal Feijóo, 1939). This was the highest infection prevalence ever reported for opossums in the Gran Chaco based on a direct parasitological method. We note that Trypanosoma freitasi was unknown at that time; other trypanosomes have been isolated from the bloodstream of Didelphis sp., and Trypanosoma rangeli has not been found in the Gran Chaco as yet. A longitudinal xenodiagnosis survey in Santiago del Estero recorded much more frequent T. cruzi infections in D. albiventris (22–43%) than in skunks (4.1–5.5%) between 1984 and 1991 (Pietrokovsky et al., 1991; Wisnivesky-Colli et al., 1992; Schweigmann et al., 1999), and a substantial decline in the infection prevalence of both hosts (7.9% and 1.1%, respectively) between 2003 and 2006 (Ceballos et al., 2006). In a dry section of Chaco province, T. cruzi infections were frequently documented in D. albiventris (35.7%) but not in armadillos examined by xenodiagnosis (Diosque et al., 2004). More recent, small-sized surveys in eastern Paraguay and northeastern Argentina detected no T. cruzi-infected opossum using xenodiagnosis and/or PCR (Bar et al., 1999; Yeo et al., 2005). Our review indicates that few surveys of T. cruzi infection in sylvatic mammals have been conducted in the Gran Chaco, especially in the humid section, and that opossum infections with T. cruzi are widespread and widely variable over time and space. In light of major differences in host and vector diversity between the dry and humid Chaco, it is premature to lump results and draw generalizations across the ecoregion.
One potential limitation of our study is that the occurrence of mixed infections of T. cruzi DTUs in the mammalian hosts might have been masked by genotype selection associated with specific in vitro culture methods (Yeo et al., 2007; Llewellyn et al., 2011); sensitivity of the PCRs used to identify parasite genotypes, and differential parasite histotropism (Burgos et al., 2005). Experimental studies have shown differential selection of T. cruzi genotypes depending on the host species (Jansen et al., 1991; Roellig et al., 2009). Therefore, this process could further enhance segregation of transmission cycles involving specific DTUs within the same area.
The putative vectors of TcI and TcIII in sylvatic transmission cycles of the Argentinean or Paraguayan Chaco have not been identified conclusively (Wisnivesky-Colli et al., 1992; Yeo et al., 2005; Ceballos et al., 2006). Despite extensive searches in Pampa del Indio, only T. sordida and P. geniculatus were collected in sylvatic habitats and none of them were infected with T. cruzi, whereas very few specimens of T. sordida collected in peridomestic habitats were infected with TcVI and TcI (Maffey et al., in press). Both species of Triatominae were implicated elsewhere in sylvatic cycles of T. cruzi involving Didelphis opossums and nine-banded armadillos (Miles, 1979; Barretto, 1979; Bar and Wisnivesky-Colli, 2001). P. geniculatus has usually been associated with armadillos as vectors of TcIII throughout the Americas but it has also been found infected with TcI, TcII and TcIV in Colombia, Brazil and Venezuela (Carrasco et al., 2005; Valente et al., 1998; Póvoa et al., 1984; Salazar et al., 2006; Marcili et al., 2009).
Other candidate vector species exist in the study region. Unlike in the dry Chaco of Bolivia, Argentina and Paraguay (Noireau et al., 2005; Ceballos et al., 2006; Rolón et al., 2011), sylvatic foci of T. infestans have yet to be identified in the humid Chaco where more thorough searches are needed. Triatoma rubrovaria–associated with T. cruzi-infected nine-banded armadillos in Uruguay (Salvatella et al., 1982) and southern Brazil–has not been recorded in the Gran Chaco (Carcavallo et al., 1998). The only other known vectors of T. cruzi present in Chaco province are Panstrongylus guentheri, T. guasayana, Triatoma platensis and Triatoma delpontei (Martínez et al., 1988; Carcavallo et al., 1998). Among them, only a few adults of T. platensis were detected during several domestic and peridomestic surveys conducted throughout Pampa del Indio municipality between 2007 and 2010 (Gurevitz et al., 2011; unpublished results). Identifying the vector species implicated in the sylvatic transmission of T. cruzi is crucial to provide a more thorough understanding of system components and dynamics.
Research Highlights.
We identified the main sylvatic hosts of Trypanosoma cruzi in northeastern Argentina.
Didelphis albiventris opossums and Dasypus novemcinctus armadillos were infected.
