Summary
Dendritic cells (DCs) play a key role in defense against infections and also in preventing inflammatory and autoimmune diseases. The response of DCs to pathogens is tightly regulated by many mechanisms to allow for appropriate, but not pathogenic, responses. We previously showed that DCs with deficiencies for two ITAM-bearing signaling adapters, DAP12 and FcRγ, produce more inflammatory cytokines upon treatment with Toll-like receptor (TLR) agonists than wild-type (WT) DCs. Here, we investigated whether the TREM-2 receptor pairs with DAP12 to inhibit TLR responses in DCs. TREM-2-deficient BMDCs showed increased inflammatory cytokine and type 1 IFN production in response to TLR ligation. Additionally, TREM-2-deficient BMDCs had increased TLR-induced maturation and were more efficient at inducing antigen-specific T cell-proliferation upon CpG DNA stimulation compared with WT BMDCs. Finally, we showed that a TREM-2 ligand is expressed on the surface of BMDCs, suggesting that the TREM-2 receptor transduces inhibitory signals due to recognition of an endogenous ligand.
Keywords: TREM-2, TLR, dendritic cell, DAP12, ITAM signaling
Introduction
DCs link the innate and adaptive immune system [1–3] and play an important role in host-defense by producing pro-inflammatory cytokines and chemokines after pathogen recognition through pattern recognition receptors such as Toll-like receptors (TLR) [4, 5]. TLR recognize pathogen-associated molecular patterns (PAMPs) using the extracellular leucine-rich repeat region [6]. After TLR ligation, TLR recruit MyD88 and/or TRIF via the TLR-IL-1R (TIR) domain in the cytoplasmic region resulting in the initiation of downstream signaling [6]. TLR signaling is essential for the function of DCs and macrophages in response to infection with many pathogens.
Many receptors on leukocytes associate non-covalently with signaling adapters that contain Immunoreceptor tyrosine-based activation motif (ITAM) sequences [7]. After ligation of a receptor paired with an ITAM-containing signaling adapter, the ITAM tyrosines are phosphorylated by src family kinases leading to the recruitment and activation of the Syk family kinases Syk or ZAP70 [8, 9]. In myeloid cells such as macrophages and DCs, there are two ITAM-containing adapters, DAP12 and FcεRIγ (referred to as FcRγ) [10, 11]. DAP12 and FcRγ can pair with many different receptors in macrophages and DCs. In our previous studies, we found that DAP12 negatively regulates TLR responses in macrophages [12–14]. DAP12-deficient macrophages exhibit higher pro-inflammatory cytokine production than WT macrophages upon stimulation with a panel of TLR agonists [14]. This increased pro-inflammatory cytokine production of DAP12-deficient macrophages was suppressed by transducing a chimeric receptor consisting of the extracellular domain of TREM-2 and the cytoplasmic domain of DAP12 [15]. Consistent with this finding, reduction of TREM-2 levels by knockdown or knockout caused hyperresponsiveness to TLR stimulation in macrophages [15, 16].
ITAM-bearing signaling adapters also negatively regulate TLR responses in DCs [12]. DAP12 or FcRγ-deficient DCs produced higher amounts of pro-inflammatory cytokines and showed increased maturation in response to TLR agonists than WT DCs. Interestingly, DCs deficient in both DAP12 and FcRγ had the highest TLR responses when compared with WT, DAP12-deficient and FcRγ-deficient DCs, indicating that specific receptors associated with both DAP12 and FcRγ are expressed on DCs and may be cooperatively involved in the negative regulation of TLR responses in these cells [12]. This is distinct from macrophages where we have not seen a role for FcRγ in inhibiting TLR responses (J.A. Hamerman, unpublished observation).
Based upon these studies we hypothesized that TREM-2 may contribute to the inhibition of TLR responses by DAP12 and/or FcRγ in DCs. Here, we show that BMDCs lacking TREM-2 were hyper-responsive to TLR stimulation as assessed by inflammatory cytokine production, type I IFN production and maturation. The phenotype of TREM-2-deficient DCs was similar to that of DAP12-deficient DCs. Furthermore, we demonstrate that BMDCs express an endogenous ligand for TREM-2 on their cell surface. Taken together, we conclude that TREM-2 negatively regulates TLR responses by interaction with an endogenous TREM-2 ligand in DCs.
Results
TREM-2 is expressed on DCs in a DAP12-dependent manner
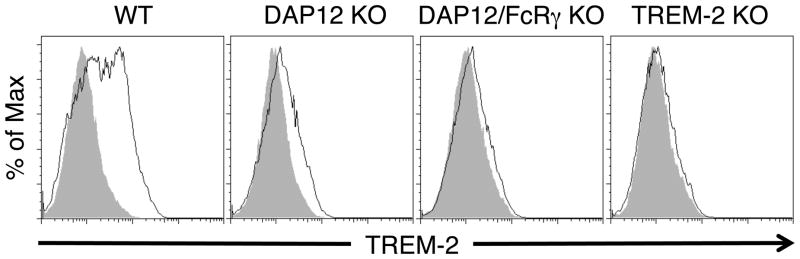
We previously have reported that TREM-2 is a DAP12-coupled receptor that negatively regulates TLR responses in macrophages [14, 15]. Here we investigated whether TREM-2 acts as a negative regulator of TLR responses in DCs. We first examined the expression of TREM-2 on BMDCs. TREM-2 was expressed on the surface of WT BMDCs cultured for 6 days in GM-CSF (Fig. 1), consistent with a previous study showing TREM-2 mRNA in BMDCs [17]. The TREM-2 cell-surface expression was reduced in DAP12-deficient BMDCs, indicating that DAP12 pairs with TREM-2 and is required for maximal TREM-2 expression on the cell surface in BMDCs (Fig. 1). BMDCs lacking both DAP12 and FcRγ had no staining for TREM-2 similar to those grown from TREM-2-deficient bone marrow, suggesting that FcRγ may minimally contribute to cell surface expression of TREM-2 in these cells.
Figure 1. TREM-2 is expressed on the cell surface of BMDCs in a DAP12-dependent manner.
BMDCs were cultured from WT, DAP12-deficient and DAP12/FcRγ-deficient BM cells for 6 days with GM-CSF, and then stained for CD11c and TREM-2. The histograms are gated on CD11c-positive cells. The gray shaded histogram is the isotype control and the thin black line is the staining for TREM-2. These data are representative of at least three independent experiments.
