Abstract
Purpose
To evaluate the safety and activity of 6 months of treatment with lenalidomide at 5 or 25 mg/d in nonmetastatic biochemically relapsed prostate cancer.
Experimental Design
Sixty men with non-castrate, nonmetastatic, biochemically relapsed prostate cancer were stratified by prostate-specific antigen (PSA) doubling time, surgery/radiation therapy, prior androgen deprivation therapy (ADT), and randomized to lenalidomide 5 mg (n = 26) or 25 mg/d (n = 34) for 3 weeks repeated monthly for 6 months or until dose-limiting toxicity or disease progression. Toxicity was evaluated monthly, and PSAs and X-rays/scans every 6 months. Study size was determined to detect a progression rate of 40% at 6 months in either arm with 85% power (compared with a rate of 80% in the population receiving no treatment). Changes in PSA slopes were calculated using the regression of the log PSA for each patient before and during the initial 6 months and compared by t test.
Results
Baseline variables were balanced between arms. Grade 3/4 toxicity rates were 12% (n = 3) with 5 mg and 29% (n = 10) with 25 mg (P = 0.1), most commonly neutropenia (five patients, all on 25 mg). Two patients per arm had thromboembolic events. The change in PSA slope was greater with 25 mg versus 5 mg [−0.172 (−0.24 to −0.11) versus −0.033 (−0.11 to 0.04); P = 0.005]. With a mean follow-up of 31.4 months (range 14–44), five patients on 25 mg and one patient on 5 mg remain on the study.
Conclusions
Lenalidomide has acceptable toxicity and is associated with long-term disease stabilization and PSA declines. Randomized studies evaluating conventional clinical disease end points in this patient population are planned.
Prostate cancer is the most common noncutaneous cancer in American men with over 210,000 new cases diagnosed annually in the United States (1). Although patients with localized disease are often cured by local modalities of treatment, approximately 30% show evidence of biochemical relapse at 10 years. The optimal management of these patients remains elusive at the present time. Salvage radiation therapy after radical prostatectomy may provide long-term benefit in a significant proportion of patients, mainly those with positive prognostic variables [i.e., Gleason score, preradiotherapy prostate-specific antigen (PSA) level, surgical margins, PSA doubling time (PSADT), and seminal vesicle invasion], and the use of androgen deprivation treatment (ADT) remains controversial.
Although ADT is effective in reducing serum PSA levels in most patients, its long-term benefits on survival and quality of life remain undefined. Recent data emphasize the incidence of cumulative toxicities with ADT which may offset any potential survival benefit from early intervention and could affect quality of life (2, 3).
The natural history of patients with biochemically relapsed, non-castrate prostate cancer is heterogeneous. Patients may remain asymptomatic and free of clinical evidence of disease for many years (4, 5). Data on the natural history of patients relapsing after local treatment indicate that time to biochemical relapse, Gleason score, and the PSADT predict the probability of metastasis-free and prostate cancer–specific survival (4, 6–9). These data have been used in our study design including patient selection, criteria for stratification, and definition of end points.
The evaluation of new compounds in this patient population remains challenging because of the lack of validated clinical trial methodology. The follow-up time required until conventional clinical and radiological end points occur is often long. Given the significance of PSADT and other dynamic measurements of PSA in predicting the outcome of this group of patients, changes in dynamic values observed during treatment have been a popular approach applied in clinical studies designed for screening potentially active compounds.
Preliminary data suggest that thalidomide has some clinical activity in patients with advanced castrate-resistant prostate cancer (10–12). A significant limitation of thalidomide is the incidence of neurotoxicity. Lenalidomide (Revlimid; Celgene Corporation) is an oral thalidomide analogue which has been shown to produce immunomodulation (13, 14), modulation of tumor cell microenvironment (15), and inhibition of angiogenesis (16, 17) and proliferation (18). Preliminary data suggest that lenalidomide may have clinical activity in patients with metastatic castrate-resistant prostate cancer (18, 19), and it is currently undergoing testing in a phase III trial, in combination with docetaxel. The possible clinical activity of lenalinomide, supports further testing of this compound for delaying progression in patients with nonmetastatic biochemically relapsed prostate cancer.
To assess a potential signal for clinical activity of lenalidomide in this early disease state, we employed previously reported methodology (20, 21) for evaluating the safety and preliminary efficacy of nonhormonal compounds on the progression of relapsed, nonmetastatic prostate cancer patients. The results of our randomized, double-blinded, phase I/II study are reported herein.
Materials and Methods
All patients on this study were treated and followed at the Johns Hopkins Hospital. Funding support for this study was provided by Celgene Corporation, Summit, NJ. Blinded lenalidomide (25 mg versus 5 mg) given to patients in blistered packages was provided by the sponsor.