Opossums were infected with T. cruzi I and armadillos with T. cruzi III.
Panstrongylus geniculatus and Triatoma sordida were found in sylvatic habitats.
Two independent sylvatic transmission cycles of T. cruzi occur in the humid Chaco.
Acknowledgements
We are grateful to Leonardo Lanati, Marta Lauricella, Marina Leporace, Lucía Maffey, Margarita Bisio, Juan M. Burgos, Pablo Teta, Emiliano Muschetto, Luis Leyes, Joaquín Maidana, Pollo Lescano, Blanco bros. and Raúl Stariolo for field and laboratory assistance. Reference strains of TcI-VI were kindly provided by Patricio Diosque, Miguel A. Basombrío and Michel Tibayrenc. This study was supported by awards from the International Development Research Center (EcoHealth Program), Tropical Disease Research (UNICEF/PNUD/WB/WHO), University of Buenos Aires, and National Institutes of Health/National Science Foundation Ecology of Infectious Disease program award R01 TW05836 funded by the Fogarty International Center and the National Institute of Environmental Health Sciences to UK, REG, and Joel Cohen. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. REG, MVC and AGS are members of CONICET Researcher’s Career.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarcón de Noya B, Díaz-Bello Z, Colmenares C, Ruiz-Guevara R, Mauriello L, Zavala-Jaspe R, Suarez JA, Abate T, Naranjo L, Paiva M, Rivas L, Castro J, Márques J, Mendoza I, Acquatella H, Torres J, Noya O. Large urban outbreak of orally acquired acute Chagas’ disease at a school in Caracas, Venezuela. J. Infect. Dis. 2010;201:1308–1315. doi: 10.1086/651608. [DOI] [PubMed] [Google Scholar]
- Bar ME, Alvarez BM, Oscherov EB, Pieri Damborsky M, Jörg ME. Contribution to knowledge of reservoirs of Trypanosoma cruzi (Chagas, 1909) in Corrientes Province, Argentina. Rev. Soc. Bras. Med. Trop. 1999;32:271–276. doi: 10.1590/s0037-86821999000300008. [DOI] [PubMed] [Google Scholar]
- Bar ME, Wisnivesky-Colli C. Triatoma sordida Stål 1859 (Hemiptera, Reduviidae: Triatominae) in palms of northeastern Argentina. Mem. Inst. Oswaldo Cruz. 2001;96:895–899. doi: 10.1590/s0074-02762001000700002. [DOI] [PubMed] [Google Scholar]
- Barnabé C, Brisse S, Tibayrenc M. Population structure and genetic typing of Trypanosoma cruzi, the agent of Chagas disease: a multilocus enzyme electrophoresis approach. Parasitology. 2000;120:513–526. doi: 10.1017/s0031182099005661. [DOI] [PubMed] [Google Scholar]
- Barretto MP. Epidemiologia. In: Brener Z, Andrade ZA, Barral-Netto M, editors. Trypanosoma cruzi. E–Guanabara Koogan: e Doença de Chagas; 1979. pp. 89–151. [Google Scholar]
- Brenière SF, Bosseno MF, Noireau F, Yacsik N, Liegeard P, Aznar C, Hontebeyrie M. Integrated study of a Bolivian population infected by Trypanosoma cruzi, the agent of Chagas Disease. Mem. Inst. Oswaldo Cruz. 2002;97:289–295. doi: 10.1590/s0074-02762002000300002. [DOI] [PubMed] [Google Scholar]
- Brisse S, Barnabé C, Tibayrenc M. Identification of six Trypanosoma cruzi phylogenetic lineages by random amplified polymorphic DNA and multilocus enzyme electrophoresis. Int. J. Parasitol. 2000;30:35–44. doi: 10.1016/s0020-7519(99)00168-x. [DOI] [PubMed] [Google Scholar]
- Brisse S, Verhoef J, Tibayrenc M. Characterisation of large and small subunit rRNA and mini-exon genes further supports the distinction of six Trypanosoma cruzi lineages. Int. J. Parasitol. 2001;31:1218–1226. doi: 10.1016/s0020-7519(01)00238-7. [DOI] [PubMed] [Google Scholar]
- Bucher EH, Huszar PC. Sustainable management of the Gran Chaco of South America. J. Environ. Manage. 1999;57:99–108. [Google Scholar]
