Abstract
The management of men with metastatic castration-resistant prostate cancer (CRPC) has taken several leaps forward in the past year, with the demonstration of improved overall survival with three novel agents (sipuleucel-T, cabazitaxel with prednisone and abiraterone acetate with prednisone), and a significant delay in skeletal-related events observed with denosumab. The pipeline of systemic therapies in prostate cancer remains strong, as multiple agents with a diverse array of mechanisms of action are showing preliminary signs of clinical benefit, leading to more definitive phase III confirmatory trials. In this review, which represents part 1 of a two-part series on metastatic CRPC, we will summarize the mechanisms of resistance to hormonal and chemotherapies and discuss the evolving landscape of treatment options for men with CRPC, with a particular focus on currently approved and emerging treatment options following docetaxel administration, as well as prognostic factors in this post-docetaxel state. As docetaxel remains the standard initial systemic therapy for men with metastatic CRPC for both palliative and life-prolonging purposes, knowledge of these evolving standards will help to optimize delivery of care and long-term outcomes.
Keywords: castrate-resistant prostate cancer, docetaxel-refractory, cabazitaxel, abiraterone, sipuleucel-T
Introduction
Although much of the recent focus on prostate cancer relates to the over-diagnosis and over-treatment of this disease, each year approximately 30 000 men still die of advanced prostate cancer in the United States, making it the second most common cause of cancer-related death in American men.1 Androgen deprivation therapy is the most effective systemic treatment for recurrent prostate cancer; however, the vast majority of these patients will eventually develop resistance to hormonal approaches, necessitating other forms of therapy. Although several chemotherapeutic strategies have been employed to treat castration-resistant prostate cancer (CRPC), it was not until 2004 that one such approach was shown to be lifeprolonging. In that year, two phase III clinical trials reported a survival advantage with the use of docetaxel chemotherapy in men with metastatic CRPC,2,3 resulting in the US Food and Drug Administration (FDA) approval of this agent. However, while docetaxel is both palliative and life-prolonging, it is not the ultimate answer for patients with CRPC, as virtually all men develop eventual resistance to this chemotherapy agent or are unable to tolerate its toxicities long term.
Until 2010, there were no treatment options conferring a survival benefit for patients with docetaxel-refractory CRPC, although mitoxantrone was often employed in this setting for its palliative effects on bone pain.4 That unmet clinical need was filled in 2010, when a randomized phase III trial (TROPIC) showed a survival advantage for a novel taxane, cabazitaxel, over mitoxantrone in men with metastatic CRPC that had progressed after previous docetaxel therapy.5 Based on these results, cabazitaxel was approved by the FDA in June 2010 for the second-line treatment of metastatic CRPC. In the same year, an oral agent with the ability to suppress androgen synthesis, abiraterone, was also reported to improve survival in a phase III study when evaluated against placebo in men with docetaxel-refractory metastatic CRPC.6 This drug is anticipated to gain FDA approval in the next several months. Finally, an autologous immunotherapy product, sipuleucel-T, was FDA approved in April 2010 for the treatment of minimally symptomatic metastatic CRPC, based on the results of a randomized phase III trial comparing this agent against placebo.7 Given this abundance of new drugs, a new question emerges: How do we select men for each new therapy, and in what sequence should these agents rationally be given?
This review is the first article in a series of two papers discussing the management of docetaxel-refractory CRPC. The first review will highlight the molecular mechanisms of castration resistance and docetaxel resistance in patients with advanced prostate cancer, and will outline the currently available treatment options for these patients. Such approaches include the use of modern chemotherapy drugs, androgen-modulating agents and immunotherapy products. The second review will focus on emerging therapies for men with metastatic CRPC, highlighting some novel molecules that target other cellular pathways (such as angiogenesis, second-generation hormonal therapies, cell growth and proliferation, apoptosis, cell nutrition, DNA repair, metastasis/ invasion and epigenetic regulation).
Mechanisms of castration resistance in prostate cancer
Persistent androgen receptor signaling: androgen receptor amplifications, mutations and splice variants
Activation of the intracellular androgen receptor (AR) by androgens (for example, testosterone and dihydrotestosterone) (Table 1) stimulates cell proliferation while inhibiting apoptosis in prostate cancer cells, resulting in tumor growth and progression.8 In the absence of androgens, AR is bound to heat-shock proteins (for example, HSP90) and remains primarily in the cytoplasm. Upon activation by androgens, AR dissociates from the heat-shock proteins and translocates into the nucleus, where it binds (with co-activators and co-repressors) to androgen-response elements of DNA to induce transcriptional activation of target genes.9 During progression to castration resistance induced by persistent androgen suppression, AR signaling is maintained through a variety of mechanisms including increased expression of AR10,11 amplification of the AR gene,12 and structural changes in AR caused by genetic mutations13 or mRNA splice variants.14
Table 1.
Mechanisms of castration resistance in prostate cancer
| Persistent AR signaling |
| Amplification of the AR gene |
| Increased expression of the AR protein |
| Greater stability and nuclear localization of the AR protein |
| Genetic mutations in the AR gene |
| Promiscuous activation of the AR protein by non-androgens (for example, estrogens, progestins, tyrosine kinases) |
| Ligand-independent (constitutive) activation of the AR protein |
| Active AR mRNA splice variants |
| Ectopic androgen synthesis |
| Androgen synthesis by adrenal glands |
| Intratumoral androgen synthesis |
| Increased conversion of extra-gonadal androgens to testosterone |
| Modulation of AR co-regulators |
| Overexpression of steroid receptor co-activators (for example, p160, NCOA2) |
| Downregulation of steroid receptor co-repressors (for example, β-arrestin 2) |
| Facilitation of AR-mediated transcription |
| Activation of compensatory AR-independent pathways |
| Activation of the PI3K/Akt/mTOR pathway |
| Activation of the Ras/Raf/MEK/ERK pathway |
| Overexpression of antiapoptotic proteins (for example, Bcl-2, Bcl-XL, clusterin, survivin) |
| Activation of other pathways (for example, TGF-βR, Wnt/β-catenin, Src kinase, IL-6R) |
Abbreviations: AR, androgen receptor; ERK, extracellular signal-regulated kinase; IL, interleukin; MEK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide 3-kinase; TGF, tumor growth factor.
The increased expression, greater stability and nuclear localization of AR in CRPC are all indicative of an overactive AR, which can be stimulated by minute concentrations of circulating androgens.15 To this end, animal experiments have showed that AR overexpression is necessary and sufficient for growth of many prostate cancer cells in the setting of castrate serum androgen levels.10 Similarly, in patients with CRPC, increased transcription of the AR gene and persistence of the AR protein were found in cancer cells isolated from metastatic tissue samples.16 In addition to amplification of the wild-type AR gene, increased quantity of AR in CRPC may be caused by greater stabilization and slower turnover of AR.17 Moreover, while wild-type AR is only activated by androgens, the specificity of ligand binding can be broadened by somatic mutations usually occurring in the ligand-binding domain of AR.18 These mutations can lead to decreased specificity and inappropriate activation of the receptor by non-androgens, resulting in a promiscuous AR phenotype that may lead to the activation by estrogens, progestins, tyrosine kinases and other oncogenic signaling molecules. Finally, the castration-resistant state may promote alternative splicing of the AR gene, yielding variant mRNA transcripts lacking the ligand-binding domain, which are constitutively active.19,20 Thus, there are a variety of AR-mediated mechanisms of resistance to androgen deprivation therapy, each of which may be anticipated to require different therapeutic approaches.
Ectopic androgen synthesis
Although androgen deprivation therapy (using luteinizing hormone-releasing hormone agonists or antagonists) decreases total serum testosterone levels by approximately 95%, this intervention primarily inhibits gonadal androgen synthesis and does not affect extra-gonadal androgens. It is now established that, in CRPC, there is continuous production of androgens by the adrenal glands as well as the prostate cancer itself.21,22 Moreover, in the castrate state, intraprostatic concentrations of testosterone and dihydrotestosterone remain sufficient to stimulate AR. The main mechanisms by which CRPC is able to overcome low circulating androgen levels are local conversion of adrenal androgens (for example, androstenedione) to testosterone,23 and de novo intratumoral synthesis of androgens through increased expression of steroidogenic enzymes such as cytochrome P450 17 (CYP17).24 This enzyme is the target of several new drugs for CRPC.25
Co-regulators of AR
Co-activators (and co-repressors) function as signaling adjuncts for AR-mediated transcription, facilitating or inhibiting binding and activation of AR to and rogen-response elements in promoter and enhancer regions of DNA. Among the most important transcriptional co-regulators in prostate cancer is the p160 family of nuclear steroid receptor co-activators.26 Preclinical experiments and studies of human prostate tumors strongly suggest that overexpression of such steroid receptor co-activators is important in the emergence of the castration-resistant phenotype.27,28 In addition, another nuclear receptor co-activator, NCOA2, has recently been reported to function as an oncogene in a subset of prostate cancers.29 Finally, downregulation of AR-related co-repressors may also be involved in the development of CRPC.30
AR-independent pathways
Castration resistance may also be caused by the activation of other oncogenic survival pathways through promiscuous activation of AR by non-androgens (for example, estrogens, progestins, anti-androgens, receptor tyrosine kinases) or by alternative mechanisms including activation of compensatory signaling pathways.31 For example, it has been shown that signaling, which is normally AR-dependent, may be triggered in CRPC even at undetectable androgen levels by the activation of other receptor tyrosine kinases (for example, insulin-like growth factor-1R, epidermal growth factor-R, vascular endothelial growth factor-R) and their associated signal-transduction pathways (for example, phosphoinositide 3-kinase/Akt/mammalian target of rapamycin pathway, Ras/Raf/mitogen-activated protein kinase/extracellular signal-regulated kinase pathway).32 In addition, crosstalk has been observed between the cell-surface tyrosine kinase HER2/neu and/or HER3 and intracellular AR in CRPC, resulting in AR activation by HER2/neu in the absence of androgens.33,34 Activation of other receptors and their pathways (for example, tumor growth factor-βR, Wnt/β-catenin, Src kinase and interleukin-6R) has also been implicated in crosstalk with AR.35,36
Another potential resistance mechanism against castration involves the activation of antiapoptotic pathways associated with survival. In human beings and in animal models of advanced prostate cancer, overexpression of the antiapoptotic protein Bcl-2 has been found to confer resistance to androgen suppression.37 In addition, other antiapoptotic factors related to Bcl-2, such as Bcl-XL and survivin, are also frequently overexpressed in CRPC, but not in hormone-responsive disease.38,39
Mechanisms of docetaxel resistance in prostate cancer
Unfavorable tumor microenvironment
Docetaxel resistance in metastatic CRPC can occur by processes intrinsic to the biology of prostate cancer or by general mechanisms of drug resistance shared by multiple tumor types (Table 2). One form of chemotherapy resistance arises from impaired drug distribution.40 Anticancer drugs reach solid tumors via the bloodstream and must cross the extracellular matrix to enter cancer cells. However, tumor blood vessels are disorganized and have chaotic blood flow, leading to impaired delivery of nutrients and oxygen and resulting in regions of tumor hypoxia.41 Similarly, the poorly developed tumor vasculature impairs delivery of anticancer drugs including taxanes to the tumor site, where they must then penetrate deep into the tumor tissue.42 Increased interstitial fluid pressure caused by leaky vasculature, coupled with impaired lymphatic drainage, also impedes intratumoral drug delivery.43 Even if anticancer drugs do penetrate into the tumor, the poorly nourished cancer cells furthest from functional blood vessels are often slowly proliferating and resistant to cell cycle-dependent drugs. Moreover, areas of tumor hypoxia may not only confer chemotherapy resistance, but may also select for cancer cells with more malignant phenotypes.44 For instance, hypoxia-inducible transcription factors (for example, hypoxia-inducible factor 1a) have been shown to induce expression of genes that increase the likelihood of survival, treatment resistance and metastasis of tumor cells, including prostate cancer cells.45
Table 2.
