Abstract
Background
The reduction of malaria parasite transmission by preventing human-vector contact is critical in lowering disease transmission and its outcomes. This underscores the need for effective and long lasting arthropod/insect repellents. Despite the reduction in malaria transmission and outcomes in Tanzania, personal protection against mosquito bites is still not well investigated. This study sought to determine the efficacy of menthol propylene glycol carbonate (MR08), Ocimum suave as compared to the gold standard repellent N, N-diethyl-methylbenzamide (DEET), either as a single dose or in combination (blend), both in the laboratory and in the field against Anopheles gambiae s.l and Culex quinquefasciatus.
Methods
In the laboratory evaluations, repellents were applied on one arm while the other arm of the same individual was treated with a base cream. Each arm was separately exposed in cages with unfed female mosquitoes. Repellents were evaluated either as a single dose or as a blend. Efficacy of each repellent was determined by the number of mosquitoes that landed and fed on treated arms as compared to the control or among them. In the field, evaluations were performed by human landing catches at hourly intervals from 18:00 hr to 01:00 hr.
Results
A total of 2,442 mosquitoes were collected during field evaluations, of which 2,376 (97.30%) were An. gambiae s.l while 66 (2.70%) were Cx. quinquefaciatus. MR08 and DEET had comparatively similar protective efficacy ranging from 92% to 100 for both single compound and blends. These findings indicate that MR08 has a similar protective efficacy as DEET for personal protection outside bed nets when used singly and in blends. Because of the personal protection provided by MR08, DEET and blends as topical applicants in laboratory and field situations, these findings suggest that, these repellents could be used efficiently in the community to complement existing tools. Overall, Cx. quinquefasciatus were significantly prevented from blood feeding compared to An. gambiae s.l.
Conclusion
The incorporation of these topical repellents for protection against insect bites can be of additional value in the absence or presence of IRS and ITNs coverage. However, a combination of both the physical (bed nets) and the repellent should be used in an integrated manner for maximum protection, especially before going to bed. Additional research is needed to develop repellents with longer duration of protection.
Background
Mosquitoes are one of the major disease vectors affecting human populations worldwide. The main approaches to reducing human-vector contact include: use of physical barriers, such as bed nets [1,2]; chemical barriers, such as indoor residual spray [2]; topical application of repellents and burning insect repellent plants indoors [3-6]; house modification; [7] and other behavioral mechanisms such as zoo-prophylaxis [8]. Despite reports of reduction in the burden of malaria disease and its vectors in some African states [9,10], other personal protection methods against mosquito bites are still needed to complement the existing tools that have contributed to this declining trend. Insect repellents are one of the major sources for personal human protection against mosquito bites. An insect repellent has been defined as a substance applied to the skin [4], clothing [11] or other surfaces which discourage or inhibit insects (arthropods in general) from landing or climbing on that surface and sometimes with short or long range spatial repellence. The widely available standard approved synthetic topical repellent is DEET (N, N-diethyl-methylbenzamide), while a large number of plant based repellents are evolving. DEET has been referred to as the gold standard repellent since it has been widely marketed as a commercial repellent and showed protection efficiency of 8 hours after application [12]. Since its first use in 1954, DEET has been shown to act as a strong molecular confusant by jumbling the insect’s odour receptors activity in different concentrations both in the field and laboratory [13,14]. Plant based arthropod repellents currently in the market includes Citronella (Cymbopogonnardus or Cymbopogonwinterianus) [15] and lemon eucalyptus (Eucalyptus maculate citiodon) [16]. Since ancient times, plant products or whole plants have been used to repel or kill mosquitoes and other domestic insect pests in communities [3-6]. The methods of delivering these traditional repellents were thermal expulsion and direct burning, which have been demonstrated to cause reduction in indoor density of mosquitoes [5,6]. In other instances the essential oils from plants have been shown to last longer and have higher protective efficacy when mixed with a carrier, such as a cream, than synthetic repellents mixed with a carrier such as gylcerin [17,18]. In Tanzania, OcimumsppLantana camara,neem ( Azadirachtaindica), Eucalyptus spp and other plant species have efficiently repelled and killed the main malaria ( An.gambiae s.s, An.arabiensis) and non-malaria vectors( Cx. quinquefasciatus)[3,4,16]. Essential oils from these plant materials such as Ocimum kilimandscharicum[4], O. suave[4], L. camara[4,19], A. indica[20] and O. forskolei[21] have different chemicals in varying compositions. The variations in the protective efficacy of essential oils in different mosquito species has been attributed to differences in the concentrations of chemical constituents [3,4,21]. Menthol propyleneglycol carbonate(MR08), a derivative of naturally occurring menthol, has shown higher feeding inhibition in laboratory tests against Aedes aegypti and other arthropods, such as sand flies [22]. However, MR08 has not been evaluated against Cx. quinquefasciatus and An.gambiae s.l .. In sand flies, time to first landing was greater than 120 minutes post application with MR08 [22].
The current study assessed the repellent bioactivity of MR08 plant based product, O.suave as compared to DEET against An.gambiae s.s, An.arabiensis and Cx. quinquefasciatus in both laboratory and field conditions.
Methods
Recruitment of volunteers
Volunteers were given informed consent forms to read in order to clearly understand the study objectives and this was further re-enforced by discussions with the study teams. Those who participated in the field trials were screened for malaria parasites before being recruited into the study. Malaria parasite screening was done on a weekly basis for all participants during the course of study.
Inclusion criteria
All study participants were above 18 years of age, agreed to sign the consent form and were screened for malaria parasites before participating in the study. All mosquitoes used for evaluations in the laboratory were 3 days old post emergence and were non-blood fed.
Exclusion criteria
All persons below 18 years of age and those who did not sign the consent form and not screened for malaria parasites were excluded.
Study design
The study had two designs: controlled laboratory-based experiments and also small scale community based field trials. Laboratory study designs had five replicates in each dosage. The field study was conducted using a 5 by 5 Latin square design. During the study period, treatments were rotated in each house among the selected houses to avoid positional bias. Additionally, wind speed was recorded daily and categorized as normal, moderate or strong.
