Abstract
Molecular deletion of transglutaminase 2 (TG2) has been shown to improve function and survival in a host of neurological conditions including stroke, Huntington's disease, and Parkinson's disease. However, unifying schemes by which these cross-linking or polyaminating enzymes participate broadly in neuronal death have yet to be presented. Unexpectedly, we found that in addition to TG2, TG1 gene expression level is significantly induced following stroke in vivo or due to oxidative stress in vitro. Forced expression of TG1 or TG2 proteins is sufficient to induce neuronal death in Rattus norvegicus cortical neurons in vitro. Accordingly, molecular deletion of TG2 alone is insufficient to protect Mus musculus neurons from oxidative death. By contrast, structurally diverse inhibitors used at concentrations that inhibit TG1 and TG2 simultaneously are neuroprotective. These small molecules inhibit increases in neuronal transamidating activity induced by oxidative stress; they also protect neurons downstream of pathological ERK activation when added well after the onset of the death stimulus. Together, these studies suggest that multiple TG isoforms, not only TG2, participate in oxidative stress-induced cell death signaling; and that isoform nonselective inhibitors of TG will be most efficacious in combating oxidative death in neurological disorders.
Introduction
Transglutaminases (TG) are an inducible family of protein cross-linking or polyaminating enzymes that have been implicated in diverse neurological conditions. The best-studied enzyme of the family, TG2 (gene: Tgm2; protein: TG2), is ubiquitously expressed in the human body and is the most abundant isoform in the brain.
A role for TG2 in neurodegeneration was first invoked as a cross-linker of aggregated proteins in a host of diseases associated with protein dyshomeostasis. Indeed, its transamidating activity has been shown to be upregulated at the symptomatic stage of Huntington's disease (HD) (Karpuj et al., 1999; Dedeoglu et al., 2002; Karpuj et al., 2002), Parkinson's disease (PD) (Gibrat et al., 2010), Alzheimer's disease (AD) (Martin et al., 2011), cerebral ischemia (Ientile et al., 2004), traumatic brain injury (Tolentino et al., 2002), and spinal cord injury (Festoff et al., 2002). To establish whether TG2 transamidating activity is causally related to neurodegeneration, structurally diverse inhibitors have been developed that inhibit the cysteine catalytic site. Intense interest in TG2 has been fueled by the success of reversible broad inhibitors [e.g., cyst(e)amine] that have shown durable and reproducible protective effects in in vivo models of several chronic neurodegenerative diseases such as HD (Dedeoglu et al., 2002), PD (Gibrat et al., 2010) and intracerebral hemorrhage (Okauchi et al., 2009). Indeed, cystamine is in phase II studies in humans with HD.
While a focus on TG2 has been validated by the therapeutic success of germline deletion of TG2 in rodent models of neurodegenerative disease (Iismaa et al., 2009), two important issues remain unsettled. First, isoform nonselective inhibitors result in therapeutic benefit beyond TG2 deletion in rodent models of HD (Bailey and Johnson, 2006), suggesting the possibility that other isoforms of the TG family can compensate for deletion of a single isoform; second, TG2 deletion does not decrease protein aggregation, suggesting a more complex role for the enzyme in stress responses (Mastroberardino et al., 2002). Indeed, current studies implicate TG2 in diverse cellular functions, including autophagosome formation (D'Eletto et al., 2009), axonal BDNF trafficking (Borrell-Pagès et al., 2006), and transcriptional repression (McConoughey et al., 2010). Accordingly, the current study was designed with two specific goals: first, to elucidate the role, if any, of other TG isoforms in neuronal injury; and second, to understand whether a common putative mediator of death, oxidative stress, could induce TG message levels and activity as part of a death cascade.
We report that multiple isoforms of TG are significantly induced following stroke in vivo or oxidative stress in vitro; that forced expression of TG1 or TG2 induces cell death in cortical neurons; and that oxidative stress-induced cell loss in cortical neurons can be rescued by isoform nonselective inhibitors.
Materials and Methods
Chemicals.
Several structurally different TG inhibitors were tested in our models: cystamine dihydrochloride (broad and reversible inhibitor, Sigma); and the irreversible inhibitors B003 (Boc-DON-Gln-Ile-Val-OMe, Zedira GmbH), T101 (1,3,4,5-tetramethyl-2-[(2-oxopropyl)thio]imidazolium chloride, Zedira GmbH), D004 (1,3-dimethyl-4,5-diphenyl-2-[(2-oxopropyl)thio]imidazolium trifluorosulfonicacid salt), and TAMRA-DON (Zedira GmbH). The MEK inhibitors U0126, SL327 and the inactive analog U0124 (Calbiochem) were tested in our model. l-glutamic acid and its analog, l-homocysteic acid (HCA) were purchased from Sigma-Aldrich.
Transient middle cerebral artery occlusion.
The use of animals and procedures were approved by the Institutional Animal Care and Use Committees of Weill Medical College of Cornell University. Ten- to 12-week-old C57BL/6 male mice were subjected to transient ischemic by middle cerebral artery occlusion (MCAO) as previously described (Cho et al., 2005, 2007; Kim et al., 2008). Briefly, mice were anesthetized with a mixture of isoflurane/oxygen/nitrogen. A fiber optic probe was glued to the parietal bone (2 mm posterior and 5 mm lateral to the bregma) and connected to a laser-Doppler flowmeter (Periflux System 5010; Perimed) for continuous monitoring of cerebral blood flow (CBF) in the center of the ischemic territory. For MCAO, a 6–0 Teflon-coated black monofilament surgical suture (Doccol) was inserted into the exposed external carotid artery, advanced into the internal carotid artery, and wedged into the cerebral arterial circle to obstruct the origin of the MCA for 30 min. The filament was withdrawn to allow reperfusion. Lidocaine was administered during postischemia as analgesics. Using a rectal probe controlled by a masterflex pump and thermistor temperature controller (Cole-Parmer), animals' body temperatures were maintained at 37 ± 0.5°C during MCAO and 2 h after postischemia. Only animals that exhibited >80% reduction in CBF during MCAO and >80% reperfusion 10 min following reperfusion were included in the study.
Tissue preparation after MCAO.
Brains were excised, frozen, and sectioned using an unbiased stereological sampling strategy. Infarct typically spans ∼6 mm rostrocaudal, approximately from +2.8 to −3.8 mm from bregma. To collect tissue to reflect the infarct area, the entire infarct region was cryosectioned for gene expression (four sections at 50 μm thickness) and collected serially at 600 micron intervals. The sections were cut in half and collected for each hemisphere.
Mouse embryonic fibroblasts.
TG2 knock-out mice, designated Tgm2tm1.1Rmgr (TG2−/−), were generated on a 129S1/Sv-ImJ background as previously described (Nanda et al., 2001). Heterozygous offspring were backcrossed to wild-type C57BL/6 (B6.Cg) mice for 12 generations to generate congenic heterozygous TG2+/− mice with ∼99.95% B6.Cg-TG2+/− genomic homogeneity, respectively. These heterozygous animals were mated to generate TG2 wild-type (TG2+/+) or TG2−/− mice that were then set up in breeding pairs to generate TG2+/+ or TG2−/− embryos for experimentation. Mouse embryonic fibroblasts (MEFs) were isolated from 13.5-d-postcoitus female and male embryos and were cultured on DMEM/10% FCS [DMEM high glucose, Invitrogen); 10% (v/v) fetal calf serum (Invitrogen); 400 μm l-glutamine (Invitrogen); 0.2 U/ml penicillin (Invitrogen); and 0.2 mg/ml streptomycin (Invitrogen)]. Only MEFs of passage five or lower was used for experiments.