Mechanisms of docetaxel resistance in prostate cancer
| Unfavorable tumor microenvironment |
| Disorganized tumor blood vessels lead to impaired drug distribution |
| Increased interstitial fluid pressure impedes intra-tumoral drug delivery |
| Hypoxic areas of tumor are slowly proliferating and resistant to cell-cycle-dependent drugs |
| Tumor hypoxia may select for cancer cells with overexpression of survival pathways |
| Amplification of paracrine growth loops between tumor cells and stromal cells |
| Adhesion of cancer cells to each other and to extracellular matrix |
| EMT and acquisition of stemness properties |
| Drug efflux pump |
| Docetaxel has high substrate affinity for P-glycoprotein, the dominant drug efflux pump |
| Cancer cells that synthesize P-glycoprotein are resistant to docetaxel and paclitaxel |
| Resistance is conferred by overexpression of the MDR1 gene |
| Additional drug export pumps include ABCB4 (encoded by MDR2) and ABCC1 (encoded by MRP1) |
| Alterations in microtubule structure and/or function |
| β-Tubulin mutations that affect docetaxel binding |
| Increased total cellular β-tubulin content or overexpression of certain β-tubulin isotypes (for example, βIII-tubulin) |
| Post-translational β-tubulin modifications |
| Altered expression of microtubule-destabilizing phosphoproteins |
| Promotion of microtubule-based microtentacles |
| Overexpression of microtubule-associated proteins |
| Altered interaction between microtubules and other cytoskeletal components (for example, γ-actin) |
| Apoptotic defects |
| Upregulation of Bcl-2 and clusterin |
| Increased expression of lipid kinases (for example, sphingosine kinase-1) and serine-threonine kinases (for example, Pim-1 kinase) |
| Activation of the PTEN/PI3K/mTOR pathway |
| Activation of the MAPK/ERK pathway |
| Activation of other pathways (for example, Hedgehog, β-catenin, IL-6, EGFR, endothelin, somatostatin pathways) |
Abbreviations: ABCB4, ATP-binding cassette B4; EGFR, epidermal growth factor receptor; EMT, epithelial–mesenchymal transition; ERK, extracellular signal-regulated kinase; IL, interleukin; MAPK, mitogen-activated protein kinase; MDR1, multi-drug resistance gene; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homologue deleted on chromosome ten.
In addition, preclinical models have shown that primary drug resistance among several cancers can be induced by a paracrine amplification loop of growth factors and cytokines produced by the tumor stroma (for example, interleukin-6, stromal cell-derived factor-1), and by adhesion of cancer cells to the extracellular matrix that is mediated by integrin receptors on cancer cells.46 To this end, it has been shown that tumor cells with sensitivity to drugs in dilute culture acquire resistance when exposed to the same drug in three-dimensional multicellular tumor spheres.47 This unfavorable tumor microenvironment may promote invasiveness, through a process termed epithelial– mesenchymal transition or epithelial plasticity, as well as through the acquisition of stemness properties in prostate tumor cells, each of which has been linked to chemotherapeutic resistance.48–50 Indeed, expression of epithelial–mesenchymal transition factors in human prostate cancer has recently been linked to castration resistance.51,52 Also, prostate cancer cells as well as bone cells and bone matrix are a rich source of growth factors, which can promote the development of osteoblastic metastases.53 For example, chemokine ligand-2 is a soluble factor (produced by prostate cancer cells and various host cells) that acts as a modulator of prostate cancer growth in bone, through stimulation of tumor cells and osteoclasts.54 Secretion of this ligand can be induced by chemotherapy and may protect prostate cancer cells from docetaxel cytotoxicity by activating the phosphoinositide 3-kinase/Akt/mammalian target of rapamycin pathway.55
Drug efflux pump
Docetaxel is a taxane drug that binds to β-tubulin, which is incorporated into cytoskeletal microtubules during the G2/M phase of cell cycle, inhibiting microtubule disassembly and triggering cell death in dividing cells.56 The effectiveness of docetaxel is often limited by its high substrate affinity for P-glycoprotein, an ATP-dependent membrane-bound drug efflux pump that decreases the intracellular concentration of docetaxel, thus preventing its anti-mitotic activity.57 P-glycoprotein is a member of the ATP-binding cassette transporter family of proteins. It has been shown that cancer cells that synthesize P-glycoprotein become resistant to docetaxel as well as paclitaxel.58 This resistance is conferred by overexpression of the multi-drug resistance (MDR1) gene encoding P-glycoprotein.59 Additional drug export pumps that also promote decreased intracellular docetaxel accumulation include ABCB4 (encoded by the MDR2 gene) and ABCC1 (encoded by the MRP1 gene).60
Microtubule alterations
Alterations in microtubule structure/function represent an additional mechanism of docetaxel resistance. Examples of structural changes that may induce taxane resistance include β-tubulin mutations affecting docetaxel binding, increased total cellular β-tubulin content, altered expression of β-tubulin isotypes (for example, overexpression of βIII-tubulin) and post-translational β-tubulin modifications.61,62 Functional changes that may promote docetaxel resistance include alternative binding of docetaxel to β-tubulin, altered expression of microtubule-destabilizing phosphoproteins, promotion of micro-tubule-based microtentacles that facilitate invasion and vascular extravasation, elevated levels of kinesins and activation of the TXR1/thrombospondin pathway.63,64 Finally, taxane resistance may also develop as a result of microtubule changes induced by interactions with other cytoskeletal components (for example, γ-actin) or overexpression of microtubule-associated proteins.65
Apoptotic defects and enhanced cell survival
In addition to stabilizing microtubules, docetaxel can induce apoptosis by inhibiting the antiapoptotic protein Bcl-2 in proliferating cells.66 However, during treatment of prostate cancers with taxanes, Bcl-2 and secretory clusterin (another antiapoptotic cytoprotective protein) become upregulated, decreasing the effectiveness of docetaxel by impairing apoptosis.67,68 Moreover, increased expression of lipid kinases (for example, sphingosine kinase-1) and serine-threonine kinases (for example, Pim-1 kinase) has been observed during taxane treatment, further promoting prostate cancer cell proliferation.69,70
Activation of multiple additional signaling pathways associated with prostate cancer cell survival has also been linked to chemotherapy resistance, but a detailed discussion of these is beyond the scope of this review. This is supported by various preclinical experiments showing that treatment with agents directed against activated cellular pathways such as phosphatase and tensin homologue deleted on chromosome ten/phosphoinositide 3-kinase/mTOR, mitogen-activated protein kinase/extracellular signal-regulated kinase, nuclear factor-κB/interleukin-6, Janus kinase/signal transducers and activators of transcription, epidermal growth factor receptor/HER2/3, Hedgehog and β-catenin pathways, endothelin and the somatostatin pathway restores or increases the sensitivity of prostate tumors to taxanes and leads to delayed progression in model systems.71–77
Chemotherapy for docetaxel-refractory CRPC
Given the many ways that prostate cancer cells can adapt to cellular stressors such as androgen deprivation, taxane-based chemotherapy, and hypoxia and unfavorable tumor microenvironments, current and emerging treatments are now beginning to individually address these mechanisms. The next sections of this review will discuss the current approaches to management of men with docetaxel-resistant prostate cancer.
Cabazitaxel: a novel taxoid
Cabazitaxel (XRP6258, or TXD258) is a novel semisynthetic tubulin-binding taxoid that differs from docetaxel (and paclitaxel) because of its poor affinity for P-glycoprotein, the ATP-dependent drug efflux pump.78,79 In preclinical studies using cancer cell lines and mouse xenograft models, cabazitaxel was shown to be active in both docetaxel-sensitive tumors as well as those with primary or acquired docetaxel resistance.80
The first hint that cabazitaxel would have activity in prostate cancer came during phase I testing, where cabazitaxel was administered by intravenous infusion every 3 weeks at escalating doses of 10, 15, 20 or 25mg/m2.81 In that study, the principal dose-limiting toxicity was neutropenia (including febrile neutropenia). Non-hematological toxicities included emesis, diarrhea, neurotoxicity and fatigue, but these were generally mild to moderate. Anticancer activity was seen in two patients, both of which had metastatic CRPC. One man with mitoxantrone-refractory disease (receiving 15mg/m2 of cabazitaxel) had a 66% decline in PSA and a partial radiographic response in measurable lesions, as well as bone pain improvement. A second patient with docetaxel-refractory CRPC (receiving 25mg/m2 of cabazitaxel) had an 89% PSA reduction as well as a confirmed partial response in target lesions.81 A phase II study of cabazitaxel in men with prostate cancer was never conducted.