Laboratory study was conducted at The Tropical Pesticides Research Institute (TPRI)(Arusha, Tanzania), while the field trials were carried out in Mabogini village around Lower Moshi irrigation schemes, 10 kilometers south of Moshi town, Tanzania. This area is known to have high mosquito densities mostly throughout the year compared to other areas [23].
Concentration and blend preparations
Three repellents, MR-08, DEET and O. suave, were formulated at different dosages while DEET was used as the gold standard repellent [12] and compared to two botanical based repellents, MR08 and O. suave plant extracts. MR08 is menthol propyleneglycol carbonate [24] while O.suave extracts were made by steam distillation from O.suave shrubs. The active ingredients of the O.suave extracts have been described elsewhere [25]. DEET is well known as an effective repellent in different studies [12,26]. The dosages were made singly at a dosage of 10, 20 and 30% by volume with base cream as carrier substance for all the three repellent formulations. The base cream is a commercially available cold cream base (Rite Aid Corp, Harrisburg, PA, USA) comprised of mineral oil, water, beeswax, ceresin, sodium borate, fragrance and carbomer. Combination of two repellents at the lowest dosages where made (hereinafter referred to as blends). Three blends were made (MR08 10% + DEET 10%, hereinafter named - blend 1); (MR08 10% + O.suave 10%, hereinafter referred to as blend 2) and ( O.suave 10% + DEET 10%, hereinafter named blend 3). Both single dosage and blend were then evaluated in the laboratory and in the field. These dosages were made in volume ratio of repellent and base cream as a carrier (Repellent: Cream base ratios at 30:70; 20:80 and 10:90).
Cage repellent evaluation
Two mosquito species were used in the laboratory experiments: Cx.quinquefasciatus (Mabogini strain) and An.gambiae s.s (Kisumu strain). Twenty five mosquitoes which were 3 days old post emergence [27] were used for these experiments; sugar solution (Sucrose 10%) was taken out from the cages 30 minutes before trials. The two arms of the same individual were used, with one arm acting as treatment (applied with 2 ml of repellent on the skin surface) while the other arm was used as control and applied with base cream on the surface. The two arms of the same individual were used simultaneously to avoid bias occasioned by differential attractiveness (Figure 1) [28]. A timer was set after introducing mosquitoes into a cage and stopped after a mosquito landed on a treated or untreated arm. The blood fed mosquitoes were scored after 30 minutes based on the abdominal status as described in The WHO protocol [27]. These experiments were conducted in standard cages of 30 cm x 30 cm x 30 cm.
Figure 1 .
Picture showing a volunteer evaluating feeding succession in a (A) treatment and (B) Control.
Field evaluation of repellents
Two repellents, MR08 30% and DEET 30% were selected as single dosages for field evaluations, while blends used were blend 2 and blend 3. Each single repellent or blend was evaluated using a pair of volunteers who exposed their legs treated with either base cream alone (control) or repellent in base cream [27]. Five houses were used in 5 × 5 Latin square designs with a total of ten volunteers participating in these field trials. Evaluations were conducted outdoors in Mabogini village during the season when farmers were transplanting rice paddies and hence higher mosquito densities. Mosquitoes were collected using a mechanical hand aspirator with assistance of a hand held battery torch. Mosquitoes were collected at hourly intervals from 18:00 hr to 01:00 hr. The mosquitoes which were caught using the aspirators were taken to the field station laboratory and sorted using morphological keys to species level and separated by sexes as described by Gillies and Coetzee [29] under a dissecting microscope.
Houses selected for mosquito sampling were 500 meters apart and all houses were made of burnt bricks with an iron sheet roof. Wind speed and other parameters, such as presence or absence of rain were recorded.
Statistical analysis
Data analysis was performed using the PWAS statistics 18.0 (SPSS Inc., Chicago, IL). The comparison between control and treatments was carried out using a paired t-Test with two samples of equal variance (homoscedastic). The comparison of the mean time taken to the first landing in treated and control host was done using one-way analysis of variance (ANOVA).
Analysis of field data was done using multi-factorial analysis of variance (MANOVA). The Tukeys HSD test was applied to assess the contribution of each factor to the mosquito species density sampled by the volunteers, such as days, wind speed and house locations. Students paired t-test homoscedastic was used to compare the overall protection difference between blend treatments evaluated in the field.
Ethical issues
This study was given an ethical approval from KCM College of Tumaini University and Tropical Pesticides Research Institute Research Ethics Review Committee. All volunteers were given written consent forms signed in front of a witness who was not a study participant. All volunteers were screened for malaria parasites before the study and weekly for the period of the study. None of the volunteers were found to be infected with malaria parasites in the field study.
Results
Laboratory evaluations of repellents
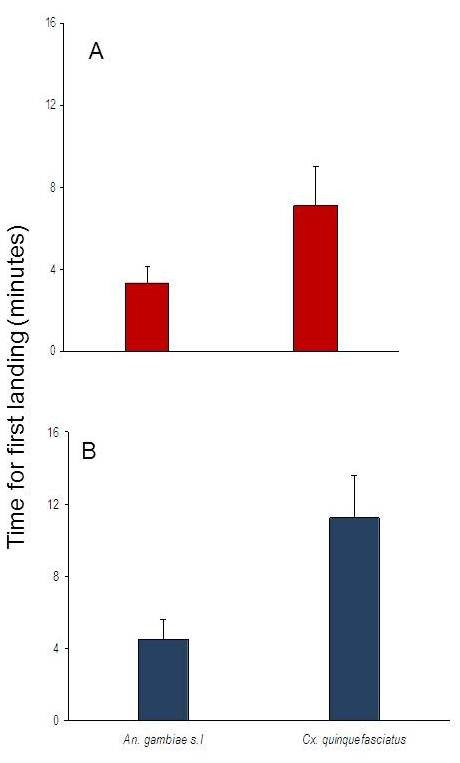
Seven dosages of repellents were evaluated singly and in three blends. Among the repellents evaluated singly, O. suave had the shortest time of 2.20 minutes before mosquitoes landed for blood feeding, while using the blends, the least time to landing on the volunteer’s arm was 1.90 minutes given by Blend 1. Overall, more protection time was observed with Cx. quinquefasciatus compared to An. gambiae for first landing (Figure 2).
