Abstract
The incidence of Campylobacter jejuni has increased during the last decade, and today it is the leading cause of bacterial enteritis in most developed countries. Still, there is a lack of knowledge about infection routes and to what extent identified sources are responsible for spreading the bacterium to humans. The major objective of this work was to explore the genetic similarity between C. jejuni isolated from different sources. C. jejuni isolated from patients (n = 95), five types of meat (n = 71), and raw water (n = 11) during the year 2000 were subtyped by pulsed-field gel electrophoresis (PFGE). The pulsotypes obtained after digestion with SmaI revealed not only that C. jejuni is genetically diverse but also that specific pulsotypes occur frequently. Five clusters comprising 88 of the 162 SmaI-digested isolates were obtained. After digestion with KpnI most isolates in four of the five clusters were still indistinguishable, while the fifth cluster was strongly dissolved. The clusters comprised high frequencies of human and meat isolates, while only one of nine water isolates belonged to a cluster. The largest cluster comprised 21 human isolates, one raw water isolate, and seven chicken meat isolates, originating from at least six different broiler flocks. Low frequencies of antibiotic resistance were revealed when the meat and water isolates were tested for sensitivity to six antibiotics. Interestingly, the five isolates resistant to quinolones displayed similar or identical pulsotypes. The results showed that PFGE has proved useful in identifying clones and will be used in future work focusing on identification and eradication of the major reservoirs for common clones.
Campylobacter jejuni has for several years been the major cause of bacterial gastroenteritis in Sweden as well as in most developed countries. The natural reservoir for Campylobacter is the intestinal tract of warm-blooded animals. Knowledge about transmission routes is limited partly because most cases occur sporadically. Contaminated food and water are believed to be the major sources of infection, while spreading between persons occurs sparsely. Poultry, but also unpasteurized milk, water, and contact with pets and cattle are usually ranked high as risk factors in case-control studies (1, 12, 23, 26).
For studying the epidemiology of Campylobacter infections, several genetic typing methods have been developed in order to differentiate isolates below species level (29). Macrorestriction profiling (MRP) by pulsed-field gel electrophoresis (PFGE) has proved useful for this purpose, and its discriminatory power can be enhanced by increasing the number of restriction enzymes used (18, 19).
C. jejuni is considered a genetically diverse species, but nevertheless a limited number of widespread and persistent clones have been identified (11, 14-16, 20). Identical genotypes are commonly present in flocks raised simultaneously on a farm, and clones have also been shown to be able to persist during successive broiler flock rotations (16, 20). As a consequence of cross-contamination during slaughter, poultry meat may be contaminated by genotypes not previously present in the flock (17). It has been shown that coinfection with different C. jejuni genotypes can occur in humans and that the PFGE pattern can change during an episode of infection (22, 25), although both occurrences are low in frequency. There are also indications that C. jejuni can undergo intensive recombination leading to different PFGE patterns. However, this phenomenon seems to occur at low frequencies and under certain unknown conditions (3, 28).
Most human infections with C. jejuni are self-limiting and do not require antimicrobial chemotherapy. However, treatment with erythromycin or a fluoroquinolone is needed in individuals with invasive or very severe disease. Also, immunocompromised patients, the very young, and the very old may be treated. The prevalence of quinolone- or macrolide-resistant Campylobacter is increasing worldwide (8). Resistance may arise during treatment of humans, but it is also believed that the use of these antibiotics in the veterinary field contributes to increased resistance among strains infecting humans. The predominant mechanism for high-level quinolone resistance in C. jejuni appears to be a C→T transition in codon 86 in the quinolone resistance-determining region (QRDR) of gyrA, which encodes a subunit of DNA gyrase (24, 27).
The objectives of the present study were to (i) evaluate if some specific genotypes of C. jejuni are common in Swedish meat and raw water and if these genotypes also are present in patients infected in Sweden and (ii) investigate the frequency of antibiotic resistance in the meat and water isolates and determine if resistance is linked to certain genotypes.