The safety and efficacy of cabazitaxel in patients with advanced prostate cancer was definitively evaluated in a pivotal randomized phase III trial (TROPIC) that was conducted between January 2007 and October 2008 in 146 institutions across 26 countries, and recruited 755 men with metastatic CRPC who had progressed during (29%) or after (71%) docetaxel-based chemotherapy.5 Of these, 377 patients were randomized to receive mitoxantrone 12mg/m2 intravenously every 3 weeks (with oral prednisone 10mg daily), and 378 patients were assigned to receive cabazitaxel 25mg/m2 intravenously every 3 weeks (plus oral prednisone). The study was powered to detect a 25% improvement in the risk of death between the two intervention arms, using an intention-to-treat analysis. This study was the basis of the FDA’s approval of cabazitaxel and prednisone for the second-line treatment of docetaxel-refractory metastatic CRPC.82
After a median follow-up of 12.8 months, overall survival in men receiving cabazitaxel was 15.1 months compared with 12.7 months in men receiving mitoxantrone (P<0.0001; hazard ratio 0.70).5 In subset analyses, the survival advantage of cabazitaxel persisted regardless of whether patients had measurable disease or pain at baseline, or whether progression had occurred while receiving docetaxel or following a treatment holiday. In addition, cabazitaxel’s survival benefit was most pronounced for men with Eastern Cooperative Oncology Group performance status 0–1 (vs 2), for men receiving only one previous chemotherapy (vs ≥2 chemotherapies), for patients ≥65 years of age (vs <65 years), for patients with rising PSA at study entry (vs non-rising PSA), for patients who had received ≥12 cycles of previous docetaxel (vs <12 cycles) and for patients with disease progression within <3 months of docetaxel initiation (vs ≥3 months of docetaxel initiation).5 The last two observations are important because they imply that cabazitaxel may be effective even in patients with disease progression early during docetaxel treatment and in those who have received high cumulative doses of docetaxel. These are patients who are unlikely to benefit from further docetaxel retreatment.
Compared with mitoxantrone, cabazitaxel also significantly lengthened progression-free survival (2.8 vs 1.4 months; P<0.0001; hazard ratio 0.74), extended time to PSA progression (6.4 vs 3.1 months; P=0.001; hazard ratio 0.75), increased radiographic tumor response rates (14.4 vs 4.4%; P=0.0005) and increased PSA response rates (39.2 vs 17.8%; P=0.0002).5 There were no differences between the two treatment arms with respect to pain responses (8–9% in each arm), or time to pain progression.
The most common serious adverse events related to cabazitaxel were hematological, including grade ≥3 neutropenia in 82% of patients (febrile neutropenia in 8%), grade ≥3 leukopenia in 68% of patients and grade ≥3 anemia in 11% of patients.5 This high degree of myelosuppression begs the question of whether a lower dose of cabazitaxel (for example, 20mg/m2) may have been more appropriate, and a randomized trial comparing the safety and efficacy of these two doses (25 vs 20mg/m2) is being planned (Table 3). In the absence of efficacy information on lower cabazitaxel doses, prophylactic or secondary use of growth factor support should be strongly considered, as reflected in several national guidelines.83 Other non-hematological toxicities included grade ≥3 diarrhea in 6% of patients and grade ≥3 fatigue in 5% of patients. Importantly, although peripheral neuropathy (all grades) was observed in 14% of patients receiving cabazitaxel, only 1% developed grade 3 neuropathy. This relative lack of cumulative neurotoxicity with cabazitaxel is encouraging, especially given the established neurological sequelae of docetaxel.
Table 3.
Selected ongoing phase II and III clinical trials of novel chemotherapy agents for men with docetaxel-pretreated metastatic CRPC
| Target/drug class | Agent | Phase | Treatment arm(s) | Identifier |
|---|---|---|---|---|
| Chemotherapeutic drugs | ||||
| Microtubules (taxane) | Cabazitaxel | III | Randomized trial: cabazitaxel 25mg/m2 i.v. every 3 weeks vs cabazitaxel 20mg/m2 i.v. every 3 weeks |
NCT01308580 |
| Tesetaxel | II | Single-arm trial: tesetaxel 40 mg orally once every 3 weeks | NCT01296243 | |
| TPI-287 | II | Single-arm trial: TPI-287 165mg/m2 i.v. every 3 weeks | NCT00479635 | |
| Microtubules (epothilone) | Patupilone | II | Single-arm trial: patupilone 8 mg/m2 i.v. every 3 weeks | NCT00407251 |
Abbreviations: CRPC, castration-resistant prostate cancer; i.v., intravenous.
Mitoxantrone
Mitoxantrone was one of the first chemotherapies to be FDA approved for CRPC, based on the observation of improved quality of life and bone pain in men with metastatic CRPC when compared against prednisone alone, although this agent did not improve survival.4 After docetaxel replaced mitoxantrone as the initial treatment-of-choice for CRPC based on an overall survival advantage shown in two pivotal trials (TAX-327 and SWOG-9916),2,3 mitoxantrone became the de facto standard for second-line chemotherapy in docetaxelrefractory disease. This was partly due to a lack of other approved agents in this setting, and partly due to its palliative effects. In a post hoc analysis of the TAX-327 study, men initially treated with docetaxel who crossed over to receive mitoxantrone upon progression had >50% PSA reductions in 15% of cases, median PSA progression-free survival of 3.4 months, and median overall survival of 10.0 months.84 Similar modest results were seen in other trials specifically designed to assess the utility of mitoxantrone as second-line therapy in docetaxel-pretreated CRPC. One such study reported >50% PSA declines in 20% of patients, partial radiographic responses in 9% and median survival of 9.8 months.85 Thus, mitoxantrone therapy leaves much to be desired in terms of improvements in survival in this setting, but remains a palliative regimen in a select group of patients, and clearly there are subgroups of men who do benefit from mitoxantrone therapy. Initiatives to identify these subgroups based on clinical or biological parameters will be important to help guide its rational use, either alone or in combination with other agents.
Docetaxel retreatment
In the absence of any approved life-prolonging options for men with docetaxel-pretreated CRPC, reintroduction of docetaxel after a drug-free interval was an alternative strategy before the emergence of cabazitaxel, and may remain a viable option for select groups of men with retained sensitivity to docetaxel following an initial response and subsequent holiday. This approach is most successful for patients with an initial favorable response to docetaxel (based on PSA, radiographic and/or pain responses) who stop therapy owing to toxicity or after completion of 10 treatment cycles.86 In phase II trials enrolling CRPC patients with previous responses to docetaxel lasting ≥4 months, docetaxel retreatment produced >50% PSA declines in 25–45% of patients, radiographic responses in about 10%, median progression-free survival of 5–6 months and median overall survival of 13–15 months.87,88 Moreover, the interval from the last cycle of docetaxel therapy to disease progression has been associated with the efficacy of subsequent docetaxel retreatment.89 Finally, while the addition of a second agent to docetaxel or the use of offlabel alternative chemotherapeutics upon disease progression is appealing (that is, carboplatin, etoposide, vinorelbine, paclitaxel), the evaluation of these agents as monotherapies or combinations90 is limited to single-arm phase II studies in select groups of men without a control group. Thus, efficacy is difficult to ascertain, particularly in the setting of newly available and approved agents such as cabazitaxel.
Other taxanes
The first taxane agent to reach the market was paclitaxel, which was FDA approved in 1992 for the treatment of refractory ovarian cancer,91 but has never gained approval for prostate cancer. Because most studies of paclitaxelbased treatment of CRPC have focused on front-line chemotherapy,92,93 the use of paclitaxel in docetaxelpretreated patients is guided mainly by retrospective analyses. One observational study investigated outcomes of men with metastatic CRPC who received paclitaxel together with carboplatin after previous docetaxel treatment, and reported ≥30% PSA reductions in 64% of patients, median progression-free survival of 2.8 months and median overall survival of 9.7 months.94 However, with the emergence of cabazitaxel, paclitaxel’s future as a second-line chemotherapy agent for CRPC is uncertain.
Tesetaxel is a novel semisynthetic taxane that represents the first oral agent in its class.95 Like cabazitaxel, tesetaxel has been shown to overcome P-glycoprotein-mediated multi-drug resistance in vitro and in vivo.96 This agent has shown partial radiographic responses in patients with advanced non-small-cell lung cancer,97 and a phase II study of tesetaxel in men with chemotherapy-naïve or -pretreated CRPC is now being planned (Table 3).
TPI-287 is a third-generation intravenous taxane that was also designed to avoid drug efflux by the P-glycoprotein pump. It has shown activity against multiple human tumor xenografts including those expressing MDR1 and showing resistance to other taxane agents.98 Phase I studies of TPI-287 in individuals with advanced solid tumors have shown clinical activity in patients with pancreatic and prostate cancers, and a phase II study99 in taxane-pretreated men with CRPC is now underway (Table 3).
Epothilones
The epothilones (macrolide agents originally extracted from myxobacteria)100 are a novel class of non-taxane tubulin polymerization drugs whose cytotoxicity is mediated through microtubule stabilization, bypassing known taxane-resistance mechanisms.101 The first agent in this class is ixabepilone, a semisynthetic analog of epothilone B with potent cytotoxic activity mediated by inducing cell cycle arrest at G2/M phase.102 Clinically, ixabepilone has shown modest antitumor activity in men with chemotherapy-naïve metastatic CRPC in phase II studies.103
In the second-line setting, a recent randomized phase II trial compared ixabepilone against mitoxantrone in men with docetaxel-refractory CRPC.85 In that study, ≥50% PSA declines were seen in 17 and 20% of men (P>0.05), partial objective responses were observed in 4 and 9% of patients (P>0.05) and median overall survival was 10.4 and 9.8 months (P>0.05). Significant adverse events related to ixabepilone were neutropenia (grade ≥3 in 54% of patients), anorexia, stomatitis, fatigue and muscle weakness. Another single-arm study examining the combination of ixabepilone plus mitoxantrone in men with docetaxel-pretreated CRPC showed ≥50% PSA reductions in 45% of patients, partial radiographic responses in 22% of men, median progression-free survival of 4.4 months and median overall survival of 12.5 months.104 With this combination, 32% of patients experienced grade ≥3 neutropenia, and 24% developed grade ≥2 neuropathy.