Primary neuronal and astrocyte cultures.
Primary rat cortical neurons were obtained from fetal Sprague Dawley rats at embryonic day 17 (E17) as previously described (Ratan et al., 1994). Primary mouse cortical neurons were obtained from C57BL/6 TG2+/− mice mated to generate male and female TG2+/+, TG2−/+, or TG2−/− embryos at day 15. Single embryonic neuronal cultures were performed, and their genotype was revealed by PCR. The astrocyte-neuronal cultures were obtained as previously described (Haskew-Layton et al., 2010).
Genotyping.
Genomic DNA for PCR was extracted from the embryos using the DNeasy genomic DNA isolation kit (Qiagen). The TG2 wild-type alleles were detected using the primer 5′-GGAGCACACAGGCCTTATGAGCTGAAG-3′. The TG2 knock-out alleles were detected using the primer 5′-CAGATAGGGATACAAGAAGCATTGAAG-3′. As a common reverse primer, we used 5′-GCCCCACAAAGGAGCAAGTGTTACTATGTC-3′.
Cell viability.
For neuronal cytotoxicity studies, cortical neurons were plated at a density of 106 cells/ml in 96-well plates (100 μl) and in 6-well plates (3 ml), and were treated with glutamate 5 mm. Specified concentrations of cystamine, B003, T101, D004, and TAMRA-DON were added at the time of glutamate treatment. In the post-treatment experiments, TG inhibitors were added at various time points after glutamate addition (14, 17, 19, and 21 h), and viability was assessed 24 h later. MEF TG2+/+ and TG2−/− cells were plated at 104 cells/ml in black 96-well plates for 16 h (+/− glutamate), and live cells were visualized with calcein AM (Invitrogen) by a Flash Cytometer (Trophos) and counted with TINA v4.8 (Trophos). In the astrocyte-neuronal cocultures, astrocytes were pretreated for 16 h with inhibitors at various concentrations. The drugs were removed, and the neurons were plated on top of the astrocytes at a concentration of 0.5 × 106 cells/ml in the presence or absence of the glutamate analog HCA. Neuronal viability was measured 48 h after neuronal plating by quantifying the neuronal-specific marker MAP2 using a horseradish peroxidase/Amplex Red assay, as previously described (Haskew-Layton et al., 2010).
RNA extraction and real-time PCR.
Total RNA was prepared from immature primary cortical neurons (E17), MEF, and brain sections, and were reversed transcribed to cDNA with a standard protocol. The expression levels of rat and mouse Tgm1, Tgm2 were quantified by real-time (RT) PCR as previously described (McConoughey et al., 2010). The primers used in rat samples are the following: Tgm1 (5′-AGAGCACCACACCGATGAGTTTGA-3′ and 5′-TCCGATGAGAAGCTCAAGGGCAAT-3′); Tgm2 (5′-GCCTTGGAACTTTGGGCAGTTTGA-3′ and 5′-TCATCATTGCAGTTGACCATGCCG-3′); and β-actin (5′-CCATTGAACACGGCATTGTCACCA-3′ and 5′-GCCACACGCAGCTCATTGTAGAAA-3′). The primers used in mouse samples are the following: Tgm1 (5′-TGTGGAGATCCTGCTCAGCTACCTA-3′ and 5′-TGTCTGTGTCGTGTGCAGAGTTGA-3′); Tgm2 (5′-TTCCGGCTGACTCTGTACTTCGAG-3′ and 5′-ACATTGTCCTGTTGGTCCAGCACT-3′); and β-actin (5′-TGAACCCTAAGGCCAACCGTGAAA-3′ and 5′-GAGTCCATCACAATGCCTGTGGTA-3′). The comparative cycle threshold (Ct) method was used to analyze the data from quantitative RT-PCR. The amount of target (Tgm1 or Tgm2) normalized to an endogenous reference (β-actin) and relative to a calibrator (Fig. 1A,B, sham contralateral; Fig. 1C,D, 0 h treatment; Fig. 2C,D, TG2+/+) is given by the 2−ΔΔCt algorithm, also known as the delta-delta-Ct or ddCt algorithm. The mean Ct and SD values were calculated by ABI Sequence Detection System software version 1.4 (Applied Biosystems). Each sample was run in triplicate, and in each experiment three or four samples per condition were analyzed. One-way ANOVA followed by Dunnett's post hoc test was calculated in Prism (GraphPad Software).
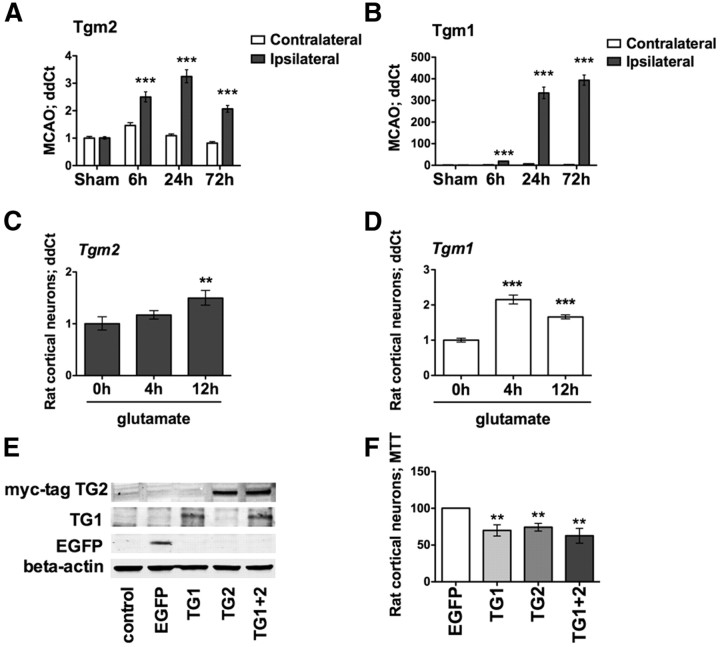
Figure 1.
TG1 and TG2 mRNA levels are upregulated in a focal model of stroke (MCAO) and in an in vitro model of neuronal oxidative stress, and they are sufficient to induce cell death. A, B, Temporal expression profile for Tgm2 (A) and Tgm1 (B) in MCAO. Both of the genes are significantly upregulated in the ipsilateral side compared with the contralateral hemisphere (***p < 0.001). A similar upregulation is revealed in an in vitro model of oxidative stress. C, D, Tgm2 (C) and Tgm1 (D) levels are significantly upregulated 12 h after glutamate (5 mm) exposure in rat neurons (**p < 0.01; ***p < 0.0001 compared with the relative glutamate 0 h). E, F, Forced expression of TG1 and/or TG2 (E) exerts neuronal toxicity, as revealed by MTT (F). Significant toxicity compared with EGFP expression. **p < 0.01. EGFP-expressing cells are calculated at 100% survival.