TREM-2 negatively regulates TLR-induced inflammatory cytokine production in DCs
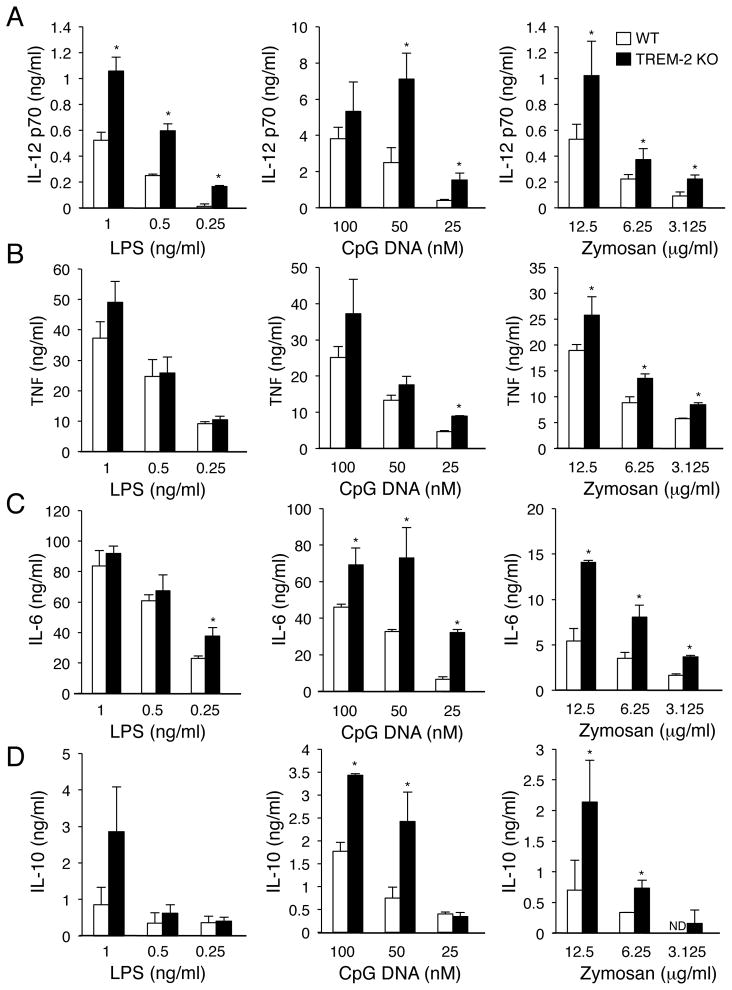
To address whether TREM-2 regulates TLR responses in DCs, we generated BMDCs from WT and TREM-2-deficient mice. We first investigated whether TREM-2 deficiency affected DC development from BM cells cultured in the presence of GM-CSF. Total cell number was decreased in TREM-2-deficient BM cell culture after 5 days of culture (Supplemental Fig. 1A), however the percentage of total cells that were CD11c+ DCs was not changed between WT and TREM-2-deficient cultures (Supplemental Fig. 1B). We next stimulated these BMDCs using various TLR ligands (LPS, CpG DNA and Zymosan) for 16 hours and performed ELISA to evaluate secretion of IL-12 p70 and TNF. Though Zymosan is a complex particle that interacts with multiple pattern recognition receptors, such as Dectin-1, it also signals through a TLR2/TLR6 heterodimer [18, 19]. TREM-2-deficient DCs produced significantly more IL-12 p70 than WT DCs after stimulation with a range of doses of LPS, CpG DNA and Zymosan (Fig. 2A). TNF secretion from TREM-2-deficient DCs was modestly increased over WT DCs (Fig. 2B). In addition to IL-12 p70 and TNF, IL-6 and IL-10 secretion was also higher in TREM-2-deficient DCs than WT DCs after stimulation with these TLR ligands (Fig. 2C, D). Interestingly, we did not see any cytokine production from unstimulated TREM-2-deficient DCs (Ito and Hamerman, unpublished observation).
Figure 2. Increased TLR-induced cytokine secretion from TREM-2-deficient DCs.
(A–D) CD11c+ BMDCs were stimulated with the indicated doses of TLR agonists (LPS, CpG DNA and Zymosan) for 16 h. (A) IL-12 p70, (B) TNF, (C) IL-6 and (D) IL-10 concentrations in the culture supernatant were determined by ELISA. Data are represented as mean + SD of triplicate wells (* p<0.05, as determined by an unpaired two-tailed students t-test). These data are representative of two (LPS) and four (CpG DNA and Zymosan) independent experiments for TNF and IL-12 p70 and two independent experiments for IL-6 and IL-10.
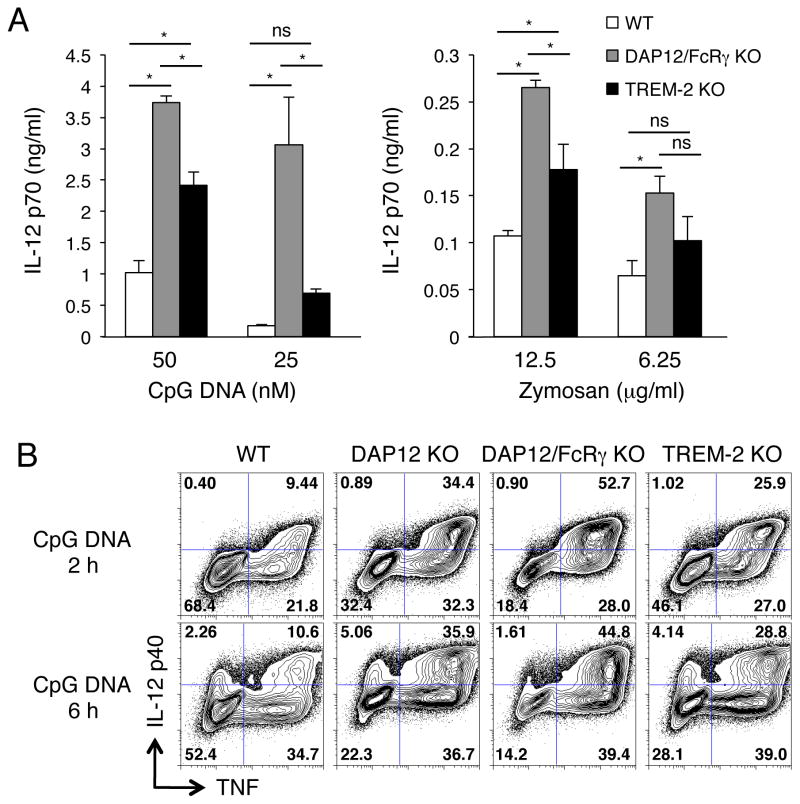
We next compared pro-inflammatory cytokine secretion between WT, DAP12/FcRγ-deficient and TREM-2-deficient DCs (Fig. 3A). DAP12/FcRγ-deficient and TREM-2-deficient DCs showed higher IL-12 p70 production than WT DCs after 16 h stimulation with CpG DNA or Zymosan (Fig. 3A). The TLR responses in TREM-2-deficient DCs were lower than DAP12/FcRγ-deficient DCs (Fig. 3A). We also compared the pro-inflammatory cytokine production of WT, DAP12-deficient, DAP12/FcRγ-deficient and TREM-2-deficient BMDCs by intracellular cytokine staining. After both 2 and 6 h stimulation with CpG DNA, the percentage of IL-12 p40+TNF+ cells was higher in TREM-2-deficient, DAP12-deficient and DAP12/FcRγ-deficient DCs than in WT DCs (Fig. 3B). Consistent with the ELISA results (Fig. 3A), DAP12/FcRγ-deficient DCs showed the highest percent of IL-12 p40+TNF+ cells after CpG DNA stimulation (Fig. 3B). Both TREM-2-deficient and DAP12-deficient DCs showed an intermediate phenotype of pro-inflammatory cytokine production in between WT and DAP12/FcRγ-deficient DCs in response to CpG DNA (Fig. 3B). Furthermore, the cytokine staining pattern of TREM-2-deficient DCs was very close to that of DAP12-deficient DCs, suggesting that TREM-2 inhibits TLR responses primarily through DAP12 in DCs.
