Abstract
Nitric oxide (NO) defends against intracellular pathogens but its synthesis must be regulated due to cell and tissue toxicity. During infection, macrophages import extracellular arginine to synthesize NO, generating the byproduct citrulline. Accumulated intracellular citrulline is thought to fuel arginine synthesis catalyzed by argininosuccinate synthase (Ass1) and argininosuccinate lyase (Asl), which would lead to abundant NO production. Instead, we find that citrulline is exported from macrophages during early stages of NO production with < 2% retained for recycling via the Ass1-Asl pathway. Later, extracellular arginine is depleted, and Ass1 expression allows macrophages to synthesize arginine from imported citrulline to sustain NO output. Ass1-deficient macrophages fail to salvage citrulline in arginine-scarce conditions, leading to their inability to control mycobacteria infection. Thus, extracellular arginine fuels rapid NO production in activated macrophages, and citrulline recycling via Ass1 and Asl is a fail-safe system that sustains optimum NO production.
Introduction
Mononuclear phagocytes (macrophages and dendritic cells) recognize, control and kill a wide variety of pathogens. Successful pathogens have made corresponding adaptations to reside and replicate within macrophages (Mycobacterium sp., Toxoplasma gondii, Leishmania sp.) while other pathogens use macrophages as a vehicle to facilitate rapid growth and dissemination (Listeria sp., Salmonella sp., Francisella sp.). Host containment and killing mechanisms activated after pathogen detection include the production of free radical anti-microbials, including reactive oxygen species (ROS) and nitric oxide (NO). NO generation requires inducible nitric oxide synthase (iNOS) expression through cooperative signals from interferons and NF-κB activation from TLR and related signaling pathways (Farlik et al., 2010). The IFN-γ-mediated activation of NO production is required for control of numerous clinically important intracellular pathogens in mouse models (Bogdan, 2001; Nathan and Shiloh, 2000). Furthermore, humans with defects in IFN-γ signaling were discovered due to their increased susceptibility to Mycobacteria (Casanova and Abel, 2002). In humans, the production of NO by phagocytes remains controversial because human macrophages cannot be readily provoked to make NO in conventional in vitro culture conditions (Martinez et al., 2006; Vogt and Nathan, 2011). However, both iNOS and NO have been detected in human tissues and alleles of NOS2 have been linked to M. tuberculosis susceptibility suggesting that human NO is needed for microbial containment (Choi et al., 2002; Facchetti et al., 1999; Moller et al., 2009; Nicholson et al., 1996; Pautz et al., 2010). Superoxide generation also contributes to control of M. tuberculosis and to several other homotropic pathogens (Ehrt and Schnappinger, 2009). Thus, free radical generation is a critical component of mammalian resistance to intracellular pathogens.
Macrophages that seed tissues during inflammation can be ‘polarized’ by cytokines, dead cells, and microbial products. Macrophages adopt M1 (also termed ‘classical’ activation, driven by TLR and IFN activation) or M2 (‘alternative’ activation, driven by IL-4 and IL-13 signaling) activation states. M1 and M2 activation is plastic and macrophages can be coerced from one state to the other depending on the local inflammatory milieu (Murray and Wynn, 2011a; Stout and Suttles, 2004). In early work to define macrophage plasticity, arginine metabolism was recognized to be significant in macrophage biology because M1 macrophages use arginine to make NO and citrulline while M2 cells do not express iNOS or produce NO, but instead convert arginine to ornithine and urea via type 1 arginase (Arg1) (Munder et al., 1998; Shearer et al., 1997). M1 macrophages are essential for controlling the growth of intracellular pathogens while M2 macrophages are required for immunity to some worms and appear critical for wound healing and tissue repair (Murray and Wynn, 2011b). NO generated from arginine in M1 macrophages is a potent inhibitor of T cell proliferation, an inducer of necrosis, and a cause of DNA damage, therefore its levels must be tightly constrained (Bogdan, 2001; Pautz et al., 2010). Collateral damage from NO production is mitigated in a myriad of ways. For example, a key pathway to suppress NO production is the induction of Arg1 expression in M1 macrophages, which consumes arginine, limiting the amount available to iNOS. Some intracellular pathogens have harnessed this pathway as a survival mechanism by inducing Arg1 in both the infected and surrounding tissue (El Kasmi et al., 2008; Qualls et al., 2010).
Arginine is required for nitrogen elimination in mammals via the urea cycle where toxic ammonia is fixed in the mitochondria of hepatocytes through the sequential reactions of carbamoyl phosphate synthase (Cps1) and ornithine transcarbamylase (Otc). This complex reaction requires ATP, bicarbonate, and ornithine to generate citrulline. Citrulline is transported out of the mitochondria and used by argnininosuccinate synthase (Ass1) and argininosuccinate lyase (Asl) to produce argininosuccinate and arginine, respectively. The urea cycle does not operate in macrophages because Cps1 and Otc are not expressed. Instead, macrophages import arginine by cationic amino acid transporters including CAT1 and CAT2 encoded by Slc7a1 and Slc7a2, respectively. Whereas CAT1 expression is constitutive, CAT2 is inducible (Thompson et al., 2008) and is considered to be the main way activated macrophages harvest arginine from their environment. Indeed, a threshold concentration of extracellular arginine is required for NO production irrespective of the amount of arginine inside the cell; this phenomenon is termed the ‘arginine paradox’ and has yet to be fully explained in biochemical terms (Lee et al., 2003).
The coordinated expression of iNOS, Ass1, and Asl in activated macrophages has led to a recycling model for NO production where the arginine converted to citrulline by iNOS is recycled to arginine by Ass1/Asl to supply the intracellular arginine for NO production; a process seemingly at odds with the ‘arginine paradox’ (Curis et al., 2005; Morris, 2007). Here we used biochemical methods to uncover advanced aspects of arginine metabolism in macrophages undergoing parasitism by intracellular pathogens where NO is required for control. We first showed that extracellular arginine directly fuels NO production in M1 activated and infected macrophages while intracellular arginine levels remain unaltered. Second, when arginine is plentiful, activated macrophages import this amino acid and expel citrulline as a by-product of NO synthesis. Third, we found that when arginine is depleted following high-output NO production, citrulline is imported and used to synthesize arginine via Ass1. Ass1 activity is required for optimal control of Mycobacteria in both in vitro and in vivo infection models suggesting that arginine depletion in chronically infected microenvironments is counteracted by citrulline recycling. Thus extracellular arginine supports the initial burst of NO, however, sustained NO production by the conversion of citrulline to arginine by Ass1 is necessary for optimal control of persistent pathogens.
Results
The arginine metabolism transcriptome in M1 and M2 polarization
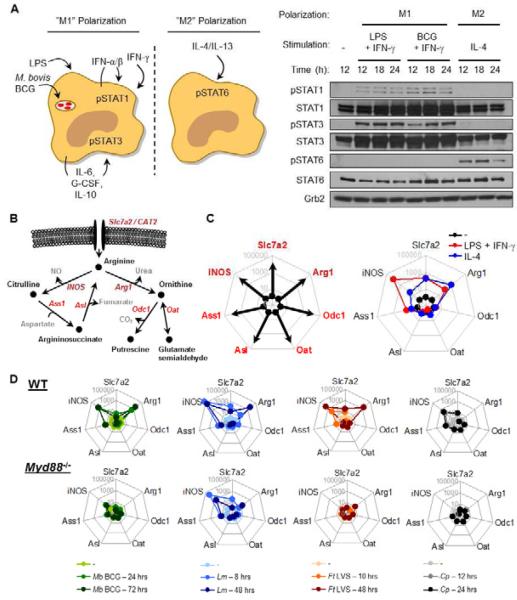
We first determined the gene expression profile of the known enzymes and transporters involved in arginine metabolism under M1 and M2 polarizing conditions (Murray and Wynn, 2011b). In primary bone marrow derived-macrophages (BMDMs) stimulated with LPS + IFN-γ for 24 hours to induce M1 activation as defined by the pattern of STAT phosphorylation (pSTAT1, pSTAT3, low or undetectable pSTAT6, Fig. 1A), iNOS, Slc7a2, Arg1 and Ass1 were induced, while Asl, Odc1, and Oat were repressed (Fig 1B, C). M2 polarized macrophages, stimulated with IL-4 (pSTAT6, low or undetectable pSTAT1, pSTAT3, Fig. 1A), displayed low NO output coupled with increased Slc7a2 and Arg1 transcription, but minimal changes in iNOS, Ass1, Asl, Odc1, and Oat (Fig 1B, C). Consistent with the gene expression data, only LPS + IFN-γ stimulation resulted in NO production (Fig. S1A), and protein levels of Asl, Odc1, and Oat remained constant during both M1 and M2 polarization (Fig. S1B).