- Burgos JM, Begher SB, Freitas JM, Bisio M, Duffy T, Altcheh J, Teijeiro R, Lopez Alcoba H, Deccarlini F, Freilij H, Levin MJ, Levalle J, Macedo AM, Schijman AG. Molecular diagnosis and typing of Trypanosoma cruzi populations and lineages in cerebral Chagas disease in a patient with AIDS. Am. J. Trop. Med. Hyg. 2005;73:1016–1018. [PubMed] [Google Scholar]
- Burgos JM, Altcheh J, Bisio M, Duffy T, Valadares HM, Seidenstein ME, Piccinali R, Freitas JM, Levin MJ, Macchi L, Macedo AM, Freilij H, Schijman AG. Direct molecular profiling of minicircle signatures and lineages of Trypanosoma cruzi bloodstream populations causing congenital Chagas disease. Int. J. Parasitol. 2007;37:1319–1327. doi: 10.1016/j.ijpara.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Canal Feijóo EJ. Actas y Trabajos VI Congreso Nacional de Medicina. Vol. 3. Argentina: Córdoba; 1939. [16 al 21 de octubre]. Vectores, depósitos parasitarios y casos clínicos de la Enfermedad de Chagas en la Provincia de Santiago del Estero; pp. 103–113. [Google Scholar]
- Canale DM, Carcavallo RU. Triatoma infestans (Klug) In: Carcavallo RU, Rabinovich JE, Tonn RJ, editors. Factores Biológicos y Ecológicos de la Enfermedad de Chagas. Vol. 28. Ministerio de Salud y Acción Social de Argentina: Buenos Aires: OPS, MSAS-SNCH; 1985. pp. 237–250. [Google Scholar]
- Canale DM, Cecere MC, Chuit R, Gürtler RE. Peridomestic distribution of Triatoma garciabesi and Triatoma guasayana in north-west Argentina. Med. Vet. Entomol. 2000;14:383–390. doi: 10.1046/j.1365-2915.2000.00254.x. [DOI] [PubMed] [Google Scholar]
- Carcavallo RU, Martínez A. Entomoepidemiología de la República Argentina. Investigaciones Científicas de las Fuerzas Armadas Argentinas. 1968;1:76–92. [Google Scholar]
- Carcavallo RU, Curto de Casas SI, Sherlock IA, Galindez Giron I, Jurberg J, Galvão C, Mena Segura C, Noireau F. Geographical distribution and alti-latitudinal dispersion. In: Carcavallo RU, Galíndez Girón I, Jurberg J, Lent H, editors. Atlas of Chagas’ Disease Vectors in the Americas. Vol. III. Fiocruz: Rio de Janeiro; 1998. pp. 747–792. [Google Scholar]
- Carpenter JW, Mashima TY, Rupiper DJ. Exotic Animal Formulary. 2nd ed. Philadelphia: W.B. Saunders Company; 2001. [Google Scholar]
- Cardinal MV, Lauricella MA, Ceballos LA, Lanati L, Marcet PL, Levin JM, Kitron U, Gürtler RE, Schijman AG. Molecular epidemiology of domestic and sylvatic Trypanosoma cruzi infection in rural northwestern Argentina. Int. J. Parasitol. 2008;38:1533–1543. doi: 10.1016/j.ijpara.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos LA, Cardinal MV, Vazquez-Prokopec GM, Lauricella MA, Orozco MM, Cortinas R, Schijman AG, Levin MJ, Kitron U, Gürtler RE. Long-term reduction of Trypanosoma cruzi infection in sylvatic mammals following deforestation and sustained vector surveillance in northwestern Argentina. Acta Trop. 2006;98:286–296. doi: 10.1016/j.actatropica.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos LA, Piccinali RV, Berkunsky I, Kitron U, Gürtler RE. First finding of melanic sylvatic Triatoma infestans (Hemiptera: Reduviidae) in the Argentine Chaco. J. Med. Entomol. 2009;46:1195–1202. doi: 10.1603/033.046.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman MD, Baggaley RC, Godfreyfausset PF, Malpas TJ, White G, Canese J, Miles MA. Trypanosoma cruzi from the Paraguayan Chaco isoenzyme profiles of strains isolated at Makthlawaiya. J. Protozool. 1984;31:482–486. doi: 10.1111/j.1550-7408.1984.tb02999.x. [DOI] [PubMed] [Google Scholar]
- Cohen JE, Gürtler RE. Modeling household transmission of American Trypanosomiasis. Science. 2001;293:694–698. doi: 10.1126/science.1060638. [DOI] [PubMed] [Google Scholar]