MATERIALS AND METHODS
Isolates. The C. jejuni isolates from water (n = 11) and meat (n = 71) originated from a prevalence study managed by the National Food Administration in the year 2000. The meat isolates originated from chicken (n = 61), turkey (n = 4), pork (n = 3), lamb (n = 2), and duck (n = 1). Ten of the meat isolates originated from imported meat. Meat samples were taken at restaurants and at retail establishments from all over Sweden and were analyzed essentially as described by the Nordic Committee on Food Analysis (18a). Briefly, the whole sample was rinsed with 100 ml of peptone-water. Twenty-five milliliters was transferred to 225 ml of Preston broth, and after microaerophilic incubation at 42°C for 24 h, a 10-μl volume was spread on Campylobacter blood-free selective medium, containing cefoperazone (32 mg/liter) and amphotericin (10 mg/liter). The plates were incubated microaerobically at 42°C for 48 h. After initial bacterial characterization, suspected colonies were confirmed as thermophilic campylobacter using a PCR method based on the 23S rRNA gene (9). For classification to C. jejuni a PCR method based on the hippuricase gene was applied (13). The raw water isolates originated from samples taken from raw (incoming) water at surface water treatment plants. Four of the raw water isolates originated from samples taken on different occasions in June, September, and November from one water plant. The remaining seven isolates originated from different water plants. The eight water plants were geographically scattered in southern Sweden.
Ninety-five human isolates, obtained from epidemiologically unrelated domestic patients in 2000, were included in the study. For collection of the human isolates, fecal samples were analyzed at hospital laboratories in Gävle and Stockholm, Sweden. Fecal swab samples were cultured on plates with Campylobacter blood-free selective medium as described for the meat samples. The 71 isolates from Gävle were collected from 19 January to 7 December, and the 24 isolates from Stockholm were sampled between 9 October and 28 November. Species identification of C. jejuni was initially based on a positive hippurate reaction. Isolates with a negative or intermediate hippurate reaction were identified to the species level by PCR combined with restriction enzyme (AluI and TspI) analysis (9).
MRP analyses.
DNA preparation was in accordance with the Campynet protocol (http://campynet.vetinst.dk). DNA from all isolates was cleaved by the endonuclease SmaI (Amersham Biosciences, Uppsala, Sweden). The running conditions for SmaI-digested DNA were as described in the Campynet protocol. DNA cleaved with KpnI was separated in 1% pulsed-field gel-certified agarose (Pharmacia Biotech, Uppsala, Sweden) gels in 0.5× Tris-borate-EDTA buffer using a CHEF DRII/III apparatus (Bio-Rad), with pulse times of 1 to 20 s for 19 h and 20 to 25 s for 3 h at 6 V/cm, a 120°C angle, and a temperature of 14°C. The gels were stained with ethidium bromide, and images were captured under UV light transillumination. The images were analyzed using the computer program GelCompar II (Applied Maths, Kortrijk, Belgium). Band position tolerance and optimization were both set to 1%. The dendrograms were constructed by using the Dice coefficient and the unweighted pair-group method with arithmetic means.
Antibiotic resistance.
Antimicrobial susceptibility testing was performed by a microdilution method (VetMIC, National Veterinary Institute, Uppsala, Sweden), in which antimicrobials were dried in serial twofold dilutions in microtiter wells. Each well was inoculated with 100 μl of Mueller-Hinton broth with an inoculum density of approximately 106 CFU/ml. C. jejuni CCUG 11284 was included as a quality control. The breakpoints were set as follows: ampicillin, >16 μg/ml; erythromycin, >16 μg/ml; nalidixic acid, >16 μg/ml; enrofloxacin, >1 μg/ml; oxytetracycline, >8 μg/ml; and gentamicin, >8 μg/ml. These are the breakpoints applied by the Swedish Veterinary Antimicrobial Resistance Monitoring program (http://www.sva.se/pdf/svarm2002.pdf).
Determination of mutations in the QRDR of the gyrA gene.
A 423-bp fragment covering the QRDR of gyrA was amplified by PCR, using the primers 5′-GATGGTTTAAAGCCTGTTCAT (forward) and 5′-CGCCATACCTACAGCTATACC (reverse) and chromosomal DNA from selected Campylobacter isolates from meat. The single band obtained after agarose gel electrophoresis from each reaction mixture was purified using a QIAEX II gel extraction kit (Qiagen, Hilden, Germany). Sequencing of the obtained fragments was performed using the forward primer, a BigDye Terminator Cycle Sequencing Ready Reaction kit, and an ABI PRISM 377 DNA sequencer (Applied Biosystems).