Inclusion criteria
Eligible patients were ≥18 years old and had histologically proven prostatic adenocarcinoma (reviewed by our institution's urological pathologists) with a confirmed rising serum PSA level of ≥1 ng/mL (at least 2 wk apart) and no evidence of locoregional or distant metastasis determined by physical exam and radiologically (bone scan and computer-assisted tomography scan of the chest abdomen and pelvis), adequate bone marrow (absolute neutrophil count ≥ 1.5 × 103/L, platelet count ≥ 100 × 103/L), renal (serum creatinine ≤ 1.5 mg/dL), and liver (total bilirubin ≤ 1.5 mg/dL, aspartate aminotransferase and alanine aminotransferase ≤ 2× upper limit of reference range) functions, and serum testosterone >150 ng/mL. All previous local modalities of treatment, including radiation and surgery, were completed at least 4 weeks prior to treatment in this study. Prior systemic treatments such as chemotherapy, ADT, and biological or vaccine therapy, had to be discontinued for at least 6 months prior to study entry. Patients with a prior history of malignancy had to be disease-free for at least 5 years with exception of treated basal cell or squamous cell carcinoma of the skin. Eastern Cooperative Oncology Group performance status was ≤2 and life expectancy >6 months at study entry. All participating patients signed an Institutional Review Board–approved consent form.
Patient stratification and randomization
Eligible patients were stratified according to the PSADT (<3 mo versus 3.0–8.9 mo versus ≥9.0 mo), prior local treatment (surgery and/or radiation therapy), and ADT >6 months prior to study entry (with evidence of testosterone recovery >150 ng/dL) versus none, and then randomly assigned to receive identically appearing oral lenalidomide at doses of 5 or 25 mg/d, packaged and dispensed in labeled blister packs. Both patients and investigators were blinded. Treatment was given on days 1 to 21 of a 28-day cycle, for a total of six cycles or until disease progression or dose-limiting toxicity.
Follow-up evaluation of toxicity and disease status
Patients underwent monthly follow-up visits for toxicities and every other month physical exams. Serial serum PSA and testosterone levels were obtained on the first day of cycles 1, 2, 4, and 6. PSA samples were frozen and processed after each 6 month period or sooner if clinically indicated. The PSA data was therefore only available to investigators and patients after each 6-month cycle. Plasma concentration levels of lenalidomide were measured at steady state on day 21 of the second cycle. Complete assessments of disease status included a bone scan and computer-assisted tomography scan of the chest/abdomen/pelvis during the rest week of the sixth cycle (days 21–28), or sooner if clinically indicated.
Duration of treatment
Protocol treatment continued until biochemical or clinical disease progression or dose-limiting toxicity. Biochemical progression was defined as ≥25% increase of PSA level at 6 months over the baseline. Clinical disease progression was defined as any new evidence of disease on monthly physical exam (including new findings on digital rectal examination) or scans at 6 months, suggestive of local or distant disease recurrence. Duration of treatment was defined as the time from date of treatment initiation to date of first observation of a termination event, death due to any cause, or early discontinuation of treatment for any reason. Patients without evidence of disease progression or dose-limiting toxicity after each 6-month cycle of treatment, were given an additional 6-month cycle until the above events occurred.
Safety evaluation, management of pertinent toxicity, and definition of dose-limiting toxicity
Toxicity was defined according to the National Cancer Institute Common Toxicity Criteria version 3.0 (http://ctep.info.nih.gov). For nonhematologic toxicity, treatment was stopped for any grade 2 cardiac or hypersensitivity reaction (including rash) or grade 3 to 4 of other toxicity, at which point patients were followed weekly until ≤grade 1 and then restarted at the next scheduled day 1. Treatment was discontinued at the first occurrence of ≥grade 3 cardiac or hypersensitivity reaction (including rash), upon recurrence of a same grade 3/4 event, for recurrent grade ≥2 hypersensitivity reactions, and for any toxicity requiring longer than 3 weeks to recover to ≤grade 1. For hematologic toxicity, treatment was stopped for any grade 3 toxicity, until resolved to ≤grade 2 for asymptomatic patients and ≤grade 1 for symptomatic patients. Treatment was discontinued at the first occurrence of grade 4 toxicity, upon recurrence of symptomatic grade 3 toxicity, and any toxicity requiring longer than 3 weeks to recover. Doselimiting toxicity was defined as any recurrent grade 3 (excluding grade 3 hematologic events) or grade 4 toxicity, any recurrent grade 2 cardiac or grade 3 hypersensitivity reaction, and pulmonary embolism. A data safety monitoring committee reviewed the efficacy and safety data in our patient population.