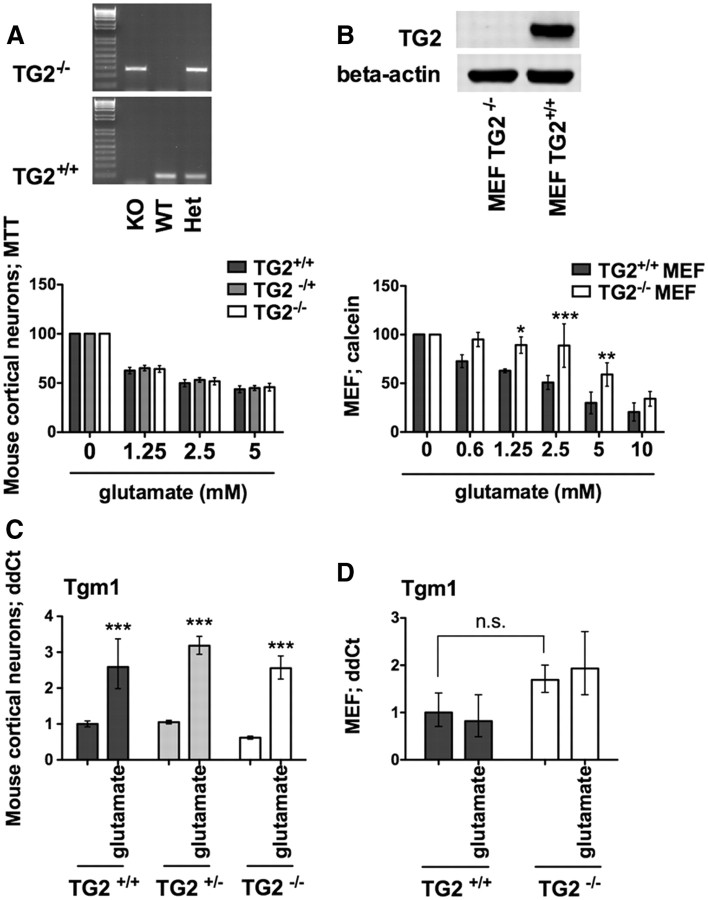
Figure 2.
TG2 is necessary for oxidative death in MEFs but not cortical neurons. Resistance of cortical neurons to TG2 knockout is associated with compensatory upregulation of Tgm1. A, B, TG2−/− in single embryonic neuronal cultures (A) and in MEFs (B) and relative MTT assay. TG2 deletion in MEFs significantly protects against glutamate-induced death. *p < 0.05; **p < 0.01; ***p < 0.001 compared with TG2+/+ MEFs; untreated controls are calculated at 100% of survival. C, D, Tgm1 levels are induced after 12 h of glutamate treatment in mouse neurons (C) (***p < 0.001 compared with controls), but not in MEFs (D).
Measurement of endogenous transamidating activity.
For the glutamate treatments, cells were treated at different time points (4, 8, and 12 h) and cotreated with cystamine (100 μm), B003 (200 μm), D004 (50 μm), T101 (50 μm), U0126 (10 μm), U0124 (10 μm), and SL327 (10 μm) for 12 h. The lysine donor biotin pentylamine (BPA; 500 μm) (Thermo Scientific) was added to the media at the same time. Cells were lysed in hypotonic buffer (10 mm Tris-HCl pH8, 1 mm KCl, 1 mm MgCl2). Equal amounts of proteins were loaded in a Bio-Dot Apparatus (Bio-Rad). Extracellular proteins were harvested from the media and centrifuged at 21,000 × g at 4°C for 10 min and then 100 μl per sample was loaded into the Bio-Dot Apparatus. The nitrocellulose membrane was probed for streptavidin and actin (loading control). The dot densitometry was calculated with the Odyssey software (Integrated Intensity, LI-COR Bioscience).
TG1 and TG2 overexpression.
Human TG2 and TG1 or EGFP sequences were introduced into cortical neurons (E17) using the Amaxa Rat neuron Nucleofector Kit (Lonza). The next day, TG2, TG1, and EGFP overexpression was confirmed by Western blot, and cell survival was measured by MTT.
TG2 antibody.
We produced a specific custom rabbit monoclonal antibody (Epitomics), using recombinant mouse TG2 as immunogen. Specificity is shown in Figure 2D.
Immunoblot analysis.
Protein extracts were obtained using 1% Triton buffer. Antibodies against Myc-tag (9E10, Covance), EGFP (Molecular Probes), TG2 (custom made), TG1 (Abcam), and β-actin (AC-74; Sigma-Aldrich) were diluted 1:1000, 1:2000; 1:1000; 1:1000, and 1:10,000, respectively. Proteins were detected using an Odyssey infrared imaging system (LI-COR Biosciences).
Statistical analysis.
Statistical analysis was conducted by two-way ANOVA followed by Bonferroni's post hoc test or one-way ANOVA followed by Dunnett's post hoc test. Statistically significant results were defined as follows: *p < 0.05; **p < 0.01; and ***p < 0.001. All the experiments presented here were repeated at least three times.
Results
Multiple TGs are induced following stroke in vivo or neuronal oxidative stress in vitro
TG inhibition has been shown to reduce damage in hemorrhagic (Okauchi et al., 2009) and ischemic stroke models (Tolentino et al., 2004; Hwang et al., 2009), but an analysis of how message levels of distinct TG isoforms change following ischemic stroke has not been performed. We analyzed a temporal profile of mRNA expression of six of the nine known TG isoforms from the ipsilateral hemisphere of rodents that underwent focal brain ischemia (MCAO; sham, and 6, 24, and 72 h after reperfusion). As expected, Tgm2 message levels were significantly induced in the ipsilateral hemisphere (Fig. 1A). Surprisingly, however, we also found a >100-fold increase in Tgm1, which peaked 72 h after the onset of ischemia (Fig. 1B).
As stroke-induced damage is characterized by nitrosative (Samdani et al., 1997) and oxidative stress (Chen et al., 2011), we used the experimental leverage of a well established in vitro model in cortical neurons. In this model, glutamate simulates oxidative stress via a non-receptor-mediated mechanism involving the inhibition of plasma membrane cystine transport and depletion of the versatile antioxidant glutathione (Ratan et al., 1994). Prior studies have shown the utility of this model for studying stroke pathogenesis and treatment (Siddiq et al., 2005; Langley et al., 2008). As expected, not only Tgm2, but also Tgm1 message levels were induced in a manner qualitatively similar to that found following stroke in vivo (Fig. 1C,D).
Conflicting data have been presented about the role of TGs in modulating cell death (Piacentini et al., 2011). However, most of the studies implicating TG2 as a positive modulator of survival have been performed in transformed cancer cells, not in primary neurons. To determine whether TG1 or TG2 is sufficient to induce cell death, we transfected immature cortical neurons with human TG1, TG2, or EGFP as control (Amaxa, Lonza). We verified expression of TG1, TG2, or EGFP in separate experiments 24 h after transfection by immunoblot (Fig. 1E). Moreover, as monitored by morphological (phase contrast) or metabolic (MTT assay) criteria, overexpression of TG1 or TG2 is sufficient to induce neuronal cell death (Fig. 1F). Of note, transfection of the two isoforms together, which resulted in the same levels of each isoform as when they are expressed individually, did not enhance cell death. These results show that TG1 and TG2 are sufficient to induce neuronal death, and suggest that they work via a similar pathway, as their effects at this level of expression are not additive or synergistic.
Our findings suggested that TG1 and TG2 might work redundantly to ensure the death of neurons following oxidative death. To establish whether TG activity is necessary for oxidative death, we took advantage of germline knockouts of TG2 (TG2−/−). Animals deficient in this isoform have shown smaller infarct volumes in stroke (Colak and Johnson, 2012) or resistance to the deleterious effects of mutant huntingtin (Mastroberardino et al., 2002; Bailey and Johnson, 2006). However, germline knockout of TG2 was not sufficient to protect cortical neurons from oxidative stress (Fig. 2A); but its complete absence in mouse embryonic fibroblasts (cultured from TG2−/− mice) increased resistance to glutamate-induced oxidative stress (Fig. 2B). The ability of TG1 and TG2 to induce death of cortical neurons raised the possibility that cortical neurons were insensitive to TG2 deletion because of compensatory upregulation of TG1, whereas MEFs were sensitive to TG2 deletion because of the absence of TG1 compensation. Consistent with this model, we found that TG1 is induced in glutamate-treated cortical neurons that are TG2−/− (Fig. 2C) but not in glutamate-treated MEFs that are TG2−/− (Fig. 2D).