- Carrasco HJ, Torrellas A, García C, Segovia M, Feliciangeli MD. Risk of Trypanosoma cruzi I (Kinetoplastida: Trypanosomatidae) transmission by Panstrongylus geniculatus (Hemiptera: Reduviidae) in Caracas (Metropolitan District) and neighboring States, Venezuela. Int. J. Parasitol. 2005;35:1379–1384. doi: 10.1016/j.ijpara.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Diosque P, Barnabé C, Padilla AM, Marco JD, Cardozo RM, Cimino RO, Nasser JR, Tibayrenc M, Basombrío MA. Multilocus enzyme electrophoresis analysis of Trypanosoma cruzi isolates from a geographically restricted endemic area for Chagas´ disease in Argentina. Int. J. Parasitol. 2003;33:997–1003. doi: 10.1016/s0020-7519(03)00139-5. [DOI] [PubMed] [Google Scholar]
- Diosque P, Padilla AM, Cimino RO, Cardozo RM, Negrette OS, Marco JD, Zacca R, Meza C, Juarez A, Rojo H, Rey R, Corrales RM, Nasser JR, Basombrío MA. Chagas disease in rural areas of Chaco province, Argentina: epidemiologic survey in humans, reservoirs, and vectors. Am. J. Trop. Med. Hyg. 2004;71:590–593. [PubMed] [Google Scholar]
- Diotaiuti L, Pereira AS, Loiola CF, Fernandes AJ, Schofield CJ, Dujardin JP, Pinto Dias JC, Chiari E. Inter-relation of sylvatic and domestic transmission of Trypanosoma cruzi in areas with and without domestic vectorial transmission in Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz. 1995;90:443–448. doi: 10.1590/s0074-02761995000400002. [DOI] [PubMed] [Google Scholar]
- Fernandes O, Sturm NR, Derre R, Campbell DA. The mini-exon gene: a genetic marker for zymodeme III of Trypanosoma cruzi. Mol. Biochem. Parasitol. 1998;95:129–133. doi: 10.1016/s0166-6851(98)00073-5. [DOI] [PubMed] [Google Scholar]
- Genoways HH, Timm RM. The xenarthrans of Nicaragua. J. Neotrop. Mammal. 2003;10:231–253. [Google Scholar]
- Gurevitz JM, Ceballos LA, Gaspe MS, Alvarado-Otegui JA, Enriquez GF, Kitron U, Gürtler RE. Factors affecting infestation by Triatoma infestans in a rural area of the humid Chaco in Argentina: a multi-model inference approach. PLoS Negl. Trop. Dis. 2011;5:e1349. doi: 10.1371/journal.pntd.0001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE. Sustainability of vector control strategies in the Gran Chaco region: current challenges and possible approaches. Mem. Inst. Oswaldo Cruz. 2009;104(Suppl. 1):52–59. doi: 10.1590/s0074-02762009000900009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE, Kitron U, Cecere MC, Segura EL, Cohen JE. Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina. PNAS. 2007a;104:16194–16199. doi: 10.1073/pnas.0700863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE, Cecere MC, Lauricella MA, Cardinal MV, Kitron U, Cohen JE. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology. 2007b;134:69–82. doi: 10.1017/S0031182006001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera L, D'Andrea PS, Xavier SC, Mangia RH, Fernández O, Jansen AM. Trypanosoma cruzi infection in wild mammals of the National Park Serra da Capivara and its surroundings (Piaui, Brazil), an area endemic for Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 2005;99:379–388. doi: 10.1016/j.trstmh.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Jansen AM, Leon L, Machado GM, da Silva MH, Souza-Leão SM, Deane MP. Trypanosoma cruzi in the opossum Didelphis marsupialis: parasitological and serological follow-up of the acute infection. Exp. Parasitol. 1991;73:249–259. doi: 10.1016/0014-4894(91)90096-f. [DOI] [PubMed] [Google Scholar]
- Lauricella MA, Stariolo R, Riarte AR, Segura EL, Gürtler RE. Distribution and pathogenicity of Trypanosoma cruzi isolated from peridomestic populations of Triatoma infestans and Triatoma guasayana from rural western Argentina. Mem. Inst. Oswaldo Cruz. 2005;100:123–129. doi: 10.1590/s0074-02762005000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisboa CV, Mangia RH, De Lima NR, Martins A, Dietz J, Baker AJ, Ramon-Miranda CR, Ferreira LF, Fernandes O, Jansen AM. Distinct patterns of Trypanosoma cruzi infection in Leontopithecus rosalia in distinct Atlantic coastal rainforest fragments in Rio de Janeiro, Brazil. Parasitol. 2004;129:703–711. doi: 10.1017/s0031182004005918. [DOI] [PubMed] [Google Scholar]