Statistical analysis
Study analysis was planned based on the initial 6-month period (cycle 1). The primary efficacy statistical end point in this study was the change in PSA slope. A regression of the log PSA on the time of PSA measurements for each patient was calculated for the “pretreatment” PSA values and for the “on-treatment” PSA values. The analysis of “on-treatment” PSA was based on the initial 6-month period of the study. The “pretreatment” slope was deducted from the “on-treatment” slope for each patient and the mean change in slope was compared between study arms with a two-sample t test. The change in PSA slope was regressed on lenalidomide-plasma concentration. The probability of relapse or progression at 6 months and the probability of toxicity outcomes were compared between arms with cross-tabulations and χ2 tests. Eligible patients for this study had confirmed rising serum PSA levels (≥1 ng/mL). The null hypothesis was based on institutional data indicating that without treatment, at least 80% of patients with rising serum PSAs would show evidence of disease progression at 6 months (Eisenberger M., Johns Hopkins Hospital prostate cancer database; refs 4, 7). Patients were randomized to receive either 5 or 25 mg/d of oral lenalidomide. The 5 mg arm was included in the study to allow for a dose-response evaluation, but was not expected to decrease the 80% historical rate of progression. We hypothesized that the 25 mg arm would be of interest if the proportion of patients with disease progression at 6 months was 50% lower than the historical experience (reduced from 80% to 40%). This would be further supported if the concomitant evaluation of the 5 mg arm in the context of a randomized study was associated with a higher proportion of disease progression at 6 months compared with the 25 mg arm. With 30 patients per study arm, the study was designed to have 85% power to detect a decrease in the 6-month progression rate, from 80% in the 5 mg arm to 40% in the 25 mg arm, using a two-sided χ2 test with a significance level of 0.05. All P values reported are two-sided. Computations were done using the Statistical Analysis System (SAS Users Guide: Statistics, version 5 edition, 1985; SAS Institute, Inc.).
Regulatory considerations
The research was carried out in accordance with approval by the Institutional Review Board committee of our institution and all toxicities as well as outcome data were reviewed by an independent data safety monitoring committee.
Results
Patients
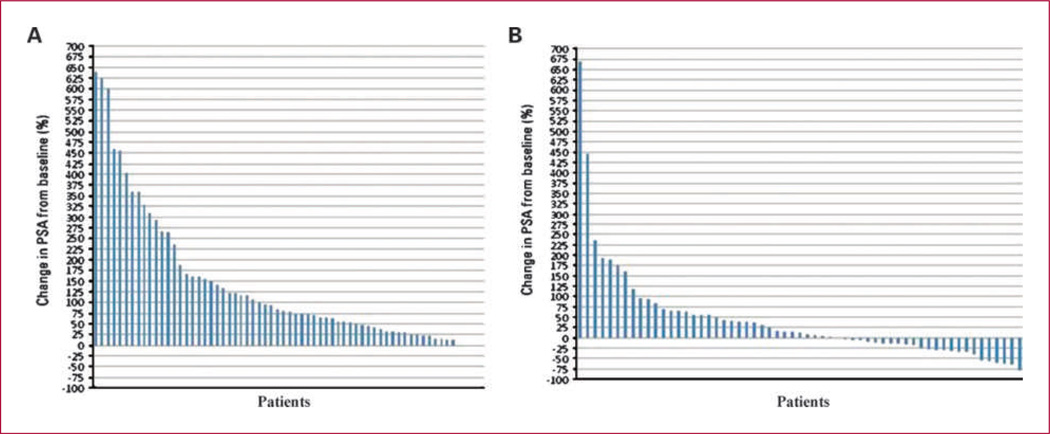
Between July 2006 and January 2009, 77 patients were screened for study entry. Seventeen patients were considered screening failures, and the reasons included metastatic disease (n = 14), local recurrence (n = 1), no evidence of a confirmed increase in PSA (n = 1), and abnormal liver function tests (n = 1). Thus, 60 eligible patients (mean age, 63 y; range, 50–81 y) were stratified, and randomized to receive oral lenalidomide 5 mg/d (n = 26) or 25 mg/d (n = 34). The slight imbalance between arms resulted from the randomization and stratification method employed. Pretreatment patient characteristics were balanced between arms, and are summarized in Table 1. Figure 1A is a waterfall plot of PSA progression in the 6-month period preceding study entry.
Table 1.
