SUMMARY
The interferon-induced host restriction factor tetherin poses a barrier for SIV transmission from primates to humans. Following cross-species transmission, the chimpanzee precursor of pandemic HIV-1 switched from the accessory protein Nef to Vpu to effectively counteract human tetherin. As we report here, the experimental reintroduction of HIV-1 into its original chimpanzee host resulted in a virus that can use both Vpu and Nef to antagonize chimpanzee tetherin. Functional analyses demonstrated that alterations in and near the highly conserved ExxxLL motif in the C-terminal loop of Nef were critical for the reacquisition of anti-tetherin activity. Strikingly, just two amino acid changes allowed HIV-1 Nef to counteract chimpanzee tetherin and promote virus release. Our data demonstrate that primate lentiviruses can reacquire lost accessory gene functions during a single in vivo passage and suggest that other functional constraints keep Nef ready to regain anti-tetherin activity.
INTRODUCTION
As a consequence of retroviral threat over millions of years, humans have evolved antiviral factors that act at different stages of the viral life cycle: TRIM5α proteins, which inactivate incoming viral capsids; cytidine deaminases (e.g. APOBEC3G), which inhibit reverse transcription and introduce hyper-mutations into the viral genome; SAM domain and HD domain-containing protein 1 (SAMHD1), which restricts HIV-1 infection of myeloid cells; and tetherin (also known as CD317 or BST2), which suppresses virion release from infected cells (reviewed in Malim and Emerman, 2008; Kirchhoff, 2010; Laguette and Benkirane, 2012). These antiviral factors are under positive selection pressure for diversification because they are engaged in an “arms race” with pathogens which themselves are adapting and evading their activity (Meyerson and Sawyer, 2011). As a consequence they act in a species-specific manner and provide protection against cross-species transmissions. However, these protective barriers can fail, as exemplified by the AIDS pandemic. One reason for this failure is that “modern” primate lentiviruses, i.e. SIV and HIV-1, have evolved effective countermeasures against the restriction factors of their respective hosts. These viral factors, which include Vif, Vpr, Vpu, Vpx and Nef, are not essential for replication in some cell lines but required for efficient viral replication and spread in vivo.
Recent studies provided surprising details into the zoonotic origins of HIV-1 groups M, N, O and P and the adaptive changes that followed during their replication in humans. It is now established that SIVcpz, the direct precursor of pandemic HIV-1, represents a recombinant of precursors of viruses nowadays found in red-capped mangabeys and Cercopithecus monkeys (Bailes et al., 2003). This chimeric virus received a vpu gene from the precursor of SIVgsn/mus/mon and a nef gene from the progenitor of SIVrcm and was thus equipped with two potential tetherin antagonists (Sauter et al., 2009). During adaptation in chimpanzees, Nef but not Vpu evolved to counteract tetherin. Nef was maintained as the tetherin antagonist after transmission of SIVcpz to gorillas, most likely because chimpanzee and gorilla tetherins are highly similar. However, this was not the case after zoonotic transmissions of SIVcpz and SIVgor to humans because the human tetherin orthologue contains a deletion that renders it resistant to Nef (Jia et al., 2009; Lim et al., 2010; Sauter et al., 2009; Zhang et al., 2009). The precursor of pandemic HIV-1 group M cleared this hurdle by switching from Nef to Vpu to counteract tetherin in the new human host (Sauter et al., 2009). In contrast, the Vpu and Nef proteins of non-pandemic HIV-1 group O and P strains remained poor tetherin antagonists and group N Vpus gained some anti-tetherin activity but lost their capacity to degrade CD4 (Sauter et al., 2009; Sauter et al., 2011; Yang et al., 2011). Thus, tetherin poses a barrier for SIV transmission from primates to humans and only HIV-1 group M has fully cleared this hurdle.
Based on the above, it seems clear that switches between Nef and Vpu mediated tetherin antagonism preceded the emergence of pandemic HIV-1 and that the reacquisition of a once lost Vpu function may have been a prerequisite for the effective spread of the AIDS pandemic. Currently, it is unknown how rapidly accessory primate lentiviral proteins can regain lost activities against host restriction factors. To address this, we examined how HIV-1 evolved to antagonize tetherin following experimental infections of chimpanzees, which were conducted over 10 years ago in the context of vaccine and pathogenesis studies (Novembre et al., 1997; Mwaengo and Novembre, 1998). We found that two closely related molecular clones of chimpanzee adapted HIV-1 regained Nef-mediated anti-tetherin activity. This came as a surprise as both viruses also maintained their Vpu-mediated counteraction of tetherin. Most strikingly, we could demonstrate that as few as two amino acid changes were sufficient to render HIV-1 Nef active against chimpanzee tetherin. Thus, after a century of replicating in humans, HIV-1 Nef reacquired anti-tetherin activity within a single passage in its original host.