Figure 1. Transcriptional regulation of arginine metabolism in activated macrophages.
(A) Left, Schematic of M1 and M2 macrophage polarization. Right, BMDMs were left untreated (-), or stimulated with LPS + IFN-γ, M. bovis BCG + IFN-γ, or IL-4 for the indicated times. Cell lysates were analyzed by immunoblot.
(B) Predicted model of arginine metabolism in macrophages.
(C) Left, Example transcriptome analysis. Right, BMDMs (n = 3) were untreated (-) or stimulated with LPS + IFN-γ or IL-4 for 24 hours. RNA was collected and analyzed by qRT-PCR.
(D) Primary macrophages from C57Bl/6 or Myd88-/- mice were untreated (-) or infected with M. bovis BCG (n=3, MOI = 100), L. monocytogenes (n=2, MOI = 10), F. tularensis LVS (n=3, MOI = 5), or C. psittaci (n≥2, MOI = 1) for the indicated times. RNA was collected and analyzed by qRT-PCR.
Data are representative of at least two experiments.
See also Figure S1.
Intracellular infection produces predictable changes in the expression of genes encoding arginine metabolizing enzymes
Whereas the above studies in M1 and M2 polarized macrophages gave a clear picture of the expression profiles of mRNAs encoding arginine metabolizing enzymes, little is known about the changes that occur in gene expression when macrophages are parasitized by different intracellular pathogens. Therefore, we expanded our gene expression analysis to model pathogens that adopt four distinct intracellular lifestyles (Table 1) (Hingley-Wilson and Lalvani, 2008; Hybiske and Stephens, 2008; Ray et al., 2009). Primary macrophages were infected with M. bovis BCG (Mb BCG), Listeria monocytogenes (Lm), Francisella tularensis live vaccine strain (Ft LVS), or Chlamydia psittaci (Cp) over time, followed by gene expression analysis by qRT-PCR (Fig 1D, bar graphs in Fig. S1D). We found that M. bovis, L. monocytogenes, and F. tularensis induced a distinct and predictable pattern of gene expression characterized by early expression of iNOS, Ass1, and Slc7a2 and delayed expression of Arg1. While macrophages can transport and metabolize arginine via Slc7a1/CAT1 and Arg2, respectively, their expression at the mRNA level was unaltered implying a predominant role for Slc7a2 and Arg1 during these infections (Fig. S1E). Using MyD88-deficient macrophages and a reciprocal supernatant transfer assay system, we found that most gene expression changes could be accounted for by MyD88-dependent production of host cytokines in combination with type I IFN signaling (Table 1, Fig. 1D, S1F,G). Although MyD88-deficient macrophages displayed decreased iNOS mRNA following L. monocytogenes infection, iNOS gene expression was absent in infected Ifnar-/- macrophages demonstrating the necessity for IFN signaling in this model (Fig. S1H), consistent with previous findings using Ifnb-/- macrophages (Toshchakov et al., 2002). Arg1, while induced following infection with M. bovis, F. tularensis, and L. monocytogenes, is regulated by two extrinsic autocrine-paracrine pathways controlled by MyD88-mediated production of STAT3-activating cytokines (M. bovis, L. monocytogenes (Fig. S1I)) or MyD88-mediated production of IL-4 and IL-13 (F. tularensis) (Qualls et al., 2010; Shirey et al., 2008). C. psittaci, an obligate intracellular pathogen that lives and develops within an inclusion membrane, caused minimal changes in gene expression of the arginine metabolizing enzymes suggesting this pathogen has evolved to induce limited activation of macrophages, similar to Leishmania sp (Zhang et al., 2010). Collectively, our data argue that the host response to intracellular infection is stereotypic and involves activation of arginine transport and the expression of genes encoding the six regulated arginine metabolizing enzymes and that this gene expression pattern is predominantly regulated by host activation of cell extrinsic pathways that are controlled by MyD88- and IFN-mediated signaling.
Table 1.
Selected Requirements for Macrophage Arginine Metabolism Transcriptome Regulation
| Slc7a2 | iNOS | Ass1 | Arg1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Intracellular Lifestyle | Exit Strategy | MyD88 Required | IFN Required | MyD88 Required | IFN Required | MyD88 Required | IFN Required | MyD88 Required | Additional Cytokine Req. |
| Mycobacterium bovis BCG | Endosomal / Phagosomal | Inhibited cellular egress (as compared to M. tuberculosis) | Yes | Yes | Yes | Yes | ? | Yes | IL-6, G-CSF, IL-10 | |
| Listeria monocytogenes | Cytosolic | Actin-based protrusion / Pyroptosis Induction | Partial | No | Partial I | Yes | No | ? | Yes | Stat3-inducing likely |
| Francisella tularensis LVS | Cytosolic | Induction of Apoptosis / Pyroptosis | Yes | ? | Yes | ? | Yes | ? | Yes | IL-4, IL-13 |
| Chlamydia psittaci | Endosomal / Inclusion membrane | Cellular lysis / Vacuolar Extrusion | Yes | ? _ | Yes | ? | Yes | ? | Not Induced | |
Quantification of arginine-derived metabolites
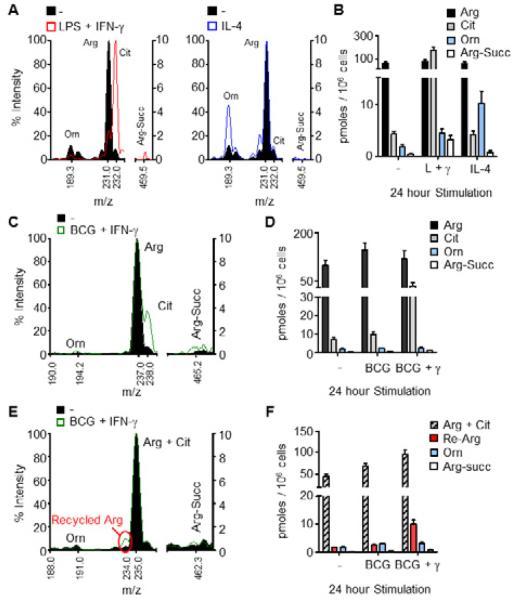
How gene expression is linked to arginine metabolism was next investigated using quantitative methodologies to measure arginine catabolism. The transcriptome data suggested that macrophages stimulated with LPS + IFN-γ or IL-4 should produce high relative amounts of intracellular citrulline or ornithine, respectively. To test this, macrophages were left untreated, or stimulated for 24 hours with LPS + IFN-γ or IL-4, and the cell lysates were subjected to mass spectrometry (MS) analysis to detect arginine and its direct metabolites (Fig. S2A). Unstimulated BMDMs contained arginine, but lower amounts of citrulline, ornithine, and argininosuccinate (Fig. 2A, B). M1 polarized macrophages had increased intracellular citrulline at the expense of arginine, while argininosuccinate and ornithine amounts were modestly increased (Fig. 2A, B). Conversely, M2 polarization resulted in an increase in ornithine production, with unchanged citrulline and argininosuccinate (Fig. 2A, B).
Figure 2. Detection of arginine metabolites by mass spectrometry (MS).
(A and B) BMDMs were untreated (-) or stimulated with LPS + IFN-γ or IL-4 for 24 hours, and cell lysates were analyzed by MS for arginine (Arg), citrulline (Cit), argininosuccinate (Arg-Succ), and ornithine (Orn). Sample mass spectra are shown (A) with the corresponding concentrations of indicated metabolites (B).