- Llewellyn MS, Lewis MD, Acosta N, Yeo M, Carrasco HJ, Segovia M, Vargas J, Torrico F, Miles MA, Gaunt MW. Trypanosoma cruzi IIc: phylogenetic and phylogeographic insights from sequence and microsatellite analysis and potential impact on emergent Chagas disease. PLoS Negl. Trop. Dis. 2009;3:e510. doi: 10.1371/journal.pntd.0000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn MS, Rivett-Carnac JB, Fitzpatrick S, Lewis MD, Yeo M, Gaunt MW, Miles MA. Extraordinary Trypanosoma cruzi diversity within single mammalian reservoir hosts implies a mechanism of diversifying selection. Int. J. Parasitol. 2011;41:609–614. doi: 10.1016/j.ijpara.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca D´Oro GM, Gardenal CN, Perret B, Crisci JV, Montamat EE. Genetic structure of Trypanosoma cruzi populations from Argentina estimated from enzyme polymorphism. Parasitol. 1993;107:405–410. doi: 10.1017/s0031182000067755. [DOI] [PubMed] [Google Scholar]
- Maffey L, Cardinal MV, Ordóñez-Krasnowski PC, Lanati LA, Lauricella MA, Schijman AG, Gürtler RE. Direct molecular identification of Trypanosoma cruzi Discrete Typing Units in domestic and peridomestic Triatoma infestans and Triatoma sordida from the Argentine Chaco. Parasitol. doi: 10.1017/S0031182012000856. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcet PL, Duffy T, Cardinal MV, Burgos JM, Lauricella MA, Levin MJ, Kitron U, Gürtler RE, Schijman AG. PCR-based identification of Trypanosoma cruzi lineages in feces of triatomine bugs from rural northwestern Argentina. Parasitology. 2006;132(Pt 1):57–65. doi: 10.1017/S0031182005008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcili A, Lima L, Valente VC, Valente SA, Batista JS, Junqueira ACV, Souza AI, Rosa JA, Campaner M, Lewis MD, Llewellyn MS, Miles MA, Teixeira MMG. Comparative phylogeography of Trypanosoma cruzi TCIIc: New hosts, association with terrestrial ecotopes, and spatial clustering. Infec. Genet. Evol. 2009;9:1265–1274. doi: 10.1016/j.meegid.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Martínez A, Carcavallo RU, Cichero JA. República Argentina. In: Carcavallo RU, Rabinovich JE, Tonn RJ, editors. Factores biológicos y ecológicos en la Enfermedad de Chagas. Vol. 29. Buenos Aires, Argentina: Ministerio de Salud y Acción Social de Argentina; 1988. pp. 345–354. [Google Scholar]
- Martínez FA, Guana Añasco LG, Resoagli EH. Las especies de Dasypodidae como reservorios de la enfermedad de Chagas en el nordeste argentino (Mammalia: Edentata) Gac. Vet. (Buenos Aires) 1983;45:376–383. [Google Scholar]
- Mazza S, Romaña C, Schurman K. Nuevas observaciones sobre la infección espontánea de armadillos del país por el Trypanosoma cruzi. La Prensa Médica Argentina. 1930;3:3–20. [Google Scholar]
- Mazza S, Schreiber F. Hallazgo en el Departamento Obligado, Santa Fe, de otra especie de mustélido naturalmente infectado por Schizotrypanum cruzi, de Triatoma infestans infectados en nidos de comadrejas, de Triatoma platensis infectados en nidos de psitácidos y de Psammolestes coreodes sin infestación en nidos de dendrocoláptidos. Misión de Estudios de Patología Regional Argentina. 1938;34:17–35. [Google Scholar]
- Miles MA. Transmission cycles and the heterogeneity of Trypanosoma cruzi. In: Lumsden WHR, Evans DA, editors. Biology of the Kinetoplastida. Vol. 1. 1979. pp. 117–196. [Google Scholar]