RESULTS
A chimpanzee-adapted HIV-1 strain utilizes both Vpu and Nef to antagonize tetherin
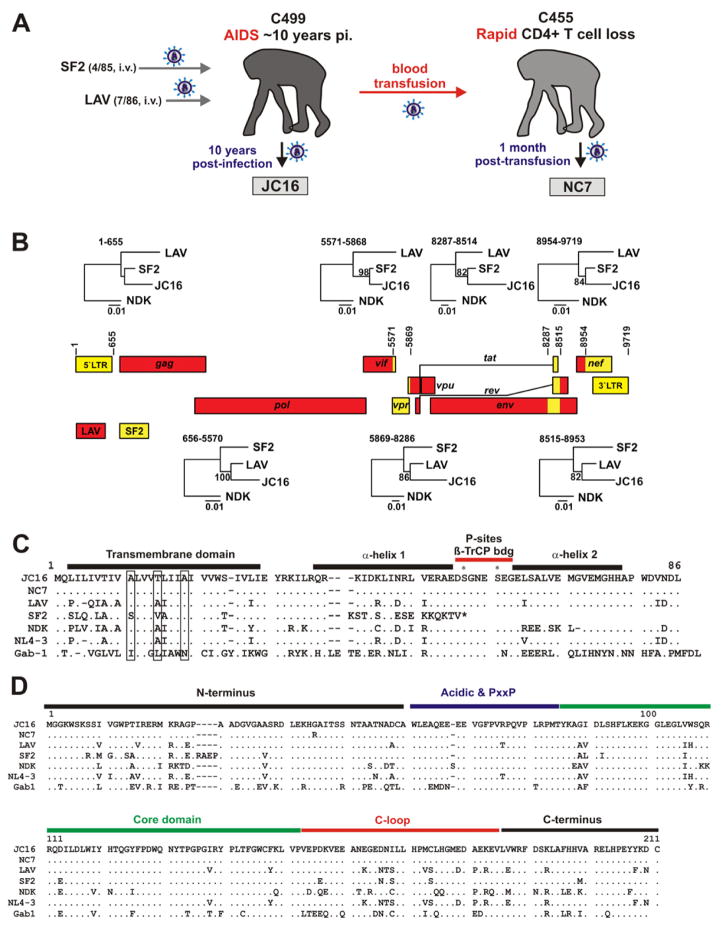
To examine how Vpu and Nef function evolved after reintroduction of HIV-1 into its original chimpanzee (CPZ) host, we took advantage of two molecular clones (JC16 and NC7) derived from chimpanzees, who were experimentally infected with HIV-1 in the mid 1980s (Novembre et al., 1997; Mwaengo and Novembre, 1998). JC16 was derived from CPZ C499, who was initially infected with the SF2 (also called ARV-2) isolate of HIV-1 in 1985 and subsequently inoculated with a CPZ-passaged LAV strain amplified in human PBMCs (Fultz et al., 1987) in 1986 (Figure 1A). Notably, a molecular clone of SF2 contains a defective vpu gene suggesting that this HIV-1 strain may not encode a functional tetherin antagonist. Cloning of JC16 was performed by PCR amplification of proviral sequences from a coculture of C499-derived PBMCs with cells from an uninfected CPZ (Mwaengo and Novembre, 1998). JC16 was obtained about 10 years after infection when C499 had developed severe CD4+ T cell depletion and AIDS (Novembre et al., 1997). NC7 was cloned from a second CPZ (C455) one month post-transfusion with blood from C499.
Figure 1. Origin and properties of the CPZ-adapted HIV-1 clones JC16 and NC7.
(A) Overview of the derivation of the CPZ-adapted HIV-1 JC16 and NC7 molecular clones. The numbers in parentheses give month and year of intravenous (i.v.) infection. See text for details.
(B) Generation of the recombinant JC16 genome from LAV and SF2 parental viruses. Open reading frames are indicated as boxes with HXB2 coordinates indicating inferred breakpoints (Korber et al. 1998). Neighbor-joining phylogenetic trees for the SF2 and LAV derived segments are shown above and below the recombination scheme, respectively. Bootstrap support ≥70% is shown above branches. Scale bars indicate genetic distance of 0.01.
(C, D) Alignment of (C) Vpu and (D) Nef amino acid sequences. The JC16 Vpu or Nef sequences are shown on top for comparison. (C) The three Ala-residues involved in tetherin interaction in the hydrophobic transmembrane (TM) domain, the two helical regions and the β-TrCP-binding motif containing two phosphorylation sites are indicated. (D) The myristoylated N-terminus, the acidic region and the PxxP-motif, as well as the conserved core domain, the flexible C-loop region and the C-terminus in the JC16 Nef are indicated. The colored bars indicate the regions used in the generation of chimera between the JC16 and LAV Nefs (see Figure 3A). Dashes indicate gaps introduced to optimize the alignment.