Figure 3. TREM-2-deficient DCs produce increased TLR-induced cytokines, similar to DAP12-deficient DCs.
(A) CD11c-purified BMDCs were stimulated with the indicated concentration of CpG DNA and Zymosan for 16 h and IL-12 p70 production was determined by ELISA. Data are represented as mean + SD of triplicate wells (* p<0.05, as determined by a one-way ANOVA with Bonferroni’s post-test). (B) BMDCs were incubated with CpG DNA (100 nM) for 2 h and 6 h, and then fixed, permeabilized, stained with anti-IL-12 p40 and anti-TNF Abs, and analyzed by flow cytometry. These data are representative of (A) four and (B) two independent experiments.
TREM-2 negatively regulates TLR-induced maturation of DCs
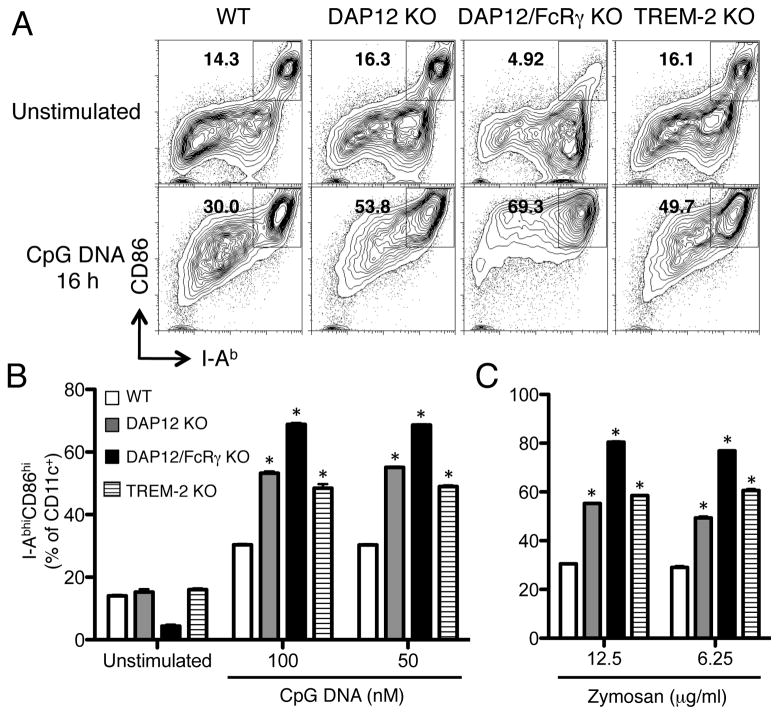
Next, we assessed maturation of WT, DAP12-deficient, DAP12/FcRγ-deficient and TREM-2-deficient BMDCs after stimulation with CpG DNA or Zymosan for 16 h. As shown in Fig. 4, TREM-2-deficient DCs had more I-AbhighCD86high mature cells than WT DCs after CpG DNA and Zymosan stimulation. Importantly, the maturation level of TREM-2-deficient DCs was very similar to that of DAP12-deficient DCs, suggesting that TREM-2 signaling is mediated by DAP12 in BMDCs. We also compared TREM-2-deficient DCs to those deficient in both DAP12 and FcRγ. Similar to what we found for cytokine production, TREM-2-deficient DCs showed less CpG DNA- and Zymosan-induced maturation than DAP12/FcRγ-deficient DCs. Interestingly, whereas WT, DAP12-deficient and TREM-2-deficient DCs had a similar amount of maturation in the absence of stimulus, DCs lacking both DAP12 and FcRγ consistently had less basal maturation even though they had the highest amount of stimulus-induced maturation (Fig. 4B). In conclusion, these results show that TREM-2/DAP12 signaling negatively regulates DC TLR responses.
Figure 4. TREM-2-deficient DCs show increased maturation in response to TLR agonists.
BMDCs were incubated with CpG DNA (100 nM) or Zymosan (12.5 μg/ml) for 16 h. After incubation, cells were stained with anti-CD11c, anti-CD86 and anti-I-Ab Abs and analyzed by flow cytometry. (A) Plots represent CD11c-positive gated cells. (B, C) The percent of CD86high/I-Abhigh CD11c+ BMDC after 16 h of (B) CpG DNA or (C) Zymosan treatment. Data are represented as mean + SD of triplicate wells. *p<0.05 versus WT, as determined by a one-way ANOVA with Dunnett’s post-test. These data are representative of at least two independent experiments.
TREM-2 negatively regulates type I IFN responses in DCs
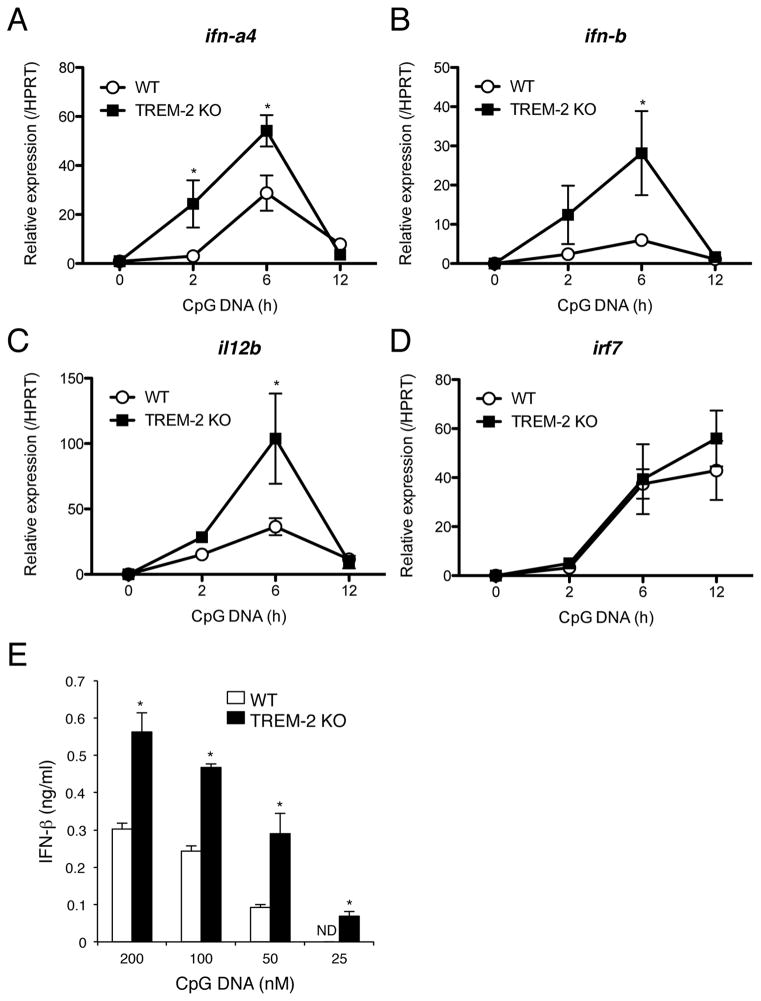
It has been reported that Siglec-H is involved in the negative regulation of type I IFN responses through DAP12 signaling in plasmacytoid DCs (pDCs) [20, 21]. Though TREM-2 is not expressed in pDCs (Ito and Hamerman, unpublished data), we hypothesized that TREM-2 may inhibit type I IFN production in conventional DCs, such as BMDCs. We assessed IFN-α4 and IFN-β expression by qRT-PCR in WT and TREM-2-deficient BMDCs after CpG DNA stimulation. Expression of mRNAs encoding both type I IFNs analyzed were higher in TREM-2-deficient BMDCs compared to WT BMDCs at 2 h and 6 h after stimulation (Fig. 5A, B). As expected, TREM-2-deficient BMDCs also expressed more mRNA encoding IL-12 p40 (il12b) at 2 h and 6 h after CpG DNA treatment than WT BMDCs (Fig. 5C). Intriguingly, IRF7 expression was not changed between WT and TREM-2-deficient BMDCs (Fig. 5D). IRF7 is induced by type I IFN stimulation and plays a major role in the positive feedback regulation of type I IFN expression [22, 23]. We also measured IFN-β secretion after 16 h of CpG DNA stimulation by ELISA. TREM-2-deficient BMDCs secreted significantly more IFN-β protein than WT BMDCs after CpG DNA stimulation (Fig. 5E). These results suggest that increased type I IFN response in TREM-2-deficient DCs was due to lack of TREM-2/DAP12 signaling at the primary TLR response phase. In conclusion, these results demonstrate that TREM-2 negatively regulates DC production of type I IFN in addition to IL-12 p70 and TNF in response to TLR ligation.