Patupilone is a second intravenous epothilone that is able to stabilize tubulin polymerization at much lower concentrations than taxanes, is not a substrate for P-glycoprotein and can overcome multi-drug resistance mechanisms in human tumor models.105 A single-arm phase II trial of patupilone in men with docetaxelrefractory CRPC has shown ≥50% PSA declines in 45% of patients, partial objective responses in 5% of men and median progression-free survival of 5.6 months.106 Overall survival results from this study are awaited (Table 3). Adverse events with patupilone differ from those of ixabepilone, and include diarrhea, emesis, fatigue and anorexia (with minimal hematological toxicities). However, to our knowledge, further studies with ixabepilone or patupilone in men with CRPC are not being planned given the approval of cabazitaxel and the limited development potential of these agents in CRPC.
Platinum compounds
The most common platinum agents tested in the second-line setting in men with metastatic CRPC are carboplatin and oxaliplatin. The combination of either carboplatin or oxaliplatin with a taxane agent in men with docetaxelrefractory CRPC has been a popular approach, yielding promising results in phase II studies (≥50% PSA declines in up to 60% of men, objective responses in up to 40%, progression-free survival times of up to 6 months and overall survival times of up to 15 months).90,107,108 In addition, carboplatin and oxaliplatin have been investigated in combination with other non-taxane agents (such as etoposide,109 pemetrexed110 and capecitabine)111 in patients with docetaxel-pretreated disease; results from these trials have also been intriguing (≥50% PSA declines in up to 50% of men, overall survival of up to 19 months). Notably, in patients with metastatic smallcell prostate carcinoma, the use of platinum agents (especially cisplatin) in combination with etoposide112 is probably the most successful approach, even in the first-line setting. However, outcomes for men with small-cell prostate carcinoma remain unacceptably poor (progression-free survival of 4–5 months; overall survival of 10–12 months),113,114 and clinical trial participation should be strongly encouraged. It remains to be seen whether men with CRPC who have adenocarcinoma with neuroendocrine differentiation may also have a selective sensitivity to platinums.115
Satraplatin is an oral platinum compound that showed promising preclinical activity in prostate cancer and tumor cell lines resistant to other platinums as well as taxanes.116 To examine the efficacy and safety of satraplatin in men with metastatic CRPC, a 950-patient placebo-controlled randomized phase III trial (SPARC) was conducted in patients with progression after one previous chemotherapy, representing the largest trial ever performed in this population. Patients were randomized (2:1) to receive either oral satraplatin with prednisone (n=635) or placebo plus prednisone (n=315). Of note, only 51% of participants had received previous docetaxel treatment, whereas 49% had received other chemotherapy agents (mainly mitoxantrone). Although satraplatin resulted in an improved progression- free survival compared with placebo (2.6 vs 2.2 months; hazard ratio 0.67; P<0.001), as well as prolonged time to pain progression (15.3 vs 5.1 months; hazard ratio 0.64; P<0.001), it did not show an overall survival advantage (14.1 vs 14.2 months; hazard ratio 0.98; P=0.80).117 However, a preplanned subgroup analysis of the 488 docetaxel-pretreated patients showed a trend towards improved overall survival favoring satraplatin after adjusting for other prognostic factors (hazard ratio 0.78; P=0.06), suggesting that satraplatin may be most efficacious following docetaxel therapy. Toxicities of satraplatin were generally manageable, and included myelosuppression and gastrointestinal disturbances. It will be important to learn from the failure of satraplatin in CRPC, perhaps through the identification of blood-based or clinical predictors of platinum sensitivity in prostate cancer, given that there are clearly subgroups of men with strong clinical benefit to platinum-based therapies.
AR-directed approaches for docetaxel-refractory CRPC
Abiraterone acetate
Abiraterone acetate (a pregnenolone derivative) is an oral, potent, selective and irreversible inhibitor of the steroidogenic enzyme CYP17, blocking both its 17α-hydroxylase and its C17,20-lyase activity.118 As a result, extra-gonadal androgen production is impaired through the inability to convert pregnenolone to dihydroepiandrostenedione and progesterone to androstenedione. In an initial phase I study in men with chemotherapy- naïve CRPC, it was shown that the primary toxicities of abiraterone (which included hypertension, hypokalemia and peripheral edema) were related to a syndrome of secondary mineralocorticoid excess due to feedback upregulation of mineralocorticoid synthesis, and were largely reversible after administration of a selective aldosterone receptor antagonist or a corticosteroid.119 For this reason, in several subsequent trials abiraterone was combined with low-dose prednisone, although the indiscriminant use of corticosteroids in all abiraterone- treated patients has been debated.120 Other adverse events with abiraterone include liver function abnormalities and cardiac complications.
The early efficacy of abiraterone in CRPC was investigated in multiple phase II trials and in multiple disease states. In patients with chemotherapy-naïve metastatic CRPC, ≥50% PSA declines were seen in approximately 65% of men, and radiographic tumor responses occurred in about 35% of men.121 Interestingly, in chemotherapy-untreated patients who had received previous ketoconazole, ≥50% PSA reductions still occurred in about 45% of cases.122 Finally, in patients with docetaxel-refractory metastatic CRPC, ≥50% PSA declines were observed in approximately 40% of men and objective tumor responses occurred in about 20% of cases.123,124 Importantly, patients progressing on abiraterone did not develop increased levels of androgenic compounds downstream of CYP17, implying that resistance probably occurs through some other mechanism. In addition, several patients treated with singleagent abiraterone who received a corticosteroid upon PSA progression subsequently experienced secondary PSA declines of ≥50%. It has been hypothesized that this phenomenon may be related to promiscuous AR activation by upstream steroidal precursors, and that addition of corticosteroids may suppress central adrenocorticotropic hormone secretion that would otherwise promote the synthesis of these upstream steroidal compounds.
Several of these trials have also evaluated abiraterone’s effect on circulating tumor cell (CTC) counts, a parameter that has been shown to correlate with overall survival in men with CRPC receiving chemotherapy.125 Specifically, patients with ≥5 CTCs per 7.5 ml of blood at baseline (unfavorable levels) have a median survival of 11.5 months compared with 21.7 months in men with baseline CTC counts <5 per 7.5 ml (favorable levels).126 Moreover, transitioning from an unfavorable to a favorable CTC count after treatment portends a similar survival to having a favorable baseline CTC count. To this end, in a study enrolling chemotherapy-naïve CRPC patients, 59% of men converted from unfavorable CTC levels at baseline to favorable CTC levels after abiraterone treatment;121 and in a trial of docetaxel-pretreated patients, 34% of men converted from unfavorable to favorable CTC counts.124
To evaluate definitively the efficacy and safety of abiraterone, a pivotal multicenter placebo-controlled blinded randomized phase III trial (COU-AA-301) was conducted in men with docetaxel-pretreated metastatic CRPC who had not received previous ketoconazole.6 This trial was powered to detect a 25% improvement in overall survival, and randomized men (2:1) to receive either abiraterone 1000mg daily plus prednisone 10mg daily (n=797), or placebo plus prednisone (n=398). The trial met its primary end point, showing a median overall survival of 14.8 months in the abiraterone arm and 10.9 months in the placebo arm (hazard ratio 0.65; P<0.0001). In addition, when compared with placebo, abiraterone prolonged radiographic progression-free survival (5.6 vs 3.6 months; hazard ratio 0.67; P<0.0001), improved time to PSA progression (10.2 vs 6.6 months; hazard ratio 0.58; P<0.0001), and produced more PSA responses (38 vs 10%; P<0.0001).6 Notably, the overall survival duration seen here (14.8 months) is similar to that of cabazitaxel/ prednisone (15.1 months) in this second-line population with similar baseline characteristics across trials, whereas the survival of men treated with prednisone alone was slightly inferior to that of mitoxantrone with prednisone in the TROPIC trial (10.9 vs. 12.7 months). Although cross-trial comparisons are problematic, this finding does suggest that there may be at least some potential benefit to mitoxantrone in this population. Based on the results of the COU-AA-301 trial, it is anticipated that the FDA will approve abiraterone in patients with metastatic CRPC in the next several months. A second randomized phase III trial (COUAA- 302) targeting men with docetaxel- and ketoconazole- naïve CRPC has completed accrual of over 1000 patients, and has also been powered to detect an overall survival advantage with abiraterone/prednisone compared with placebo/prednisone. It is very likely that abiraterone will be used clinically in both the predocetaxel and post-docetaxel settings, given the comfort level and safety of using this and other hormonal agents in prostate cancer patients before chemotherapy.
Ketoconazole
The anti-fungal ketoconazole was the first drug to target extra-gonadal (adrenal and intratumoral) androgen synthesis in men with CRPC. When used at high doses (800–1200mg daily), ketoconazole acts as a non-selective competitive inhibitor of several cytochrome P450 enzymes including CYP17, thus reducing androstenedione production as well as circulating testosterone.127 Single-arm and randomized studies using high-dose ketoconazole in men with CRPC have shown ≥50% PSA declines in 30–60% of patients, with a median response duration of approximately 5–10 months.128 However, a randomized phase III trial comparing anti-androgen withdrawal alone vs anti-androgen withdrawal plus high-dose ketoconazole in men with chemotherapy-naïve CRPC failed to show a survival benefit for ketoconazole,129 and this agent has not been FDA approved for prostate cancer, although it is still widely prescribed for this indication. The efficacy of ketoconazole in the docetaxel-refractory setting has only been examined in retrospective studies,130,131 where ≥50% PSA reductions have been observed in approximately 30% of patients and last about 3–6 months. Toxicities of ketoconazole include fatigue, anorexia, emesis, peripheral neuropathy and transaminitis, and most patients require concurrent physiological hydrocortisone replacement. Interestingly, one recent study showed that the addition of the 5α-reductase inhibitor dutasteride to ketoconazole/hydrocortisone improved the PSA response rate to 56% with a median progression-free survival of 14.5 months and an acceptable toxicity profile in a select group of heterogeneous men with CRPC, suggesting that combination approaches may enhance the clinical benefit of androgen synthesis inhibitors.132
Immunotherapy for CRPC
Sipuleucel-T
Cancer immunotherapy refers broadly to approaches that attempt to treat cancer by activating an immune response against malignant cells while overcoming tumor-induced tolerance. Although not always considered a disease amenable to immune-directed therapies, prostate cancer may in fact be an ideal target for immunological attack because it produces several tissue-specific proteins that may serve as tumor antigens: these include PSA and prostatic acid phosphatase. This notion has been applied to the development of sipuleucel-T, an autologous prostatic acid phosphatase-loaded dendritic cell immunotherapy.133 During the course of treatment with sipuleucel-T, a patient’s own antigen-presenting cells are collected by leukapheresis and co-incubated ex vivo with a fusion protein containing prostatic acid phosphatase linked to granulocyte–macrophage-colony-stimulating factor. After culturing this fusion protein with the antigen-presenting cells, the primed immunotherapy product is then reinfused into the patient, activating T cells via MHC class I and class II molecules and resulting in a prostatic acid phosphatase - directed antitumor immune response.134
Several clinical trials using this personalized prostate cancer immunotherapy have been conducted. In a randomized phase II/III study comparing sipuleucel-T against placebo in 127 men with asymptomatic metastatic CRPC, the immunotherapy did not achieve its primary end point of improving progression-free survival. Intriguingly however, the study showed an improvement in median overall survival favoring sipuleucel- T over placebo (25.9 vs 21.4 months; hazard ratio 0.59; P=0.01).135 A second phase II/III trial that randomized 98 men with asymptomatic CRPC to either sipuleucel-T or placebo also failed to show a statistically significant improvement in progression-free survival, and a survival benefit was not shown either. Encouragingly however, a post hoc pooled analysis of these two trials (n=225) suggested a survival advantage for the immunotherapy product, with a median survival of 23.2 months with sipuleucel-T and 18.9 months with placebo (hazard ratio 0.67; P=0.01).136 Adverse events related to sipuleucel-T are generally mild and include fever, chills/sweats, myalgias and headache. These usually occur during or shortly after infusion of the immunotherapy.