Phylogenetic analyses showed that JC16 represents a recombinant between the LAV and SF2 strains. Most parts of the JC16 genome clustered with LAV, except for (i) the vpr gene; (ii) a short stretch of 228 nucleotides encoding portions of the gp41 transmembrane, Tat and Rev proteins and (iii) most of the nef gene and the LTR region (Figure 1B). We found that the JC16 and NC7 Vpu amino acid sequences are identical (Figure 1C) and those of Nef differ only by a single substitution in the N-terminus (Figure 1D). However, the CPZ-adapted Vpu and Nef sequences showed multiple differences from those of molecular clones derived from the parental SF2 and LAV isolates. Notably, the AxxxAxxxA motif that is conserved in HIV-1 Vpus and critical for counteraction of human (HU) tetherin (Vigan and Neil, 2010; Skasko et al., 2011), was mutated to AxxxTxxxA (Figure 1C). The three A-residues have been shown to interact directly with the transmembrane region of tetherin (Skasko et al., 2011). Thus, a V30M substitution in the CPZ tetherin orthologue may have selected for the A15T adaptation in Vpu. The JC16 Nef exhibited multiple changes compared to the parental LAV and SF2 Nef proteins, but most of them clustered near the N-terminus and in the C-loop of Nef known to be critical for the interaction with adaptor protein (AP) complexes (Figure 1D). Although the chimpanzee-derived HIV-1 Vpu and Nef sequences varied substantially from those of the input HIV-1 strains, most functional domains, such as the β-TrCP-binding motif (DSGxxS) in Vpu and the acidic domain as well as the P(xxP)3 and ExxxLL motifs in Nef, were conserved.
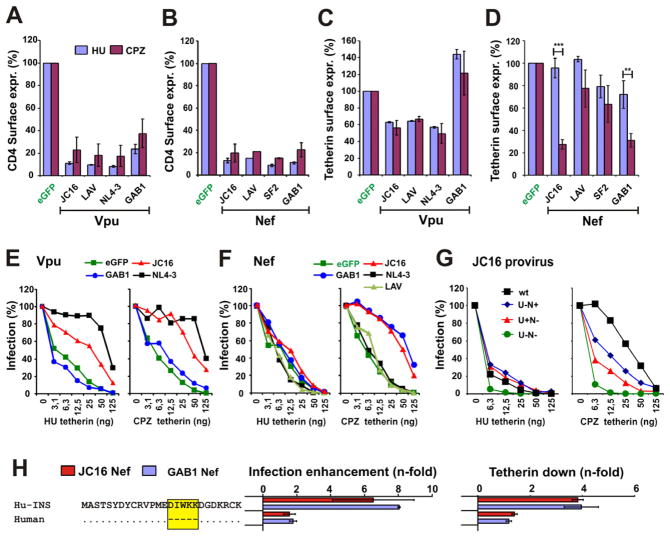
To determine how Vpu and Nef function evolved during re-adaptation of HIV-1 to chimpanzees, we cotransfected 293T cells with vectors coexpressing Vpu or Nef and eGFP together with constructs expressing HU or CPZ derived CD4. Flow cytometric analyses showed that the JC16 Vpu and Nef proteins reduced HU and CPZ CD4 cell surface expression by more than 80% and were thus fully active (Figure 2A, B). The JC16 Nef also downmodulated class I MHC and (weakly) CD28 cell surface expression in PBMCs (Figure S1A, S1B). Analyses of Vpu and Nef proteins containing a C-terminal AU-1-tag confirmed these results and showed that all constructs expressed proteins of the expected size (Figure S1C).
Figure 2. Functional activity of Vpu and Nef proteins of a CPZ-adapted HIV-1 strain.
(A–D) Levels of (A, B) CD4 and (C, D) tetherin cell surface expression in the presence of Vpu or Nef relative to levels measured in cells transfected with the GFP control vector (100%). Values are averages (±SD) from three experiments. **, p<0.01; ***, p<0.001.
(E, F) Infectious virus yield from 293T cells cotransfected with the HIV-1 NL4-3 ΔVpuΔNef construct and vectors expressing the indicated (E) vpu or (F) nef alleles in combination with various quantities of plasmids expressing HU or CPZ tetherin. Panels E to G give average values derived from triplicate infections of TZM-bl indicator cells relative to those obtained in the absence of tetherin expression vector (100%). All results were verified by p24 ELISA.
(G) The JC16 clone uses both Nef and Vpu to antagonize tetherin. Virus release from 293T cells following transfection with 2.5 μg of a proviral JC16 wt construct or mutants lacking either nef (U+N−), vpu (U−N+) or both (U−N−) and varying amounts of plasmids expressing HU or CPZ tetherin.
(H) The JC16 Nef targets amino acid residues that are missing in the human tetherin orthologue. 293T cells were transfected with plasmids expressing HU tetherin or a variant in which the N-terminal deletion was restored (HU-INS). The middle panel shows the enhancement of infectious virus release relative to the eGFP only control derived from triplicate infections of TZM-bl indicator cells. The right panel shows the reduction of the surface expression levels of the respective tetherin variant in the presence of JC16 or GAB1 Nefs relative to those measured in cells transfected with the GFP only vector. The deletion in HU tetherin is highlighted by a yellow box. (See also Figure S1).
To test whether the CPZ-adapted HIV-1 Vpu maintained its capability to counteract tetherin, we first examined its effect on the cell surface expression levels of HU and CPZ tetherin. The JC16, LAV and NL4-3 Vpus reduced the levels of HU and CPZ tetherin surface expression by about 40% to 50%, whereas the SIVcpz GAB1 Vpu was inactive (Figures 2C, S1D). In contrast, the JC16 and GAB1 Nefs efficiently reduced the surface levels of CPZ but not of HU tetherin, whereas the LAV and SF2 Nefs generally had only modest effects (Figure 2D, S1D). Thus, the CPZ-adapted HIV-1 JC16 Nef showed a functional phenotype similar to SIVcpz Nefs and distinct from HIV-1 Nefs. Next, we measured infectious virus yields from 293T cells following cotransfection of a ΔVpuΔNef HIV-1 NL4-3 proviral vector with fixed quantities of Vpu and Nef constructs and different doses of tetherin expression plasmid. Our data showed that the JC16 Vpu maintained the ability to antagonize tetherin, being even slightly more effective against the CPZ than against the HU orthologue (Figure 2E). As mentioned above, this may be due to an A15T change in the TMD of the JC16 Vpu. While the anti-tetherin activity of the JC16 Vpu was as expected, it came as a surprise that the JC16 Nef also counteracted CPZ tetherin (Figures 2F, S1E).