Figure 5. Enhanced type I IFN responses in TREM-2-deficient DCs.
(A–D) CD11c-purified BMDCs from WT and TREM-2-deficient mice were stimulated with CpG DNA (100 nM) for the indicated time and qPCR performed using specific primers against (A) IFN-α4, (B) IFN-β, (C) IL-12 p40 and (D) IRF7. HPRT was used as internal control. (E) IFN-β production from BMDCs upon CpG DNA stimulation. After 16 h of CpG stimulation, culture supernatants were recovered and IFN-β production measured by ELISA. Data are represented as mean +/− SD of triplicate wells. *p<0.03 versus WT, as determined by an unpaired two-tailed students t-test. These data are representative of two independent experiments.
TREM-2 negatively regulates antigen-specific T cell proliferation by BMDCs
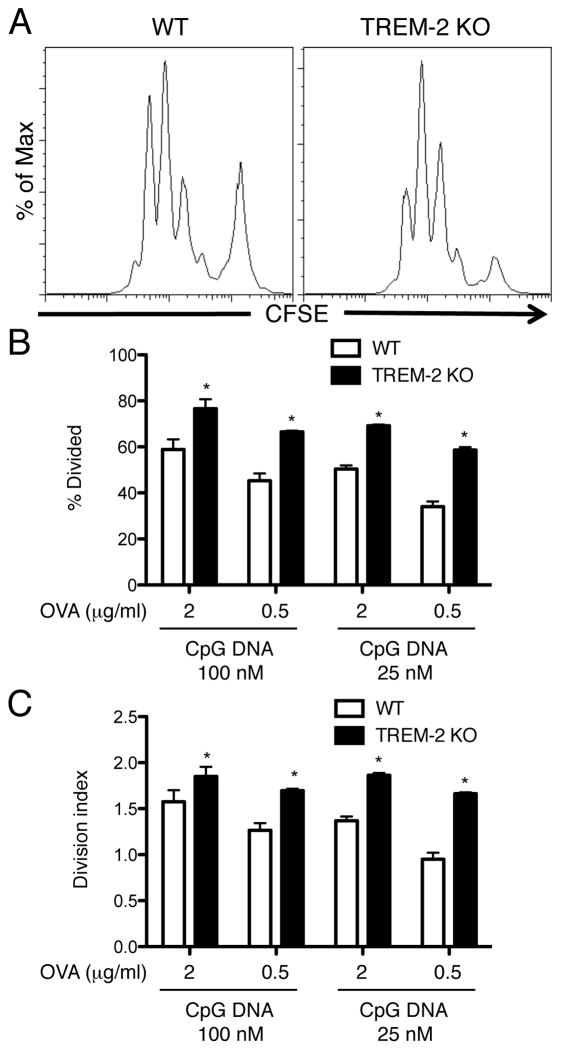
Because TREM-2-deficient BMDCs matured more efficiently than WT BMDCs, we investigated whether the antigen-presenting activity of TREM-2-deficient DCs was higher than that of WT DCs. We co-cultured OVA peptide-pulsed BMDCs in the presence of high (100 nM) and low (25 nM) doses of CpG DNA with CFSE-labeled OT-II TCR transgenic CD4+ T cells for 72 h and detected CFSE dilution of CD4+ T cells by flow cytometry (Fig. 6A). TREM-2-deficient DCs elicited more OVA-specific T cell division than WT DCs upon CpG DNA stimulation when measured as the percent of T cells that had undergone at least one division (Fig. 6B) or the average number of divisions per T cell (Fig. 6C). Therefore, along with DC maturation, TREM-2 negatively regulates the ability of BMDCs to induce antigen-specific T cell proliferation.
Figure 6. Enhanced antigen-presentation activity in TREM-2-deficient DCs.
(A) CD11c-purified BMDCs from WT and TREM-2-deficient mice were co-cultured with CFSE-labeled CD4+ OT-II T cells in the presence of OVA, CpG DNA and GM-CSF for 72 h. After co-culture, CFSE dilution of CD4+ OT-II T cells was detected by flow cytometry. (B) The percentage of divided and (C) division index of CD4+ T cells were calculated by Flowjo software. Data are represented as mean +/− SD of triplicate wells. *p<0.05 versus WT, as determined by an unpaired two-tailed students t-test. These data are representative of two independent experiments.