Figure 2 .
Time taken in (A) laboratory and (B) field first biting for An.gambiae s.l and Cx. quinquefasciatus.
The mean protection efficacy of the singly evaluated dosages ranged from 77.60% to 100% while for blends ranged from 58.80% to 98.80% in the laboratory (Figure 3). The protection from each treatment was significantly different for both An. gambiae s.s and Cx. quinquefasciatus relative to the control (Table 1).
Figure 3 .
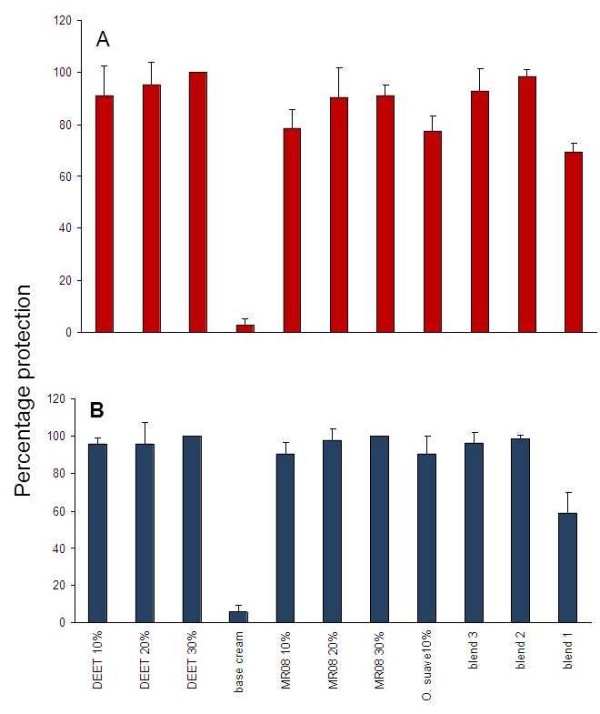
Protection efficiency of single dosage and blend evaluated in the laboratory for (A) An.gambiae s.l and (B) Cx. quinquefasciatus.
Table 1.
Proportions of An. gambiae s.s and Cx. quinquefasciatus protected from feeding on arms treated with different repellent concentrations and controls in laboratory
| Species | Repellent | % Concentrations | Treatments | Control | Paired T-test |
|---|---|---|---|---|---|
|
An. gambiae s.s |
DEET |
10 |
91.2 |
2.7 |
t = 19.64,P < 0.001 |
| |
|
20 |
95.2 |
3.6 |
t = 25.57, P < 0.001 |
| |
|
30 |
100 |
4.8 |
t =112.99, P < 0.001 |
| |
MR08 |
10 |
78.4 |
5.6 |
t = 35.02, P < 0.001 |
| |
|
20 |
90.4 |
3.7 |
t = 20.28, P < 0.001 |
| |
|
30 |
91.2 |
2.9 |
t =40.95, P < 0.001 |
| |
OS |
10 |
77.4 |
3.8 |
t = 45.19, P < 0.001 |
| |
Blend 1 |
10 |
69.4 |
2.9 |
t = 43.35, P < 0.001 |
| |
Blend 2 |
10 |
92.8 |
4.8 |
t = 70.09, P < 0.001 |
| |
Blend 3 |
10 |
98.4 |
3.4 |
t =24.32, P < 0.001 |
|
Cx. quinquefasciatus |
DEET |
10 |
95.6 |
2.7 |
t = 61.39, P < 0.001 |
| |
|
20 |
96.1 |
4.4 |
t = 18,59, P < 0.001 |
| |
|
30 |
100 |
6.9 |
t =75.91, P < 0.001 |
| |
MR08 |
10 |
90.4 |
5.8 |
t = 33.29, P < 0.001 |
| |
|
20 |
97.8 |
4.9 |
t = 39.23, P < 0.001 |
| |
|
30 |
100 |
6.7 |
t =75.91, P < 0.001 |
| |
OS |
10 |
90.2 |
5.9 |
t = 32.22, P < 0.001 |
| |
Blend 1 |
10 |
58.8 |
5.3 |
t = 13.42, P < 0.001 |
| |
Blend 2 |
10 |
98.8 |
4.9 |
t = 71.33, P < 0.001 |
| Blend 3 | 10 | 96.0 | 6.1 | t = 31.23, P < 0.001 |
The protective efficacy of MR08 10% was statistically insignificant (P = 0.347) when compared to DEET 10%; MR08 20% and DEET 20% using An.gambiae s.s. Similarly, MR08 30% gave significantly less protection than DEET 30% in volunteers (Table 2). However, comparative evaluation between the different blends against An. gambiae s.s showed that Blend 2 gave significantly higher protective efficacy than blend 1 (t = 2.78, df = 4, P ≤ 0.001); Blend 3 gave significantly higher protection than blend 1 ( P = 0.002) while protective efficacy between Blend 2 and blend 3 was statistically insignificant ( P =0.180).
Table 2.
Comparative protection of DEET and MR08 repellents against An.gambiae s.l and Cx. quinquefasciatusat different dosages
| Species | % Concentration | DEET | MR08 | Paired T-test |
|---|---|---|---|---|
|
An. gambiae s.s |
10 |
91.2 |
78.4 |
t = 2.43, P = 0.036 |
| |
20 |
95.2 |
90.4 |
t = −271.33, P = 0.054 |
| |
30 |
100 |
91.2 |
t = 5.88, P = 0.002 |
|
Cx. quinquefasciatus |
10 |
95.6 |
90.4 |
t = 1.87, P = 0.067 |
| |
20 |
96.1 |
97.8 |
t = 0.42, P = 0.347 |
| 30 | 100 | 100 | NS* |
Note: NS* mean not significant different and results could not be displayed in PWAS statistics output.