Finally, we used proviral constructs to test the function of JC16 Nef and Vpu. The analysis of nef-defective JC16 constructs with or without a functional vpu gene confirmed that this CPZ-adapted HIV-1 strain uses both Vpu and Nef to antagonize CPZ tetherin (Figure 2G). In contrast, the CPZ-adapted HIV-1 JC16 strain did not efficiently counteract HU tetherin. We also examined whether the JC16 Nef targets the same residues as Nefs from wild-type SIVcpz strains. In agreement with previous studies (Jia et al., 2009; McNatt et al., 2009; Sauter et al., 2009), introduction of five amino acids (D15IWKK19) that are missing in the N-terminal part of HU tetherin restored its sensitivity to the JC16 Nef (Figure 2H). Thus, CPZ passage restored the capability of this HIV-1 Nef to target the region missing in the HU tetherin orthologue.
Two amino acid changes confer anti-tetherin activity to a current HIV-1 Nef
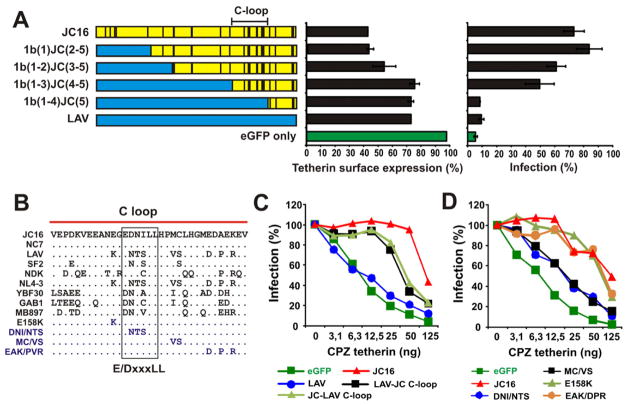
To map the determinants of anti-tetherin activity in Nef, we generated a set of recombinants between the active JC16 Nef and the inactive LAV Nef (Figure 3A). Analysis of the recombinant Nefs showed that downmodulation of tetherin and enhancement of virion release was critically dependent on the presence of the C-terminal flexible loop (C-loop) of JC16 (Figure 3A). Next, we swapped only the C-loops of HIV-1 and SIVcpz Nefs (Figure 3B) and found that the presence of the JC16 C-loop (LAV-JC C-loop) increased the anti-tetherin activity of LAV Nef, while insertion of the LAV loop (JC-LAV C-loop) reduced the functional activity of JC16 Nef (Figure 3C). Further analyses demonstrated that the central ten amino acids of the C-loop (named Ccen) play a key role in Nef-mediated anti-tetherin activity (Figures S2A, S2B). Examination of single to triple amino acid substitutions demonstrated that changes of the variable residues within the otherwise conserved E/DxxxLL motif (DNI161-163NTS) as well as changes of MC168/169VS (named JC16-VS) and a single C169S substitution downstream of this motif severely impaired the capability of the JC16 Nef to counteract CPZ tetherin (Figures 3D, S2C). Notably, none of these changes affected other Nef activities, such as the downmodulation of CD4, CD28, CD25, MHC-I or CXCR4 (examples shown in Figure S2D).
Figure 3. Mapping residues critical for the gain of anti-tetherin activity by HIV-1 Nef.
(A) The left panel indicates the chimeras between the JC16 (yellow) and LAV (blue) Nefs analyzed. Amino acid differences are indicated by vertical dashes. The effect of various Nefs on expression levels of CPZ tetherin (middle) or infectious virus release in the presence of CPZ tetherin (right) are shown as average values ±SD from triplicate experiments.
(B) Alignment of HIV-1 and SIVcpz C-loop regions of Nef. The JC16 C-loop sequence is shown on top for comparison. The E/DxxxLL motif and specific mutants analyzed in panel D are indicated.
(C, D) Effect of Nefs differing in (C) their C-loop sequences or (D) specific amino acids on infectious virus release in the presence of CPZ tetherin. LAV-JC C-loop represents the LAV Nef containing the C-loop of the JC16 Nef and vice versa JC-LAV C-loop represents the JC16 Nef containing the C-loop of the LAV Nef. The mutant JC16 Nefs contain changes of E158K, DNS161-163NTS, MC169/169VS, and EAK174/176/178DPR as shown in panel B. Numbers give amino acid positions in the JC16 Nef. Curves represent the average infection values (n=3) relative to those obtained in the absence of tetherin. (See also Figure S2).