RESULTS
MRP on food, raw water, and human isolates.
To get an idea of how frequently a chicken carcass can carry more than one genetically distinguishable isolate, we initially conducted MRP on two to five isolates from the same fresh chicken sample. A total of 33 isolates from 10 samples were tested, and in all cases, isolates originating from the same sample displayed an identical pulsotype (data not shown). In the following, only one isolate per sample was analyzed.
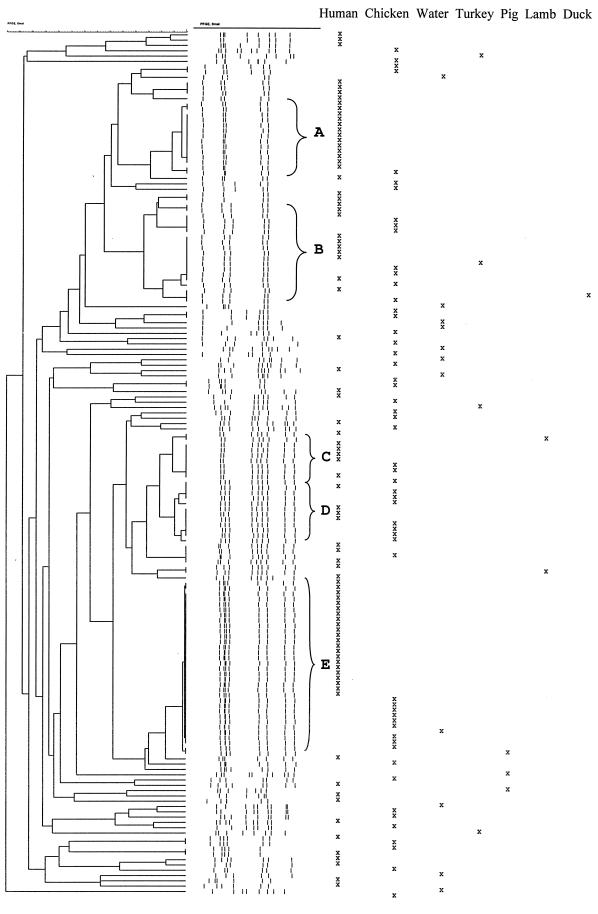
All meat (n = 71), raw water (n = 11), and human (n = 95) isolates were subjected to MRP using the restriction enzyme SmaI (Fig. 1). Fifty-three isolates displayed unique pulsotypes, and eight pulsotypes were shared by two isolates. Two pulsotypes comprised three and four isolates, respectively. The remaining 86 isolates belonged to one of five clusters, designated A, B, C, D, and E (Fig. 1). The isolates within the clusters A to E were digested with KpnI and subjected to a second round of PFGE in order to investigate the diversity within the groups (Fig. 2). Despite several attempts, six isolates from chicken and nine human isolates were refractory to digestion with SmaI.
FIG. 1.
Dendrogram based on SmaI-digested DNA from C. jejuni isolated from meats, water, and humans. Clusters A to E are grouped by braces.
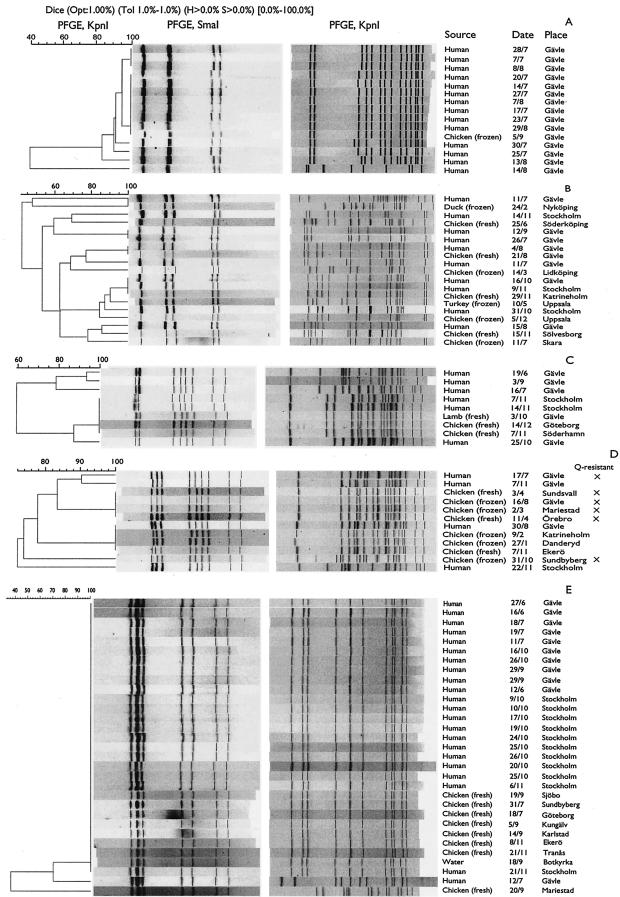
FIG. 2.