Pretreatment patients characteristics
| Variables | Group | P | |
|---|---|---|---|
| Revlimid, 5 mg (n = 26) |
Revlimid, 25 mg (n = 34) |
||
| Age (y) | 63.7 (7.1) | 63.1 (7.2) | 0.76 |
| Mean (SD), range | 50–75 | 52–81 | |
| Gleason | 7.1 (1.0) | 7.3 (1.1) | 0.51 |
| Mean (SD), range | 6–9 | 5–9 | |
| Local therapy | |||
| Radical prostatectomy | 12 | 12 | 0.62 |
| Radiation therapy | 4 | 8 | |
| Surgery + RT | 10 | 14 | |
| Prior ADT | 8 | 11 | |
| Prestudy serum testosterone (ng/dL): mean (range) | 374 (148–564) | 342 (175–569) | 1.0 |
| Prestudy PSA (ng/mL) | 11.9 (11.9) | 12.4 (13.6) | |
| Mean (SD), range | 1.4–43.6 | 1.2–66.2 | 0.88 |
| Prestudy PSADT (mo) | |||
| <3 m | 6 | 10 | 0.85 |
| 3–8.9 | 13 | 16 | |
| ≥9 | 7 | 8 | |
| Mean (range), 95% CI | 0.16 (0.11–0.21) | 0.18 (0.13–0.23) | |
| Pretreatment PSA slopes | 0.0–0.45 | 0.03–0.72 | 0.55 |
Fig. 1.
A waterfall plot of PSA progression preceding study entry (A), and of PSA outcome during the study (B).
Toxicity
The most common severe (grade 3/4) treatment-related adverse events are shown in Table 2. Their incidence was 12% (n = 3) and 29% (n = 10) with the 5 mg/d and 25 mg/d doses, respectively, (P = 0.1). The main grade 3/4 adverse events were neutropenia (five patients, all on lenalidomide, 25 mg/d; no episodes of febrile neutropenia occurred), thromboembolic events (n = 4), fatigue (n = 2), skin toxicity (n = 2), and appendicitis (n = 1). Overall, nine patients discontinued treatment due to serious adverse events, 12% (n = 3) in the 5 mg arm and 18% (n = 6) in the 25 mg arm. There were no dose reductions and no treatment-related fatal or irreversible toxicities. All patients with thromboembolic complications were removed from the study, and all recovered. The most frequent grade 1 or 2 adverse events are shown in Table 3. These were reversible and seen more frequently in the 25 mg arm. Twelve percent (n = 4) of the patients in the 25 mg arm and 0% on the 5 mg arm required interruption of lenalidomide treatment due to any adverse event.
Table 2.
Grades 3 and 4 adverse events
| Type of adverse event |
5 mg (n = 26) |
25 mg (n = 34) |
P |
|---|---|---|---|
| Neutropenia | 0% | 15% (n = 5) | 0.041 |
| Venous thromboembolism | 8% (n =2) | 6% (n =2) | 0.78 |
| Fatigue | 4% (n =1) | 3% (n =1) | 0.85 |
| Skin | 0% | 6% (n =2) | 0.38 |