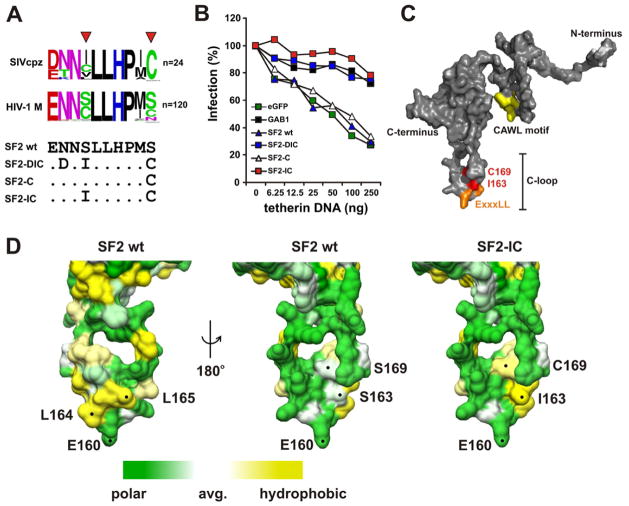
We next examined the frequency of amino acid (aa) residues in the E/DxxxLL motif and the four C-terminally adjacent residues in SIVcpz and HIV-1 Nef proteins. Overall, this 10-aa region is well conserved. However, SIVcpz Nefs usually contained an Ile (163I) just next to the di-leucine motif, whereas HIV-1 Nefs contained a Ser or Cys residue at this position (Figure 4A). Furthermore, an adjacent Cys (169C) residue was highly conserved in SIVcpz Nefs, whereas HIV-1 M Nefs frequently encoded Ser or Asn. Strikingly, these residues were not only critical for anti-tetherin activity of the JC16 Nef, but the combination of the S163I and S169C changes (SF2-IC) also rendered the HIV-1 SF2 Nef fully active against CPZ tetherin (Figures 4B). Similarly, SF2 Nef proteins containing 163C or 163V residues, which are present in about 50% of all SIVcpz Nefs (Figure 4A), together with the S169C substitution (SF2-CC, SF2-VC) also antagonized CPZ tetherin (Figure S3A, S3B). None of these mutations in the SF2 Nef had significant effect on the CD4 downmodulation function (Figure S3C). Examination of the localization of these residues in the SF2 Nef crystal structure, which includes the entire C-terminal loop (Horenkamp et al., 2011), showed that despite their close physical proximity the critical 163I and 169C residues and the ExxxLL AP interaction site form distinct surfaces (Figure 4C, 4D). Moreover, the interaction site of Nef with CD4 (CAWL motif), is spatially well separated from the ExxxLL motif (Figure 4C). Altogether, these results suggest that residues 163I and 169C are specifically involved in the interaction of Nef with tetherin.
Figure 4. Two amino acid changes confer anti-tetherin activity to the HIV-1 SF2 Nef.
(A) The upper panel gives frequency plots of amino acid residues in and next to the ExxxLL motif of HIV-1 M and SIVcpz Nef proteins and the lower panel shows the mutations in the SF2 Nef analyzed in panel B. The triangles indicate residues critical for anti-tetherin activity.
(B) Infectious virus yield from 293T cells cotransfected with the NL4-3 ΔVpuΔNef construct, vectors expressing the indicated nef alleles, and various quantities of plasmid expressing CPZ tetherin. The SF2 Nef mutants contain changes of NSS161/163/169DIC (SF2-DIC), S169C (SF2-C) and SS163/169IC (SF2-IC). All values represent averages of three measurements and are shown relative to those obtained in the absence of tetherin expression vector (100%). Results were confirmed in two independent experiments and by measuring p24 release.
(C) Structure of the HIV-1 Nef protein. The I163 and C169 (red) residues critical for tetherin antagonism and the acidic residue and two leucines (orange) in the E/DxxxLL motif involved in the interaction with AP complexes as well as the CAWL motif (yellow) involved in CD4 binding are indicated.
(D) Localization of residues critical for tetherin antagonism and AP interaction (all indicated by dots) and electrostatic surface potential of the C-terminal flexible loop of the SF2 Nef. Changes of S163I and S169C that are critical for the anti-tetherin activity of Nef generate a hydrophobic groove in the otherwise largely polar C-loop. (See also Figure S3)
A minority of HIV-1 nef alleles encode a cystein at position 169, just like the vast majority of SIVcpz Nefs (Figure 4A). To examine whether a subset of contemporary HIV-1 Nefs is active against CPZ tetherin, we selected twelve previously described patient-derived nef alleles (Specht et al., 2010) that encoded 169C either together with 163S (as in most HIV-1 Nefs) or with 163C (as in some SIVcpz Nefs) (Figure S3D). All these HIV-1 nef alleles were substantially less potent in antagonizing CPZ tetherin than the CPZ-adapted JC16 Nef (Figure S3E). However, HIV-1 nef alleles encoding 163C/169C were somewhat more active in promoting virus release in the presence of CPZ tetherin than those encoding 163S/169C (Figure S3F). Thus, some contemporary HIV-1 Nefs that contain the same residues as SIVcpz Nefs at amino acid positions 163 and 169 exhibit a modest activity against CPZ tetherin.