Cx. quinquefasciatus feeding protection was performed using dosages made singly;MR08 10%, DEET 10%, MR08, 20%,DEET 20%,MR08 and DEET 30% which in comparisons had no significant differences in protection efficacy (Table 2).
The results of comparative protection between An. gambiae s.s and Cx. quinquefasciatus in different dosages, indicated that in MR08 10% more Cx. quinquefasciatus were prevented from feeding than An. gambiae s.s but this was statistically insignificant (P > 0.05). Experiments conducted with DEET 10%, indicated that both Cx. quinquefasciatus and An. gambiae s.s were equally inhibited from feeding but were statistically insignificant too. ForMR08 20%, both Cx. quinquefasciatus and An. gambiae s.s were equally inhibited from feeding and were not statistically significant. For DEET 20% both Cx. quinquefasciatus and Angambiae s.s showed equal proportions of feeding inhibition and were not significantly different. Using MR08. 30%, Cx. quinquefasciatus were significantly inhibited from feeding compared to An. gambiae s.s. Cx. quinquefasciatus and An. gambiae s.s were not statistically different in feeding protection against DEET 30%.They were 100% protected from feeding. OS protected significantly more Cx. quinquefasciatus from feeding than An. gambiae s.s and this was significantly different (Table 3). The protective efficacy between blend 2 and 3 was statistically insignificant, however Blend 1 showed significantly higher protection, with An. gambiae s.s being inhibited more from feeding compared to Cx. quinquefasciatus (Table 3).
Table 3.
Percentage of An.gambiae s.s and Cx. quinquefasciatus repelled from feeding on treated arms in laboratory trials
| Repellent | % Concentration | An.gambiaes.s | Cx. quinquefasciatus | Paired T-test |
|---|---|---|---|---|
| DEET |
10 |
91.2 |
95.6 |
t = 1.06, P = 0.175 |
| |
20 |
95.2 |
96.1 |
t = 0.209, P = 0.422 |
| |
30 |
100 |
100 |
NS* |
| MR08 |
10 |
78.4 |
90.4 |
t = 2.78, P = 0.025 |
| |
20 |
90.4 |
97.8 |
t = 1.69, P = 0.083 |
| |
30 |
91.2 |
100 |
t = 5.88, P = 0.002 |
| OS |
10 |
77.4 |
90.2 |
t = 4.38, P = 0.006 |
| Blend 1 |
10 |
69.4 |
58.8 |
t = 2.82, P = 0.036 |
| Blend 2 |
10 |
92.8 |
98.8 |
t = 0.39, P = 0.359 |
| Blend 3 | 10 | 98.4 | 96.0 | t = 1.55, P = 0.098 |
Note: NS* mean not significant different and results could not be displayed in PWAS statistics output.
Field evaluations
After laboratory evaluations, use of blend 1 was discontinued due to the poor performance observed in protection against both An. gambiae s.s and Cx. quinquefasciatus. Blend 2 and 3 were used in field trials together with MR08 30% and DEET 30% against wild mosquito populations.
A total of 2,442 mosquitoes were collected during field evaluations. A total of 600 (24.57%), mosquitoes were collected by volunteers from treatment groups, while 1,842 (75.43%), were from control groups. Out of the mosquitoes collected, 97.30% (2376/2442), were An. gambiae s.l and 2.70% (66/2442) were Cx. quinquefaciatus.
In the analysis, the four repellents evaluated had significant vector reduction in comparison to controls for both An. gambiae s.l (F = 195.95, df = 4, P < 0.001) and Cx. quinquefasciatus (F = 8.861, df = 4, P < 0.001). Overall, when mosquitoes were taken as independent variables, house position had no effect on repellent efficacy since houses had no mosquito density variation (F = 0.786, df. = 4, P = 0.672). The density of mosquitoes sampled from all houses was statistically insignificant when wind speed categories was used as a grouping factor (F = 0.624, df. = 4, P = 0.645). Despite variations in wind speed during the sampling days, there were no statistical differences in mosquito densities (Figure 4). Tukeys HSD test analysis for all the dosages used showed that the protective efficacy was dosage dependent (Figure 5). Differences in protective efficacies against An. gambiae s.l and Cx. quinquefasciatus were also noted among the single repellent and the blends . The treatments were able to reduce the mosquito density on treated volunteers relative to control in the evening biting cycles (Figure 6). Cx. quinquefasciatus took significantly longer time to land and probe on the volunteers than An. gambiae s.l both under laboratory and field conditions (F = 15.42, df = 1, P < 0.001) (Figure 2).
Figure 4 .
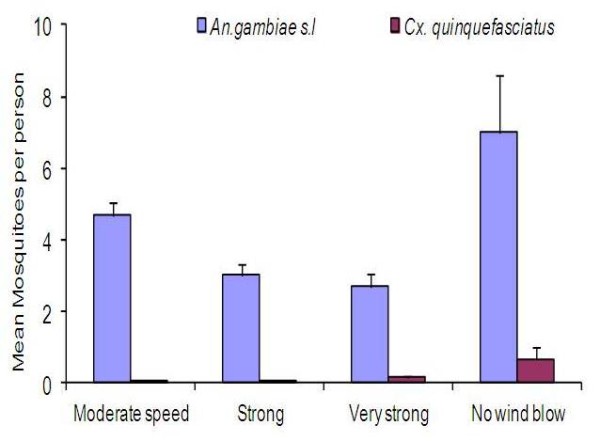
The effect of wind speed on the number of caught (A) An.gambiae s.l and (B) Cx. quinquefasciatus densities during field evaluation of repellents.
Figure 5 .