NOTE: Two additional patients (both on the 25 mg arm) had thromboembolism on subsequent treatment.
Table 3.
Most common (occurring in ≥20% of patients in any of the arms) grades 1 and 2 adverse events
| Type of adverse event | Lenalidomide, 5 mg (n = 26) | Lenalidomide, 25 mg (n = 34) | P |
|---|---|---|---|
| Anemia | 27% (n = 7) | 29% (n = 10) | 0.83 |
| Constipation | 38% (n = 10) | 44% (n = 15) | 0.66 |
| Fatigue | 46% (n = 12) | 85% (n = 29) | 0.0012 |
| Hyperglycemia | 42% (n = 11) | 41% (n = 14) | 0.93 |
| Increase of aspartate aminotransferase | 8% (n = 2) | 24% (n = 8) | 0.1 |
| Leg and/or hand muscular cramping | 19% (n = 5) | 41% (n = 14) | 0.07 |
| Lymphopenia | 4% (n = 1) | 24% (n = 8) | 0.03 |
| Neutropenia | 19% (n = 5) | 26% (n = 9) | 0.5 |
| Pruritus | 31% (n = 8) | 44% (n = 15) | 0.29 |
| Rash | 27% (n = 7) | 44% (n = 15) | 0.17 |
| Thrombocytopenia | 15% (n = 4) | 24% (n = 8) | 0.43 |
PSA changes and disease progression
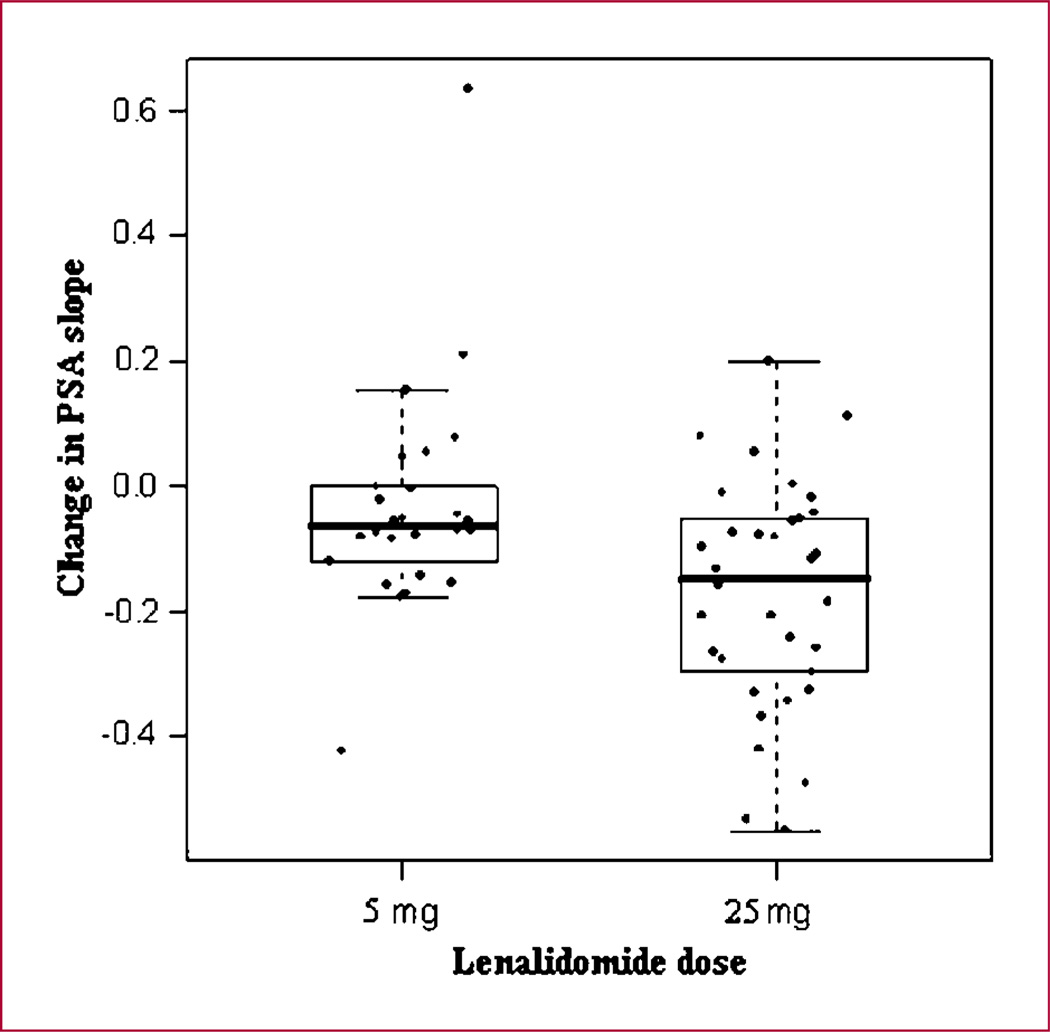
The mean [range; 95% confidence interval (CI)] pretreatment PSA slopes were 0.16 (0.11–0.21; 0.0–0.45) and 0.18 (0.13–0.23; 0.03–0.72) in the 5 and 25 mg dose groups, respectively (P = 0.55). The mean PSA slope change (range; 95% CI, on study minus pretreatment) in the two groups was −0.033 (−0.11 to 0.04) and −0.172 (−0.24 to −0.11) in the 5 and 25 mg dose groups, respectively. The median PSA slope change in the two study groups was −0.064 for the 5 mg arm and −0.146 for the 25 mg arm. The mean change in slope with treatment was significantly greater in the 25 mg arm compared with the 5 mg group (P = 0.005; Fig. 2). Figure 1A and B are waterfall plots of PSA outcome, showing the percentage of change in PSA in all patients in the 6 months preceding study entry (Fig. 1A) and during the 6 months on-study (Fig. 1B). The “pretreatment” and “on-treatment” waterfall plots in each arm are very similar (data not shown). Among the patients on 25 mg dose, 18% (n = 6) had a decrease of the PSA >50% of the baseline, whereas none was seen on the 5 mg arm. Overall, 10 of 26 patients in the 5 mg arm and 13 of 34 patients in the 25 mg arm progressed at 6 months (Table 4). Seven of 26 patients in the 5 mg arm and 5 of 34 patients in the 25 mg arm showed a ≥25% PSA increase, and 3 of 26 patients in the 5 mg arm versus 8 of 34 patients in the 25 mg arm showed either local relapse or new distant metastasis on scans.
Fig. 2.