(A to E) Dendrogram based on KpnI-digested DNA from the isolates in clusters A to E, respectively, shown in Fig. 1. The crosses in panel D indicate isolates resistant to both nalidixic acid and enrofloxacin (Q-resistant).
Group A.
Besides an isolate from a frozen chicken, the 15 isolates within group A were from humans and were isolated in July or August. The group comprised six unique KpnI pulsotypes, of which two pulsotypes were identical for three and eight isolates, respectively (Fig. 2A). Five of the six KpnI pulsotypes were highly similar, indicating that only one isolate was distantly related.
Group B.
Group B, consisting of 19 isolates, was strongly dissolved after digestion with KpnI, and only three subgroups, each comprising two or three isolates, remained indistinguishable (Fig. 2B).
Group C.
Five isolates (two human, two chicken, and one lamb) in group C, which consisted of nine isolates, were still indistinguishable from each other after KpnI digestion, while the remaining four isolates each displayed a unique pulsotype (Fig. 2C). The time between slaughter of the two chickens carrying indistinguishable isolates was more than 1 month.
Group D.
Restriction with KpnI split up group D, which consisted of 12 isolates, into two subgroups with four identical isolates in each (Fig. 2D). One subgroup comprised four chicken isolates, and the other one comprised three chicken and one human isolate. The remaining four isolates all displayed unique pulsotypes. The four chicken isolates from one of the subgroups originated from chickens that had been slaughtered at an interval of at least 9 months.
Group E.
Twenty-nine of the 31 isolates in group E were still indistinguishable from each other after digestion with KpnI (Fig. 2E). Almost 25% of the digested human isolates belonged to this group. Thirteen of the 21 indistinguishable human isolates were isolated from October to November, but no known relationships between the cases existed according to case histories. The slaughter interval between the first and last chicken with indistinguishable isolates was 4 months, and the seven chicken isolates were from at least six different slaughter groups. All eight meat isolates in this group were from fresh chicken, although 38% of the chicken isolates in the study were from frozen chicken.
Antibiotic resistance.
The 71 meat and 11 water isolates were analyzed for susceptibility to six antibiotics (Table 1). Despite several attempts, two isolates from chicken and two isolates from water did not grow in the microtiter wells, including the wells without antibiotics. Thus, MICs could not be recorded for these four isolates. None of the tested isolates was resistant to gentamicin or erythromycin. Two isolates were resistant to oxytetracycline, and four isolates were resistant to ampicillin. Five isolates were resistant to both nalidixic acid and enrofloxacin, including an isolate which, in addition, was resistant to ampicillin and oxytetracycline. The five quinolone-resistant isolates all belonged to cluster D, and after digestion with KpnI only the multiresistant isolate displayed a separate pulsotype (Fig. 2D). The four indistinguishable resistant isolates were all from Swedish chicken while the multiresistant isolate originated from a Dutch chicken. Testing the four human isolates belonging to cluster D revealed that one isolate had an antimicrobial susceptibility pattern identical to the multiresistant isolate while the other three isolates were all susceptible to the antibiotics tested (Fig. 2D).
TABLE 1.