DISCUSSION
High mutation rates are a key feature of primate lentiviruses and allow them to efficiently adapt to their respective host environments. One might expect that this high adaptability comes at a cost in that viral genes which function in a species-specific manner should rapidly accumulate disruptive mutations. Surprisingly, however, we found that HIV-1 reacquired the ability to use its Nef protein to counteract CPZ tetherin within a single passage following reintroduction into its original chimpanzee host. Moreover, just two amino acid changes (S163I and S169C) in the C-loop of HIV-1 Nef conferred full anti-tetherin activity (Figure 4). This is quite striking given that the chimpanzee-human transmission event of SIVcpz that resulted in the emergence of pandemic HIV-1 group M strains, which led to the disruption of the Nef mediated anti-tetherin activity, is estimated to have occurred a century ago (Korber et al., 2000; Worobey et al., 2008).
To our current knowledge both Nef and Vpu lost and regained anti-tetherin activity several times during the cross-species transmission events that preceded the emergence of AIDS. Nef is well known for its multi-functionality and most of its activities, such as downmodulation of CD4 and MHC-I or enhancement of viral infectivity, are species-independent (reviewed in Kirchhoff et al., 2008). Similarly, Vpu not only antagonizes tetherin in a species-specific manner but also degrades CD4 independently of the host species (Sauter et al., 2009). Thus, even when Nef or Vpu lose a host specific function after a cross-species transmission event, the preservation of the remaining activities will ensure the maintenance of genes that also remain predisposed to quickly regain the disrupted functions. It is thus tempting to speculate that a common by-product of the multi-functionality of lentiviral accessory genes is an increased potential of these viruses to overcome species barriers. In fact, recent evidence suggests that Vpr and Vpx are also multi-functional (Planelles and Barker, 2010) and that the accessory viral proteins may generally interact with multiple cellular factors (Jäger et al., 2011).
Our finding that after reintroduction into its original chimpanzee host, HIV-1 not only evolved Nef-mediated activity against CPZ tetherin, but also maintained Vpu as a tetherin antagonist, adds to the evidence that tetherin antagonism is advantageous for viral spread in vivo (Liberatore and Bieniasz, 2011; Serra-Moreno et al., 2011). Perhaps more importantly, the present data are derived from an animal model that represents the closest approximation of AIDS in humans, i.e. infection of our closest relative, the chimpanzee, with pandemic HIV-1 group M strains. In comparison, previous in vivo results were obtained either in mouse models that required IFN induction to demonstrate definitive effects of tetherin or the SIVmac/macaque model (Liberatore and Bieniasz, 2011; Serra-Moreno et al., 2011).
The finding that the CPZ-adapted HIV-1 strain maintained two tetherin antagonists was unexpected since most primate lentiviruses use either Nef, Vpu or Env to antagonize this restriction factor. Chimpanzee C499 was productively infected with two different HIV-1 isolates, i.e. ARV-2 (SF2) in 1985 and LAV in 1986. The SF2 molecular clone, which is derived from ARV-2, encodes a grossly truncated Vpu (Figure 1C). Thus, it is tempting to speculate that the SF2 strain initially evolved Nef as an antagonist of CPZ tetherin because it lacks a functional Vpu. Later, LAV recombined with this virus and the resulting CPZ-adapted JC16 strain received the critical region of the nef gene from SF2 (Figure 1B). Importantly, JC16 was particularly fit to replicate in chimpanzees since C499 ultimately developed high viral loads and AIDS. Furthermore, transfusion of its blood into a second ape (C455) was associated with high plasma viral loads and a rapid and sustained decline of CD4+ T cells (Novembre et al., 1997). Similarly, virus derived from C455 (termed NC) was pathogenic in two additional chimpanzees, whereas other HIV-1-infected chimpanzees did not show signs of disease progression (Novembre et al., 2001; O’Neil et al., 2000). Thus, the CPZ-adapted HIV-1 JC and NC strains may be particularly pathogenic in chimpanzees and it is tempting to speculate that the evolution of two effective tetherin antagonists may contribute to their virulence.
In summary, we show here that just two amino acid changes in the C-loop of Nef are sufficient to fully restore the activity of a contemporary HIV-1 group M strain against the tetherin orthologue of its original chimpanzee host. Nef seems to be highly prone to regain anti-tetherin activity because it uses similar domains and mechanisms to down-modulate CD4 and to antagonize tetherin. In comparison, the functional constraints on Vpu may be less stringent because multiple changes in the TM region of SIVcpz Vpus seem to be required to confer activity against HU Tetherin (Lim et al., 2010). This may help to explain why tetherin antagonism by the HIV-1 Nef protein was restored within a single-ape passage, whereas HIV-1 group O and P Vpus have thus far been unable to gain anti-tetherin activity in humans (Sauter et al., 2009; Sauter et al., 2011; Yang et al., 2011). It will be of interest to further elucidate whether viral proteins that counteract antiviral factors in a species-specific manner have also commonly evolved functions or interactions that are species independent to avoid their complete inactivation and elimination after cross-species transmission.
EXPERIMENTAL PROCEDURES
JC16 and NC7 molecular clones
Generation of the JC16 and NC7 infectious molecular clones was performed as described previously (Mwaengo and Novembre, 1998). Splice overlap extension PCR was used to generate vpu- and/or nef-defective mutants. See the Supplemental Experimental Procedures for details.