Changes of PSA slope in the two dose groups of lenalidomide. *, the length of the box is the spread of the middle 50% of the observations. The line inside each box is the median. The lines extend to the upper quartile plus 1.5 times the interquartile range and the lower quartile minus 1.5 times the interquartile range.
Table 4.
Treatment characteristics and response at 6 mo
| Revlimid, 5 mg (n = 26) | Revlimid, 25 mg (n = 34) | P | |
|---|---|---|---|
| Percentage with no progression at 6 mo | 50% (n = 13) | 44% (n = 15) | |
| Reason for stopping lenalidomide | |||
| Toxicity | 11.5% (n = 3) | 17.5% (n = 6) | |
| Progression of PSA ≥25% | 27% (n = 7) | 15% (n = 5) | |
| Progression of scans | 11.5% (n = 3) | 23.5% (n = 8) | |
| No PSA progression (in all patients) | 50% (n = 13) | 56% (n = 19) | |
| Negative PSA slope at 6 mo | 38% (n = 10) | 38% (n = 13) | |
| Mean PSA slope change (range, 95% CI) | −0.033 (−0.11 to 0.04) | −0.172 (−0.24 to −0.11) | 0.005 |
| Mean (range, 95% CI) steady state (at 2 mo), lenalidomide-plasma concentration (ng/mL) | 12.67 (6.1–19.2) | 65.14 (24.3–106.0) | 0.01 |
Lenalidomide pharmacokinetics
Blood samples for measurement of lenalidomide plasma concentration at steady state (day 21 of the second treatment cycle) were available in 20 of 26 and 27 of 34 patients on 5 and 25 mg of lenalidomide, respectively. Mean lenalidomide plasma concentrations (range, 95% CI) were 12.67 ng/mL (6.1–19.2) and 65.14 ng/mL (24.3–106.0), in the 5 and 25 mg arm, respectively (P = 0.01). Plasma concentrations did not correlate with the change in PSA slope (Pearson correlation coefficient, ρ = −0.19; P = 0.20).
Long-term follow-up, post-6 month (cycle 1) period
Thirteen of 26 (50%) and 15 of 34 (44%) of the patients in the 5 and 25 mg arms, respectively, stayed on treatment for longer than 6 months. The rate of progressive disease, as defined by the protocol, in subsequent cycles is similar in both arms, and significantly lower than historical data. With a mean follow-up of 31.4 ± 10.4 months (range, 14–44), six patients are still on study, five patients on the 25 mg arm and one patient on the 5 mg arm. At present, the average duration of treatment is 13.1 months (range, 0.25–40) and 10.5 months (range, 1–35), in the 5 and 25 mg arm, respectively.
Discussion
The present trial was designed to evaluate the feasibility, safety, and potential benefit of lenalidomide in early (nonmetastatic) prostate cancer. Because eligible patients have no other evidence of active disease, we relied on changes in PSA dynamics as a potential signal for antitumor activity. Based on our prior experience, any expression of PSA dynamics (PSADT, PSA slopes, etc.) represents the strongest predictor of outcome in this patient population (4, 7). Because the risk stratification grouping of this patient population by PSADT (7) is, at present, a noncontinuous variable (<3, 3–9, ≥9 mo), the closely related PSA slope (4) was chosen as the primary efficacy statistical end point in this study. It has better properties for analysis purposes as using all the available data points, being continuous, and not subject to some of the constraints of PSADT calculations. We hypothesized that a dose-response relationship on PSA dynamics could support a potential therapeutic benefit by delaying the rate of progression to clinically evident metastasis. To test this hypothesis, we assumed that the 25 mg dose was more likely to have activity than the low dose based on existing data with this compound in other malignancies (22, 23). The randomized phase I/II trial design was selected to minimize patient selection differences between arms by controlling (stratifying) for various factors that are known to predict for time-dependent variables in this patient population and other end points that could affect the outcome to be evaluated (type of primary treatment, PSADT, and prior ADT). To maximize patient compliance for the 6-month study period, all serum samples in these patients were frozen and PSA determinations were analyzed in batches at the end of every 6-month interval. The sample size for each study arm was calculated to detect a 50% decrease in the rate of disease progression from 80% (seen in a large patient population, refs. 4, 7; with similar baseline characteristics as the present study population) to 40% observed at 6 months. These end points were selected based on our prior experience in this population indicating that 80% of the patients eligible for this study would continue to show evidence of progression at 6 months without treatment. It is hard to keep patients blinded to their PSA for a prolonged period of time. We feel that the 6-month period is a reasonable period of exposure, allowing enough PSA measurements to evaluate its dynamics. Based on the known PSA dynamics without treatment in this patient population (4, 7), biochemical progression was defined as an increase of PSA ≥25% at 6 months. Although we recognize that the biochemical progression and the 50% decrease in progression rate at 6 months, as defined in this study, has not been validated in relation to clinically relevant events (such as bone metastasis or survival for example), we feel that this represents a reasonable choice of potential clinical significance to employ in initial screening for a signal of activity. Moreover, although the value of PSA kinetic changes to predict clinically meaningful end points as objective scan progression is uncertain at present, preliminary results from a retrospective combined analysis of three prospective phase II studies seems to suggest that changes in PSA kinetics (PSA slope, PSADT, and PSA velocity) may be correlated with metastasis-free survival (24). Further validation of this approach will require specially designed phase III trials.