- Miles MA, Souza AA, Póvoa MM. Mammal tracking and nest location in Brazilian forest with an improved spool-and-line device. J. Zool. (London) 1981;195:331–347. [Google Scholar]
- Miles MA, Yeo M, Gaunt M. Genetic diversity of Trypanosoma cruzi and the epidemiology of Chagas disease. In: Kelly JM, editor. Molecular Mechanisms in the Pathogenesis of Chagas Disease. Eurekah.com & Kluwer Academic/Plenum Publishers; 2003. pp. 1–15. [Google Scholar]
- Montamat EE, Luca d'Oro G, Perret B, Rivas C. Characterization of Trypanosoma cruzi from Argentina by electrophoretic zymograms. Acta Trop. 1992;50:125–133. doi: 10.1016/0001-706x(91)90005-5. [DOI] [PubMed] [Google Scholar]
- Noireau F, Cortez MG, Monteiro FA, Jansen AM, Torrico F. Can wild Triatoma infestans foci in Bolivia jeopardize Chagas disease control efforts? Trends Parasitol. 2005;2:17–10. doi: 10.1016/j.pt.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Noireau N, Diosque P, Jansen AM. Trypanosoma cruzi: adaptation to its vectors and its hosts. Vet. Res. 2009;40:26. doi: 10.1051/vetres/2009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrokovsky S, Schweigmann NJ, Riarte A, Alberti A, Conti O, Montoya S, Wisnivesky-Colli C. The skunk Conepatus chinga as new host of Trypanosoma cruzi in Argentina. J. Parasitol. 1991;77:643–645. [PubMed] [Google Scholar]
- Póvoa MM, De Sousa AA, Naiff RD, Arias JR, Biancardi CB, Miles MA. Chagas' disease in the Amazon Basin IV. Host records of Trypanosoma cruzi zymodemes in the State of Amazonas and Rondonia, Brazil. Ann. Trop. Med. Parasitol. 1984;78:479–487. [PubMed] [Google Scholar]
- Ramírez JD, Guhl F, Rendón LM, Rosas F, Marin-Neto JA, Morillo CA. Chagas cardiomyopathy manifestations and Trypanosoma cruzi genotypes circulating in chronic Chagasic patients. PLoS Negl. Trop. Dis. 2010;4:e899. doi: 10.1371/journal.pntd.0000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roellig DM, Ellis AE, Yabsley MJ. Genetically different isolates of Trypanosoma cruzi elicit different infection dynamics in raccoons (Procyon lotor) and Virginia opossums (Didelphis virginiana) Int. J. Parasitol. 2009;39:1603–1610. doi: 10.1016/j.ijpara.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolón M, Vega MC, Román F, Gómez A, de Arias AR. First report of colonies of sylvatic Triatoma infestans (Hemiptera: Reduviidae) in the Paraguayan Chaco, using a trained dog. PLoS Negl. Trop. Dis. 2011;5:e1026. doi: 10.1371/journal.pntd.0001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaña C, Schürman K. La infección espontánea y la experimental del tatú del Chaco santafesino por el Trypanosoma cruzi. 7ma Reunión de la Sociedad Argentina de Patología Regional del Norte, 5, 6 y 7 de Octubre de 1931, Bs. As. Imprenta de la Universidad 1932. 1931 [Google Scholar]
- Salazar A, Schijman AG, Triana-Chávez O. High variability of Colombian Trypanosoma cruzi lineage I stocks as revealed by low-stringency single primer-PCR minicircle signatures. Acta Trop. 2006;100:110–118. doi: 10.1016/j.actatropica.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Salvatella R, González JC, Franca Rodríguez ME. Hallazgo de Trypanosoma cruzi (Chagas, 1909) en Dasypus novemcinctus novemcinctus (Linne) de Uruguay. Rev. Ur. Patol. Clín. 1982;18:17–21. [Google Scholar]
- Schijman AG, Altcheh J, Burgos JM, Biancardi M, Bisio M, Levin M, Freilij H. Aetiological treatment of congenital Chagas disease diagnosed and monitored by the polymerase chain reaction. J. Antimicrob. Chemother. 2003;52:441–449. doi: 10.1093/jac/dkg338. [DOI] [PubMed] [Google Scholar]
- Schmidt KA, Ostfeld RA. Biodiversity and the dilution effect in disease ecology. Ecology. 2001;82:609–619. [Google Scholar]