To evaluate definitively the impact of sipuleucel-T on survival, a pivotal multicenter double-blind placebo-controlled randomized phase III trial (IMPACT) was conducted in men with asymptomatic or minimally symptomatic metastatic CRPC,7 leading to the FDA approval of this agent. In this trial, 512 patients were randomized (2:1) to receive either sipuleucel-T or placebo, and only 14% of men had received previous treatment with docetaxel chemotherapy. This study notably did not enroll men with visceral metastatic disease or men who were taking narcotics for cancer pain or immunosuppressive agents. Impressively, median overall survival was 25.8 months in the sipuleucel-T group vs 21.7 months in the placebo group (hazard ratio 0.78; P=0.03), despite 64% of patients on placebo crossing over to receive salvage sipuleucel-T (prepared from cryopreserved antigen-presenting cells collected at the time of placebo preparation). In the subset of patients with previous chemotherapy treatment, overall survival trended in favor of sipuleucel-T, but this effect was not statistically significant. Therefore, although this immunotherapy is approved for all patients with asymptomatic or minimally symptomatic CRPC, it will likely have its largest impact in the pre-chemotherapy setting given the greater ease of administration before chemotherapy, and the broader group of men in this setting who are able to benefit from this therapy.
Similar to previous studies with sipuleucel-T, the IMPACT study found no difference in progression-free survival between the two treatment arms. Some investigators attribute the discordance between progression-free and overall survival to a possible class effect of immunotherapy agents, relating to their mechanism of action which is distinct from cytotoxic therapies. To this end, a similar phenomenon has been observed in a study using a PSA-directed poxviral-based immunotherapy product in men with metastatic CRPC.137 Problematic end points such as progression-free survival in CRPC (which may be confounded by both PSA and bone scan flare or delayed-onset effects) may perhaps be better addressed by revised guidelines using end points that are tailored to immunological agents.138
Conclusion
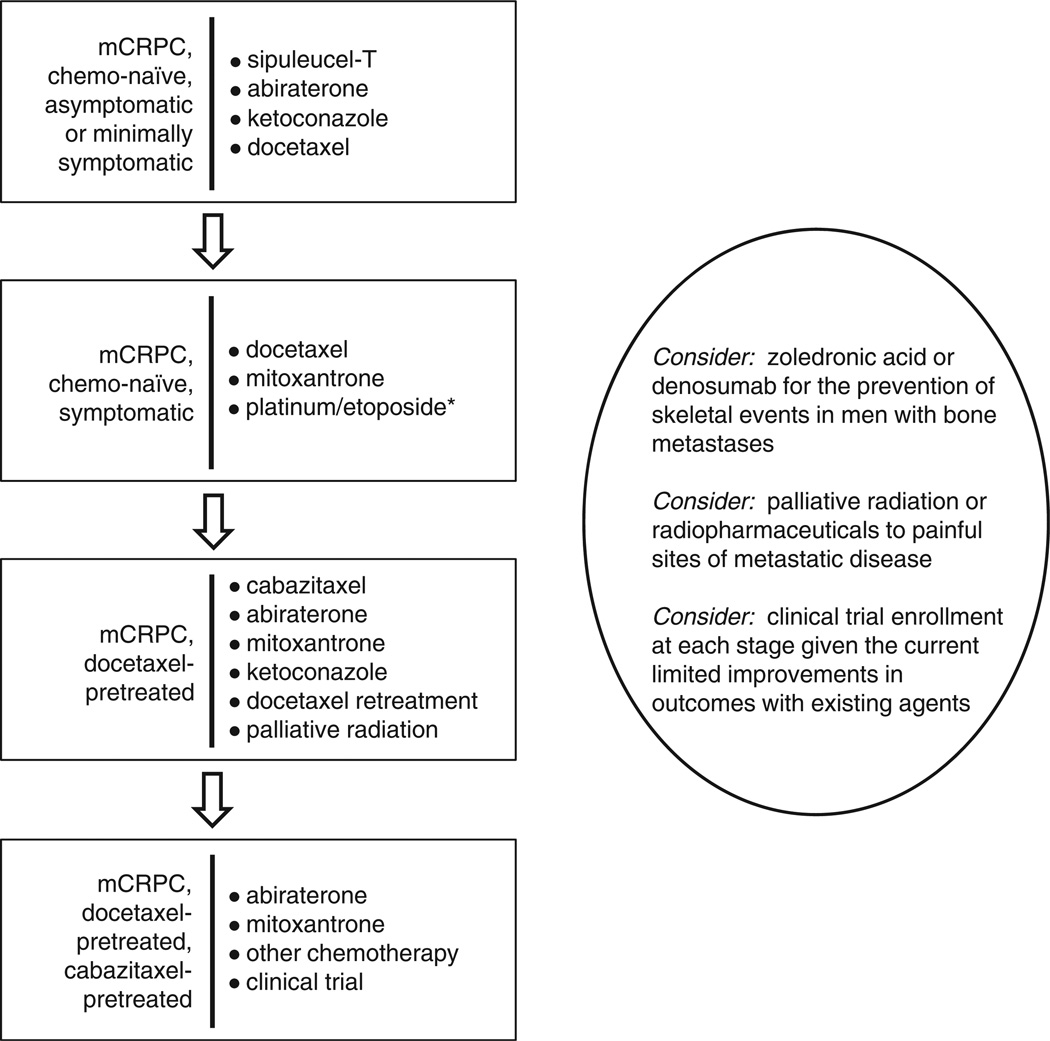
In the past year, three treatment modalities have been shown to improve survival in men with metastatic CRPC: cabazitaxel, abiraterone and sipuleucel-T. However, the rapid emergence of effective drugs for patients with CRPC has also resulted in some uncertainly about the optimal sequencing (or combination) of these agents. Figure 1 shows one plausible algorithm for the management of men with metastatic CRPC, although other treatment paradigms (including those recommended by the National Comprehensive Cancer Network)83 may also be reasonable.
Figure 1.
Proposed treatment paradigm for metastatic castration-resistant prostate cancer (mCRPC). Note: *Patients with small-cell (and possibly neuroendocrine) carcinoma.
Understanding the natural history of the disease in this post-docetaxel state may help facilitate treatment decisions, and may also provide important prognostic information. To this end, we have previously developed a multivariate prognostic model for men who had progressed following docetaxel chemotherapy, which showed a reasonable predictive accuracy for overall survival after accounting for several important prognostic factors.139 These included traditional prognostic parameters such as performance status and the presence of significant pain, the number of metastatic sites, alkaline phosphatase levels, degree of anemia and the presence/absence of liver metastases. However, we also identified novel prognostic factors that became important in this disease state, including time from original diagnosis (a measure of disease aggressiveness), how progression occurred (on docetaxel or following completion of a planned course), what type of progression was present (pain, radiographic or PSA progression) and the number of docetaxel cycles that a man had received (a measure of progression-free survival with previous docetaxel). Using this nomogram, median overall survival was found to range from 5 to 50 months,139 providing vital prognostic information with reasonable accuracy. Although this model may not directly aid a practitioner in deciding which treatment to use following docetaxel, it may provide useful information on prognosis to help better gauge treatment goals and how to individually tailor either aggressive or palliative therapies to these men.
In our view, sipuleucel-T is most appropriate for patients with asymptomatic to minimally symptomatic bone- or lymph node-metastatic CRPC who have not yet received cytotoxic chemotherapy, although select men may be able to receive this therapy during a prolonged chemotherapy holiday. Abiraterone with prednisone would also be a reasonable choice in this setting, although the results of the COU-AA-302 trial are still awaited, and there is some concern about the use of immunosuppressive corticosteroids early in the disease course, particularly concurrently with immunologic therapies such as sipuleucel-T. Ketoconazole and hydrocortisone (with or without dutasteride) would be an alternative to abiraterone. In patients with symptomatic disease or visceral metastases, or in those who have rapidly progressive disease at imminent risk of symptoms, chemotherapy should be initiated sooner rather than later, and docetaxel with prednisone is the preferred first choice. In men who may not be able to tolerate docetaxel, mitoxantrone may be used, especially in those with bone pain in whom a palliative benefit might be achieved. Alternatively, a platinum-etoposide doublet may be beneficial and palliative for men with metastatic prostatic small cell (or neuroendocrine) carcinoma. In docetaxel-pretreated patients, best evidence supports the use of cabazitaxel or abiraterone (once approved) each given with prednisone, whereas mitoxantrone may be reasonable in men at high risk of neutropenic complications or in those with significant peripheral neuropathy or taxane intolerance. Docetaxel retreatment may also be appropriate, especially in patients with a good initial response to docetaxel and subsequent discontinuation for reasons other than disease progression. Finally, in patients with multiply chemotherapy-refractory disease, treatment with abiraterone may be entertained but, in the absence of life-prolonging therapies in this setting, clinical trial participation should be encouraged in patients with good performance status.