The statistically significant difference in the change in slope of PSA (pre-study versus study period) favoring the 25 mg dose would support the predefined hypothesis that lenalidomide treatment might result in a dose-dependent effect on the progression of these patients (Table 4; Fig. 2). This is further evidenced by a greater negative change in the slope of PSA declines with the 25 mg dose. Interestingly, although the higher dose had a greater effect on the slope of PSA, the observed progression rates at 6 months in both arms were lower than the expected progression rate in our historical data. Even at the 12-month point, 58% (n = 15) and 50% (n = 17) of patients on the 5 and 25 mg arms, respectively, were considered to have disease progression which, again, is better than our historical experience.
A number of correlative studies are under way and will be reported separately. However, a preliminary analysis assessing autoantibody responses in a panel of commonly recognized prostate cancer–associated antigens in stored sera samples revealed a robust autoantibody response at 6 months compared with the baseline (25). Among the antigens included are testis cancer antigens, antigenic proteins recognized by IgG in patients with chronic prostatitis, and MAD-Pro-34, MAD-CT-1, and LAGE-1, all of which have previously been shown to be the targets of autoantibody responses in prostate cancer patients. These data, coupled with the pattern of clinical responses, suggest that lenalidomide-associated general immunomodulation could be the basis for its potential clinical benefit manifested by a delay in disease progression. Current data on file at Celgene as well as published data (22, 26) suggest that the 5 mg dose may be above the threshold for immune stimulation, and associated with clinical activity, which could support a benefit even at the lower dose. Additional studies in progress will aim to characterize the autoantibody profile in these patients, as well as potential correlations between cytokines and other immunologic variables with study outcome measures.
Overall, the incidence and severity of adverse drug-related reactions was modest and reversible, which indicates that this drug is appropriate for long-term treatment. The incidence of grade 1/2 adverse events was similar between the arms except for fatigue and lymphopenia, which were more common with the 25 mg dose (Table 3). A higher proportion of patients (29%) receiving the 25 mg dose developed grade 3/4 and dose-limiting toxicity during the treatment period, when compared with the 5 mg dose arm (12%, P = 0.1; Table 2). This consisted mainly of neutropenia, venous thromboembolic events (n = 4, 7%), fatigue, and skin rash. Prophylactic measures to prevent venous thromboembolism should be considered in future trials in this disease.
In summary, the present study suggests that lenalidomide may be active in this patient population as evidenced by dose-dependent changes in PSA slope, and lower than expected rates of disease progression compared with historical data. Long-term treatment is feasible and overall reasonably well tolerated. For more definitive conclusions regarding the efficacy of lenalidomide in this disease, further testing in prospective randomized studies evaluating more conventional disease end points is warranted. Overall, the lack of significant differences in progression between dose levels at 6 months, and the toxicity profile favoring the lower dose, support testing both dose levels in a larger cohort. Based on PSADT risk stratification in this patient population and the associated prostate cancer mortality we feel that patients with a PSADT of <9 months may be appropriate candidates for future trials.
Translational Relevance.
There is no standard treatment in nonmetastatic biochemically relapsed prostate cancer. The present study suggests a potential benefit of lenalidomide in the progression rate of these patients.