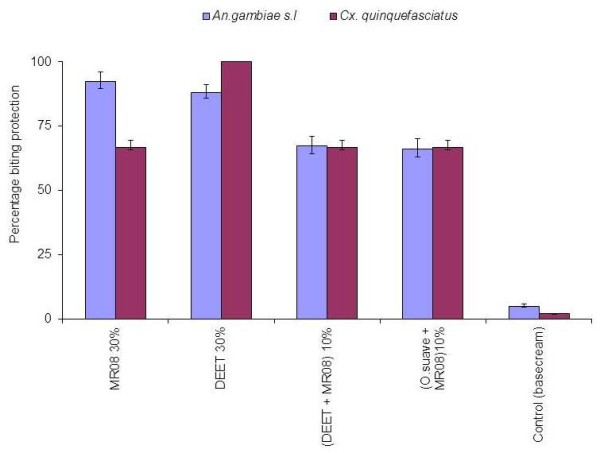
The mean protection of single repellents and blends for (A) An.gambiae s.l and (B) Cx.quinquefasciatusin field situation.
Figure 6 .
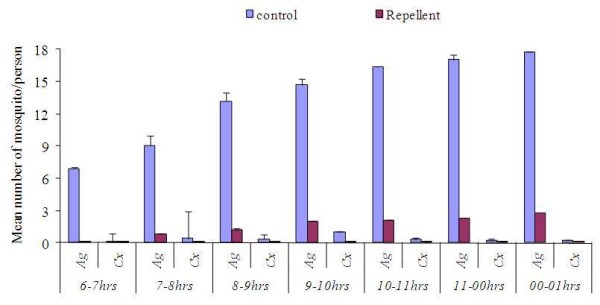
Effect of repellents on mean mosquito densities caught in treatments and controls for 7 hours.
Discussion
This study has demonstrated that MR08had nearly the same results compared to the gold standard repellent DEET in both laboratory and field situations. In the laboratory, DEET 30% (v/v) had protection efficiencies of 100% for the two mosquito species while MR08 had a protection efficiency of 100% and 91.2% for Cx. quinquefasciatus and An. gambiae s.s respectively, against mosquito bites 1 hour post exposure. Under field conditions, 30% MR08 had the highest protection efficiency (92.39%) while DEET 30% had a protection efficiency of 88.17% for seven hours (from 18:00 hrs to 01:00 hrs). These hourly intervals coincided with the first effective host seeking cycles of malaria vectors, An. gambiae s.l. In both laboratory and field evaluation of various repellent dosages, protection was found to be dosage dependent as found elsewhere [30,31] while with blends, the synergistic effect was dosage mixture dependent [11,32]. The strategy of using repellents in combination (Blends) conferred a better protection with little amount of repellent used (Blend 2 and Blend 3) compared to DEET and MR08 30% alone. Both blend 2 and 3 protected volunteers by 82 and 85%, and 98.4 and 92.8% in the field and laboratory respectively up to a period of 7 hours. Mosquito densities and efficacy of compound evaluated in the field had the same environmental variability, such as wind and house locations, thus these findings are likely to be as a result of the chemical compounds in the repellents. The application of repellents reduced the density of mosquito landing on the treatment group compared to the control volunteers (Figure 4).
In northern Tanzania, high distribution and usage of ITNs have shown increased protection to communities against malaria vectors when more than 80% of the population own and use bed nets properly [33,34]. In other studies, bed net utilization has proved to reduce malaria infections when used properly as personal protection tools [35,36]. There is increased exposure risk to those who are out of bed net either in the evening or morning during the peak biting cycles of the malaria vectors [37]. Due to high coverage of ITN and IRS programmes, malaria vector feeding and resting behaviours’ are likely to have changed to maximize available feeding opportunities. Reports suggest that An. gambiae s.s has changed from being endophagic and endophilic to exophagic and exophilic respectively [2,24,38,39]. This behavioural adaptation may present a problem in the personal protection in individuals when outdoors [39].
Application of MR08 as topical repellent could reduce the biting risk to those outdoors, hence adding protective value for individuals who are outside the bed nets during earlier and late biting cycles. These findings suggest that the protective efficacy which is maintained for a period of 7 hours is believed to be realistic for users who retire late to bed under the protection of the bed net. Field evaluations of repellents (18:00 to 01:00 hrs) were conducted during the first host seeking cycles of mosquitoes [40]. Therefore, one can extrapolate the efficacy of these products in protecting individuals against random opportunistic host seeking mosquitoes. Thus, MR08 can complement the effects of ITNS and IRS for the unprotected individuals when used as the topical repellent [41]. Currently in western Kenya, the decrease of relative abundance of vector species have been observed due to high implementation of intervention tools against malaria vectors [42] while in other places the displacement of the vector species composition have been reported [43]. In Dar-es-salaam Tanzania, outdoor feeding among malaria vectors has been reported to be increasing due to wide ITNs coverage, hence increasing biting pressure on unprotected individuals outdoors [44].
In the current study, the observed reduction in biting rates both in laboratory and field evaluations may have great impact on infective bite reduction when incorporated with wide use of IRS and ITNs in the community. Malaria decline in different parts of Africa is associated with high ITN and IRS coverage [45,46] and reliable diagnostic and treatment services [45]. On the other hand, it has been observed that, when vector density declines, communities have a tendency to useless personal protection tools, such as bed nets, against disease vectors [47]. It is necessary for the community to become more aware of using topical repellents.
In controlling African malaria vectors, ITN and IRS have been deployed with the assumption that vector behaviour remains endophilic and endophagic. This assumption is derived from classical behavioural studies by Gillies and Coetzee [29]. These vectors have changed behaviour and tend to feed outdoors (exophagic) due to massive IRS, house modifications and ITN coverage [7,39,48]. In most malaria endemic areas, covering all households with IRS and ITNs is practically impossible [40,49]. Another important limitation of ITN and IRS is that many people do not retire indoors or to bed earlier and miss the benefit of the protection offered by these methods during the earlier biting cycles of malaria vectors [44]. Additional tools, such as repellents, should be considered to supplement existing vector protection tools.