In addition to the above approaches, the use of bone-health-promoting agents such as denosumab or zole-dronate should be strongly considered for men with bone metastatic disease to prevent pathological fractures, spinal cord compression or the need for radiation or surgery for skeletal complications. Palliative radiation or radiopharmaceuticals (for example, samarium, strontium or investigational radium) also have a significant role in the approach to men with symptomatic disease, both before or after systemic therapies. Several additional active agents are currently in phase III development (see the second review in this series), and some of these therapies are also likely to further expand the treatment options for men with metastatic CRPC in the near future.
In summary, cabazitaxel is the first agent to be approved by the FDA for use in men with metastatic CRPC who have progressed after previous docetaxel chemotherapy, and abiraterone acetate is also anticipated to gain FDA approval in this same patient population. In addition, sipuleucel-T may be beneficial in selected docetaxel-pretreated patients, so long as they remain minimally or asymptomatic and do not have visceral (especially hepatic) metastases. Clinical trial participation remains another excellent option for docetaxelrefractory patients.
Footnotes
Conflict of interest
Dr Antonarakis declares no conflicts of interest. Dr Armstrong has served as a consultant for Novartis and Amgen; he is on the speaker’s bureau for Pfizer, sanofi-aventis and Dendreon; and he has received research funding from Bristol-Myers Squibb, Imclone, Pfizer/Wyeth, Active Biotech, Medivation, Dendreon, Novartis and sanofi-aventis.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 3.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–1764. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 5.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 6.de Bono JS, Logothetis CJ, Fizazi K, North S, Chu L, Chi KN, et al. Abiraterone acetate plus low-dose prednisone improves overall survival in patients with metastatic castration-resistant prostate cancer who have progressed after docetaxel-based chemotherapy: results of COU-AA-301, a randomized double-blind placebo-controlled phase III study. Ann Oncol. 2010;21(Suppl 8) abstract LBA5. [Google Scholar]
- 7.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 8.Feldman BJ, Feldman D. The development of androgenindependent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 9.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 11.Tomlins SA, Mehra R, Rhodes DR, Cao X, Wang L, Dhanasekaran SM, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- 12.Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–319. [PubMed] [Google Scholar]
- 13.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 14.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nupponen N, Visakorpi T. Molecular biology of progression of prostate cancer. Eur Urol. 1999;35:351–354. doi: 10.1159/000019907. [DOI] [PubMed] [Google Scholar]
- 16.Tamura K, Furihata M, Tsunoda T, Ashida S, Takata R, Obara W, et al. Molecular features of hormone-refractory prostate cancer cells by genome-wide gene expression profiles. Cancer Res. 2007;67:5117–5125. doi: 10.1158/0008-5472.CAN-06-4040. [DOI] [PubMed] [Google Scholar]
- 17.Gregory CW, Johnson RT, Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001;61:2892–2898. [PubMed] [Google Scholar]
- 18.Brooke GN, Bevan CL. The role of androgen receptor mutations in prostate cancer progression. Curr Genomics. 2009;10:18–25. doi: 10.2174/138920209787581307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci USA. 2010;107:16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 22.Stigliano A, Gandini O, Cerquetti L, Gazzaniga P, Misiti S, Monti S, et al. Increased metastatic lymph node 64 and CYP17 expression are associated with high stage prostate cancer. J Endocrinol. 2007;194:55–61. doi: 10.1677/JOE-07-0131. [DOI] [PubMed] [Google Scholar]
- 23.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reid AH, Attard G, Barrie E, de Bono JS. CYP17 inhibition as a hormonal strategy for prostate cancer. Nat Clin Pract Urol. 2008;5:610–620. doi: 10.1038/ncpuro1237. [DOI] [PubMed] [Google Scholar]
- 26.Zhou HJ, Yan J, Luo W, Ayala G, Lin SH, Erdem H, et al. SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res. 2005;65:7976–7983. doi: 10.1158/0008-5472.CAN-04-4076. [DOI] [PubMed] [Google Scholar]
- 27.Shi XB, Xue L, Zou JX, Gandour-Edwards R, Chen H, deVere White RW. Prolonged androgen receptor loading onto chromatin and the efficient recruitment of p160 co-activators contribute to androgen-independent growth of prostate cancer cells. Prostate. 2008;68:1816–1826. doi: 10.1002/pros.20849. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Wu RC, O’Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9:615–630. doi: 10.1038/nrc2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lakshmikanthan V, Zou L, Kim JI, Michal A, Nie Z, Messias NC, et al. Identification of betaArrestin2 as a corepressor of androgen receptor signaling in prostate cancer. Proc Natl Acad Sci USA. 2009;106:9379–9384. doi: 10.1073/pnas.0900258106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Attar RM, Takimoto CH, Gottardis MM. Castration-resistant prostate cancer: locking up the molecular escape routes. Clin Cancer Res. 2009;15:3251–3255. doi: 10.1158/1078-0432.CCR-08-1171. [DOI] [PubMed] [Google Scholar]
- 32.Zhu ML, Kyprianou N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr Relat Cancer. 2008;15:841–849. doi: 10.1677/ERC-08-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 34.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 35.Wang G, Wang J, Sadar MD. Crosstalk between the androgen receptor and beta-catenin in castrate-resistant prostate cancer. Cancer Res. 2008;68:9918–9927. doi: 10.1158/0008-5472.CAN-08-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desai SJ, Ma AH, Tepper CG, Chen HW, Kung HJ. Inappropriate activation of the androgen receptor by non-steroids: involvement of the Src kinase pathway and its therapeutic implications. Cancer Res. 2006;66:10449–10459. doi: 10.1158/0008-5472.CAN-06-2582. [DOI] [PubMed] [Google Scholar]
- 37.Raffo AJ, Perlman H, Chen MW, Day ML, Streitman JS, Buttyan R. Overexpression of Bcl-2 protects prostate cancer cells from apoptosis in vitro and confers resistance to androgen depletion in vivo. Cancer Res. 1995;55:4438–4445. [PubMed] [Google Scholar]
- 38.Yang CC, Lin HP, Chen CS, Yang YT, Tseng PH, Rangnekar VM, et al. Bcl-xL mediates a survival mechanism independent of the phosphoinositide 3-kinase/Akt pathway in prostate cancer cells. J Biol Chem. 2003;278:25872–25878. doi: 10.1074/jbc.M301744200. [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Latham DE, Delaney MA, Chakravarti A. Survivin mediates resistance to antiandrogen therapy in prostate cancer. Oncogene. 2005;24:2474–2482. doi: 10.1038/sj.onc.1208490. [DOI] [PubMed] [Google Scholar]
- 40.Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor micro-environment. J Natl Cancer Inst. 2007;99:1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 41.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 42.Kyle AH, Huxham LA, Yeoman DM, Minchinton AI. Limited tissue penetration of taxanes: a mechanism for resistance in solid tumors. Clin Cancer Res. 2007;13:2804–2810. doi: 10.1158/1078-0432.CCR-06-1941. [DOI] [PubMed] [Google Scholar]
- 43.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure: an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 44.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maxwell PJ, Gallagher R, Seaton A, Wilson C, Scullin P, Pettigrew J, et al. HIF-1 and NFκB-mediated upregulation of CXCR1 and CXCR2 expression promotes cell survival in hypoxic prostate cancer cells. Oncogene. 2007;26:7333–7345. doi: 10.1038/sj.onc.1210536. [DOI] [PubMed] [Google Scholar]
- 46.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi H, Man S, Graham CH, Kapitain SJ, Teicher BA, Kerbel RS. Acquired multicellular-mediated resistance to alkylating agents in cancer. Proc Natl Acad Sci USA. 1993;90:3294–3298. doi: 10.1073/pnas.90.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70:6945–6956. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 49.Mani SA, GuoW, Liao MJ, Eaton EN, Ayyanan A, Zhou AYet al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial-to-mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13:7003–7011. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka H, Kono E, Tran CP, Miyazaki H, Yamashiro J, Shimomura T, et al. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat Med. 2010;16:1414–1420. doi: 10.1038/nm.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Logothetis CJ, Navone NM, Lin SH. Understanding the biology of bone metastases: key to the effective treatment of prostate cancer. Clin Cancer Res. 2008;14:1599–1602. doi: 10.1158/1078-0432.CCR-07-4603. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Lu Y, Pienta KJ. Multiple roles of chemokine (C-C motif) ligand 2 in promoting prostate cancer growth. J Natl Cancer Inst. 2010;102:522–528. doi: 10.1093/jnci/djq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qian DZ, Rademacher BL, Pittsenbarger J, Huang CY, Myrthue A, Higano CS, et al. CCL2 is induced by chemotherapy and protects prostate cancer cells from docetaxel-induced cytotoxicity. Prostate. 2010;70:433–442. doi: 10.1002/pros.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer. 2010;10:194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- 57.Bradshaw DM, Arceci RJ. Clinical relevance of transmembrane drug efflux as a mechanism of multidrug resistance. J Clin Oncol. 1998;16:3674–3690. doi: 10.1200/JCO.1998.16.11.3674. [DOI] [PubMed] [Google Scholar]