(C-F) BMDMs were infected with BCG with or without IFN-γ in the presence of heavy 13C-arginine (C, D) or 15N-arginine (E, F) for 24 hours, and cell lysates were analyzed by MS as above. The right y-axis was used for MS analysis of argininosuccinate (C, E). Data are representative of at least 3 experiments (A, B) or one experiment (C-F) with BMDMs pooled from at least 4 mice. Recycled arginine (Re-Arg).
Error bars, SEM.
See also Figure S2.
Citrulline is exported from NO-producing macrophages
We followed arginine and its metabolites after infection with M. bovis BCG to explore the arginine metabolic signature during intracellular infection. M. bovis BCG was chosen as the model system for the majority of the subsequent studies because unlike L. monocytogenes, F. tularensis, or M. tuberculosis, BCG does not induce apoptosis or necrosis in vitro in the time frame of our experiments, but is still controlled by the host IFN-γ-NO pathway (Hingley-Wilson and Lalvani, 2008; Ramakrishnan, 2012). BMDMs cultured in 13C-arginine as the sole source of arginine were left untreated, or infected with M. bovis BCG with and without IFN-γ for 24 hours. The peak for 12C-arginine disappeared and the expected peak for 13C-arginine appeared when heavy arginine was substituted for normal arginine in the medium (Fig. 2C, D, S2A). BCG-infected BMDMs contained similar amounts of arginine metabolites as the unstimulated BMDMs. Only when IFN-γ was added did intracellular citrulline increase (Fig. 2C, D). iNOS-mediated arginine metabolism results in one molecule of nitric oxide (NO) plus one molecule of citrulline. Yet, when comparing NO production to the amount of intracellular citrulline, the estimated NO output was greater than 100-fold that of intracellular citrulline (Fig. S2B, S2C). Although the Griess assay used to measure NO production detects NO2- and not additional NO derivatives, this estimation of NO output led us to believe that we were not optimally detecting citrulline production. One possibility was that following macrophage stimulation and NO production, citrulline was utilized by Ass1 in arginine recycling, which would result in continuous NO accumulation. Secondly, citrulline may be exported from macrophages following NO production (Fig. S2D).
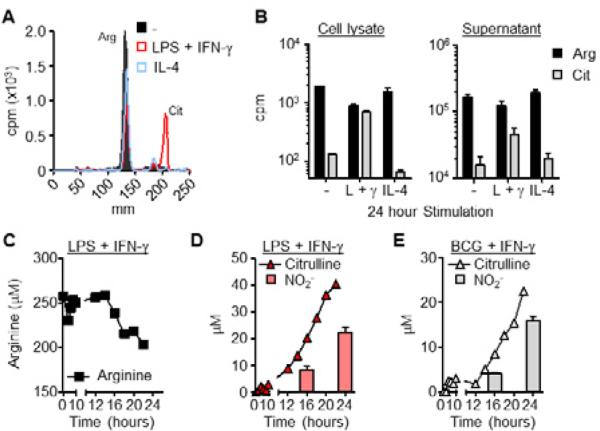
The contribution of recycling to arginine metabolism was addressed by culturing BMDMs infected with M. bovis BCG in media with 15N-arginine the sole source of arginine, resulting in a fragment ion m/z of 235 for imported arginine and citrulline, and 234 for recycled arginine. (Fig. S2A, E). Only a minute amount of intracellular arginine was derived from the recycling pathway (Fig. 2E, F). We next tested if citrulline was exported from macrophages following NO production using radioactive arginine tracing and thin layer chromatography (TLC). BMDMs, cultured in media containing 3H-arginine, were stimulated for 24 hours with LPS + IFN-γ or with IL-4 as a control. Arginine metabolites were analyzed from cell lysates and supernatants. Similar to the data obtained by MS, intracellular citrulline was increased in LPS + IFN-γ stimulated BMDMs compared to unstimulated and IL-4 stimulated controls (Fig. 3A, B). However, >98% of the synthesized citrulline was exported from macrophages into the extracellular milieu (cell lysate citrulline = 6.8×102 +/- 0.6×102 cpm, supernatant citrulline = 4.6×104 +/- 1.1×104 cpm), confirming the preference for citrulline export versus arginine recycling under these conditions (Fig. 3B). We then determined the kinetics of citrulline export following stimulation with LPS + IFN-γ. As expected there was a decrease in extracellular arginine over time that began between 12 and 16 hours post-stimulation (Fig. 3C). This correlated with an increase in extracellular citrulline and NO that matched the kinetics of arginine depletion from the media (Fig. 3D). We observed the same trend in IFN-γ–stimulated BMDMs infected with M. bovis BCG (Fig. 3E). These data were verified by MS analysis of citrulline export under the same stimulation conditions in both BMDMs and thioglycolate elicited peritoneal-derived macrophages (PDMs) (Fig. S3). Thus, macrophages import extracellular arginine and export citrulline, rather than recycle arginine, for NO production following pathogenic stimuli or intracellular infection.
Figure 3. Citrulline is exported from macrophages following iNOS-mediated arginine metabolism.
(A and B) BMDMs (n=3) were untreated (-) or stimulated with LPS + IFN-γ or IL-4 in the presence of 3H-arginine for 24 hours. Levels of arginine and its metabolites were determined by thin layer chromatography (TLC) followed by scintillation counts per minute (cpm). A sample TLC is shown (A) with the corresponding relative amounts of intracellular and extracellular arginine and citrulline (B). Note the difference in scale from the cell lysate and supernatant graphs. Data are the mean cpm.
(C-E) BMDMs (3 mice pooled) were stimulated with LPS + IFN-γ or BCG + IFN-γ in the presence of 3H-arginine over time. Extracellular levels of arginine (C) and citrulline (D and E) were determined by TLC as above. NO production was determined by assaying supernatant NO2- (D and E).
Error bars, SD.
See also Figure S3.
Arginine starvation primes macrophages for citrulline metabolism
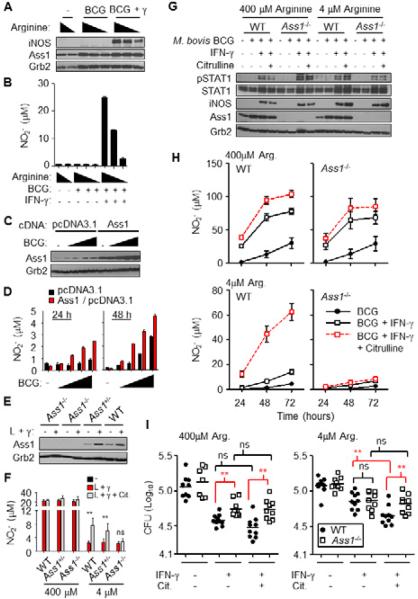
Ass1 is the enzyme responsible for citrulline metabolism in mammals (Curis et al., 2005; Morris, 2007). As predicted from qRT-PCR data, Ass1 protein increased following infection with M. bovis BCG and was not regulated either by the addition of IFN-γ or the amount of arginine in the media, whereas iNOS protein production required both BCG and IFN-γ (Fig. 4A). Ass1 production following M. bovis BCG infection itself was not sufficient for NO production, but as expected required iNOS protein to facilitate the arginine to citrulline reaction (Fig. 4A, B). However, during infection with M. bovis BCG, Ass1 overexpression in RAW 264.7 macrophages was sufficient to enhance NO production at 24 and 48 hours post-infection compared to vector only controls (Fig. 4C, D).
Figure 4. Ass1 regulates NO production and is required for optimal control of M. bovis BCG in arginine-deprived macrophages.
(A) BMDMs (3 mice pooled) cultured in media with 400μM, 4μM, or no added arginine, were infected with BCG with and without IFN-γ, or left untreated (-) for 24 hours. Cell lysates were analyzed by immunoblot (A) and NO production was determined by assaying supernatant NO2- in duplicate (B). Data are representative of at least two experiments.
(C and D) RAW 264.7 macrophages overexpressing Ass1 or the vector backbone (pcDNA3.1) were infected with BCG or left untreated (-) over time. (D) Overexpression of Ass1 was determined by immunoblot at 48 hrs post-treatment. (D) NO production was determined by assaying supernatant NO2-. Data are representative of at least two experiments.