BMDCs express a ligand on their cell surface for TREM-2
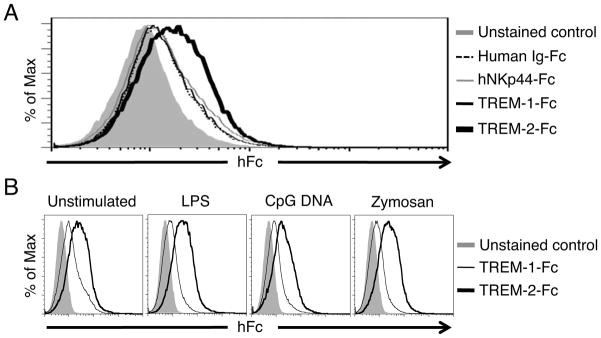
TREM-2 has been described to have both endogenous mammalian ligands and exogenous ligands on some bacteria and fungi [24–28]. We have shown that macrophages express an endogenous ligand for TREM-2, which we believe constitutively ligates TREM-2 in macrophages to allow for inhibition of TLR responses [15]. We therefore examined whether DCs also express a ligand on their surface for TREM-2 using a TREM-2-Fc fusion protein consisting of the TREM-2 extracellular domain fused to the human IgG Fc domain [15]. We found that TREM-2-Fc, but not TREM-1-Fc, could bind to the cell surface of BMDCs (Fig. 7A). We used several negative controls, e.g. human IgG1 Fc alone and human NKp44-Fc, to validate the TREM-2-Fc staining was positive (Fig. 7A). TREM-1-Fc staining was not any different than any of the negative controls used. Treatment of BMDCs for 16 h with LPS, CpG DNA or Zymosan did not change the staining with the TREM-2 Fc reagent (Fig. 7B). These data show that DCs express a ligand for TREM-2 and support a model whereby TREM-2 constitutively interacts with its ligand, transducing a signal through DAP12 to inhibit DC TLR responses.
Figure 7. TREM-2 ligand is expressed on the cell surface of BMDCs.
(A) Unstimulated BMDCs were incubated with human Ig-Fc, TREM-1-Fc, NKp44-Fc or TREM-2-Fc protein and then stained with anti-CD11c Ab. The histogram shows CD11c-positive gated cells. The solid gray histogram is the unstained control, the dashed black line is human Ig-Fc binding, the thin gray line is NKp44-Fc binding, the thin black line is TREM-1-Fc binding and the bold black line is TREM-2-Fc binding. (B) WT BMDCs were stimulated with LPS (0.5 ng/ml), CpG DNA (100 nM) or Zymosan (12.5 μg/ml) for 16 h. These BMDCs were incubated with TREM-1-Fc or TREM-2-Fc protein and then stained with anti-CD11c Ab. The histogram shows CD11c-positive gated cells. The solid gray histogram is the unstained control, the thin line is TREM-1-Fc binding, and the bold line is TREM-2-Fc binding. These data are representative of five (unstimulated) and four (LPS, CpG DNA, Zymosan treated) independent experiments.
Discussion
DAP12 and FcRγ are ITAM-containing signaling adapters expressed in myeloid and natural killer (NK) cells [10, 29]. We and others have focused on the function of these ITAM-containing adapters in macrophages and DCs and found that, surprisingly, DAP12 has a critical role in negative regulation of macrophage and DC activation upon TLR ligation [12–14, 29, 30]. In macrophages, the TREM-2 receptor pairs with DAP12 to inhibit TLR-induced inflammatory cytokine production [15]. In this study, we show that TREM-2 also plays a negative role in TLR responses in bone marrow-derived DCs. In BMDCs, TREM-2 inhibits inflammatory cytokine production, type I IFN production, maturation and the ability to induce antigen-specific T cell proliferation. Additionally, we found the expression of endogenous TREM-2 ligand on DCs. Taken together, we conclude that the TREM-2 receptor specifically pairs with DAP12 to inhibit TLR responses in BMDCs.
The phenotype of the TREM-2-deficient BMDCs was very similar to that of DAP12-deficient BMDCs, and distinct from those lacking both DAP12 and FcRγ, which had much higher responses than those lacking TREM-2 or DAP12. These data suggest that signaling through DAP12 is required for inhibition of TLR responses by TREM-2, and that there is an additional FcRγ-coupled receptor that can inhibit TLR responses in BMDCs. Several FcRγ-pairing receptors that inhibit TLR responses have been identified in human pDCs [31–33]. To date, no receptors that signal through FcRγ to inhibit TLR responses have been identified in mouse pDCs or in mouse or human conventional DCs. It is striking that TREM-2-deficient BMDCs are better at inducing antigen-specific T cell priming, whereas DAP12-deficient mice have been shown to have defects in Th1 priming during EAE [34]. This suggests that the DCs that are key for inducing these Th1 responses in vivo likely express a distinct DAP12-associated receptor or receptors from TREM-2 that can promote the differentiation of T cells into Th1 effectors by DCs.
Interestingly, we found that TREM-2 cell surface expression was greatly reduced in DAP12-deficient BMDCs compared to WT DCs, whereas we have previously shown that TREM-2 surface expression is only slightly reduced in DAP12-deficient macrophages [15]. This difference between DCs and macrophages is interesting and could possibly be due to differences in the availability of DAP10, a related signaling adapter, in macrophages and DCs. DAP10 has recently been shown to associate with TREM-2 in WT macrophages, and we postulate that the robust surface expression of TREM-2 in DAP12-deficient macrophages is due to the availability of DAP10 to pair with TREM-2 in these macrophages [35]. It is possible that there is less available DAP10 to pair with TREM-2 and allow surface expression in BMDCs than in macrophages, either because of lower expression of DAP10 or a higher ratio of DAP10 to DAP12 pairing receptors in BMDCs than macrophages.
TREM-2 and DAP12 have been implicated positively in the development and function of several macrophage populations in mouse and human. Mutations in TREM-2 and DAP12 cause the rare recessive disease Nasu-Hakola syndrome (also called PLOSL), which is characterized by bone cysts and fractures, and progressive dementia and eventual CNS failure [36]. These phenotypes of Nasu-Hakola patients suggest dysfunction of osteoclasts and microglia, the TREM-2 and DAP12 expressing resident macrophage-like cells in the bone and brain, respectively. DAP12-deficient mice have mild osteopetrosis and have defects in the development of osteoclasts from bone marrow precursors in vitro [37]. Similarly, human peripheral blood monocytes lacking DAP12 or TREM-2 from patients with Nasu-Hakola disease have a reduced ability to differentiate into mature, functional osteoclasts [38, 39].
In osteoclasts and DCs, it has been shown that the cell surface receptor Plexin-A1 associates with TREM-2. Interestingly, treatment of BMDCs with Semaphorin 6D (Sema6D), a ligand of Plexin-A1, induces IL-12 p40 production, and optimal IL-12 p40 secretion after Sema6D treatment requires TREM-2 and DAP12 expression [40]. These data suggest that Sema6D/Plexin-A1 positively regulate osteoclast and DC function in the absence of TLR ligation. Also in support of a positive role for TREM-2 in DC function, Bouchon et. al. showed that monoclonal antibody cross-linking of TREM-2 on human monocyte-derived DCs results in partial maturation of the DCs [41]. This is in contrast to our findings that TREM-2 inhibits the ability of mouse BMDCs to mature and induce antigen-specific T cell proliferation after TLR stimulation. Further studies will need to address why the TREM-2/DAP12 receptor complex may sometimes inhibit and other times activate DC function. We speculate that direct activation of TREM-2/DAP12, such as with crosslinking antibody or with Sema6D/PlexinA1, leads to activation of DC cytokine production, but that the constitutive TREM-2/DAP12 signal present in DCs and macrophages in conjunction with a TLR response leads to inhibition. This inhibition may be caused by a constitutive signal downstream of the DAP12 ITAM and Syk, the sequestration of signaling components by constitutive signaling through DAP12 and Syk, or by the induction of negative regulators of the TLR signal transduction pathway [13].