Intracellular transglutaminase transamidating activity is increased following oxidative stress and structurally diverse TG inhibitors protect neurons from oxidative stress-driven cell death
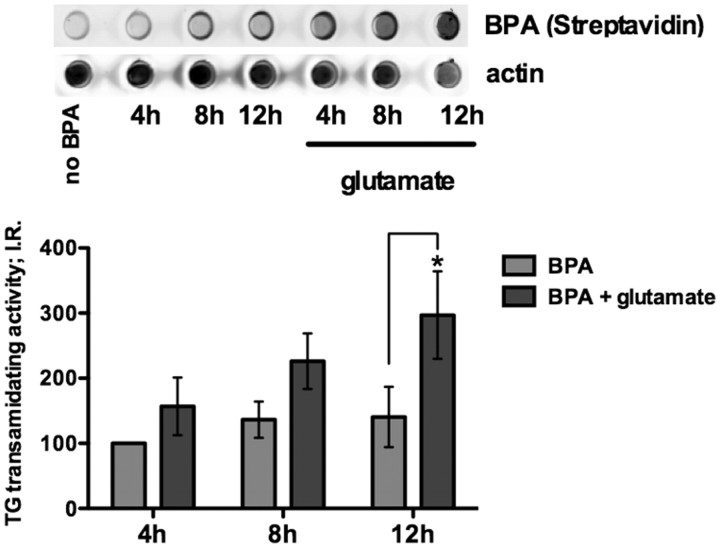
To establish whether TG1 and TG2 are both necessary for oxidative neuronal death, we used a set of structurally diverse small molecules whose commonality is that they inhibit the transamidating site cysteine in both TG1 and TG2 (Griffin et al., 2002). The use of diverse compounds makes it likely if common biological effects are observed, that these effects can be attributed to inhibition of the transamidating activity of TGs, rather than to an off-target effect of any one class of compounds. Moreover, use of small molecules allows us to target the transamidating activity without affecting TG GTPase activity or other domains. Before testing the compounds, we verified that the transamidating activity is increased following oxidative stress using BPA, a lysine donor whose incorporation into protein has been shown to be dependent on transamidating activity (Fig. 3) (Lee et al., 1992).
Figure 3.
Intracellular TG transamidating activity is increased upon glutamate treatment. Endogenous transamidating activity is significantly increased after 12 h of glutamate treatment compared with control by dot blot assay. **p < 0.01.
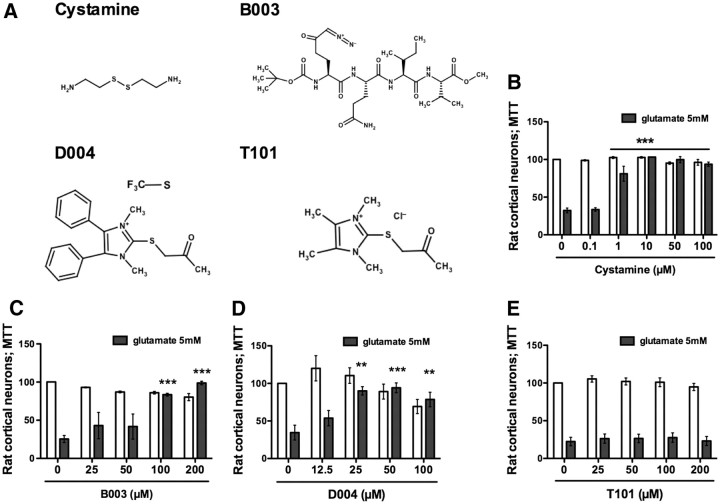
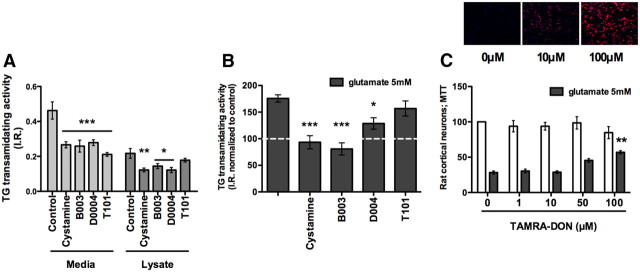
We first evaluated the effect of a commonly used TG inhibitor, cystamine, that was recently shown not to have significant preference for TG2 over TG1 or TG3 in in vitro assays (Schaertl et al., 2010). Cystamine is the disulfide form of the free thiol cysteamine, and it reversibly inhibits TG activity by acting as an alternative substrate for the enzyme. It is therefore considered as a pseudo-inhibitor substrate (Fig. 4A). As expected, we found that cystamine not only inhibited TG activity (Fig. 5A,B), but also potently protected cortical neurons from oxidative death (Fig. 4B). As cystamine has been shown to have off-target effects including caspase inhibition (Lesort et al., 2003), we tested an irreversible peptide inhibitor, B003, which has similar IC50 values for recombinant TG1 (1.1 μm) as those for TG2 (0.3 μm), but almost no activity toward caspase 3 (McConoughey et al., 2010). B003 forms a covalent bond with cysteine in the active site and is channeled specifically to TG by the amino acids that surround the reactive DON moiety (Fig. 4A). Similar to cystamine, B003 protected cortical neurons from oxidative death (Fig. 4C) and reduced cell associated transamidating activity (Fig. 5A,B). Together, these structurally diverse, isoform-nonselective TG inhibitors suggest that the transamidating activity is necessary for the death of cortical neurons in response to glutathione depletion.
Figure 4.
TG inhibition protects immature cortical neurons from oxidative stress-mediated cell death. A, Structures of four diverse, reversible or irreversible, isoform-nonselective TG inhibitors tested in an in vitro model of oxidative stress. B–D, Cystamine (1–100 μm) (B), B003 (100, 200 μm) (C), and D004 (25–100 μm) (D) protect primary immature cortical neurons against oxidative stress-induced cell death. E, T101, a cell-impermeable TG inhibitor, fails to provide protection. **p < 0.01; ***p < 0.001 compared with glutamate treatment alone; untreated controls are calculated at 100% of survival.
Figure 5.
Inhibition of intracellular TG activity correlates with neuroprotection. A, All inhibitors tested are able to significantly reduce extracellular transamidating activity (A). A, B, T101 fails to inhibit intracellular TG transamidating activity. B, All the protective inhibitors are able to significantly downregulate TG2 activity to control levels. C, TAMRA-DON, a peptide-based, fluorescent inhibitor analog to B003, enters the cells (10–100 μm) (top), and it protects against oxidative stress (100 μm) (bottom). **p < 0.01 compared with glutamate treatment alone; untreated controls are calculated at 100% of survival.