Acknowledgments
Grant Support
The authors acknowledge Celgene Corporation for providing lenalidomide and funding support.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 2.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 3.Moul JW, Bañez LL, Freedland SJ. Rising PSA in nonmetastatic prostate cancer. Oncology (Williston Park) 2007;21:1436–1445. (Review). [PubMed] [Google Scholar]
- 4.Pound C, Partin A, Eisenberger M, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 5.Zelefsky MJ, Ben-Porat L, Scher HI, et al. Outcome predictors for the increasing PSA state after definitive external-beam radiotherapy for prostate cancer. J Clin Oncol. 2005;23:826–831. doi: 10.1200/JCO.2005.02.111. [DOI] [PubMed] [Google Scholar]
- 6.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 7.Antonarakis ES, Trock BJ, Feng Z, et al. The natural history of meta-static progression in men with PSA-recurrent prostate cancer after radical prostatectomy: 25-year follow-up. J Clin Oncol. 2009;27:15s. (suppl; abstr 5008). [Google Scholar]
- 8.D'Amico AV, Moul JW, Carroll PR, Sun L, Lubeck D, Chen MH. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst. 2003;95:1376–1383. doi: 10.1093/jnci/djg043. [DOI] [PubMed] [Google Scholar]
- 9.Zhou P, Chen MH, McLeod D, Carroll PR, Moul JW, D'Amico AV. Predictors of prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Clin Oncol. 2005;23:6992–6998. doi: 10.1200/JCO.2005.01.2906. [DOI] [PubMed] [Google Scholar]
- 10.Figg W, Dahut W, Duray P, et al. A randomized phase II trial of thalidomide, an angiogenesis inhibitor, in patients with androgen independent prostate cancer. Clin Cancer Res. 2001;7:1888–1893. [PubMed] [Google Scholar]
- 11.Drake MJ, Robson W, Mehta P, Schofield I, Neal DE, Leung HY. An open label phase II study of low dose thalidomide in androgen independent prostate cancer. Br J Cancer. 2003;88:822–827. doi: 10.1038/sj.bjc.6600817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahut WL, Gulley JL, Arlen PM, et al. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J Clin Oncol. 2004;22:2532–2539. doi: 10.1200/JCO.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 13.Gaidarova s, Corral LG, Gleizer E, et al. Lenalidomide enhances anti-tumor effect of γδ T cells against mantle cell lymphoma. Blood. 2008;112:906. (Abstract 2616). [Google Scholar]
- 14.Gorgun G, Ramsay AG, Holderried TA, et al. E(mu)-TCL1 mice represent a model for immunotherapeutic reversal of chronic lymphocytic leukemia-induced T-cell dysfunction. Proc Natl Acad Sci U S A. 2009;106:6250–6255. doi: 10.1073/pnas.0901166106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-α. J Immunol. 1999;163:380–386. [PubMed] [Google Scholar]
- 16.Lentzsch S, LeBlanc R, Podar K, et al. Immunomodulatory analogs of thalidomide inhibit growth of Hs Sultan cells and angiogenesis in vivo. Leukemia. 2003;17:41–44. doi: 10.1038/sj.leu.2402745. [DOI] [PubMed] [Google Scholar]
- 17.Lu L, Payvandi F, Wu L, et al. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc Res. 2009;77:78–86. doi: 10.1016/j.mvr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Dahut WL, Aragon-Ching JB, Woo S, et al. Phase I study of oral lenalidomide in patients with refractory metastatic cancer. J Clin Pharmacol. 2009;49:650–660. doi: 10.1177/0091270009335001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu D, Corral LG, Fleming YW, Stein B. Immunomodulatory drugs Revlimid (lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer Immunol Immunother. 2008;57:1849–1859. doi: 10.1007/s00262-008-0512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenbaum E, Zahurak M, Sinibaldi V, et al. Marimastat in the treatment of patients with biochemically relapsed prostate cancer: a prospective randomized, double-blind, phase I/II trial. Clin Cancer Res. 2005;11:4437–4443. doi: 10.1158/1078-0432.CCR-04-2252. [DOI] [PubMed] [Google Scholar]
- 21.Laufer M, Pound CR, Carducci MA, Eisenberger MA. Management of patients with rising prostate-specific antigen after radical prostatectomy. Urology. 2000;55:309–315. doi: 10.1016/s0090-4295(99)00465-3. [DOI] [PubMed] [Google Scholar]
- 22.Richardson PG, Schlossman RL, Weller E, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100:3063–3067. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 23.Ferrajoli A, Lee BN, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonarakis ES, Lin J, Keizman D, Carducci MA, Eisenberger MA. The effect of changes in PSA kinetics on metastasis-free survival (MFS) in patients with PSA-recurrent prostate cancer (PC) treated with nonhormonal agents: combined analysis of three randomized trials. J Clin Oncol. 2010;28:15s. doi: 10.1002/cncr.26437. (suppl;abstr 4549). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maricque BB, Smith HA, Drake C, et al. Detection of autoantibody responses to prostate cancer-associated antigens following treatment of patients with androgen deprivation therapy. American Association for Cancer Research (ACCR) 101st Annual Meeting; Washington (DC). 2010. (Abstract). [Google Scholar]
- 26.Idler I, Giannopoulos K, Zenz T, et al. Lenalidomide treatment of chronic lymphocytic leukaemia patients reduces regulatory T cells and induces Th17 T helper cells. Br J Haematol. 2010;148:948–950. doi: 10.1111/j.1365-2141.2009.08014.x. [DOI] [PubMed] [Google Scholar]