TREM-2/DAP12 signaling also plays a positive role in phagocytosis [25, 27, 42]. Knockdown of TREM-2 or DAP12 in microglia reduced the phagocytosis of apoptotic neurons, whereas overexpression of TREM-2 increased phagocytosis [42]. Apoptosis has been shown to induce expression of an unknown TREM-2 ligand on the surface of several cell types, including neurons [24, 25]. These facts suggest that microglia recognize and phagocytose apoptotic neurons via TREM-2 ligation. This TREM-2 ligation upon phagocytosis of apoptotic cells may help protect against any inadvertent TLR-induced inflammatory response to self-DNA released from the apoptotic neurons. Consistent with this idea, knockdown of TREM-2 in microglia causes an increase in TNF and NOS2 transcription when the cells are exposed to apoptotic neurons [42]. Interestingly, TREM-2 can also recognize and bind to several species of bacteria and fungi [26–28] and is involved in phagocytosis of these bacteria [27]. These observations indicate that TREM-2 binds both endogenous and exogenous ligands to induce phagocytosis.
Our data demonstrate that TREM-2 negatively regulates DC and macrophage function in the presence of TLR ligands derived from bacteria and viruses, such as LPS and CpG DNA. TREM-2 also inhibited DC responses to the fungal particle Zymosan, which contains ligands for the TLR2/TLR6 heterodimer as well as ligands for additional receptors such as Dectin-1 and Nod2 [18, 19, 43]. We propose that DCs require continuous TREM-2 ligation for suppression of TLR responses to keep immune responses in check. The same endogenous and exogenous ligands that induce phagocytosis may also be able to cause the inhibitory signals we describe here, though these ligands have not been characterized at a molecular level. Indeed, though we have detected TREM-2 Fc binding to BMDCs, we have no direct evidence that the putative TREM-2 ligands bound by TREM-2 Fc participate in inhibitory signaling through TREM-2. Current studies in our laboratory aim to identify the endogenous TREM-2 ligands that cause inhibitory signals.
The fact that TREM-2 has ligands on both cell types found to express TREM-2, macrophages and BMDCs, suggests that whenever TREM-2 is expressed it will be cross-linked and transduce inhibitory signals. We have extensively examined resting DC populations in lymphoid organs for TREM-2 surface expression, yet have not detected it by flow cytometry (Ito and Hamerman, unpublished observations). Additionally, TREM-2 mRNA is not found in the many DC populations from lymphoid and non-lymphoid tissues in the steady state used for microarray analysis at Immgen.org. It is possible that during inflammation, TREM-2 may be induced on DC populations in vivo and there serve to turn off the inflammatory response. We have investigated one recently-described inflammatory DC population that differentiates in response to LPS injection and has been suggested to be an in vivo correlate of BMDCs grown in GM-CSF [44], but we did not find TREM-2 mRNA expression on these cells (Ito and Hamerman, unpublished observation). Interestingly, human TREM-2 expression is found in both immature and activated DCs and macrophages, all differentiated from monocytes in culture, but not on monocytes themselves [41]. Future studies will aim to identify what DC populations express TREM-2 during inflammation or infection in vivo.
Similar to how TREM-2 binds an endogenous ligand, ILT7, a FcRγ–associated receptor predominantly expressed on human pDCs, binds a pDC-expressed ligand bone marrow stromal cell antigen 2 (BST2) [31, 32]. Cross-linking of ILT7 using a monoclonal antibody or BST2 inhibits TLR7 and TLR9-mediated IFN-α and TNF production from human pDCs. BST2 was also found on several human cancer cell lines and human pDCs [31]. This suggests that there is the possibility for a cis interaction between ILT7 and BST2 on human pDCs, similar to what we suggest here for TREM-2 and its ligand on DCs and on macrophages [15]. Interestingly, BST2 expression was dramatically induced in IFN-α stimulated cell lines that do not express BST2 under steady state conditions [31], suggesting that ILT7/BST2 ligation on pDCs contributes to the attenuation or termination of IFN-α responses via FcRγ signaling after virus infection. Taken together with the data presented here, the regulation by inhibitory receptor-ligand pairs expressed on the same cells appears to be a widely used strategy for tuning the responses of innate inflammatory cells such as macrophages and DCs. Whether these receptor-ligand interactions occur in cis with both receptor and ligand on the same cell, or whether they occur in trans by neighboring cells remains to be determined, both for the TREM-2/TREM-2 ligand interaction and the ILT7/BST2 interaction.
In conclusion, TREM-2 has both activating and inhibitory functions in DCs as well as in other myeloid cells such as macrophages and microglia. TREM-2 binds both endogenous and exogenous ligands and may play an important role in regulating the magnitude of DC responses to infection. Identification of a TREM-2 ligand with inhibitory effects on TLR responses will be important for understanding the mechanism of DC regulation by TREM-2 and DAP12, and may lead to specific therapies that modulate DC function.
Materials & Methods
Mice
C57BL/6 mice were purchased from Charles River. DAP12-deficient mice (Tyrobp−/−) were backcrossed 12 generations against C57BL/6 mice [34]. DAP12/FcRγ-deficient mice were generated by crossing these DAP12-deficient mice with FcRγ-deficient mice generated with C57BL/6 ES cells (FcεR1γ −/−), provided by Dr. Takashi Saito (RIKEN, Yokohama, Japan) [45]. TREM-2-deficient mice were provided by Dr. Marco Colonna (Washington University, St. Louis) [16]. All mice were housed in specific-pathogen-free barrier animal facilities. All experiments were performed under an Institutional Animal Care and Use Committee (IACUC)-approved protocol.
Antibodies and Reagents
The following antibodies (Abs) were used: anti-FcγRII/III (2.4G2), anti-CD11c (N418), anti-I-Ab (M5/114.15.2), anti-CD86 (GL-1), anti-TREM-2 (78.18) [46], anti-IL12 p40 (C17.8), anti-TNF-α (MP6-XT22), PE-conjugated Streptavidin (eBioscience) and PE-conjugated anti-human IgG Fc (Jackson ImmunoResearch). TREM-1-Fc and TREM-2-Fc proteins were kindly provided by Dr. J.P. Houchins (R&D Systems). Recombinant murine (rm) GM-CSF was purchased from Peprotech. LPS (List Biological Laboratories), CpG DNA (ODN1826; Invivogen) and Zymosan (SIGMA-Aldrich) were used to stimulate BMDCs.
BMDC preparation
DC medium consisted of RPMI 1640 (Hyclone) supplemented with 10% fetal bovine serum (FBS; SIGMA), 2 mM L-glutamine (GIBCO), 1 mM sodium pyruvate (GIBCO), 0.1 mM nonessential amino acid (GIBCO), 10 mM HEPES (LONZA), Penicillin/Streptomycin (GIBCO), and 10 ng/ml GM-CSF (Peprotech). In brief, we took BM cells from femurs and tibias and lysed red blood cells by using ACK buffer (LONZA). The BM cells were plated into 10 cm Petri dish (5 per mouse) using 10 ml of DC medium in 37°C CO2 incubator. After 2 days of culture, we added 10 mls of DC medium and cultured for 3 days, and then changed half the volume of the culture medium to fresh DC medium. At Day 6, we collected the cultured cells and in some cases purified CD11c+ cells by MACS.