(E and F) FLDMs from Ass1+/+ (WT, n = 4), Ass1+/- (n = 8), and Ass1-/- (n = 4), were cultured in media with 400μM or 4μM arginine with or without 1mM citrulline. Macrophages stimulated for 24 hours with LPS + IFN-γ (L + γ) were assayed for Ass1 protein by immunoblot (E) and NO production (F). Data are combined from 2 experiments.
(G-I) FLDMs from Ass1-/- mice or littlermate Ass1+/+ controls (WT) were cultured in media with 400 M or 4 M arginine. FLDMs were infected with BCG with or without IFN-γ for 72 hours in the presence or absence of 1mM citrulline. Cell lysates were assayed by immunoblot (G), and NO production was determined by assaying supernatant NO2- (H). BCG from lysed macrophages were grown for 2-3 weeks on LH10 agar (I). Data from (I) are the BCG colony forming units (CFU) from individual FLDM lysates of two independent experiments combined (n = 10).
Error bars, SD. ** p < 0.01 by Student's t test; ns, not significant.
See also Figure S4.
We next used conventional Ass1-/- mice (Patejunas et al., 1994). This mutation results in plasma hypercitrullinemia and death shortly following birth in mice as Ass1 is required in the urea cycle. We generated fetal liver-derived macrophages (FLDMs) from Ass1-/-, Ass1+/-, and Ass1+/+ (WT) embryos and stimulated them with LPS + IFN-γ for 24 hours under “arginine plentiful” concentrations (400 μM) or “arginine starved” conditions (4 μM arginine). While plasma arginine amounts average 100 μM, concentrations in other tissues have not been thoroughly examined. We therefore set our experimental conditions to test two extremes of arginine availability. We found no differences in NO production between Ass1 and control FLDMs when arginine was plentiful. However, when citrulline was added to the low arginine media, WT and Ass1+/- FLDMs displayed enhanced NO production compared to FLDMs without added citrulline. FLDMs from Ass1-/- mice, on the other hand, failed to produce more NO when citrulline was added to the media. Moreover, the effect of citrulline addition on increased NO production occurred in arginine starved macrophages and not in arginine plentiful conditions, suggesting citrulline metabolism contributes to macrophage arginine biosynthesis and NO production only under conditions of arginine scarcity (Fig. 4E, F).
We next extended our studies to test the requirement for Ass1 activity during anti-microbial functions of macrophages. Under arginine plentiful and starved conditions, FLDMs from Ass1-/- and Ass1+/+ controls were infected with M. bovis BCG without and with the addition of IFN-γ and citrulline. Infection of Ass1+/+ FLDMs resulted in increased Ass1 protein compared to uninfected FLDMs, and its expression was not altered further by the addition of IFN-γ or citrulline (Fig 4G). While some NO was detected from BCG infection alone, this response was amplified in conditions with IFN-γ (Fig. 4H). In arginine plentiful conditions, both Ass1+/+ and Ass1-/- FLDMs produced NO following BCG infection. Whereas Ass1+/+ FLDMs utilize extracellular citrulline to regenerate arginine for NO production, Ass1-/- FLDMs were unable to perform this reaction (Fig. 4H).
72 hrs following infection, macrophage lysates were plated for BCG growth to determine the effect of added citrulline on mycobacterial killing. In arginine plentiful conditions, Ass1+/+ FLDMs increased their capacity to kill BCG in the presence of IFN-γ, yet added citrulline had no significant anti-microbial effect (Fig. 4I). Ass1-/- FLDMs had less killing capacity, which correlated with the reduced NO production during infection (Fig. 4H, I). In arginine starved cultures there was a significant increase in killing when extracellular citrulline was added to the Ass1+/+ FLDMs, yet Ass1-/- FLDMs did not show enhanced killing, consistent with previous data showing Trypanosoma cruzi can be killed by macrophages when exogenous citrulline is added to media containing low arginine (Norris et al., 1995). Similar data were obtained from Ass1-deficient BMDMs infected with M. bovis BCG (Fig. S4A, B), yet Ass1-deficient macrophages infected with either L. monocytogenes actA/inlB mutant or F. tularensis LVS displayed no significant difference in bacterial control compared to WT macrophages even though they were inhibited in NO production in low arginine media supplemented with citrulline (Fig. S4C, D). While NO is required for optimal control of mycobacterial infection, conflicting data exist on the necessity of NO for macrophage control of Listeria and Francisella, likely reflecting the differences we see in our experiments (Edwards et al., 2010; Lindgren et al., 2005; MacMicking et al., 1997a; Nathan and Shiloh, 2000; Polsinelli et al., 1994). Also, we cannot exclude the possibility of NO-independent mechanisms for Ass1 function during intracellular pathogenesis in macrophages. Nevertheless, these data imply that Ass1-mediated citrulline metabolism is not required for macrophage control of all intracellular pathogen types, thus we focused on the requirement for Ass1 during mycobacterial infection.
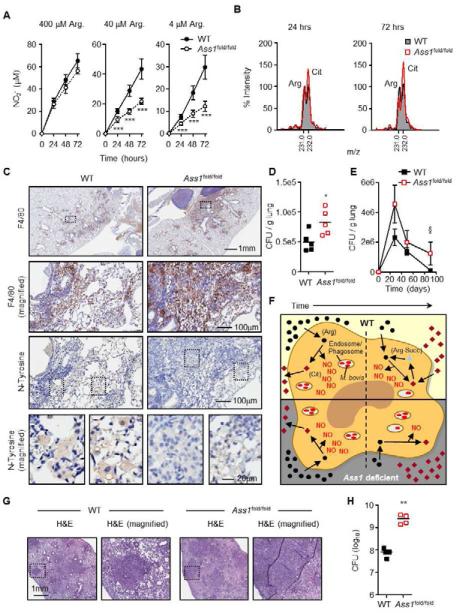
The Ass1 fold mutation is a hypomorphic allele of Ass1 that is not as severe as the mutation in Ass1-/- mice. Ass1fold/fold mice still succumb to death a few weeks following birth resulting from pathologies associated with liver and brain dysfunction but provided us with a convenient experimental tool to establish bone marrow (BM) chimeras (Perez et al., 2010). Therefore, we generated chimeric mice by BM transplantation from Ass1+/+ and Ass1fold/fold donors (CD45.2) to lethally irradiated recipient mice (CD45.1). Greater than 90% chimerism was obtained by 5 weeks post-transplant and macrophages from these mice responded similarly to Ass1-/- FLDMs following M1 stimulation without and with added citrulline (Fig. S5A, B). As with Ass1-/- FLDMs, 24 hours following M1 stimulation there were no differences in NO output between WT and Ass1fold/fold macrophages (Fig. 5A). However, at 48 and 72 hours post-stimulation when extracellular arginine becomes limiting, Ass1fold/fold macrophages were impaired in NO generation when cultured in 40 M or 4 M arginine compared to WT cells, confirming that NO output from recycled arginine is only required in low extracellular arginine concentrations (Fig. 5A). As expected, citrulline in supernatants from Ass1fold/fold macrophages was increased compared to WT since WT macrophages use citrulline for arginine biosynthesis (Fig. 5B).
Figure 5. Ass1 is required for optimal NO output and control of infection in vivo.
(A and B). BMDMs from WT or Ass1fold/fold bone marrow chimeras (n = 3) were cultured in media with 400 μM, 40 μM, or 4 μM arginine and stimulated for the indicated times with LPS + IFN-γ. Culture supernatants were assayed for NO production (A) and relative amino acid levels by MS (B). Data in (B) are from cells cultured in 40 μM arginine and are normalized to WT arginine levels equal to 100% at each time point. Error bars, SD.(C and D). WT and Ass1fold/fold bone marrow chimeras (n = 6) were infected with an intranasal dose of M. bovis BCG containing 2-5×105 CFUs. 4-5 weeks following infection, lungs were analyzed by immunohistochemistry (C), and homogenized to determine CFUs (D). Data are representative of 3 experiments. (E). WT and Ass1fold/fold bone marrow chimeras (n = 3 per time point) were infected with an intranasal dose of M. bovis BCG containing ~10×106 CFUs. Lungs were homogenized at the indicated timepoints to determine CFUs. Data are from 1 experiment. Error bars, SEM. (F) Model of macrophage NO production via Ass1-mediated citrulline metabolism. In infected macrophages, either WT (top) or Ass1-deficient (bottom), extracellular arginine is preferentially utilized to generate NO via iNOS, and citrulline is exported. Over time, arginine becomes limiting, and WT macrophages utilize Ass1-mediated citrulline metabolism to regenerate arginine as a secondary source for NO production – a mechanism that is lost in Ass1-deficient macrophages, resulting in decreased NO levels and increased bacterial load.