- 58.Rowinsky EK, Smith L, Wang YM, Chaturvedi P, Villalona M, Campbell E, et al. Phase I and pharmacokinetic study of paclitaxel in combination with biricodar, a novel agent that reverses multidrug resistance conferred by overexpression of both MDR1 and MRP1. J Clin Oncol. 1998;16:2964–2976. doi: 10.1200/JCO.1998.16.9.2964. [DOI] [PubMed] [Google Scholar]
- 59.Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 60.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 61.Berrieman HK, Lind MJ, Cawkwell L. Do β-tubulin mutations have a role in resistance to chemotherapy? Lancet Oncol. 2004;5:158–164. doi: 10.1016/S1470-2045(04)01411-1. [DOI] [PubMed] [Google Scholar]
- 62.Sève P, Dumontet C. Is class III β-tubulin a predictive factor in patients receiving tubulin-binding agents? Lancet Oncol. 2008;9:168–175. doi: 10.1016/S1470-2045(08)70029-9. [DOI] [PubMed] [Google Scholar]
- 63.van Amerongen R, Berns A. TXR1-mediated thrombospondin repression: a novel mechanism of resistance to taxanes? Genes Dev. 2006;20:1975–1981. doi: 10.1101/gad.1460806. [DOI] [PubMed] [Google Scholar]
- 64.Whipple RA, Matrone MA, Cho EH, Balzer EM, Vitolo MI, Yoon JR, et al. Epithelial-to-mesenchymal transition promotes tubulin detyrosination and microtentacles that enhance endothelial engagement. Cancer Res. 2010;70:8127–8137. doi: 10.1158/0008-5472.CAN-09-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verrills NM, Po’uha ST, Liu ML, Liaw TY, Larsen MR, Ivery MT, et al. Alterations in γ-actin and tubulin-targeted drug resistance in childhood leukemia. J Natl Cancer Inst. 2006;98:1363–1374. doi: 10.1093/jnci/djj372. [DOI] [PubMed] [Google Scholar]
- 66.Haldar S, Basu A, Croce CM. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997;57:229–233. [PubMed] [Google Scholar]
- 67.Yamanaka K, Rocchi P, Miyake H, Fazli L, So A, Zangemeister-Wittke U, et al. Induction of apoptosis and enhancement of chemosensitivity in human prostate cancer LNCaP cells using bispecific antisense oligonucleotide targeting Bcl-2 and Bcl-xL genes. BJU Int. 2006;97:1300–1308. doi: 10.1111/j.1464-410X.2006.06147.x. [DOI] [PubMed] [Google Scholar]
- 68.Zoubeidi A, Chi K, Gleave M. Targeting the cytoprotective chaperone, clusterin, for treatment of advanced cancer. Clin Cancer Res. 2010;16:1088–1093. doi: 10.1158/1078-0432.CCR-09-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pchejetski D, Doumerc N, Golzio M, Naymark M, Teissié J, Kohama Tet al. Chemosensitizing effects of sphingosine kinase-1 inhibition in prostate cancer cell and animal models. Mol Cancer Ther. 2008;7:1836–1845. doi: 10.1158/1535-7163.MCT-07-2322. [DOI] [PubMed] [Google Scholar]
- 70.Mumenthaler SM, Ng PY, Hodge A, Bearss D, Berk G, Kanekal S, et al. Pharmacologic inhibition of Pim kinases alters prostate cancer cell growth and resensitizes chemoresistant cells to taxanes. Mol Cancer Ther. 2009;8:2882–2893. doi: 10.1158/1535-7163.MCT-09-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu L, Birle DC, Tannock IF. Effects of the mammalian target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res. 2005;65:2825–2831. doi: 10.1158/0008-5472.CAN-04-3137. [DOI] [PubMed] [Google Scholar]
- 72.Boldt S, Weidle UH, Kolch W. The role of MAPK pathways in the action of chemotherapeutic drugs. Carcinogenesis. 2002;23:1831–1838. doi: 10.1093/carcin/23.11.1831. [DOI] [PubMed] [Google Scholar]
- 73.Zelivianski S, Spellman M, Kellerman M, Kakitelashvilli V, Zhou XW, Lugo E, et al. ERK inhibitor PD98059 enhances docetaxel-induced apoptosis of androgen-independent human prostate cancer cells. Int J Cancer. 2003;107:478–485. doi: 10.1002/ijc.11413. [DOI] [PubMed] [Google Scholar]
- 74.Domingo-Domenech J, Oliva C, Rovira A, Codony-Servat J, Bosch M, Filella X, et al. Interleukin 6, a nuclear factor-kappaB target, predicts resistance to docetaxel in hormone-independent prostate cancer and nuclear factor-kappaB inhibition by PS-1145 enhances docetaxel antitumor activity. Clin Cancer Res. 2006;12:5578–5586. doi: 10.1158/1078-0432.CCR-05-2767. [DOI] [PubMed] [Google Scholar]
- 75.Mimeault M, Johansson SL, Vankatraman G, Moore E, Henichart JP, Depreux P, et al. Combined targeting of epidermal growth factor receptor and hedgehog signaling by gefitinib and cyclopamine cooperatively improves the cytotoxic effects of docetaxel on metastatic prostate cancer cells. Mol Cancer Ther. 2007;6:967–978. doi: 10.1158/1535-7163.MCT-06-0648. [DOI] [PubMed] [Google Scholar]
- 76.Banerjee S, Hussain M, Wang Z, Saliganan A, Che M, Bonfil D, et al. In vitro and in vivo molecular evidence for better therapeutic efficacy of ABT-627 and taxotere combination in prostate cancer. Cancer Res. 2007;67:3818–3826. doi: 10.1158/0008-5472.CAN-06-3879. [DOI] [PubMed] [Google Scholar]
- 77.LoNigro C, Maffi M, Fischel JL, Formento P, Milano G, Merlano M. The combination of docetaxel and the somatostatin analogue lanreotide on androgen-independent docetaxel-resistant prostate cancer: experimental data. BJU Int. 2008;102:622–627. doi: 10.1111/j.1464-410X.2008.07706.x. [DOI] [PubMed] [Google Scholar]
- 78.Pivot X, Koralewski P, Hidalgo JL, Chan A, Gonçalves A, Schwartsmann G, et al. A multicenter phase II study of XRP6258 administered as a 1-h intravenous infusion every 3 weeks in taxane-resistant metastatic breast cancer patients. Ann Oncol. 2008;19:1547–1552. doi: 10.1093/annonc/mdn171. [DOI] [PubMed] [Google Scholar]
- 79.Galsky MD, Dritselis A, Kirkpatrick P, Oh WK. Cabazitaxel. Nat Rev Drug Discov. 2010;9:677–678. doi: 10.1038/nrd3254. [DOI] [PubMed] [Google Scholar]
- 80.Attard G, Greystoke A, Kaye S, de Bono J. Update on tubulinbinding agents. Pathol Biol. 2006;54:72–84. doi: 10.1016/j.patbio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 81.Mita AC, Denis LJ, Rowinsky EK, de Bono JS, Goetz AD, Ochoa L, et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-h infusion every 3 weeks in patients with advanced solid tumors. Clin Cancer Res. 2009;15:723–730. doi: 10.1158/1078-0432.CCR-08-0596. [DOI] [PubMed] [Google Scholar]
- 82.FDA CfDEaR. [accessed: 4 March 2011];Approval Package for Jevtana (NDA 20-1023) http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/201023s000Approv.pdf. (Published: 17 June 2010;
- 83.Mohler J, Bahnson RR, Boston B, Busby JE, D’Amico A, Eastham JA, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Cancer Netw. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 84.Berthold DR, Pond GR, de Wit R, Eisenberger M, Tannock IF. Survival and PSA response of patients in the TAX 327 study who crossed over to receive docetaxel after mitoxantrone or vice versa. Ann Oncol. 2008;19:1749–1753. doi: 10.1093/annonc/mdn288. [DOI] [PubMed] [Google Scholar]
- 85.Rosenberg JE, Weinberg VK, Kelly WK, Michaelson D, Hussain MH, Wilding G, et al. Activity of second-line chemotherapy in docetaxel-refractory hormone-refractory prostate cancer patients: randomized phase 2 study of ixabepilone or mitoxantrone and prednisone. Cancer. 2007;110:556–563. doi: 10.1002/cncr.22811. [DOI] [PubMed] [Google Scholar]
- 86.Eymard JC, Oudard S, Gravis G, Ferrero JM, Theodore C, Joly F, et al. Docetaxel reintroduction in patients with metastatic castration-resistant docetaxel-sensitive prostate cancer: a retrospective multicentre study. BJU Int. 2010;106:974–978. doi: 10.1111/j.1464-410X.2010.09296.x. [DOI] [PubMed] [Google Scholar]
- 87.Beer TM, Ryan CW, Venner PM, Petrylak DP, Chatta GS, Ruether JD, et al. Intermittent chemotherapy in patients with metastatic androgen-independent prostate cancer: results from ASCENT, a double-blinded, randomized comparison of high-dose calcitriol plus docetaxel with placebo plus docetaxel. Cancer. 2008;112:326–330. doi: 10.1002/cncr.23163. [DOI] [PubMed] [Google Scholar]
- 88.Di Lorenzo G, Buonerba C, Faiella A, Rescigno P, Rizzo M, Autorino R, et al. Phase II study of docetaxel re-treatment in docetaxel-pretreated castration-resistant prostate cancer. BJU Int. 2010;107:234–239. doi: 10.1111/j.1464-410X.2010.09498.x. [DOI] [PubMed] [Google Scholar]
- 89.Loriot Y, Massard C, Gross-Goupil M, Di Palma M, Escudier B, Bossi A, et al. The interval from the last cycle of docetaxel-based chemotherapy to progression is associated with the efficacy of subsequent docetaxel in patients with prostate cancer. Eur J Cancer. 2010;46:1770–1772. doi: 10.1016/j.ejca.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 90.Ross RW, Beer TM, Jacobus S, Bubley GJ, Taplin ME, Ryan CW, et al. A phase 2 study of carboplatin plus docetaxel in men with metastatic hormone-refractory prostate cancer who are refractory to docetaxel. Cancer. 2008;112:521–526. doi: 10.1002/cncr.23195. [DOI] [PubMed] [Google Scholar]
- 91.Trimble EL, Adams JD, Vena D, Hawkins MJ, Friedman MA, Fisherman JS, et al. Paclitaxel for platinum-refractory ovarian cancer: results from the first 1000 patients registered to National Cancer Institute Treatment Referral Center 9103. J Clin Oncol. 1993;11:2405–2410. doi: 10.1200/JCO.1993.11.12.2405. [DOI] [PubMed] [Google Scholar]
- 92.Berry W, Friedland D, Fleagle J, Jackson D, Ilegbodu D, Boehm KA, et al. A phase II study of weekly paclitaxel/ estramustine/carboplatin in hormone-refractory prostate cancer. Clin Genitourin Cancer. 2006;5:131–137. doi: 10.3816/CGC.2006.n.029. [DOI] [PubMed] [Google Scholar]