MICs for C. jejuni isolates from meat (n = 69) and raw water (n = 9)
| Substance | Origin of isolate | No. of isolates for which MIC (mg/liter) wasa:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | >128 | ||
| Nalidixic acid | Meat | — | 3 | 44 | 14 | 3 | — | — | — | 5 | |||||
| Water | — | 1 | 7 | 1 | — | — | — | — | |||||||
| Enrofloxacin | Meat | — | 4 | 51 | 9 | — | — | — | — | 5 | |||||
| Water | — | 2 | 7 | — | — | — | — | — | |||||||
| Ampicillin | Meat | 6 | 5 | 9 | 33 | 9 | 3 | 1 | 2 | 1 | |||||
| Water | — | — | 2 | 7 | — | — | — | — | |||||||
| Oxytetracycline | Meat | 47 | 15 | 2 | 1 | — | 3 | — | — | 1 | |||||
| Water | 4 | 2 | 2 | — | — | — | — | 1 | |||||||
| Erythromycin | Meat | — | 6 | 26 | 23 | 13 | 1 | — | — | ||||||
| Water | 1 | 1 | 3 | 2 | 2 | — | — | — | |||||||
| Gentamicin | Meat | — | 13 | 51 | 5 | — | — | ||||||||
| Water | — | 6 | 3 | — | — | — | |||||||||
Data in lightface type are for the range of concentrations tested for each substance. Data for MICs above the range (shown in boldface type) are given under the concentration closest to the range, and data for MICs equal to or less than the lowest concentration tested are given under the lowest tested concentration. —, no isolates. Breakpoints for resistance were as follows: for nalidixic acid, ampicillin, and erythromycin, >16 mg/liter; for oxytetracycline and gentamicin, >8 mg/liter; for enrofloxacin, >1 mg/liter.
Sequence analysis of the QRDR of the gyrA gene.
The QRDRs from 10 food and water isolates were amplified and sequenced. The five quinolone-resistant isolates displayed a transition from C to T in codon 86, resulting in a threonine-to-isoleucine substitution in the functional protein. No other mutation was observed in any of the isolates at codons 70, 86, and 90 of gyrA.
DISCUSSION
Usually, only one or two genotypes are identified in cloacal samples from broiler flocks (2, 5, 16). However, recently Dickins et al. (6) showed that 67% of Campylobacter-positive retail chicken from the south-central United States was contaminated with more than one strain distinguishable by PFGE. Another study recognized 14 different but closely related pulsotypes from 30 packages of Dutch poultry meat collected in numerical order directly after packaging (28). A third study in the United Kingdom observed that infected flocks usually harbor three or fewer subtypes, but in one case, 10 isolates obtained from a single carcass displayed six different PFGE patterns (17). This study also found genotypes on carcasses at different steps in the slaughter process that were not present in the flock. Even the crates used to transport the birds harbored several genotypes, although a Campylobacter-free flock was transported. Our results, although limited, indicate that contaminated Swedish retail chicken does not usually carry more than one isolate distinguishable by PFGE.
The 88 isolates belonging to one of the five clusters (cluster A through E) after digestion with SmaI were further analyzed with a second restriction enzyme. Cluster B, comprising 19 isolates, displayed 15 different MRPs after digestion with KpnI, while most isolates in the other clusters were still indistinguishable after digestion with this enzyme. This clearly underlines the recommendation by On et al. (19) that a second enzyme should be used before relatedness between isolates is considered.
In accordance with earlier studies, our results show that C. jejuni from meat, raw water, and humans are genetically diverse but that some common clones are widely spread. Interestingly, only 1 of the 11 water isolates but 29 of the 65 (45%) meat isolates and 56 of the 86 (65%) human isolates were placed in any of the five clusters. Recently, a Swedish study compared MRPs from isolates originating from humans, broilers, and black-headed gulls (Larus ribibundus) (4). The dendrogram presented contained clusters with identical or very similar MRPs. The human and broiler isolates were fairly evenly distributed between the clusters, whereas the largest cluster comprised 43 of the 76 gull isolates and contained only one isolate from another source. A Danish study found a statistically significant difference in serotype distribution of isolates from wild mammals and birds compared to human and chicken isolates (20). By using multilocus sequence typing, Dingle et al. (7) divided 748 of 814 C. jejuni from patients, food animals, food, and sand beaches into 17 clonal complexes. Also, in this case there was a nonrandom source distribution among the complexes; for example, a majority of the isolates from sand beaches were clustered with themselves into two complexes, and in two other complexes beef isolates were overrepresented. In contrast, most clusters displayed a similar proportion of human and chicken isolates, and five clusters contained only isolates from these two sources. Taken together, and with the assumption that isolates from raw water plants and sand beaches usually originate from wildlife, this suggests that human isolates are generally more related to chicken isolates than to wildlife isolates.