Inhibition of intracellular but not extracellular transamidating activity protects neurons from oxidative stress
By contrast to our study, a prior study in non-neural cells failed to show that B003 could inhibit cell-based TG activity, raising the possibility that extracellular TG might contribute to death (Schaertl et al., 2010). Indeed, TG2 can be secreted to exert biological changes on the extracellular matrix (Belkin, 2011). To establish whether TG activity must be inhibited intracellularly, extracellularly, or both to induce neuroprotection, we took advantage of two structurally similar inhibitors, D004 and T101 (Fig. 4A), where the nucleophilic thiol of the active site attacks the carbon between the sulfur and the carbonyl group, leading to an irreversible TG2 acetonylation. The compounds differ in their membrane permeability. T101 has two methyl groups, while D004 presents two phenyl groups. Accordingly, D004 is more hydrophobic, and it tends to penetrate preferentially into cells. As expected, the hydrophilic T101 was recently shown to act only extracellularly (Antonyak et al., 2011). We tested these compounds in our system and found that only the cell-permeable TG inhibitor D004 exerted dose-dependent protection against glutamate toxicity (Fig. 4D). T101 failed to inhibit intracellular activity (Fig. 5A,B) and was not able to protect neurons from glutamate toxicity at any of the concentrations tested (Fig. 4E). We conclude that cystamine, B003, and D004 all inhibit intracellular TG in neurons to exert protection from oxidative stress. This conclusion was further reinforced by two additional observations: first, the ability to detect a fluorescently conjugated peptide-based inhibitor, TAMRA-DON, to penetrate inside neurons in a dose-dependent manner (Fig. 5C, top) and the ability of this inhibitor to protect against oxidative stress at 100 μm (Fig. 5C, bottom); and second, the ability of TG inhibitors to protect hippocampal neuroblasts (grown in the absence of astrocytes) from oxidative death (data not shown). Our results do not exclude the possibility that both extracellular and intracellular TG must be inhibited to protect neurons.
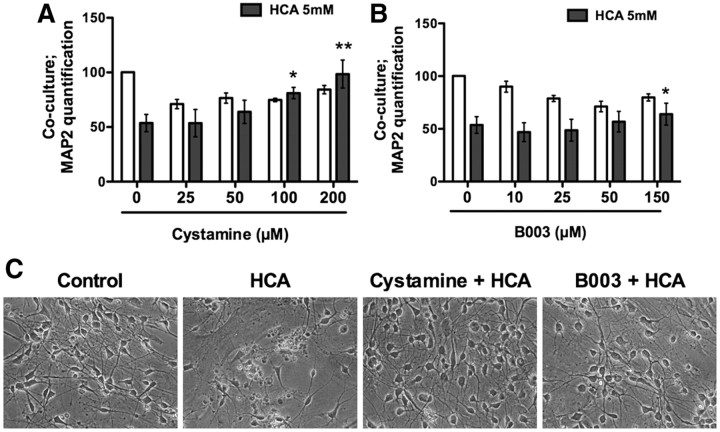
A recent study from our group showed that low, protective doses of hydrogen peroxide can induce a “state” change in astrocytes that facilitates their ability to protect adjacent neurons from glutathione depletion-induced death (Haskew-Layton et al., 2010). A targeted microarray demonstrated that Tgm2 was among the small number of genes whose downregulation correlated with neuroprotection (Haskew-Layton et al., 2010). To determine whether TG inhibition in astrocytes can non-cell autonomously protect neurons, we pretreated astrocytes with the isoform-nonselective TG inhibitors cystamine or B003. The inhibitors were washed off, cortical neurons were added, and the coculture was exposed to the glutamate analog HCA. Without pretreatment with the TG inhibitors, a substantial number of neurons died in response to the glutamate analog (Fig. 6A–C; HCA treatments). In contrast, TG inhibition in astrocytes led to a significant sparing of the neurons (Fig. 6A–C; cystamine and B003 treatments). These findings demonstrate that TG inhibition in neurons or astrocytes is sufficient to confer resistance to oxidative death.
Figure 6.
TG inhibition in astrocytes non-cell autonomously protects neurons. A, B, Astrocytes are treated overnight with the TG inhibitors Cystamine (A) or B003 (B). Following washoff of the inhibitors, neurons were plated with or without the glutamate analog HCA (5 mm) for 48 h: *p < 0.05; **p < 0.01 neuronal survival compared with glutamate treatment alone, untreated controls are calculated at 100% of survival. C, Representative phase contrast pictures for A and B; cystamine, 200 μm; B003, 150 μm.
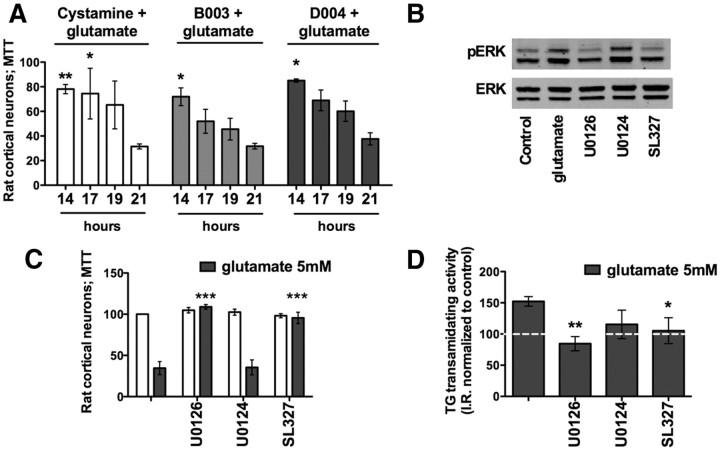
Inhibition of the ERK pathway suppresses TG2 activation
Previous studies from our laboratory and others have shown that glutathione levels reach a steady state (∼60% below control) at 6 h following glutamate treatment (Zaman et al., 1999). Cells become irreversibly committed to cell death much later, around 18 h. The increase of TGs transcription and activity within 8–12 h after glutamate exposure raised the possibility that TG inhibition, well after the onset of glutamate treatment, would suppress increased TG activity and protect neurons downstream of glutathione depletion. Indeed, addition of the cell-permeant TG inhibitors (cystamine 100 μm, B003 100 μm, D004 50 μm) up to 14–17 h after glutamate treatment, completely protected neurons from cell death (Fig. 7A), and this protection happened without an effect on glutathione depletion by glutamate (data not shown). TG inhibitors can thus be used well after depletion of glutathione and still protect neurons, and this may, in part, explain their effectiveness in multiple disease models. Increased TG activity well after the onset of oxidative stress and just before the cells are irreversibly committed to death led us to ask whether TG transamidating activity lies upstream or downstream of events known to be causally related to oxidative death that occur just before cell death commitment. Among the signaling molecules defined, the ERK family of kinases caught our attention. Prior elegant work from Ho et al. (2007) revealed a delayed and sustained activation of ERK1/2 owing to redox-mediated MKP-1 (MAPK phosphatase 1) or PP2A [4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine] inhibition. Intriguingly, prior data also suggest that ERK can activate TGs (Akimov and Belkin, 2003), and the transamidating activity may induce heterodimerization of ERK proteins into a complex in the nucleus (Lundquist and Dudek, 2006). We thus examined whether TG activity is downstream of pathological ERK activation in glutathione depletion-induced oxidative stress. To understand the relationship between TG activity and sustained ERK activation, we examined TG activity in the presence of a well established MEK1/2 (which works upstream of ERK) inhibitor U0126, its structurally similar but inactive analog U0124, and a brain-permeant MEK inhibitor, SL327 (Wang et al., 2003). These compounds were tested for activity (Fig. 7B) and protection (Fig. 7C) in our system. U0124 failed to block MEK (Fig. 7B) activation and failed to protect cells from oxidative stress (Fig. 7C). As expected, we found that protective concentrations of U0126 and SL327 significantly reduced TG activity and U0124 did not (Fig. 7D). Moreover, U0126 does not prevent death mediated by forced TG expression (data not shown). Altogether these findings place TG activity downstream of pathological ERK activation in the oxidative stress cascade and suggest that TG may be a distal point in the affector pathway of oxidative death.
Figure 7.