Expression vectors
Bi-cistronic CMV-based pCGCG expression vectors coexpressing vpu, nef, CD4 or tetherin and eGFP or DsRed2 have been described previously (Sauter et al., 2009). Splice-overlap-extension PCR with primers introducing XbaI and MluI restriction sites flanking the reading frames was used to generate chimeric nef alleles and nef mutants. PCR fragments were purified from agarose gels and inserted into the pCGCG vector using standard cloning techniques. All PCR-derived inserts were sequenced to confirm their accuracy.
Recombination analysis of JC16
The recombination breakpoints of JC16 were determined as described in the Supplemental Experimental Procedures.
Cell culture and transfections
Cells were cultured and transfected as described in the Supplemental Experimental Procedures.
Flow cytometric analysis
To determine the effect of Vpu and Nef on CD4 and tetherin cell surface expression, 293T cells were transfected by the calcium phosphate method with 1 μg of a CD4 or tetherin expression vector coexpressing eGFP and 5 μg of pCGCG eGFP/Vpu or Nef constructs expressing eGFP alone or together with Vpu or Nef. Two days post-transfection CD4 or tetherin expression was examined by FACS analysis. See Supplemental Experimental Procedures for details.
Tetherin antagonism
The capability of Vpu and Nef to antagonize tetherin was determined essentially as described previously (Sauter et al., 2009). For details, see the Supplemental Experimental Procedures.
Western Blot
Expression of Vpu, Nef and tetherin was examined as described in the Supplemental Experimental Procedures.
Sequence analyses
Vpu or Nef amino acid sequences were aligned using multiple sequence alignment with hierarchical clustering (www.multalin.toulouse.inra.fr). Vpu and Nefs sequences were drawn from the HIV Sequence Database (www.hiv.lanl.gov).
Nef model building
Mutations of S163C and S169I were inserted into the SF2 Nef structure as specified in the Supplemental Experimental Procedures.
Statistical analysis
All statistical calculations were performed with a two-tailed unpaired Students-t-test using Graph Pad Prism Version 5.0. P values <0.05 were considered significant. Correlations were calculated with the linear regression module.
Supplementary Material
HIGHLIGHTS.
HIV-1 reacquired Nef-mediated tetherin antagonism in its original chimpanzee host
The chimpanzee-adapted HIV-1 strain uses both Nef and Vpu to counteract tetherin
Just two changes in a contemporary HIV-1 Nef conferred full anti-tetherin activity
Acknowledgments
We thank Susanne Engelhart, Kerstin Regensburger and Martha Mayer for excellent technical assistance, Sebastian Lülf for help with structure depiction, and Jan Münch for critical reading of the manuscript. TZM-bl cells were obtained through the NIH AIDS Research and Reference Reagent Program. This work was supported in part by the National Institutes of Health (R21 AI080364, R01 AI50529, R01 AI58715) and by the Deutsche Forschungsgemeinschaft (DFG) (FA 378/11-1 to OTF and Leibniz award to FK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailes E, Gao F, Bibollet-Ruche F, Courgnaud V, Peeters M, Marx PA, Hahn BH, Sharp PM. Hybrid origin of SIV in chimpanzees. Science. 2003;300:1713. doi: 10.1126/science.1080657. [DOI] [PubMed] [Google Scholar]
- Fultz PN, Srinivasan A, Greene CR, Butler D, Swenson RB, McClure HM. Superinfection of a chimpanzee with a second strain of human immunodeficiency virus. J Virol. 1997;61:4026–4029. doi: 10.1128/jvi.61.12.4026-4029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenkamp FA, Breuer S, Schulte A, Lülf S, Weyand M, Saksela K, Geyer M. Conformation of the dileucine-based sorting motif in HIV-1 Nef revealed by intermolecular domain assembly. Traffic. 2011;12:867–877. doi: 10.1111/j.1600-0854.2011.01205.x. [DOI] [PubMed] [Google Scholar]
- Jäger S, Cimermancic P, Gulbahce N, Johnson JR, McGovern KE, Clarke SC, Shales M, Mercenne G, Pache L, Li K, et al. Global landscape of HIV-human protein complexes. Nature. 2011;481:365–370. doi: 10.1038/nature10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B, Serra-Moreno R, Neidermyer W, Rahmberg A, Mackey J, Fofana IB, Johnson WE, Westmoreland S, Evans DT. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 2009;5:e1000429. doi: 10.1371/journal.ppat.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff F, Schindler M, Specht A, Arhel N, Münch J. Role of Nef in primate lentiviral immunopathogenesis. Cell Mol Life Sci. 2008;65:2621–2636. doi: 10.1007/s00018-008-8094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff F. Immune evasion and counteraction of restriction factors by HIV-1 and other primate lentiviruses. Cell Host Microbe. 2010;8:55–67. doi: 10.1016/j.chom.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Korber BT, Foley BT, Kuiken CL, Pillai SK, Sodroski JK. Numbering positions in HIV relative to HXB2CG. Human Retroviruses and AIDS III. 1998:102–111. [Google Scholar]