- Schofield CJ, Jannin J, Salvatella R. The future of Chagas disease control. Trends. Parasitol. 2006;22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Schweigmann NJ, Pietrokovsky S, Bottazi V, Conti O, Bujas M, Wisnivesky-Colli C. Estudio de la prevalencia de la infección por Trypanosoma cruzi en zarigüeyas (Didelphis albiventris) en Santiago del Estero, Argentina. Rev. Panam. Salud Públ. 1999;6:371–377. doi: 10.1590/s1020-49891999001100001. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 10.1. College Station: Stata Corp; 2007. [Google Scholar]
- Sturm NR, Vargas NS, Westenberger SJ, Zingales B, Campbell DA. Evidence for multiple hybrid groups in Trypanosoma cruzi. Int. J. Parasitol. 2003;33:269–279. doi: 10.1016/s0020-7519(02)00264-3. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Nature Conservancy (TNC); Fundación Vida Silvestre Argentina (FVSA); Fundación para el Desarrollo Sustentable del Chaco (DeSdel Chaco); Wildife Conservation Society Bolivia (WCS) Vol. 12. Buenos Aires: Fundación Vida Silvestre Argentina; 2005. [accessed June 12, 2009]. Evaluación Ecorregional del Gran Chaco Americano / Gran Chaco AmericanoEcoregional Assessment. http://www.vidasilvestre.org.ar/descargables/bosques_selvas/chaco/dossier.pdf. [Google Scholar]
- Tibayrenc M. Genetic epidemiology of parasitic protozoa and other infectious agents: the need for an integrated approach. Int. J. Parasitol. 1998;28:85–104. doi: 10.1016/s0020-7519(97)00180-x. [DOI] [PubMed] [Google Scholar]
- Valente VC, Valente SA, Noireau F, Carrasco HJ, Miles MA. Chagas disease in the Amazon Basin: association of Panstrongylus geniculatus (Hemiptera: Reduviidae) with domestic pigs. J. Med. Entomol. 1998;35:99–103. doi: 10.1093/jmedent/35.2.99. [DOI] [PubMed] [Google Scholar]
- Vaz VC, D'Andrea PS, Jansen AM. Effects of habitat fragmentation on wild mammal infection by Trypanosoma cruzi. Parasitology. 2007;134:1785–1793. doi: 10.1017/S003118200700323X. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prokopec GM, Ceballos LA, Kitron U, Gürtler RE. Active dispersal of natural populations of Triatoma infestans (Hemiptera: Triatominae) in rural northwestern Argentina. J. Med. Entomol. 2004;41:614–621. doi: 10.1603/0022-2585-41.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnivesky-Colli C, Schweigmann NJ, Alberti A, Pietrokovsky SM, Conti O, Montoya S, Riarte A, Rivas C. Sylvatic American trypanosomiasis in Argentina. Trypanosoma cruzi infection in mammals from the Chaco forest in Santiago del Estero. Trans. R. Soc. Trop. Med. Hyg. 1992;86:38–41. doi: 10.1016/0035-9203(92)90433-d. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Geneva: World Health Organization; 2002. Control of Chagas Disease: Second Report of the WHO Expert Committee; pp. 57–58. [Google Scholar]
- Yeo M, Acosta M, Llewellyn M, Sánchez H, Adamson S, Miles G, López E, González N, Patterson J, Gaunt M, Rojas de Arias A, Miles MA. Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int. J. Parasitol. 2005;35:225–233. doi: 10.1016/j.ijpara.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Yeo M, Lewis MD, Carrasco HJ, Acosta N, Llewellyn M, da Silva Valente SA, de Costa Valente V, Arias AR, Miles MA. Resolution of multiclonal infections of Trypanosoma cruzi from naturally infected triatomine bugs and from experimentally infected mice by direct plating on a sensitive solid medium. Int. J. Parasitol. 2007;37:111–120. doi: 10.1016/j.ijpara.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Zingales B, Andrade SG, Briones MR, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MM, Schijman AG, Llewellyn MS, Lages-Silva E, Machado CR, Andrade SG, Sturm NR. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect. Genet. Evol. 2012;12:240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]