TG transamidating activity is a downstream target of the ERK pathway. A, TG inhibitors exerted protection when added up to 14–17 h post-glutamate exposure. B–D, Glutamate induces phosphorylated ERK (pERK) (B); MEK inhibitors U0126 and SL327 block pERK activation (B) and protect against glutamate toxicity (C) by reducing TG activation to the control level (D). *p < 0.05; **p < 0.01 compared with glutamate treatment alone; untreated controls are calculated at 100% of survival. U0124 is a negative control for U0126.
Discussion
Most schemes for TG's role in acute and chronic neurodegeneration have centered around the ability of these enzymes to cross-link mutated and/or accumulated proteins in a host of diseases, including AD, HD, and PD (Caccamo et al., 2010). And while this model unifies diseases associated with proteotoxicity, it fails to account for the benefits of molecular or pharmacological TG deletion in ischemic (Hwang et al., 2009; Colak et al., 2011) or hemorrhagic stroke (Okauchi et al., 2009). Indeed, exciting new data on the role of TG in autophagosome formation (D'Eletto et al., 2009), in inhibiting axonal transport of growth factors such as BDNF (Borrell-Pagès et al., 2006), in repressing adaptive gene expression (McConoughey et al., 2010), and on influencing nuclear actin dynamics (Munsie et al., 2011) have focused attention on biological roles of these fascinating enzymes other than cross-linking. Here, we demonstrate that TG is a necessary component of oxidative stress-induced death signaling in cortical neurons (Figs. 4–6). As oxidative stress has been implicated in almost every neurological condition, the findings suggest that even in diseases characterized by proteotoxicity TG's major role may not be limited to cross-linking but also may include cell death signaling.
Because of TG2's abundance in the brain, its transcriptional induction in a host of disease states, and the neuroprotective effects of its germline deletion, there has been almost exclusive focus on this isoform as a perpetrator of neuronal loss. Our results in a model of stroke in vivo and in a model of oxidative stress in vitro amplify recent studies by others showing that in addition to TG2, TG1 is also dramatically regulated by ischemia and/or oxidative stress. Unexpectedly, we showed that TG1 was as effective in inducing death in cortical neurons as TG2 (Fig. 1). The results help to explain the broad salutary effects of currently available small-molecule inhibitors of TG including in our in vitro model of oxidative stress, as all of these have been shown to inhibit TG1, TG2, and other TG isoforms (Schaertl et al., 2010). They also provide one explanation for why in the R6/2 mouse model of HD isoform-nonselective TG inhibition prolongs survival (19.8%) longer than germline deletion of TG2 (12%) (Bailey and Johnson, 2006). Future studies will examine the effect of dual TG1 and TG2 deletion on disease outcomes in models of stroke and HD.
TGs have been implicated in oxidative stress-induced death outside of the nervous system, but most of these studies, which implicate TG as an inhibitor of cell death, involve the addition of nonphysiological oxidants to non-neural cells. By contrast, the model we used in this study involves depletion of the versatile cellular antioxidant, glutathione—a theoretically more physiological way to induce an imbalance in oxidants and antioxidants above a toxic threshold, the operational definition of “oxidative stress.” Indeed, exposure of enriched cultures of neurons or astrocyte-neuronal cocultures to high concentrations of glutamate or glutamate analog (5 mm) leads to selective degeneration of neurons over 24–48 h (Murphy et al., 1989; Haskew-Layton et al., 2010). In this paradigm, glutamate toxicity results not from hyperactivation of ionotropic glutamate receptors, but rather via competitive inhibition of cyst(e)ine transport, leading to diminished glutathione levels and oxidative death. Accordingly, neurons undergo antioxidant-responsive activation of an ERK-dependent, caspase-independent cell death mechanism that involves 12-lipoxygenase, truncated BH3 interacting domain (tBID) and apoptosis-inducing factor (AIF); Seiler et al., 2008. Of note, disrupted calcium homeostasis, which would be expected to activate TGs, has also been implicated in oxidative glutamate toxicity (Tan et al., 1998); our findings place TG activation as an event downstream of ERK signaling, but before cell death commitment. Indeed, ERK inhibitors cannot block death due to forced expression of TG2. In conjunction with the findings herein, recent studies from our laboratory (McConoughey et al., 2010) suggest that TG acts to repress prosurvival gene expression to ensure oxidative death; but our studies do not preclude a role for TG in the effector pathway of death following glutathione depletion in cortical neurons, including AIF translocation.
The in vitro model of oxidative death used in this study has been used by many laboratories to identify a number of targets whose inhibition is protective from stroke (Siddiq et al., 2005), HD (Ferrante et al., 2003, 2004), PD (Lee et al., 2009), or AD (Sagara et al., 1998) in vivo, including hypoxia-inducible factor prolyl hydroxylases (Siddiq et al., 2005), class I and II histone deacetylases (Langley et al., 2008), activating transcription factor-4 (Lange et al., 2008), Sp1 (Ryu et al., 2003; Sleiman et al., 2011), Myc (Sleiman et al., 2011), and Keap (a negative regulator of Nrf2) (Smirnova et al., 2011). Indeed, we found a similar induction of Tgm1 and Tgm2 levels in cortical neurons undergoing oxidative death as we did in the ipsilateral hemisphere following stroke (Fig. 1). Although our in vivo studies do not distinguish neuronal versus non-neural activation of TG, we present in vitro data showing that inhibition of TG in astrocytes or neurons can abrogate neuronal oxidative death (Figs. 4–6).
In conclusion, our study highlights the role that oxidative stress, a putative mediator of many neurological conditions, can play in activating multiple TG isoforms downstream of pathological ERK signaling. The findings provide a unifying model for the role of TG in numerous neurological conditions, and the broad salutary effects of isoform-nonselective TG inhibitors. As ERK has both physiological and pathological roles (Chu et al., 2004), we propose that TG inhibition represents a more specific way to inhibit pathological ERK signaling without affecting physiologically important ERK activation downstream of growth factor receptor activation.
Footnotes
This work was supported by the National Institute of Health (Grant P01 NIA AG014930, Project 1 to R.R.R.). We thank Dr. Sara Holman for her valuable suggestions on the MEF experiments. We thank Dr. Ralf Pasternack for the precious advice on transglutaminase inhibitors and Dr. Gail Johnson for her help in transferring the TG2 knock-out animals from Dr. Siiri Iismaa to our laboratory.
The authors declare no competing financial interests.