- Korber B, Muldoon M, Theiler J, Gao F, Gupta R, Lapedes A, Hahn BH, Wolinsky S, Bhattacharya T. Timing the ancestor of the HIV-1 pandemic strains. Science. 2000;288:1789–1796. doi: 10.1126/science.288.5472.1789. [DOI] [PubMed] [Google Scholar]
- Laguette N, Benkirane M. How SAMHD1 changes our view of viral restriction. Trends Immunol. 2012;33:26–33. doi: 10.1016/j.it.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore RA, Bieniasz PD. Tetherin is a key effector of the antiretroviral activity of type I interferon in vitro and in vivo. Proc Natl Acad Sci U S A. 2011;108:18097–18101. doi: 10.1073/pnas.1113694108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ES, Malikm HS, Emerman M. Ancient adaptive evolution of tetherin shaped the functions of Vpu and Nef in human immunodeficiency virus and primate lentiviruses. J Virol. 2010;84:7124–7134. doi: 10.1128/JVI.00468-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim MH, Emerman M. HIV-1 accessory proteins--ensuring viral survival in a hostile environment. Cell Host Microbe. 2008;3:388–398. doi: 10.1016/j.chom.2008.04.008. [DOI] [PubMed] [Google Scholar]
- McNatt MW, Zang T, Hatziioannou T, Bartlett M, Fofana IB, Johnson WE, Neil SJD, Bieniasz PD. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog. 2009;5:e1000300. doi: 10.1371/journal.ppat.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson NR, Sawyer SL. Two-stepping through time: mammals and viruses. Trends Microbiol. 2011;19:286–294. doi: 10.1016/j.tim.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwaengo DM, Novembre FJ. Molecular cloning and characterization of viruses isolated from chimpanzees with pathogenic human immunodeficiency virus type 1 infections. J Virol. 1998;72:8976–8987. doi: 10.1128/jvi.72.11.8976-8987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novembre FJ, Saucier M, Anderson DC, Klumpp SA, O’Neil SP, Brown CR, 2nd, Hart CE, Guenthner PC, Swenson RB, McClure HM. Development of AIDS in a chimpanzee infected with human immunodeficiency virus type 1. J Virol. 1997;71:4086–4091. doi: 10.1128/jvi.71.5.4086-4091.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novembre FJ, de Rosayro J, Nidtha S, O’Neil SP, Gibson TR, Evans-Strickfaden T, Hart CE, McClure HM. Rapid CD4(+) T-cell loss induced by human immunodeficiency virus type 1(NC) in uninfected and previously infected chimpanzees. J Virol. 2001;75:1533–1539. doi: 10.1128/JVI.75.3.1533-1539.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil SP, Novembre FJ, Hill AB, Suwyn C, Hart CE, Evans-Strickfaden T, Anderson DC, deRosayro J, Herndon JG, Saucier M, McClure HM. Progressive infection in a subset of HIV-1-positive chimpanzees. J Infect Dis. 2000;182:1051–1062. doi: 10.1086/315823. [DOI] [PubMed] [Google Scholar]
- Planelles V, Barker E. Roles of Vpr and Vpx in modulating the virus-host cell relationship. Mol Aspects Med. 2010;31:398–406. doi: 10.1016/j.mam.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter D, Hue S, Petit SJ, Plantier JC, Towers GJ, Kirchhoff F, Gupta RK. HIV-1 Group P is unable to antagonize human tetherin by Vpu, Env or Nef. Retrovirology. 2011;8:103. doi: 10.1186/1742-4690-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter D, Schindler M, Specht A, Landford WN, Münch J, Kim KA, Votteler J, Schubert U, Bibollet-Ruche F, Keele BF, et al. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe. 2009;6:409–421. doi: 10.1016/j.chom.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Moreno R, Jia B, Breed M, Alvarez X, Evans DT. Compensatory changes in the cytoplasmic tail of gp41 confer resistance to tetherin/BST-2 in a pathogenic nef-deleted SIV. Cell Host Microbe. 2011;9:46–57. doi: 10.1016/j.chom.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skasko M, Wang Y, Tian Y, Tokarev A, Munguia J, Ruiz A, Stephens EB, Opella SJ, Guatelli J. HIV-1 Vpu protein antagonizes innate restriction factor BST-2 via lipid-embedded helix-helix interactions. J Biol Chem. 2012;287:58–67. doi: 10.1074/jbc.M111.296772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht A, Telenti A, Martinez R, Fellay J, Bailes E, Evans DT, Carrington M, Hahn BH, Goldstein DB, Kirchhoff F. Counteraction of HLA-C-mediated immune control of HIV-1 by Nef. J Virol. 2010;84:7300–7311. doi: 10.1128/JVI.00619-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worobey M, Gemmel M, Teuwen DE, Haselkorn T, Kunstman K, Bunce M, Muyembe JJ, Kabongo JMM, Kalengayi RM, Van Marck E, et al. Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960. Nature. 2008;455:661–664. doi: 10.1038/nature07390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SJ, Lopez LA, Exline CM, Haworth KG, Cannon PM. Lack of adaptation to human tetherin in HIV-1 group O and P. Retrovirology. 2011;8:78. doi: 10.1186/1742-4690-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wilson SJ, Landford WC, Virgen B, Gregory D, Johnson MC, Munch J, Kirchhoff F, Bieniasz PD, Hatziioannou T. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe. 2009;6:54–67. doi: 10.1016/j.chom.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.