For MACS sorting, GM-CSF-cultured cells were blocked with 2.4G2 in MACS buffer (1% FBS/15% Cell Dissociation Buffer/PBS) and then stained with anti-CD11c microbeads (N418; Miltenyi Biotech). After washing, the prepared cells were sorted according to the manufacturer’s protocol. The purity of CD11c positive cells was more than 95% for all genotypes.
Flow cytometry
CD11c+ BMDCs were suspended in FACS buffer (1% FBS/0.05% Sodium Azide/PBS), FcR blocked with 2.4G2 for 15 min, then incubated with Abs as indicated in text. After 30 min incubation on ice, cells were washed with FACS buffer, and analyzed on a FACSCalibur (BD Bioscience) and FlowJo software (TreeStar). For intracellular cytokine staining, we added Golgiplug (BD Bioscience) for the last 2 h of culture. Cultured cells were fixed and permeabilized using BD Cytofix/Cytoperm Fixation/Permeabilization Kit (BD Bioscience) according to the manufacturer’s protocol. For TREM-2-Fc staining, we incubated the Fc protein and PE-labeled anti-human IgG Fc Ab for 20 min at room temperature, then the Fc/anti-Fc Ab complex was used for immunostaining of BMDCs as described previously [15].
ELISA
BMDCs were plated in 96 well plate (5 × 104 cells/well) for at least 2 h in DC media, then cultured in the presence of TLR agonists at doses indicated for 16 h, after which culture supernatants were collected. Cytokine concentrations in the culture supernatants were determined using mouse IL-12 p70, TNF, IL-6 and IL-10 ELISA kits (eBioscience) and VeriKine Mouse IFN-β ELISA kit (PBL interferon source) according to the manufacturer’s protocol. The OD450/570 was measured using a VERSAmax microplate reader and Softmax Pro software (Molecular Devices).
Quantitative RT-PCR (qRT-PCR)
Total RNA prepared by using RNeasy plus mini kit (QIAGEN) was reverse-transcribed with Superscript III Reverse Transcriptase (Invitrogen) using oligo dT primer according to the manufacturer’s protocol. Quantitative PCR was performed using the Power SYBR Green PCR Master Mix (Applied Biosystems) and 7900HT (Applied Biosystems) according to the manufacturer’s protocol. The sequences of IFN-α4, IFN-β, IL-12 p40 and IRF7 primers were as described previously [23, 47–49]. HPRT was used as an internal control (Hprt-F: 5′-TGA AGA GCT ACT GTA ATG ATC AGT CAA C-3′; Hprt-AS: 5′-AGC AAG CTT GCA ACC TTA ACC A-3′).
Antigen-specific T cell proliferation assay
OVA-specific T cell response induced by BMDCs was determined by CFSE dilution. Briefly, WT and TREM-2-deficient BMDCs were isolated by MACS after 6 days of culture and plated at 1×104 cells per well of a round bottom 96 well plate with OVA323–339 (2 or 0.5 μg/ml) and CpG DNA (100 or 25 nM) in the presence of GM-CSF (10 ng/ml) for 4 h. CD4+ T cells from spleen and lymph node of OT-II transgenic mice were isolated by using Dynal Mouse CD4 Negative Isolation Kit (Invitrogen) and stained with CFSE (final 0.8μM). After 4 h of DC culture, 1×105 CFSE-labeled CD4+ OT-II T cells were added into each well and incubated for 72 h. After culture, cells were stained with anti-CD4 mAb and we performed flow cytometry to detect CFSE dilution of gated CD4+ OT-II T cells. Data analysis to calculate the percentage of divided and division index was performed by Flowjo software (Treestar).
Statistics
Significant differences of each genotype of DCs in comparison with WT DCs were determined by using Prism 5 software (Graphpad). Specific statistical tests for each figure are indicated in the figure legends.
Supplementary Material
Acknowledgments
We thank Dr. Marco Colonna for providing TREM-2-deficient mice, Dr. Takashi Saito for providing the FcRγ-deficient mice, J.P. Houchins for providing TREM-1-Fc and TREM-2-Fc reagents, Dr. Dan Campbell for providing OVA peptide and Dr. Estelle Bettelli for providing OT-II mice. We also thank Dr. Dan Campbell and members of our laboratory for helpful discussions and review of the manuscript. H. Ito is supported by an Irvington Institute Fellowship Program of the Cancer Research Institute. J. A. Hamerman is supported by a Cancer Research Institute Investigator Award and NIH AI073441 and AI081948.
Abbreviations
- TREM-2
triggering receptor expressed on myeloid cell-2
- DAP12
DNAX activating protein 12
- FcRγ
Fc receptor gamma chain
- ITAM
immunoreceptor tyrosine-based activation motif
- BMDC
bone marrow-derived dendritic cell
Footnotes
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 3.Steinman R, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, Iyoda T, Ravetch J, Dhodapkar M, Inaba K, Nussenzweig M. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 4.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 5.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochemical and Biophysical Research Communications. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 8.Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nature Reviews Immunology. 2006;6:283–294. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- 9.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanier LL. DAP10- and DAP12-associated receptors in innate immunity. Immunol Rev. 2009;227:150–160. doi: 10.1111/j.1600-065X.2008.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nimmerjahn F, Ravetch JV. Fc|[gamma]| receptors as regulators of immune responses. Nature Reviews Immunology. 2008;8:34. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 12.Chu C, Yu Y, Shen K, Lowell C, Lanier L, Hamerman J. Increased TLR responses in dendritic cells lacking the ITAM-containing adapters DAP12 and FcRgamma. Eur J Immunol. 2008;38:166–173. doi: 10.1002/eji.200737600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamerman J, Lanier L. Inhibition of Immune Responses by ITAM-Bearing Receptors. Science Signaling. 2006:re1. doi: 10.1126/stke.3202006re1. [DOI] [PubMed] [Google Scholar]
- 14.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nature immunology. 2005;6:579–586. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamerman J, Jarjoura J, Humphrey M, Nakamura M, Seaman W, Lanier L. Cutting Edge: Inhibition of TLR and FcR Responses in Macrophages by Triggering Receptor Expressed on Myeloid Cells (TREM)-2 and DAP12. The Journal of Immunology. 2006;177:2051. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- 16.Turnbull I, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, Hernandez M, Colonna M. Cutting Edge: TREM-2 Attenuates Macrophage Activation. The Journal of Immunology. 2006;177:3520. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 17.Terme M, Tomasello E, Maruyama K, Crépineau F, Chaput N, Flament C, Marolleau JP, Angevin E, Wagner EF, Salomon B, Lemonnier FA, Wakasugi H, Colonna M, Vivier E, Zitvogel L. IL-4 confers NK stimulatory capacity to murine dendritic cells: a signaling pathway involving KARAP/DAP12-triggering receptor expressed on myeloid cell 2 molecules. Journal of immunology. 2004;172:5957–5966. doi: 10.4049/jimmunol.172.10.5957. [DOI] [PubMed] [Google Scholar]