(G and H) WT (n = 5) and Ass1fold/fold (n = 4) bone marrow chimeras were infected with an aerosol dose of M. tuberculosis containing ~ 100 CFUs. Mice were sacrificed 4 weeks post-infection and lungs were analyzed by histology (G) and homogenized to determine CFUs (H). Data are from 1 experiment.
Error bars, SD (SEM in E). *** p < 0.001, ** p < 0.01, * p < 0.05 by Student's t test. § p < 0.05 by two-way ANOVA.
See also Figure S5.
To determine the in vivo role of Ass1 during infection, we intranasaly infected WT and Ass1fold/fold BM chimeras with M. bovis BCG. Mice showed no clinical symptoms of distress while infected. Mice were euthanized 4-5 weeks following infection and displayed lung pathology associated with infection, yet Ass1fold/fold chimeras had increased inflammation and macrophage F4/80 staining compared to WT (Fig. 5C, S5C-E). Most striking was that in the macrophage rich areas, Ass1fold/fold chimeras had reduced ability to produce NO to the same degree as in WT as determined by nitrotyrosine staining (Fig 5C, S5F). Proper regulation of NO production is required for optimal control of mycobacteria in vivo (El Kasmi et al., 2008; MacMicking et al., 1997b), so we tested whether Ass1fold/fold chimeras are impaired in controlling BCG. Lungs from chimera mice infected with M. bovis BCG were homogenized and plated to determine CFUs (Fig. 5D). Ass1fold/fold chimeric mice were significantly impaired at controlling M. bovis BCG, supporting the key role for NO in regulating mycobacterial infection. We expanded these experiments to test the the efficiency of mycobacterial clearance. Mice were infected with a high dose of M. bovis BCG, and lung CFUs were determined across time. While WT and Ass1fold/fold BM chimera mice displayed decreased mycobacterial loads across time, Ass1fold/fold mice were significantly slower in clearing BCG compared to WT (Fig. 5E). Data from these experiments lead to the model depicted in Fig. 5F, in which we imply the necessity for Ass1-mediated citrulline metabolism for NO production and optimal control of M. bovis BCG under arginine scarcity. We next tested this model during virulent M. tuberculosis infection. WT and Ass1fold/fold bone marrow chimeras were infected with an aerosol dose of M. tuberculosis and lungs were analyzed for pathology and CFUs 4 weeks post infection. As with BCG infection there were no obvious clinical differences between WT and Ass1fold/fold mice during the course of infection, however, Ass1fold/fold mice displayed increased lung inflammation combined with decreased infection control (Fig. 5G, H).
Discussion
Macrophage activation stimulates arginine import, which fuels the initial burst of NO synthesis to suppress intracellular pathogens consistent with older studies performed on the CAT2 system (Mori and Gotoh, 2000). Concurrent to arginine import and oxidation to NO, we found that citrulline is exported during the early stages of NO biosynthesis from arginine rather than being used to regenerate arginine. As time progresses, arginine is depleted from the milieu and citrulline import followed by its conversion to arginine by Ass1 and Asl is required to sustain NO biosynthesis. Thus, we concluded that Ass1/Asl expression functions as a fail-safe system to sustain NO production in the event extracellular arginine is depleted from the environment. In the absence of Ass1 activity, the initial phase of NO synthesis is supported by extracellular arginine, but the resulting citrulline cannot be scavenged from the environment to sustain NO production for longer periods of time (Fig. 5F). The early induction of Ass1 following macrophage activation suggests that the switch from arginine to citrulline utilization for NO production may be frequently encountered in tissue microenvironments where pathogens are encountered. The impaired pathogen control in the Ass1fold/fold chimeric mice provide compelling in vivo data to support this idea, but critically testing this concept by measuring intracellular and extracellular arginine and citrulline concentrations within restricted host microenvironments will require high-resolution MS tissue imaging capabilities that are in the early stages of development.
Four separate intracellular infections – M. bovis BCG, F. tularensis LVS, L. monocytogenes, and C. psittaci – induce the arginine metabolism transcriptome in macrophages to varying degrees, yet one commonality present in all infection types was the pattern of Ass1 induction (Fig. 1, S1). These data suggest that macrophages prime for arginine recycling early during infection to prepare for limiting extracellular arginine. This type of regulation would be expected for a gene involved in the anti-microbial response. Consistent with this idea, multiple studies have separated LPS-induced genes in macrophages as either “tolerizeable” (genes turned off following extensive LPS stimulation) or “non-tolerizeable” (genes remaining elevated, or further induced following extensive LPS stimulation) (Foster et al., 2007; Henricson et al., 1993). Ass1 was classified as non-tolerizeable by Foster et al., suggesting its importance in prolonged exposure to microbial products or infection. Even though Ass1 expression is induced via TLR stimulation and M. bovis BCG infection, arginine recycling does not occur in substantial amount during the first 24 hours when extracellular arginine is readily available (Fig. 2), and citrulline produced from iNOS is exported following its formation (Fig. 3), similar to that observed in rat alveolar macrophages (Hammermann et al., 1998).
Citrulline detection in culture supernatants from macrophages stimulated with TLR ligands was reported over two decades ago (Benninghoff et al., 1991; Hibbs et al., 1988), and shortly thereafter citrulline conversion into arginine at the cellular level was described in primary rat macrophages (Wu and Brosnan, 1992). Also from this time the initial gene regulation of Ass1 and Asl following inflammatory stimuli and TLR ligation in primary macrophages and macrophage cell lines was described (Mori, 2007; Nussler et al., 1994). Aside from a key report from Morris and colleagues, which showed that media citrulline accounted for NO-dependent killing of Trypanosoma cruzi parasites (Norris et al., 1995), little work has been performed to connect arginine recycling and pathogenesis in macrophages in vitro or in vivo. Why do activated macrophages expel citrulline? There are several plausible answers to this question. First, in macrophages generating high amounts of NO, citrulline could act as a feedback inhibitor of iNOS and thereby reduce the efficiency of the enzyme. Expelling citrulline from the cell would remove the inhibitor and potentially allow faster conversion of arginine to NO. To our knowledge this facet of iNOS enzymology has not been documented in kinetic assays using purified protein. However, most NOS inhibitors are chemical variants of arginine and citrulline. A second possibility is that citrulline is expelled to return to the liver for shunting into the urea cycle. Alternatively, citrulline may be toxic to other macrophage cellular processes or components. However, this seems unlikely given that macrophages re-import and metabolize citrulline once arginine becomes limiting. T cells also utilize extracellular citrulline to sustain proliferation during arginine starvation, suggesting intercellular sharing of this amino acid between macrophages and lymphocytes (Bansal et al., 2004). Full resolution of the citrulline export problem will first require the identification of the mechanisms involved in transport of citrulline to the extracellular milleu. While citrulline transport has been examined previously, no exclusive citrulline transporter has been identified. Instead, entry of citrulline occurs via broad neutral amino acid soluble carriers that are highly tissue specific (Curis et al., 2005; Hediger et al., 2004). Macrophages use both sodium-dependent and -independent transporters for citrulline import (Baydoun et al., 1994), yet whether citrulline export utilizes a similar set of transporters, or if an exclusive citrulline transporter exists in macrophages is yet to be determined.