The 21 human isolates in cluster E that were indistinguishable from each other also after KpnI digestion were isolated in the period from June to November. The cluster also harbored seven isolates from fresh chickens slaughtered in July, September, and November. An interesting question is whether broilers were the main source of transmission to humans or if there was a common source responsible for spreading Campylobacter to both broilers and humans. The chicken isolates originated from at least six different slaughter groups. Whether this clone is so common in the environment that it repeatedly infects broiler flocks or whether it has the potential to persist on farms or in slaughterhouses needs to be investigated. Three of the seven chicken samples that carried indistinguishable isolates came from abattoir A. For another three of the chicken samples the abattoir could not be identified. The seventh chicken isolate in this group came from abattoir B, located close to abattoir A. Around 10% of the chickens produced in Sweden are slaughtered in abattoir A. Thus, it is unlikely that at least three of the seven indistinguishable isolates would by chance originate from chickens slaughtered in this abattoir. Occasionally, it happens that producers normally delivering to abattoir A also deliver to abattoir B, and thus it is possible that all chickens carrying this subtype were produced at the same farm. A Danish study recently showed that C. jejuni can persist during successive broiler flock rotations (21). Identical or highly similar MRPs were found in at least four broiler flock rotations in 7 of 12 broiler houses at 10 breeders. In the present study, it is conceivable that the indistinguishable chicken isolates persisted in the environment at a breeder and there infected several broiler flocks. In such a way, a commonly isolated clone could in fact originate from a single breeder. Since large abattoirs distribute chickens to retailers all over Sweden, a certain clone can be widely spread and possibly infect humans all over the country.
Surface water is another possible source of infection. Eleven of the 23 digested human isolates from Stockholm belonged to cluster E. These isolates were all collected between 9 October and 21 November, and during this time only five isolates with another pulsotype were collected. The hospital and the water plant from which the raw water isolate in cluster E originate are both located in Stockholm. Whether these human patients contracted the infection after eating contaminated chicken or were part of a waterborne outbreak that passed unnoticed was not further investigated. However, the patients lived in different parts of Stockholm and received water from at least four different water plants.
The results show that Campylobacter isolates from meat and humans in Sweden are genetically diverse but that some widely spread clones exist. One or several isolates from all represented sources displayed a pulsotype identical to a human isolate, although the number of isolates originating from pork, turkey, duck, and lamb were few. Thus, independent of the source, isolates of C. jejuni should be regarded as potentially pathogenic. Furthermore, this study demonstrates the value of subtyping in order to detect clusters of certain clones of C. jejuni. The clusters might be responsible for smaller outbreaks that are not detected by mandatory notification of human cases or routine epidemiological investigations.
Antimicrobial resistance was found in our study at relatively low frequencies, with resistance to quinolones being the most prevalent. A mutation in codon 86 of the gyrA gene has been reported to be the major cause of high-level resistance to quinolones. Also, mutations at codons 70 and 90 result in nalidixic acid resistance but have less effect on fluoroquinolones (27). The five quinolone-resistant isolates all had the predicted mutation in codon 86, while no mutation was detected in codon 70 or 90. The low level of resistance among the meat isolates is consistent with the level found in C. jejuni from chicken at slaughter in Sweden. No general differences in sensitivity were seen between the water and meat isolates. In a study in the United States, C. jejuni isolates from retail meat were recently shown to be resistant to several antibiotics. Of 88 isolates 81, 42, and 32% were resistant to tetracycline, erythromycin, and nalidixic acid, respectively (10). Antibiotics put a selective pressure on the bacteria, resulting in increased resistance. Increased resistance is due to origin and transmission of resistance genes and/or propagation and spreading of resistant clones. The five quinolone-resistant isolates in the present study were all from cluster D, and four of these five isolates displayed an identical pulsotype also after KpnI digestion. This may indicate that propagation of resistant clones plays a role in the increase of resistant Campylobacter strains, which has been noted in many countries (8).
Acknowledgments
The laboratory staff at the Department of Bacteriology, National Veterinary Institute, Uppsala, is thanked for help with the human isolates.