- 18.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown GD, Gordon S. Immune recognition: A new receptor for |[beta]|-glucans. Nature. 2001;413:36. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 20.Blasius A, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blasius A, Vermi W, Krug A, Facchetti F, Cella M, Colonna M. A cell-surface molecule selectively expressed on murine natural interferon-producing cells that blocks secretion of interferon-alpha. Blood. 2004;103:4201. doi: 10.1182/blood-2003-09-3108. [DOI] [PubMed] [Google Scholar]
- 22.Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 24.Hemmi H, Idoyaga J, Suda K, Suda N, Kennedy K, Noda M, Aderem A, Steinman RM. A new triggering receptor expressed on myeloid cells (Trem) family member, Trem-like 4, binds to dead cells and is a DNAX activation protein 12-linked marker for subsets of mouse macrophages and dendritic cells. J Immunol. 2009;182:1278–1286. doi: 10.4049/jimmunol.182.3.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, Seaman WE. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem. 2009;109:1144–1156. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daws M, Sullam P, Niemi E, Chen T, Tchao N, Seaman W. Pattern Recognition by TREM-2: Binding of Anionic Ligands. The Journal of Immunology. 2003;171:594. doi: 10.4049/jimmunol.171.2.594. [DOI] [PubMed] [Google Scholar]
- 27.N’Diaye EN, Branda CS, Branda SS, Nevarez L, Colonna M, Lowell C, Hamerman JA, Seaman WE. TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria. J Cell Biol. 2009;184:215–223. doi: 10.1083/jcb.200808080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quan DN, Cooper MD, Potter JL, Roberts MH, Cheng H, Jarvis GA. TREM-2 Binds to Lipooligosaccharides of Neisseria gonorrhoeae and is Expressed on Reproductive Tract Epithelial Cells. Mucosal immunology. 2008;1:229. doi: 10.1038/mi.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamerman JA, Ni M, Killebrew JR, Chu CL, Lowell CA. The expanding roles of ITAM adapters FcRgamma and DAP12 in myeloid cells. Immunol Rev. 2009;232:42–58. doi: 10.1111/j.1600-065X.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turnbull IR, Colonna M. Activating and inhibitory functions of DAP12. Nature Reviews Immunology. 2007;7:155–161. doi: 10.1038/nri2014. [DOI] [PubMed] [Google Scholar]
- 31.Cao W, Bover L, Cho M, Wen X, Hanabuchi S, Bao M, Rosen DB, Wang YH, Shaw JL, Du Q, Li C, Arai N, Yao Z, Lanier LL, Liu YJ. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. The Journal of Experimental Medicine. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G, Yao Z, Zhang L, Lanier LL, Liu YJ. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. The Journal of Experimental Medicine. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao W, Zhang L, Rosen DB, Bover L, Watanabe G, Bao M, Lanier LL, Liu YJ. BDCA2/Fc epsilon RI gamma complex signals through a novel BCR-like pathway in human plasmacytoid dendritic cells. PLoS Biol. 2007;5:e248. doi: 10.1371/journal.pbio.0050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakker AB, Hoek RM, Cerwenka A, Blom B, Lucian L, McNeil T, Murray R, Phillips LH, Sedgwick JD, Lanier LL. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity. 2000;13:345–353. doi: 10.1016/s1074-7613(00)00034-0. [DOI] [PubMed] [Google Scholar]
- 35.Peng Q, Malhotra S, Torchia J, Kerr W, Coggeshall K, Humphrey M. TREM2- and DAP12-Dependent Activation of PI3K Requires DAP10 and Is Inhibited by SHIP1. Science Signaling. 2010;3:ra38. doi: 10.1126/scisignal.2000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paloneva J, Kestilä M, Wu J, Salminen A, Böhling T, Ruotsalainen V, Hakola P, Bakker AB, Phillips JH, Pekkarinen P, Lanier LL, Timonen T, Peltonen L. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet. 2000;25:357–361. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- 37.Kaifu T, Nakahara J, Inui M, Mishima K, Momiyama T, Kaji M, Sugahara A, Koito H, Ujike-Asai A, Nakamura A, Kanazawa K, Tan-Takeuchi K, Iwasaki K, Yokoyama WM, Kudo A, Fujiwara M, Asou H, Takai T. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J Clin Invest. 2003;111:323–332. doi: 10.1172/JCI16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cella M, Buonsanti C, Strader C, Kondo T, Salmaggi A, Colonna M. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. The Journal of Experimental Medicine. 2003;198:645–651. doi: 10.1084/jem.20022220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paloneva J, Mandelin J, Kiialainen A, Bohling T, Prudlo J, Hakola P, Haltia M, Konttinen YT, Peltonen L. DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. The Journal of Experimental Medicine. 2003;198:669–675. doi: 10.1084/jem.20030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, Mizui M, Yamamoto M, Prasad DVR, Suzuki K, Ishii M, Terai K, Moriya M, Nakatsuji Y, Sakoda S, Sato S, Akira S, Takeda K, Inui M, Takai T, Ikawa M, Okabe M, Kumanogoh A, Kikutani H. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol. 2006;8:615–622. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
- 41.Bouchon A, Hernández-Munain C, Cella M, Colonna M. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J Exp Med. 2001;194:1111–1122. doi: 10.1084/jem.194.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi K, Rochford CDP, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. The Journal of Experimental Medicine. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenzweig HL, Clowers JS, Nunez G, Rosenbaum JT, Davey MP. Dectin-1 and NOD2 mediate cathepsin activation in zymosan-induced arthritis in mice. Inflamm Res. 2011;60:705–714. doi: 10.1007/s00011-011-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, Koh H, Rodriguez A, Idoyaga J, Pack M, Velinzon K, Park CG, Steinman RM. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park SY, Ueda S, Ohno H, Hamano Y, Tanaka M, Shiratori T, Yamazaki T, Arase H, Arase N, Karasawa A, Sato S, Ledermann B, Kondo Y, Okumura K, Ra C, Saito T. Resistance of Fc receptor- deficient mice to fatal glomerulonephritis. J Clin Invest. 1998;102:1229–1238. doi: 10.1172/JCI3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Humphrey MB, Daws MR, Spusta SC, Niemi EC, Torchia JA, Lanier LL, Seaman WE, Nakamura MC. TREM2, a DAP12-associated receptor, regulates osteoclast differentiation and function. J Bone Miner Res. 2006;21:237–245. doi: 10.1359/JBMR.051016. [DOI] [PubMed] [Google Scholar]
- 47.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 48.Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, Kano S-i, Honda K, Ohba Y, Mak TW, Taniguchi T. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 49.Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, Savitsky D, Ronfani L, Akira S, Bianchi ME, Honda K, Tamura T, Kodama T, Taniguchi T. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.