We anticipated that macrophages with modified Ass1 would display altered citrulline metabolism because the only known enzyme that utilizes citrulline in mammalian cells is Ass1 (Curis et al., 2005; Morris, 2007). Overexpression of Ass1 in RAW 264.7 cells increased NO production, even in arginine-rich media, demonstrating that Ass1 protein levels affect arginine biosynthesis in macrophages. Conversely, genetic ablation of Ass1 had little effect on NO production in primary macrophages in arginine plentiful conditions, yet Ass1-deficient macrophages were unable to produce NO in arginine starved conditions when citrulline was present in the media (Fig. 4). This loss of arginine biosynthesis was relevant during infection because Ass1-deficient FLDMs and BMDMs were less able to control M. bovis BCG growth in vitro (Fig. 4, S4). We determined that Ass1 activity was critical during lung infection with M. bovis BCG by showing that Ass1fold/fold bone marrow chimeras produced stunted levels of NO, determined in vitro and by nitrotyrosine staining on lung sections, and were impaired at suppressing M. bovis BCG. Finally, we established the requirement for Ass1 during virulent M. tuberculosis infection as Ass1-deficient mice were significantly impaired at controlling infection compared to WT mice (Fig. 5). These data suggest an opportunity for molecular targeting as a form of therapy in mycobacteria-infected patients. We speculate the upregulation of Ass1, or possibly Asl, in alveolar macrophages in conjunction with current anti-tuberculosis therapy may allow increased NO production and assist in controlling M. tuberculosis.
Methods and Materials
In vitro pathogen/macrophage cocultures
BMDMs or thioglycollate elicited PDMs were prepared and infected in vitro with M. bovis bacillus Calmette-Guérin Pasteur strain (Mb BCG), Listeria monocytogenes LO28 (Lm), Francisella tularensis live vaccine strain (Ft LVS), and Chlamydia psittaci (Cp) as described (Miyairi et al., 2007; Qualls et al., 2010; Reutterer et al., 2008; Shirey et al., 2008). Reciprocal supernatant transfers: Sterile supernatants from the above infections were used to stimulate BMDMs from C57Bl/6 or Myd88-/- mice. FLDMs were obtained by collecting livers from Ass1+/+, Ass1+/-, and Ass1-/- embryos at E14 and culturing in L-cell conditioned media for 5-6 days. For killing assays, Mb BCG-macrophage co-cultures were washed with PBS, and cell lysates were prepared in 1 ml PBS + 0.25% Tween 20. Lysates received four, 30 second-rounds of sonication at 25-30 W, and 200 μl of the sonicate was plated on OADC enriched 7H10 Middlebrook agar plates. CFUs were counted following 12 to 16 days incubation at 37°C + 5% CO2. Macrophage stimulant concentration: E. coli LPS (100 ng/ml, Sigma), IFN-γ (2 ng/ml, BD Biosciences), and IL-4 (10 ng/ml, BD Biosciences).
In vivo mycobacterial infection
M. bovis BCG was administered intranasally in a 50 μl inoculum. Tissues were analyzed 4-13 weeks post infection. Lungs were homogenized in PBS and plated in 10-fold serial dilutions on 7H10 Middlebrook agar plates supplemented with OADC. CFUs were counted following 2.5 weeks incubation at 37°C. Lungs from some mice were inflated with 10% buffered formalin for histology. A low dose aerosol infection with Erdman M. tuberculosis (Trudeau Institute) was performed using a whole body inhalation exposure system (Glas-Col, Terre Haute, IN) that routinely yields ~100 CFU day 1 post infection. At 4 weeks post-infection, the animals were sacrificed and the right superior lobe of the lung was homogenized in PBS containing 0.05% Tween-80. Serial dilutions of the homogenates were plated onto 7H11 agar. The plates were incubated at 37°C and colonies counted after 21 d. For histopathological analysis, the left lung lobe was fixed in 4% paraformaldehyde and paraffin embedded until further processing.
Mass spectrometry (MS)
Cell and supernatant preparation
Intracellular: 10 × 106 to 15 × 106 BMDMs were plated on 10 cm tissue culture dishes in C-DMEM and stimulated as described in the text. Cells were washed twice with cold PBS on ice, and lysed with 1 ml cold methanol per plate. Cell debris was pelleted, and methanol supernatants were collected. Extracellular: 0.5 × 106 to 1.5 × 106 BMDMs or PDMs were stimulated as described in the text. Supernatants were collected and mixed with methanol (1:1, v/v). [U-13C]-, and [U-15N]-Arginine were obtained from Cambridge Isotope Laboratories, Inc. (Andover, MA). Preparation of butyl esters of amino acids. Methanol from the samples was evaporated and the residue was resuspended in 300 μl of 100% ethanol. Ethanol was evaporated and the amino acid butyl esters were made (Johnson, 2001). The residue was resuspended in 200 μl of n-Butanol /3N hydrogen chloride. The mixture was heated at 65°C for 15 minutes and evaporated. Solvents were evaporated by speed-vac (Savant). The residue was dissolved in 400 μl of acetonitrile/water/formic acid (50:50:0.1, v/v/v). The sample was filtered through a 0.22 μm syringe filter and stored at 4°C until further use. For MS the samples were diluted 1:10 in methanol/water (50:50, v/v). Propyl ester of arginine and heavy arginine were used as internal standards at 300 nM final concentration. These were prepared similar to the butyl ester except n-Propanol/3N hydrogen chloride was used to make the ester. Electrospray ionization and tandem MS. MS data was obtained using a Thermo Finnigan TSQ Quantum Ultra triple quadrupole mass spectrometer (Thermo Fisher Scientific). Analysis was carried out in the positive ESI selected reaction monitoring (SRM) mode with unit resolutions at both Q1 and Q3 set at 0.70 full width at half maximum. A parent scan for the product size of 70 was done for the light arginine and 74 for the heavy arginine experiments. The parent compounds analyzed were ornithine, arginine, citrulline and argininosuccinic acid (for MS parameters, see Supplemental Methods). The data were acquired and analyzed using QuantumTune software version 1.2 (Thermo Fisher Scientific). Data were analyzed by calculating the area under the curve of the ion current peaks of the internal standard and sample.
Thin layer chromatography (TLC)
Macrophages were grown in DMEM media with 10% FBS supplemented with 400 μM arginine (containing 40 μCi 3H-arginine) and stimulated with LPS + IFN-γ or IL-4. Cells were lysed in methanol, evaporated to dryness in a speedvac and resuspended in 10 μl of 50% methanol-water (v/v). The neat supernatant and the cell fraction were spotted on pre-activated Silica gel-G plates. TLC plates were developed with chloroform: methanol: ammonium hydroxide: water (1:4:2:1 v/v/v/v). For the time course experiments with 3H-arginine the supernatants were dried and made into butyl esters as described above. Samples were resuspended in 25 μl of methanol: water (50:50 v/v) and 5 μl was spotted on Silica gel-G plates. The TLC was developed with chloroform: methanol: ammonium hydroxide (3:1:0.02 v/v/v). Standards of arginine, citrulline, and ornithine were run in parallel along with the samples and detected by spraying with ninhydrin for all TLC experiments. Radioactive arginine derivatives in the TLC plates were detected using a radio-TLC Imaging Scanner (Bioscan AR-2000), scanning for 10 minutes per lane.
RNA analysis
RNA was collected using Trizol (Invitrogen) or TriPure (Roche). cDNA was synthesized using SuperScript II reverse transcriptase (Invitrogen) and analyzed by qRT-PCR. Data are presented as the mean amplicon values normalized to Gapdh and presented as fold change to untreated.
Cell culture with defined arginine
Dulbecco's Modified Eagle's Medium (DMEM) lacking arginine, leucine, and lysine (Sigma, D9443) was supplemented to nutrient levels in standard DMEM (Mediatech, 10-013-CV) used for cell culture. Arginine was supplemented at either 400 μM, 40 μM, or 4 μM as needed. Media was made “complete” by adding 10% dialyzed fetal bovine serum (Gibco, 26400) which lacked additional arginine. Arginine in the media were verified by mass spectrometry.
NO and Urea production
Nitric oxide production was determined my measuring supernatant NO2- with Greiss reagent. Titrated sodium nitrite was used as a standard. Urea production (QuantiChrom Urea Assay Kit, BioAssay Systems USA) was not assayed as extracellular citrulline inhibited urea detection (Fig. S1C).