This study was partly financed by the Swedish Board of Agriculture.
REFERENCES
- 1.Adak, G. K., J. M. Cowden, S. Nicholas, and H. S. Evans. 1995. The Public Health Laboratory Service national case-control study of primary indigenous sporadic cases of campylobacter infection. Epidemiol. Infect. 115:15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayling, R. D., M. J. Woodward, S. Evans, and D. G. Newell. 1996. Restriction fragment length polymorphism of polymerase chain reaction products applied to the differentiation of poultry campylobacters for epidemiological investigations. Res. Vet. Sci. 60:168-172. [DOI] [PubMed] [Google Scholar]
- 3.Boer, P., J. A. Wagenaar, R. P. Achterberg, J. P. Putten, L. M. Schouls, and B. Duim. 2002. Generation of Campylobacter jejuni genetic diversity in vivo. Mol. Microbiol. 44:351-359. [DOI] [PubMed] [Google Scholar]
- 4.Broman, T., H. Palmgren, S. Bergstrom, M. Sellin, J. Waldenstrom, M. L. Danielsson-Tham, and B. Olsen. 2002. Campylobacter jejuni in black-headed gulls (Larus ridibundus): prevalence, genotypes, and influence on C. jejuni epidemiology. J. Clin. Microbiol. 40:4594-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuma, T., K. Makino, K. Okamoto, and H. Yugi. 1997. Analysis of distribution of Campylobacter jejuni and Campylobacter coli in broilers by using restriction fragment length polymorphism of flagellin gene. J. Vet. Med. Sci. 59:1011-1015. [DOI] [PubMed] [Google Scholar]
- 6.Dickins, M. A., S. Franklin, R. Stefanova, G. E. Schutze, K. D. Eisenach, I. Wesley, and M. D. Cave. 2002. Diversity of Campylobacter isolates from retail poultry carcasses and from humans as demonstrated by pulsed-field gel electrophoresis. J. Food Prot. 65:957-962. [DOI] [PubMed] [Google Scholar]
- 7.Dingle, K. E., F. M. Colles, R. Ure, J. A. Wagenaar, B. Duim, F. J. Bolton, A. J., Fox, D. R. A. Wareing, and M. C. J. Maiden. September 2002, posting date. Molecular characterization of Campylobacter jejuni clones: a basis for epidemiological investigation. Emerg. Infect. Dis. 8:1-73. [Online.] http://www.cdc.gov/ncidod/EID/vol8no9/02-0122.htm. [DOI] [PMC free article] [PubMed]
- 8.Engberg, J., F. M. Aarestrup, D. E. Taylor, P. Gerner-Smidt, and I. Nachamkin. 2001. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 7:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fermer, C., and E. O. Engvall. 1999. Specific PCR identification and differentiation of the thermophilic campylobacters, Campylobacter jejuni, C. coli, C. lari, and C. upsaliensis. J. Clin. Microbiol. 37:3370-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge, B., D. G. White, P. F. McDermott, W. Girard, S. Zhao, S. Hubert, and J. Meng. 2003. Antimicrobial-resistant campylobacter species from retail raw meats. Appl. Environ. Microbiol. 69:3005-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanninen, M. L., P. Perko-Makela, A. Pitkala, and H. Rautelin. 2000. A three-year study of Campylobacter jejuni genotypes in humans with domestically acquired infections and in chicken samples from the Helsinki area. J. Clin. Microbiol. 38:1998-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapperud, G., G. Espeland, E. Wahl, A. Walde, H. Herikstad, S. Gustavsen, I. Tveit, O. Natas, L. Bevanger, and A. Digranes. 2003. Factors associated with increased and decreased risk of Campylobacter infection: a prospective case-control study in Norway. Am. J. Epidemiol. 158:234-242. [DOI] [PubMed] [Google Scholar]
- 13.Linton, D., A. J. Lawson, R. J. Owen, and J. Stanley. 1997. PCR detection, identification to species level, and fingerprinting of Campylobacter jejuni and Campylobacter coli direct from diarrheic samples. J. Clin. Microbiol. 35:2568-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manning, G., B. Duim, T. Wassenaar, J. A. Wagenaar, A. Ridley, and D. G. Newell. 2001. Evidence for a genetically stable strain of Campylobacter jejuni. Appl. Environ. Microbiol. 67:1185-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsui, T., J. E. Moore, C. Patterson, B. C. Millar, and M. Matsuda. 2002. Molecular characterisation of human campylobacteriosis in Northern Ireland: evidence of clonal stability. Ir. J. Med. Sci. 171:33-36. [DOI] [PubMed] [Google Scholar]