- 93.Cabrespine A, Guy L, Khenifar E, Curé H, Fleury J, Penault-Llorca F, et al. Randomized Phase II study comparing paclitaxel and carboplatin versus mitoxantrone in patients with hormone-refractory prostate cancer. Urology. 2006;67:354–359. doi: 10.1016/j.urology.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 94.Jeske S, Tagawa ST, Olowokure O, Selzer J, Giannakakou P, Nanus DM. Carboplatin plus paclitaxel therapy after docetaxel in men with metastatic castrate resistant prostate cancer. Urol Oncol. 2010 doi: 10.1016/j.urolonc.2009.12.023. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 95.Roche M, Kyriakou H, Seiden M. Drug evaluation: tesetaxel, an oral semi-synthetic taxane derivative. Curr Opin Invest Drugs. 2006;7:1092–1099. [PubMed] [Google Scholar]
- 96.Shionoya M, Jimbo T, Kitagawa M, Soga T, Tohgo A. DJ-927, a novel oral taxane, overcomes P-glycoprotein-mediated multidrug resistance in vitro and in vivo. Cancer Sci. 2003;94:459–466. doi: 10.1111/j.1349-7006.2003.tb01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baas P, Szczesna A, Albert I, Milanowski J, Juhász E, Sztancsik Z, et al. Phase I/II study of a 3-weekly oral taxane (DJ-927) in patients with recurrent, advanced non-small cell lung cancer. J Thorac Oncol. 2008;3:745–750. doi: 10.1097/JTO.0b013e31817c73ff. [DOI] [PubMed] [Google Scholar]
- 98.Modiano MR, Plezia P, Basche M, Cohn AL, Baram Y, Tapolsky G, et al. A phase I study of TPI-287, a novel taxane, administered every 21 days in patients with advanced cancer. J Clin Oncol. 2008;26(Suppl) abstract 13510. [Google Scholar]
- 99.Gross M, Pendergrass K, Leitner S, Leichman G, Pugliese L, Silberman S. TPI-287, a third-generation taxane, is active and well tolerated as 2nd line therapy after failure of docetaxel in hormone-refractory prostate cancer. J Clin Oncol. 2008;26(Suppl) abstract 16130. [Google Scholar]
- 100.Gerth K, Bedorf N, Hofle G, Irschik H, Reichenbach H. Epothilones A and B: antifungal and cytotoxic compounds from Sorangium cellulosum (myxobacteria). Production, physicochemical and biological properties. J Antibiot. 1996;49:560–563. doi: 10.7164/antibiotics.49.560. [DOI] [PubMed] [Google Scholar]
- 101.Lee JJ, Kelly WK. Epothilones: tubulin polymerization as a novel target for prostate cancer therapy. Nat Clin Pract Oncol. 2009;6:85–92. doi: 10.1038/ncponc1281. [DOI] [PubMed] [Google Scholar]
- 102.Rivera E, Lee J, Davies A. Clinical development of ixabepilone and other epothilones in patients with advanced solid tumors. Oncologist. 2008;13:1207–1223. doi: 10.1634/theoncologist.2008-0143. [DOI] [PubMed] [Google Scholar]
- 103.Hussain M, Tangen CM, Lara PN, Vaishampayan UN, Petrylak DP, Colevas AD, et al. Ixabepilone (epothilone B analogue BMS- 247550) is active in chemotherapy-naive patients with hormone- refractory prostate cancer: a Southwest Oncology Group trial S0111. J Clin Oncol. 2005;23:8724–8729. doi: 10.1200/JCO.2005.02.4448. [DOI] [PubMed] [Google Scholar]
- 104.Harzstark AL, Rosenberg JE, Weinberg VK, Sharib J, Ryan CJ, Smith DC, et al. Ixabepilone, mitoxantrone, and prednisone for metastatic castration-resistant prostate cancer after docetaxel-based therapy: a phase 2 study of the department of defense prostate cancer clinical trials consortium. Cancer. 2010 doi: 10.1002/cncr.25810. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 105.Bystricky B, Chau I. Patupilone in cancer treatment. Expert Opin Invest Drugs. 2011;20:107–117. doi: 10.1517/13543784.2011.542148. [DOI] [PubMed] [Google Scholar]
- 106.Beardsley EK, Saad F, Eigl B, Venner P, Hotte S, Winquist E, et al. A phase II study of patupilone in patients with metastatic castration-resistant prostate cancer who have progressed after docetaxel. J Clin Oncol. 2009;27(Suppl) doi: 10.1093/annonc/mdr336. abstract 5139. [DOI] [PubMed] [Google Scholar]
- 107.Chatta GS, Feinstein TM, Appleman LJ, Friedland DM, Jacobs SA, Evans TL, et al. Oxaliplatin and docetaxel in castration-resistant prostate cancer patients treated with up to two prior chemotherapeutic regimens: updated results of a phase II trial. J Clin Oncol. 2008;26(Suppl) abstract 5148. [Google Scholar]
- 108.Sella A, Yarom N, Zisman A, Kovel S. Paclitaxel, estramustine and carboplatin combination chemotherapy after initial docetaxel-based chemotherapy in castration-resistant prostate cancer. Oncology. 2009;76:442–446. doi: 10.1159/000217264. [DOI] [PubMed] [Google Scholar]
- 109.Loriot Y, Massard C, Gross-Goupil M, Di Palma M, Escudier B, Bossi A, et al. Combining carboplatin and etoposide in docetaxel-pretreated patients with castration-resistant prostate cancer: a prospective study evaluating also neuroendocrine features. Ann Oncol. 2009;20:703–738. doi: 10.1093/annonc/mdn694. [DOI] [PubMed] [Google Scholar]
- 110.Dorff TB, Groshen SG, Wei D, Korn C, Goldkorn A, Quinn DI, et al. Interim results of a phase II trial of pemetrexed and oxaliplatin as 2nd/3rd-line therapy in hormone-refractory prostate cancer. J Clin Oncol. 2008;26(Suppl) abstract 5139. [Google Scholar]
- 111.Blesa JM, Marco VG, Giner-Bosch V, Cerezuela P, Candel VA. Phase II trial of oxaliplatin and capecitabine after progression of first-line chemotherapy in androgen-independent prostate cancer patients. Am J Clin Oncol. 2010;34:155–159. doi: 10.1097/COC.0b013e3181d6b453. [DOI] [PubMed] [Google Scholar]
- 112.Papandreou CN, Daliani DD, Thall PF, Tu SM, Wang X, Reyes A, et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J Clin Oncol. 2002;20:3072–3080. doi: 10.1200/JCO.2002.12.065. [DOI] [PubMed] [Google Scholar]
- 113.Spiess PE, Pettaway CA, Vakar-Lopez F, Kassouf W, Wang X, Busby JE, et al. Treatment outcomes of small cell carcinoma of the prostate: a single-center study. Cancer. 2007;110:1729–1737. doi: 10.1002/cncr.22971. [DOI] [PubMed] [Google Scholar]
- 114.Brennan SM, Gregory DL, Stillie A, Herschtal A, MacManus M, Ball DL. Should extrapulmonary small cell cancer be managed like small cell lung cancer? Cancer. 2010;116:888–895. doi: 10.1002/cncr.24858. [DOI] [PubMed] [Google Scholar]
- 115.Oh WK, Tay MH, Huang J. Is there a role for platinum chemotherapy in the treatment of patients with hormone-refractory prostate cancer? Cancer. 2007;109:477–486. doi: 10.1002/cncr.22439. [DOI] [PubMed] [Google Scholar]
- 116.Sternberg CN. Satraplatin in the treatment of hormone-refractory prostate cancer. BJU Int. 2005;96:990–994. doi: 10.1111/j.1464-410X.2005.05799.x. [DOI] [PubMed] [Google Scholar]
- 117.Sternberg CN, Petrylak DP, Sartor O, Witjes JA, Demkow T, Ferrero JM, et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J Clin Oncol. 2009;27:5431–5438. doi: 10.1200/JCO.2008.20.1228. [DOI] [PubMed] [Google Scholar]
- 118.O’Donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, et al. Hormonal impact of the 17α-hydroxylase/ C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90:2317–2325. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 120.Attard G, Reid AH, de Bono JS. Abiraterone acetate is well tolerated without concomitant use of corticosteroids. J Clin Oncol. 2010;28:e560–e561. doi: 10.1200/JCO.2010.29.5170. [DOI] [PubMed] [Google Scholar]
- 121.Attard G, Reid AH, A’Hern R, Parker C, Oommen NB, Folkerd E, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ryan CJ, Smith MR, Fong L, Rosenberg JE, Kantoff P, Raynaud F, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–1488. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–1495. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scher HI, Jia X, de Bono JS, Fleisher M, Pienta KJ, Raghavan D, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–239. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 127.Trachtenberg J, Zadra J. Steroid synthesis inhibition by ketoconazole: sites of action. Clin Invest Med. 1988;11:1–5. [PubMed] [Google Scholar]
- 128.Ryan CJ, Eisenberger M. Chemotherapy for hormone-refractory prostate cancer: now it’s a question of when? J Clin Oncol. 2005;23:8242–8246. doi: 10.1200/JCO.2005.03.3092. [DOI] [PubMed] [Google Scholar]
- 129.Small EJ, Halabi S, Dawson NA, Stadler WM, Rini BI, Picus J, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) J Clin Oncol. 2004;22:1025–1033. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 130.Galsky MD, Simon K, Sonpavde G, Hutson TE, Fleming M, Kondagunta GV, et al. Ketoconazole retains activity in patients with docetaxel-refractory prostate cancer. Ann Oncol. 2009;20:965–966. doi: 10.1093/annonc/mdp199. [DOI] [PubMed] [Google Scholar]
- 131.Nakabayashi M, Oh WK, Jacobus S, Regan MM, Taplin ME, Kantoff PW, et al. Activity of ketoconazole after taxane-based chemotherapy in castration-resistant prostate cancer. BJU Int. 2010;105:1392–1396. doi: 10.1111/j.1464-410X.2009.08971.x. [DOI] [PubMed] [Google Scholar]
- 132.Taplin ME, Regan MM, Ko YJ, Bubley GJ, Duggan SE,Werner L, et al. Phase II study of androgen synthesis inhibition with ketoconazole, hydrocortisone, and dutasteride in asymptomatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7099–7105. doi: 10.1158/1078-0432.CCR-09-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 135.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 136.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 137.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Armstrong AJ, Garrett-Mayer E, de Wit R, Tannock I, Eisenberger M. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2010;16:203–211. doi: 10.1158/1078-0432.CCR-09-2514. [DOI] [PubMed] [Google Scholar]