Ass1 overexpression in RAW 264.7 cells
1×107 RAW 264.7 cells mixed with either 20 μg pcDNA3.1 (empty vector) or 20 μg pcDNA3.1 containing Ass1 cDNA were electroporated at 975 μF, 320 V. RAW 264.7 cell lines stably expressing these vectors were generated by growing in selection media (C-RPMI) containing 200 μg/ml hygromycin B. Colonies surviving hygromycin B selection were used in cell culture as described in the text and Ass1 overexpression was determined by immunoblot.
Immunoblot analysis and immunohistochemistry
Protein lysates were separated by Tris-HCl buffered 4-15% gradient SDS PAGE, followed by transfer to Protran membrane. Membranes were blocked in 3% milk in Tris-buffered saline plus 0.05% Tween and immunoblotted for respective proteins (see Supplemental Methods). Histology sections were stained by the St. Jude Veterinary Pathology Core using hematoxylin and eosin, standard staining for acid fast bacilli, and immunohistochemistry using antibodies against F4/80, CD3, B220 and nitrotyrosine.
Supplementary Material
Citrulline, a byproduct of nitric oxide (NO) synthesis, is exported from macrophages
Arginine is synthesized from imported citrulline to fuel NO synthesis in late infection
Macrophages lacking Ass1 fail to synthesize arginine from citrulline
Ass1 is required for optimal control of M. bovis BCG and M. tuberculosis
Acknowledgements
We thank D. Bush and P. Johnson of the Veterinary Pathology Core at SJCRH for immunohistochemistry, M. O'Riordan for RFP-expressing L. monocytogenes actA/inlB mutant, and J. Haverkamp for discussion and editing this manuscript. Work was supported by the National Institutes of Health (AI062921 to PJM, GM033496 to COR, CA138064 to JEQ), Cancer Center Core Grant (PJM), Hartwell Foundation Individual Biomedical Award (PJM), and the American Lebanese Syrian Associated Charities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bansal V, Rodriguez P, Wu G, Eichler DC, Zabaleta J, Taheri F, Ochoa JB. Citrulline Can Preserve Proliferation and Prevent the Loss of Cd3 Zeta Chain under Conditions of Low Arginine. JPEN J. Parenter. Enteral. Nutr. 2004;28:423–430. doi: 10.1177/0148607104028006423. [DOI] [PubMed] [Google Scholar]
- Baydoun AR, Bogle RG, Pearson JD, Mann GE. Discrimination between Citrulline and Arginine Transport in Activated Murine Macrophages: Inefficient Synthesis of No from Recycling of Citrulline to Arginine. Br. J. Pharmacol. 1994;112:487–492. doi: 10.1111/j.1476-5381.1994.tb13099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninghoff B, Lehmann V, Eck HP, Droge W. Production of Citrulline and Ornithine by Interferon-Gamma Treated Macrophages. Int. Immunol. 1991;3:413–417. doi: 10.1093/intimm/3.5.413. [DOI] [PubMed] [Google Scholar]
- Bogdan C. Nitric Oxide and the Immune Response. Nat. Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Casanova JL, Abel L. Genetic Dissection of Immunity to Mycobacteria: The Human Model. Annu. Rev. Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- Choi HS, Rai PR, Chu HW, Cool C, Chan ED. Analysis of Nitric Oxide Synthase and Nitrotyrosine Expression in Human Pulmonary Tuberculosis. Am. J. Respir. Crit. Care Med. 2002;166:178–186. doi: 10.1164/rccm.2201023. [DOI] [PubMed] [Google Scholar]
- Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Benazeth S, Cynober L. Almost All About Citrulline in Mammals. Amino Acids. 2005;29:177–205. doi: 10.1007/s00726-005-0235-4. [DOI] [PubMed] [Google Scholar]
- Edwards JA, Rockx-Brouwer D, Nair V, Celli J. Restricted Cytosolic Growth of Francisella Tularensis Subsp. Tularensis by Ifn-Gamma Activation of Macrophages. Microbiology. 2010;156:327–339. doi: 10.1099/mic.0.031716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrt S, Schnappinger D. Mycobacterial Survival Strategies in the Phagosome: Defence against Host Stresses. Cell. Microbiol. 2009;11:1170–1178. doi: 10.1111/j.1462-5822.2009.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kasmi KC, Qualls JE, Pesce JT, Smith AM, Thompson RW, Henao-Tamayo M, Basaraba RJ, Konig T, Schleicher U, Koo MS, et al. Toll-Like Receptor-Induced Arginase 1 in Macrophages Thwarts Effective Immunity against Intracellular Pathogens. Nat. Immunol. 2008;9:1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchetti F, Vermi W, Fiorentini S, Chilosi M, Caruso A, Duse M, Notarangelo LD, Badolato R. Expression of Inducible Nitric Oxide Synthase in Human Granulomas and Histiocytic Reactions. Am. J. Pathol. 1999;154:145–152. doi: 10.1016/S0002-9440(10)65261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlik M, Reutterer B, Schindler C, Greten F, Vogl C, Muller M, Decker T. Nonconventional Initiation Complex Assembly by Stat and Nf-Kappab Transcription Factors Regulates Nitric Oxide Synthase Expression. Immunity. 2010;33:25–34. doi: 10.1016/j.immuni.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster SL, Hargreaves DC, Medzhitov R. Gene-Specific Control of Inflammation by Tlr-Induced Chromatin Modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- Hammermann R, Bliesener N, Mossner J, Klasen S, Wiesinger H, Wessler I, Racke K. Inability of Rat Alveolar Macrophages to Recycle L-Citrulline to L-Arginine Despite Induction of Argininosuccinate Synthetase Mrna and Protein, and Inhibition of Nitric Oxide Synthesis by Exogenous L-Citrulline. Naunyn Schmiedebergs Arch. Pharmacol. 1998;358:601–607. doi: 10.1007/pl00005300. [DOI] [PubMed] [Google Scholar]
- Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The Abcs of Solute Carriers: Physiological, Pathological and Therapeutic Implications of Human Membrane Transport Proteinsintroduction. Pflugers Arch. 2004;447:465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- Henricson BE, Manthey CL, Perera PY, Hamilton TA, Vogel SN. Dissociation of Lipopolysaccharide (Lps)-Inducible Gene Expression in Murine Macrophages Pretreated with Smooth Lps Versus Monophosphoryl Lipid A. Infect. Immun. 1993;61:2325–2333. doi: 10.1128/iai.61.6.2325-2333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs JB, Jr., Taintor RR, Vavrin Z, Rachlin EM. Nitric Oxide: A Cytotoxic Activated Macrophage Effector Molecule. Biochem. Biophys. Res. Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hingley-Wilson S, Lalvani A. An Exit Strategy for the Tubercle Bacillus? Nat. Rev. Microbiol. 2008;6:954. doi: 10.1038/nrmicro1821-c1. [DOI] [PubMed] [Google Scholar]
- Hybiske K, Stephens RS. Exit Strategies of Intracellular Pathogens. Nat. Rev. Microbiol. 2008;6:99–110. doi: 10.1038/nrmicro1821. [DOI] [PubMed] [Google Scholar]
- Johnson DW. Analysis of Amino Acids as Formamidene Butyl Esters by Electrospray Ionization Tandem Mass Spectrometry. Rapid. Commun. Mass. Spectrom. 2001;15:2198–2205. doi: 10.1002/rcm.501. [DOI] [PubMed] [Google Scholar]