Protection against infective bites from arthropods can be achieved by either avoiding infested areas, protective gear usage (cloth with repellents and ITNs), house modifications or applying topical insect repellents and use of IRS [7,11,50]. Most commercially available repellents and formulations have up to 40% DEET, which is preferred for use in areas with high biting pressure or environmental conditions that promote the loss of repellent on skin surface [51-53]. This amount of DEET (40%) seemed to be higher than MR08 blends (of 10% and 20%), which could reduce the production costs and be affordable to the large populations.
Thousands of plant resources have been tested as sources of insect repellents [4,11,20,54,55]. ITN, treated curtains and IRS coverage have critically reduced entomological inoculation rates [36,47] and integrating the evaluated MR08 repellents in reducing human-vector contact might further reduce EIR to even lower rates than currently reported at0.54 ib/trap/year in the study area [56]. But this reduction of EIR can only be estimated for the indoor biting mosquitoes and not for the outdoor biting ones, where other tools, such as ITN and IRS cannot be implemented [57]. Therefore, during outdoor movements and late retirement to bed, application of topical repellents should be emphasized [58], together with house design modification for indoor vector reduction [7]. MR08 and other repellents could be taken into consideration to fill the gap to reduce transmission rate during this unprotected time.
Currently, slow release and vaporization methods are being tested to enhance the effectiveness of MR08 in preventing mosquito bites inside the household.
The appropriate method of delivering MR08 repellent to be protective and effective for all house occupants for longer duration of time is still on-going.
Conclusion
This study suggests that MR08 is an effective compound against bites from both malaria and filarial vectors for unprotected community members. The integrated vector control involving the conventional control tools and these repellents is necessary to enhance personal protection and significantly reduce human-vector contact.
Competing interest
EJK, AMM, SM, EJK, AQA declare to have no competing interest. JRM holds a patent on use of MR08 as an insect repellent.
Authors’ contribution
EJK and JRM conceived and designed the study, carried out data analysis and results interpretation. EJK and SM drafted the manuscript. AMM and SM did both laboratory and field data collection. FM, AMM, SM, EJK, AQA and JRM revised the manuscript. All authors approved the final version of the manuscript.
Contributor Information
Eliningaya J Kweka, Email: pat.kweka@gmail.com.
Stephen Munga, Email: munga_os@yahoo.com.
Aneth M Mahande, Email: anethf@yahoo.co.uk.
Shandala Msangi, Email: shandalamsangi@yahoo.com.
Humphrey D Mazigo, Email: humphreymazigo@gmail.com.
Araceli Q Adrias, Email: aras@poseidonsciences.com.
Jonathan R Matias, Email: jrmatias@poseidonsciences.com.
Acknowledgement
Authors wish to thank Mr. Augustine Mtui and Charles Masenga for supervision of the field work and mosquito identification in the laboratory and Ms. Coleen P. Sucgang for preparation of the graphics. Home owners who allowed experiments to be conducted in their premises are highly appreciated. This study received financial support from Poseidon Science Foundation through JRM.
References
- Dunn CE, Le Mare A, Makungu C. Malaria risk behaviours, socio-cultural practices and rural livelihoods in southern Tanzania: implications for bed net usage. Soc Sci Med. 2011;72:408–417. doi: 10.1016/j.socscimed.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Yakob L, Dunning R, Yan G. Indoor residual spray and insecticide-treated bed nets for malaria control: theoretical synergisms and antagonisms. J R Soc Interface. 2010;8:799–806. doi: 10.1098/rsif.2010.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweka EJ, Mosha F, Lowassa A, Mahande AM, Kitau J, Matowo J, Mahande MJ, Massenga CP, Tenu F, Feston E. et al. Ethnobotanical study of some of mosquito repellent plants in north-eastern Tanzania. Malaria J. 2008;7:152. doi: 10.1186/1475-2875-7-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweka EJ, Mosha FW, Lowassa A, Mahande AM, Mahande MJ, Masenga CP, Tenu F, Lyatuu EE, Mboya MA, Temu EA. Longitudinal evaluation of Ocimum and other plants effects on the feeding behavioral response of mosquitoes (Diptera: Culicidae) in the field in Tanzania. ParasitVectors. 2008;1:42. doi: 10.1186/1756-3305-1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyoum A, Killeen GF, Kabiru EW, Knols BG, Hassanali A. Field efficacy of thermally expelled or live potted repellent plants against African malaria vectors in western Kenya. Trop Med Int Health. 2003;8:1005–1011. doi: 10.1046/j.1360-2276.2003.01125.x. [DOI] [PubMed] [Google Scholar]
- Seyoum A, Palsson K, Kung'a S, Kabiru EW, Lwande W, Killeen GF, Hassanali A, Knols BG. Traditional use of mosquito-repellent plants in western Kenya and their evaluation in semi-field experimental huts against Anopheles gambiae: ethnobotanical studies and application by thermal expulsion and direct burning. Trans R Soc Trop Med Hyg. 2002;96:225–231. doi: 10.1016/S0035-9203(02)90084-2. [DOI] [PubMed] [Google Scholar]
- Lindsay SW, Emerson PM, Charlwood JD. Reducing malaria by mosquito-proofing houses. Trends Parasitol. 2002;18:510–514. doi: 10.1016/S1471-4922(02)02382-6. [DOI] [PubMed] [Google Scholar]