References
- Akimov SS, Belkin AM. Opposing roles of Ras/Raf oncogenes and the MEK1/ERK signaling module in regulation of expression and adhesive function of surface transglutaminase. J Biol Chem. 2003;278:35609–35619. doi: 10.1074/jbc.M303488200. [DOI] [PubMed] [Google Scholar]
- Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL, Holowka DA, Cerione RA. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci U S A. 2011;108:4852–4857. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CD, Johnson GV. The protective effects of cystamine in the R6/2 Huntington's disease mouse involve mechanisms other than the inhibition of tissue transglutaminase. Neurobiol Aging. 2006;27:871–879. doi: 10.1016/j.neurobiolaging.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Belkin AM. Extracellular TG2: emerging functions and regulation. FEBS J. 2011;278:4704–4716. doi: 10.1111/j.1742-4658.2011.08346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell-Pagès M, Canals JM, Cordelières FP, Parker JA, Pineda JR, Grange G, Bryson EA, Guillermier M, Hirsch E, Hantraye P, Cheetham ME, Néri C, Alberch J, Brouillet E, Saudou F, Humbert S. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J Clin Invest. 2006;116:1410–1424. doi: 10.1172/JCI27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo D, Currò M, Condello S, Ferlazzo N, Ientile R. Critical role of transglutaminase and other stress proteins during neurodegenerative processes. Amino Acids. 2010;38:653–658. doi: 10.1007/s00726-009-0428-3. [DOI] [PubMed] [Google Scholar]
- Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. Roles of oxidative stress, apoptosis, PGC-1alpha and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci. 2011;12:7199–7215. doi: 10.3390/ijms12107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Park EM, Febbraio M, Anrather J, Park L, Racchumi G, Silverstein RL, Iadecola C. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J Neurosci. 2005;25:2504–2512. doi: 10.1523/JNEUROSCI.0035-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Szeto HH, Kim E, Kim H, Tolhurst AT, Pinto JT. A novel cell-permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. J Biol Chem. 2007;282:4634–4642. doi: 10.1074/jbc.M609388200. [DOI] [PubMed] [Google Scholar]
- Chu CT, Levinthal DJ, Kulich SM, Chalovich EM, DeFranco DB. Oxidative neuronal injury. The dark side of ERK1/2. Eur J Biochem. 2004;271:2060–2066. doi: 10.1111/j.1432-1033.2004.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colak G, Johnson GV. Complete transglutaminase 2 ablation results in reduced stroke volumes and astrocytes that exhibit increased survival in response to ischemia. Neurobiol Dis. 2012;45:1042–1050. doi: 10.1016/j.nbd.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colak G, Keillor JW, Johnson GV. Cytosolic guanine nucledotide binding deficient form of transglutaminase 2 (R580a) potentiates cell death in oxygen glucose deprivation. PLoS One. 2011;6:e16665. doi: 10.1371/journal.pone.0016665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeoglu A, Kubilus JK, Jeitner TM, Matson SA, Bogdanov M, Kowall NW, Matson WR, Cooper AJ, Ratan RR, Beal MF, Hersch SM, Ferrante RJ. Therapeutic effects of cystamine in a murine model of Huntington's disease. J Neurosci. 2002;22:8942–8950. doi: 10.1523/JNEUROSCI.22-20-08942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Eletto M, Farrace MG, Falasca L, Reali V, Oliverio S, Melino G, Griffin M, Fimia GM, Piacentini M. Transglutaminase 2 is involved in autophagosome maturation. Autophagy. 2009;5:1145–1154. doi: 10.4161/auto.5.8.10040. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Kubilus JK, Lee J, Ryu H, Beesen A, Zucker B, Smith K, Kowall NW, Ratan RR, Luthi-Carter R, Hersch SM. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington's disease mice. J Neurosci. 2003;23:9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante RJ, Ryu H, Kubilus JK, D'Mello S, Sugars KL, Lee J, Lu P, Smith K, Browne S, Beal MF, Kristal BS, Stavrovskaya IG, Hewett S, Rubinsztein DC, Langley B, Ratan RR. Chemotherapy for the brain: the antitumor antibiotic mithramycin prolongs survival in a mouse model of Huntington's disease. J Neurosci. 2004;24:10335–10342. doi: 10.1523/JNEUROSCI.2599-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festoff BW, SantaCruz K, Arnold PM, Sebastian CT, Davies PJ, Citron BA. Injury-induced “switch” from GTP-regulated to novel GTP-independent isoform of tissue transglutaminase in the rat spinal cord. J Neurochem. 2002;81:708–718. doi: 10.1046/j.1471-4159.2002.00850.x. [DOI] [PubMed] [Google Scholar]
- Gibrat C, Bousquet M, Saint-Pierre M, Lévesque D, Calon F, Rouillard C, Cicchetti F. Cystamine prevents MPTP-induced toxicity in young adult mice via the up-regulation of the brain-derived neurotrophic factor. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:193–203. doi: 10.1016/j.pnpbp.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem J. 2002;368:377–396. doi: 10.1042/BJ20021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskew-Layton RE, Payappilly JB, Smirnova NA, Ma TC, Chan KK, Murphy TH, Guo H, Langley B, Sultana R, Butterfield DA, Santagata S, Alldred MJ, Gazaryan IG, Bell GW, Ginsberg SD, Ratan RR. Controlled enzymatic production of astrocytic hydrogen peroxide protects neurons from oxidative stress via an Nrf2-independent pathway. Proc Natl Acad Sci U S A. 2010;107:17385–17390. doi: 10.1073/pnas.1003996107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y, Logue E, Callaway CW, DeFranco DB. Different mechanisms account for extracellular-signal regulated kinase activation in distinct brain regions following global ischemia and reperfusion. Neuroscience. 2007;145:248–255. doi: 10.1016/j.neuroscience.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang IK, Yoo KY, Yi SS, Kim IY, Hwang HS, Lee KY, Choi SM, Lee IS, Yoon YS, Kim SY, Won MH, Seong JK. Expression of tissue-type transglutaminase (tTG) and the effect of tTG inhibitor on the hippocampal CA1 region after transient ischemia in gerbils. Brain Res. 2009;1263:134–142. doi: 10.1016/j.brainres.2009.01.038. [DOI] [PubMed] [Google Scholar]
- Ientile R, Caccamo D, Marciano MC, Currò M, Mannucci C, Campisi A, Calapai G. Transglutaminase activity and transglutaminase mRNA transcripts in gerbil brain ischemia. Neurosci Lett. 2004;363:173–177. doi: 10.1016/j.neulet.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Iismaa SE, Mearns BM, Lorand L, Graham RM. Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev. 2009;89:991–1023. doi: 10.1152/physrev.00044.2008. [DOI] [PubMed] [Google Scholar]
- Karpuj MV, Garren H, Slunt H, Price DL, Gusella J, Becher MW, Steinman L. Transglutaminase aggregates huntingtin into nonamyloidogenic polymers, and its enzymatic activity increases in Huntington's disease brain nuclei. Proc Natl Acad Sci U S A. 1999;96:7388–7393. doi: 10.1073/pnas.96.13.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpuj MV, Becher MW, Steinman L. Evidence for a role for transglutaminase in Huntington's disease and the potential therapeutic implications. Neurochem Int. 2002;40:31–36. doi: 10.1016/s0197-0186(01)00060-2. [DOI] [PubMed] [Google Scholar]