- Lee J, Ryu H, Ferrante RJ, Morris SM, Jr., Ratan RR. Translational Control of Inducible Nitric Oxide Synthase Expression by Arginine Can Explain the Arginine Paradox. Proc. Natl. Acad. Sci. USA. 2003;100:4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren H, Stenman L, Tarnvik A, Sjostedt A. The Contribution of Reactive Nitrogen and Oxygen Species to the Killing of Francisella Tularensis Lvs by Murine Macrophages. Microbes Infect. 2005;7:467–475. doi: 10.1016/j.micinf.2004.11.020. [DOI] [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric Oxide and Macrophage Function. Annu. Rev. Immunol. 1997a;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of Nitric Oxide Synthase as a Protective Locus against Tuberculosis. Proc. Natl. Acad. Sci. USA. 1997b;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional Profiling of the Human Monocyte-to-Macrophage Differentiation and Polarization: New Molecules and Patterns of Gene Expression. J. Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- Miyairi I, Tatireddigari VR, Mahdi OS, Rose LA, Belland RJ, Lu L, Williams RW, Byrne GI. The P47 Gtpases Iigp2 and Irgb10 Regulate Innate Immunity and Inflammation to Murine Chlamydia Psittaci Infection. J. Immunol. 2007;179:1814–1824. doi: 10.4049/jimmunol.179.3.1814. [DOI] [PubMed] [Google Scholar]
- Moller M, Nebel A, Valentonyte R, van Helden PD, Schreiber S, Hoal EG. Investigation of Chromosome 17 Candidate Genes in Susceptibility to Tb in a South African Population. Tuberculosis (Edinb) 2009;89:189–194. doi: 10.1016/j.tube.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Mori M. Regulation of Nitric Oxide Synthesis and Apoptosis by Arginase and Arginine Recycling. J. Nutr. 2007;137:1616S–1620S. doi: 10.1093/jn/137.6.1616S. [DOI] [PubMed] [Google Scholar]
- Mori M, Gotoh T. Regulation of Nitric Oxide Production by Arginine Metabolic Enzymes. Biochem. Biophys. Res. Commun. 2000;275:715–719. doi: 10.1006/bbrc.2000.3169. [DOI] [PubMed] [Google Scholar]
- Morris SM., Jr. Arginine Metabolism: Boundaries of Our Knowledge. J. Nutr. 2007;137:1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- Munder M, Eichmann K, Modolell M. Alternative Metabolic States in Murine Macrophages Reflected by the Nitric Oxide Synthase/Arginase Balance: Competitive Regulation by Cd4+ T Cells Correlates with Th1/Th2 Phenotype. J. Immunol. 1998;160:5347–5354. [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Obstacles and Opportunities for Understanding Macrophage Polarization. J. Leukoc. Biol. 2011a;89:557–563. doi: 10.1189/jlb.0710409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and Pathogenic Functions of Macrophage Subsets. Nat. Rev. Immunol. 2011b doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Shiloh MU. Reactive Oxygen and Nitrogen Intermediates in the Relationship between Mammalian Hosts and Microbial Pathogens. Proc. Natl. Acad. Sci. USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson S, Bonecini-Almeida Mda G, Lapa e Silva JR, Nathan C, Xie QW, Mumford R, Weidner JR, Calaycay J, Geng J, Boechat N, et al. Inducible Nitric Oxide Synthase in Pulmonary Alveolar Macrophages from Patients with Tuberculosis. J. Exp. Med. 1996;183:2293–2302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris KA, Schrimpf JE, Flynn JL, Morris SM., Jr. Enhancement of Macrophage Microbicidal Activity: Supplemental Arginine and Citrulline Augment Nitric Oxide Production in Murine Peritoneal Macrophages and Promote Intracellular Killing of Trypanosoma Cruzi. Infect. Immun. 1995;63:2793–2796. doi: 10.1128/iai.63.7.2793-2796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussler AK, Billiar TR, Liu ZZ, Morris SM., Jr. Coinduction of Nitric Oxide Synthase and Argininosuccinate Synthetase in a Murine Macrophage Cell Line. Implications for Regulation of Nitric Oxide Production. J. Biol. Chem. 1994;269:1257–1261. [PubMed] [Google Scholar]
- Patejunas G, Bradley A, Beaudet AL, O'Brien WE. Generation of a Mouse Model for Citrullinemia by Targeted Disruption of the Argininosuccinate Synthetase Gene. Somat. Cell. Mol. Genet. 1994;20:55–60. doi: 10.1007/BF02257486. [DOI] [PubMed] [Google Scholar]
- Pautz A, Art J, Hahn S, Nowag S, Voss C, Kleinert H. Regulation of the Expression of Inducible Nitric Oxide Synthase. Nitric Oxide. 2010;23:75–93. doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Perez CJ, Jaubert J, Guenet JL, Barnhart KF, Ross-Inta CM, Quintanilla VC, Aubin I, Brandon JL, Otto NW, DiGiovanni J, et al. Two Hypomorphic Alleles of Mouse Ass1 as a New Animal Model of Citrullinemia Type I and Other Hyperammonemic Syndromes. Am. J. Pathol. 2010;177:1958–1968. doi: 10.2353/ajpath.2010.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polsinelli T, Meltzer MS, Fortier AH. Nitric Oxide-Independent Killing of Francisella Tularensis by Ifn-Gamma-Stimulated Murine Alveolar Macrophages. J. Immunol. 1994;153:1238–1245. [PubMed] [Google Scholar]
- Qualls JE, Neale G, Smith AM, Koo MS, DeFreitas AA, Zhang H, Kaplan G, Watowich SS, Murray PJ. Arginine Usage in Mycobacteria-Infected Macrophages Depends on Autocrine-Paracrine Cytokine Signaling. Sci. Signal. 2010;3:ra62. doi: 10.1126/scisignal.2000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan L. Revisiting the Role of the Granuloma in Tuberculosis. Nat. Rev. Immunol. 2012;12:352–366. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- Ray K, Marteyn B, Sansonetti PJ, Tang CM. Life on the Inside: The Intracellular Lifestyle of Cytosolic Bacteria. Nat. Rev. Microbiol. 2009;7:333–340. doi: 10.1038/nrmicro2112. [DOI] [PubMed] [Google Scholar]
- Reutterer B, Stockinger S, Pilz A, Soulat D, Kastner R, Westermayer S, Rulicke T, Muller M, Decker T. Type I Ifn Are Host Modulators of Strain-Specific Listeria Monocytogenes Virulence. Cell. Microbiol. 2008;10:1116–1129. doi: 10.1111/j.1462-5822.2007.01114.x. [DOI] [PubMed] [Google Scholar]
- Shearer JD, Richards JR, Mills CD, Caldwell MD. Differential Regulation of Macrophage Arginine Metabolism: A Proposed Role in Wound Healing. Am. J. Physiol. 1997;272:E181–190. doi: 10.1152/ajpendo.1997.272.2.E181. [DOI] [PubMed] [Google Scholar]
- Shirey KA, Cole LE, Keegan AD, Vogel SN. Francisella Tularensis Live Vaccine Strain Induces Macrophage Alternative Activation as a Survival Mechanism. J. Immunol. 2008;181:4159–4167. doi: 10.4049/jimmunol.181.6.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RD, Suttles J. Functional Plasticity of Macrophages: Reversible Adaptation to Changing Microenvironments. J. Leukoc. Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RW, Pesce JT, Ramalingam T, Wilson MS, White S, Cheever AW, Ricklefs SM, Porcella SF, Li L, Ellies LG, et al. Cationic Amino Acid Transporter-2 Regulates Immunity by Modulating Arginase Activity. PLoS Pathog. 2008;4:e1000023. doi: 10.1371/journal.ppat.1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshchakov V, Jones BW, Perera PY, Thomas K, Cody MJ, Zhang S, Williams BR, Major J, Hamilton TA, Fenton MJ, et al. Tlr4, but Not Tlr2, Mediates Ifn-Beta-Induced Stat1alpha/Beta-Dependent Gene Expression in Macrophages. Nat. Immunol. 2002;3:392–398. doi: 10.1038/ni774. [DOI] [PubMed] [Google Scholar]
- Vogt G, Nathan C. In Vitro Differentiation of Human Macrophages with Enhanced Antimycobacterial Activity. J. Clin. Invest. 2011;121:3889–3901. doi: 10.1172/JCI57235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GY, Brosnan JT. Macrophages Can Convert Citrulline into Arginine. Biochem. J. 1992;281(Pt 1):45–48. doi: 10.1042/bj2810045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Kim CC, Batra S, McKerrow JH, Loke P. Delineation of Diverse Macrophage Activation Programs in Response to Intracellular Parasites and Cytokines. PLoS Negl. Trop. Dis. 2010;4:e648. doi: 10.1371/journal.pntd.0000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.