- 16.Nadeau, E., S. Messier, and S. Quessy. 2002. Prevalence and comparison of genetic profiles of Campylobacter strains isolated from poultry and sporadic cases of campylobacteriosis in humans. J. Food Prot. 65:73-78. [DOI] [PubMed] [Google Scholar]
- 17.Newell, D. G., J. E. Shreeve, M. Toszeghy, G. Domingue, S. Bull, T. Humphrey, and G. Mead. 2001. Changes in the carriage of Campylobacter strains by poultry carcasses during processing in abattoirs. Appl. Environ. Microbiol. 67:2636-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen, E. M., J. Engberg, V. Fussing, L. Petersen, C. H. Brogren, and S. L. On. 2000. Evaluation of phenotypic and genotypic methods for subtyping Campylobacter jejuni isolates from humans, poultry, and cattle. J. Clin. Microbiol. 38:3800-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Nordic Committee on Food Analysis. 1990. NMKL document 119, 2nd ed. Nordic Committee on Food Analysis, Oslo, Norway. http://www.nmkl.org.
- 19.On, S. L., E. M. Nielsen, J. Engberg, and M. Madsen. 1998. Validity of SmaI-defined genotypes of Campylobacter jejuni examined by SalI, KpnI, and BamHI polymorphisms: evidence of identical clones infecting humans, poultry, and cattle. Epidemiol. Infect. 120:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen, L., E. M. Nielsen, J. Engberg, S. L. On, and H. H. Dietz. 2001. Comparison of genotypes and serotypes of Campylobacter jejuni isolated from Danish wild mammals and birds and from broiler flocks and humans. Appl. Environ. Microbiol. 67:3115-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen, L., and A. Wedderkopp. 2001. Evidence that certain clones of Campylobacter jejuni persist during successive broiler flock rotations. Appl. Environ. Microbiol. 67:2739-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson, J. F., J. A. Frost, J. M. Kramer, R. T. Thwaites, F. J. Bolton, D. R. Wareing, and J. A. Gordon. 2001. Coinfection with Campylobacter species: an epidemiological problem? J. Appl. Microbiol. 91:206-211. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues, L. C., J. M. Cowden, J. G. Wheeler, D. Sethi, P. G. Wall, P. Cumberland, D. S. Tompkins, M. J. Hudson, J. A. Roberts, and P. J. Roderick. 2001. The study of infectious intestinal disease in England: risk factors for cases of infectious intestinal disease with Campylobacter jejuni infection. Epidemiol. Infect. 127:185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz, J., P. Goni, F. Marco, F. Gallardo, B. Mirelis, T. Jimenez De Anta, and J. Vila. 1998. Increased resistance to quinolones in Campylobacter jejuni: a genetic analysis of gyrA gene mutations in quinolone-resistant clinical isolates. Microbiol. Immunol. 42:223-226. [DOI] [PubMed] [Google Scholar]
- 25.Steinbrueckner, B., F. Ruberg, and M. Kist. 2001. Bacterial genetic fingerprint: a reliable factor in the study of the epidemiology of human campylobacter enteritis? J. Clin. Microbiol. 39:4155-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Studahl, A., and Y. Andersson. 2000. Risk factors for indigenous campylobacter infection: a Swedish case-control study. Epidemiol. Infect. 125:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, Y., W. M. Huang, and D. E. Taylor. 1993. Cloning and nucleotide sequence of the Campylobacter jejuni gyrA gene and characterization of quinolone resistance mutations. Antimicrob. Agents Chemother. 37:457-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wassenaar, T. M., B. Geilhausen, and D. G. Newell. 1998. Evidence of genomic instability in Campylobacter jejuni isolated from poultry. Appl. Environ. Microbiol. 64:1816-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wassenaar, T. M., and D. G. Newell. 2000. Genotyping of Campylobacter spp. Appl. Environ. Microbiol. 66:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]