- Mahande A, Mosha F, Mahande J, Kweka E. Feeding and resting behaviour of malaria vector, Anopheles arabiensis with reference to zooprophylaxis. Malaria J. 2007;6:100. doi: 10.1186/1475-2875-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrowitsch DW, Pedersen EM, Alifrangis M, Scheike TH, Malecela MN, Magesa SM, Derua YA, Rwegoshora RT, Michael E, Simonsen PE. Is the current decline in malaria burden in sub-Saharan Africa due to a decrease in vector population? Malaria J. 2011;10:188. doi: 10.1186/1475-2875-10-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis. 2010;10:545–555. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- Pennetier C, Chabi J, Martin T, Chandre F, Rogier C, Hougard JM, Pages F. New protective battle-dress impregnated against mosquito vector bites. ParasitVectors. 2010;3:81. doi: 10.1186/1756-3305-3-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe E, Barthel WF, Gertler SI, Hall S. Insect repellents, III. N, N-diethylamides. J Org Chem. 1954;19:493–498. doi: 10.1021/jo01369a003. [DOI] [Google Scholar]
- Bohbot JD, Dickens JC. Insect repellents: modulators of mosquito odorant receptor activity. PLoS One. 2010;5:e12138. doi: 10.1371/journal.pone.0012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino M, Steinbach N, Stensmyr MC, Hansson BS, Vosshall LB. A natural polymorphism alters odour and DEET sensitivity in an insect odorant receptor. Nature. 2011;478:511–514. doi: 10.1038/nature10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phasomkusolsil S, Soonwera M. Insect repellent activity of medicinal plant oils against Aedes aegypti (Linn.), Anopheles minimus (Theobald) and Culex quinquefasciatus Say based on protection time and biting rate. Southeast Asian J Trop Med Public Health. 2010;41:831–840. [PubMed] [Google Scholar]
- Trigg JK. Evaluation of a eucalyptus-based repellent against Anopheles spp. in Tanzania. J Am Mosq Control Assoc. 1996;12:243–246. [PubMed] [Google Scholar]
- Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13–18. doi: 10.1056/NEJMoa011699. [DOI] [PubMed] [Google Scholar]
- Kweka E, Nkya HM LL, Kimaro EE, Mwang’onde B, Mahande AM. Efficacy of Ocimum kilimandscharicum plant extracts after four years of storage against Anopheles gambiae s.s. J Cell Anim Biol. 2009;3:171–174. [Google Scholar]
- Abdel-Hady NM, Abdei-Halim AS, Al-Ghadban AM. Chemical composition and insecticidal activity of the volatile oils of leaves and flowers of Lantana camara L. cultivated in Egypt. J Egypt Soc Parasitol. 2005;35:687–698. [PubMed] [Google Scholar]
- Trudel RE, Bomblies A. Larvicidal effects of Chinaberry (Melia azederach) powder on Anopheles arabiensis in Ethiopia. ParasitVectors. 2011;4:72. doi: 10.1186/1756-3305-4-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker T, Ignell R, Ghebru M, Glinwood R, Hopkins R. Identification of mosquito repellent odours from Ocimum forskolei. ParasitVectors. 2011;4:183. doi: 10.1186/1756-3305-4-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poseidon Science Foundation. Poseidon Insect repellent. Poseidon Sciences Group, New York, NY 10168 USA; 2005. p. 16. available at http://www.poseidonsciences.com/MR-08_nontoxic_repellent_menthol_mosquitoes_flies_termites.pdf. [Google Scholar]
- Kweka EJ, Mwang'onde BJ, Mahande AM. Optimization of odour-baited resting boxes for sampling malaria vector, Anopheles arabiensis Patton, in arid and highland areas of Africa. ParasitVectors. 2010;3:75. doi: 10.1186/1756-3305-3-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, Slotman MA. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malaria J. 2011;10:184. doi: 10.1186/1475-2875-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chogo JB, Crank G. Chemical composition and biological activity of the Tanzanian plant Ocimum suave. Jnatural prod. 1981;44:308–311. doi: 10.1021/np50015a012. [DOI] [PubMed] [Google Scholar]
- Rowland M, Freeman T, Downey G, Hadi A, Saeed M. DEET mosquito repellent sold through social marketing provides personal protection against malaria in an area of all-night mosquito biting and partial coverage of insecticide-treated nets: a case–control study of effectiveness. Trop Med Int Health. 2004;9:343–350. doi: 10.1046/j.1365-3156.2003.01183.x. [DOI] [PubMed] [Google Scholar]
- WHO. Part II. Methods and Techniques. Division of Malaria and Other Parasitic Diseases, Geneva; 1975. Manual on practical entomology in malaria. [Google Scholar]
- WHO. CONTROL OF NEGLECTED TROPICAL DISEASES WHO PESTICIDE EVALUATION SCHEME. WHO, Geneva; 2009. Guidelines for efficacy testing of mosquito repellents for human skin. [Google Scholar]
- Gillies TM, Coetzee M. Supplement of the Anopheles of Africa South of Sahara (Afrotropical Region) Republic of South Africa Publication of The S. Afr. Insti. Med Research, Johannesburg; 1987. [Google Scholar]
- Kramer M, Feldlaufer MF, Chauhan KR. Mosquito biting behavior: statistical power and sources of variation in toxicity and repellent bioassays. J Med Entomol. 2010;47:199–204. doi: 10.1603/ME09155. [DOI] [PubMed] [Google Scholar]
- Licciardi S, Herve JP, Darriet F, Hougard JM, Corbel V. Lethal and behavioural effects of three synthetic repellents (DEET, IR3535 and KBR 3023) on Aedes aegypti mosquitoes in laboratory assays. Medvet entomol. 2006;20:288–293. doi: 10.1111/j.1365-2915.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- Pennetier C, Costantini C, Corbel V, Licciardi S, Dabire RK, Lapied B, Chandre F, Hougard JM. Synergy between repellents and organophosphates on bed nets: efficacy and behavioural response of natural free-flying An. gambiae mosquitoes. PLoS One. 2009;4:e7896. doi: 10.1371/journal.pone.0007896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell CA, Msuya E, Sudi M, Njunwa KJ, Carneiro IA, Curtis CF. Effect of community-wide use of insecticide-treated nets for 3–4 years on malarial morbidity in Tanzania. Trop Med Int Health. 2002;7:1003–1008. doi: 10.1046/j.1365-3156.2002.00966.x. [DOI] [PubMed] [Google Scholar]