- Kim E, Tolhurst AT, Qin LY, Chen XY, Febbraio M, Cho S. CD36/fatty acid translocase, an inflammatory mediator, is involved in hyperlipidemia-induced exacerbation in ischemic brain injury. J Neurosci. 2008;28:4661–4670. doi: 10.1523/JNEUROSCI.0982-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange PS, Chavez JC, Pinto JT, Coppola G, Sun CW, Townes TM, Geschwind DH, Ratan RR. ATF4 is an oxidative stress-inducible, prodeath transcription factor in neurons in vitro and in vivo. J Exp Med. 2008;205:1227–1242. doi: 10.1084/jem.20071460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley B, D'Annibale MA, Suh K, Ayoub I, Tolhurst A, Bastan B, Yang L, Ko B, Fisher M, Cho S, Beal MF, Ratan RR. Pulse inhibition of histone deacetylases induces complete resistance to oxidative death in cortical neurons without toxicity and reveals a role for cytoplasmic p21(waf1/cip1) in cell cycle-independent neuroprotection. J Neurosci. 2008;28:163–176. doi: 10.1523/JNEUROSCI.3200-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Rajagopalan S, Siddiq A, Gwiazda R, Yang L, Beal MF, Ratan RR, Andersen JK. Inhibition of prolyl hydroxylase protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity: model for the potential involvement of the hypoxia-inducible factor pathway in Parkinson disease. J Biol Chem. 2009;284:29065–29076. doi: 10.1074/jbc.M109.000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KN, Maxwell MD, Patterson MK, Jr, Birckbichler PJ, Conway E. Identification of transglutaminase substrates in HT29 colon cancer cells: use of 5-(biotinamido)pentylamine as a transglutaminase-specific probe. Biochim Biophys Acta. 1992;1136:12–16. doi: 10.1016/0167-4889(92)90078-p. [DOI] [PubMed] [Google Scholar]
- Lesort M, Lee M, Tucholski J, Johnson GV. Cystamine inhibits caspase activity. Implications for the treatment of polyglutamine disorders. J Biol Chem. 2003;278:3825–3830. doi: 10.1074/jbc.M205812200. [DOI] [PubMed] [Google Scholar]
- Lundquist JJ, Dudek SM. Differential activation of extracellular signal-regulated kinase 1 and a related complex in neuronal nuclei. Brain Cell Biol. 2006;35:267–281. doi: 10.1007/s11068-008-9018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, De Vivo G, Gentile V. Possible role of the transglutaminases in the pathogenesis of Alzheimer's disease and other neurodegenerative diseases. Int J Alzheimers Dis. 2011;2011:865432. doi: 10.4061/2011/865432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroberardino PG, Iannicola C, Nardacci R, Bernassola F, De Laurenzi V, Melino G, Moreno S, Pavone F, Oliverio S, Fesus L, Piacentini M. “Tissue” transglutaminase ablation reduces neuronal death and prolongs survival in a mouse model of Huntington's disease. Cell Death Differ. 2002;9:873–880. doi: 10.1038/sj.cdd.4401093. [DOI] [PubMed] [Google Scholar]
- McConoughey SJ, Basso M, Niatsetskaya ZV, Sleiman SF, Smirnova NA, Langley BC, Mahishi L, Cooper AJ, Antonyak MA, Cerione RA, Li B, Starkov A, Chaturvedi RK, Beal MF, Coppola G, Geschwind DH, Ryu H, Xia L, Iismaa SE, Pallos J, et al. Inhibition of transglutaminase 2 mitigates transcriptional dysregulation in models of Huntington disease. EMBO Mol Med. 2010;2:349–370. doi: 10.1002/emmm.201000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsie L, Caron N, Atwal RS, Marsden I, Wild EJ, Bamburg JR, Tabrizi SJ, Truant R. Mutant huntingtin causes defective actin remodeling during stress: defining a new role for transglutaminase 2 in neurodegenerative disease. Hum Mol Genet. 2011;20:1937–1951. doi: 10.1093/hmg/ddr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. 1989;2:1547–1558. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Nanda N, Iismaa SE, Owens WA, Husain A, Mackay F, Graham RM. Targeted inactivation of Gh/tissue transglutaminase II. J Biol Chem. 2001;276:20673–20678. doi: 10.1074/jbc.M010846200. [DOI] [PubMed] [Google Scholar]
- Okauchi M, Xi G, Keep RF, Hua Y. Tissue-type transglutaminase and the effects of cystamine on intracerebral hemorrhage-induced brain edema and neurological deficits. Brain Res. 2009;1249:229–236. doi: 10.1016/j.brainres.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini M, D'Eletto M, Falasca L, Farrace MG, Rodolfo C. Transglutaminase 2 at the crossroads between cell death and survival. Adv Enzymol Relat Areas Mol Biol. 2011;78:197–246. doi: 10.1002/9781118105771.ch5. [DOI] [PubMed] [Google Scholar]
- Ratan RR, Murphy TH, Baraban JM. Oxidative stress induces apoptosis in embryonic cortical neurons. J Neurochem. 1994;62:376–379. doi: 10.1046/j.1471-4159.1994.62010376.x. [DOI] [PubMed] [Google Scholar]
- Ryu H, Lee J, Zaman K, Kubilis J, Ferrante RJ, Ross BD, Neve R, Ratan RR. Sp1 and Sp3 are oxidative stress-inducible, antideath transcription factors in cortical neurons. J Neurosci. 2003;23:3597–3606. doi: 10.1523/JNEUROSCI.23-09-03597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara Y, Dargusch R, Chambers D, Davis J, Schubert D, Maher P. Cellular mechanisms of resistance to chronic oxidative stress. Free Radic Biol Med. 1998;24:1375–1389. doi: 10.1016/s0891-5849(97)00457-7. [DOI] [PubMed] [Google Scholar]
- Samdani AF, Dawson TM, Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997;28:1283–1288. doi: 10.1161/01.str.28.6.1283. [DOI] [PubMed] [Google Scholar]
- Schaertl S, Prime M, Wityak J, Dominguez C, Munoz-Sanjuan I, Pacifici RE, Courtney S, Scheel A, Macdonald D. A profiling platform for the characterization of transglutaminase 2 (TG2) inhibitors. J Biomol Screen. 2010;15:478–487. doi: 10.1177/1087057110366035. [DOI] [PubMed] [Google Scholar]
- Seiler A, Schneider M, Förster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Rådmark O, Wurst W, Bornkamm GW, Schweizer U, Conrad M. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase-dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Siddiq A, Ayoub IA, Chavez JC, Aminova L, Shah S, LaManna JC, Patton SM, Connor JR, Cherny RA, Volitakis I, Bush AI, Langsetmo I, Seeley T, Gunzler V, Ratan RR. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J Biol Chem. 2005;280:41732–41743. doi: 10.1074/jbc.M504963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman SF, Langley BC, Basso M, Berlin J, Xia L, Payappilly JB, Kharel MK, Guo H, Marsh JL, Thompson LM, Mahishi L, Ahuja P, MacLellan WR, Geschwind DH, Coppola G, Rohr J, Ratan RR. Mithramycin is a gene-selective Sp1 inhibitor that identifies a biological intersection between cancer and neurodegeneration. J Neurosci. 2011;31:6858–6870. doi: 10.1523/JNEUROSCI.0710-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova NA, Haskew-Layton RE, Basso M, Hushpulian DM, Payappilly JB, Speer RE, Ahn YH, Rakhman I, Cole PA, Pinto JT, Ratan RR, Gazaryan IG. Development of Neh2-luciferase reporter and its application for high throughput screening and real-time monitoring of Nrf2 activators. Chem Biol. 2011;18:752–765. doi: 10.1016/j.chembiol.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Sagara Y, Liu Y, Maher P, Schubert D. The regulation of reactive oxygen species production during programmed cell death. J Cell Biol. 1998;141:1423–1432. doi: 10.1083/jcb.141.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolentino PJ, DeFord SM, Notterpek L, Glenn CC, Pike BR, Wang KK, Hayes RL. Up-regulation of tissue-type transglutaminase after traumatic brain injury. J Neurochem. 2002;80:579–588. doi: 10.1046/j.0022-3042.2001.00726.x. [DOI] [PubMed] [Google Scholar]
- Tolentino PJ, Waghray A, Wang KK, Hayes RL. Increased expression of tissue-type transglutaminase following middle cerebral artery occlusion in rats. J Neurochem. 2004;89:1301–1307. doi: 10.1111/j.1471-4159.2004.02436.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang H, Xu L, Rozanski DJ, Sugawara T, Chan PH, Trzaskos JM, Feuerstein GZ. Significant neuroprotection against ischemic brain injury by inhibition of the MEK1 protein kinase in mice: exploration of potential mechanism associated with apoptosis. J Pharmacol Exp Ther. 2003;304:172–178. doi: 10.1124/jpet.102.040246. [DOI] [PubMed] [Google Scholar]
- Zaman K, Ryu H, Hall D, O'Donovan K, Lin KI, Miller MP, Marquis JC, Baraban JM, Semenza GL, Ratan RR. Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J Neurosci. 1999;19:9821–9830. doi: 10.1523/JNEUROSCI.19-22-09821.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]