- Soremekun S, Maxwell C, Zuwakuu M, Chen C, Michael E, Curtis C. Measuring the efficacy of insecticide treated bed nets: the use of DNA fingerprinting to increase the accuracy of personal protection estimates in Tanzania. Trop Med Int Health. 2004;9:664–672. doi: 10.1111/j.1365-3156.2004.01250.x. [DOI] [PubMed] [Google Scholar]
- Binka F, Akweongo P. Prevention of malaria using ITNs: potential for achieving the millennium development goals. Curr Mol Med. 2006;6:261–267. doi: 10.2174/156652406776055203. [DOI] [PubMed] [Google Scholar]
- Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;2:CD000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- Braimah N, Drakely C, Kweka E, Mosha F, Helinski M, Pates H, Maxwell C, Massawe T, Kenward MG, Curtis C. Tests of bed net traps (Mbita traps) for monitoring mosquito populations and time of biting in Tanzania and possible impact of prolonged ITN use. Int J Trop Insect Sci. 2005;25:208–213. doi: 10.1079/IJT200576. [DOI] [Google Scholar]
- Roberts DR, Alecrim WD, Hshieh P, Grieco JP, Bangs M, Andre RG, Chareonviriphap T. A probability model of vector behavior: effects of DDT repellency, irritancy, and toxicity in malaria control. J Vector Ecol. 2000;25:48–61. [PubMed] [Google Scholar]
- Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malaria J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pates H, Curtis C. Mosquito behavior and vector control. Annreview entomol. 2005;50:53–70. doi: 10.1146/annurev.ento.50.071803.130439. [DOI] [PubMed] [Google Scholar]
- Killeen GF, Smith TA. Exploring the contributions of bed nets, cattle, insecticides and excitorepellency to malaria control: a deterministic model of mosquito host-seeking behaviour and mortality. Trans R Soc Trop Med Hyg. 2007;101:867–880. doi: 10.1016/j.trstmh.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, Vulule JM, Hawley WA, Hamel MJ, Walker ED. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malaria J. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies M, Smith A. Effect of a residual house-spraying campaign on species balance in the Anopheles funestus group: The replacement of Anopheles gambiae Giles with Anopheles rivulorum Leeson. Bull Entomol Res. 1960;51:248–252. [Google Scholar]
- Geissbühler Y. Ecology and epidemiology of integrated malaria vector management in Dar es Salaam, Tanzania. PhD Thesis. University of Basel, Basel; 2008. [Google Scholar]
- Aregawi MW, Ali AS, Al-mafazy AW, Molteni F, Katikiti S, Warsame M, Njau RJ, Komatsu R, Korenromp E, Hosseini M. et al. Reductions in malaria and anaemia case and death burden at hospitals following scale-up of malaria control in Zanzibar, 1999–2008. Malaria J. 2011;10:46. doi: 10.1186/1475-2875-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegan GW, Noor AM, Akhwale WS, Cousens S, Snow RW. Effect of expanded insecticide-treated bed net coverage on child survival in rural Kenya: a longitudinal study. Lancet. 2007;370:1035–1039. doi: 10.1016/S0140-6736(07)61477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillinger U, Ndenga B, Githeko A, Lindsay SW. Integrated malaria vector control with microbial larvicides and insecticide-treated nets in western Kenya: a controlled trial. Bull World Health Organ. 2009;87:655–665. doi: 10.2471/BLT.08.055632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen GF, Okumu FO, N'Guessan R, Coosemans M, Adeogun A, Awolola S, Etang J, Dabire RK, Corbel V. The importance of considering community-level effects when selecting insecticidal malaria vector control products. ParasitVectors. 2011;4:160. doi: 10.1186/1756-3305-4-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweka EJ, Nkya WM, Mahande AM, Assenga C, Mosha FW, Lyatuu EE, Massenga CP, Nyale EM, Mwakalinga SB, Lowassa A. Mosquito abundance, bed net coverage and other factors associated with variations in sporozoite infectivity rates in four villages of rural Tanzania. Malaria J. 2008;7:59. doi: 10.1186/1475-2875-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CF, Mnzava AE. Comparison of house spraying and insecticide-treated nets for malaria control. Bull World Health Organ. 2000;78:1389–1400. [PMC free article] [PubMed] [Google Scholar]
- Maekawa E, Aonuma H, Nelson B, Yoshimura A, Tokunaga F, Fukumoto S, Kanuka H. The role of proboscis of the malaria vector mosquito Anopheles stephensi in host-seeking behavior. ParasitVectors. 2011;4:10. doi: 10.1186/1756-3305-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maibach HI, Akers WA, Johnson HL, Khan AA, Skinner WA. Insects. Topical insect repellents. Clin Pharmacol Ther. 1974;16:970–973. doi: 10.1002/cpt1974165part2970. [DOI] [PubMed] [Google Scholar]
- Schwantes U, Dautel H, Jung G. Prevention of infectious tick-borne diseases in humans: Comparative studies of the repellency of different dodecanoic acid-formulations against Ixodes ricinus ticks (Acari: Ixodidae) ParasitVectors. 2008;1:8. doi: 10.1186/1756-3305-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweka EJ, Nyindo M, Mosha F, Silva AG. Insecticidal activity of the essential oil from fruits and seeds of Schinus terebinthifolia Raddi against African malaria vectors. ParasitVectors. 2011;4:129. doi: 10.1186/1756-3305-4-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango G, Bagavan A, Kamaraj C, Abduz Zahir A, Abdul Rahuman A. Oviposition-deterrent, ovicidal, and repellent activities of indigenous plant extracts against Anopheles subpictus Grassi (Diptera: Culicidae) Parasitol Res. 2009;105:1567–1576. doi: 10.1007/s00436-009-1593-8. [DOI] [PubMed] [Google Scholar]
- Mahande A, Matias JR, Dusfour I, Kweka EJ. Knock-down resistance, rdl alleles and the annual entomological inoculation rate of wild mosquito populations from lower Moshi, Northern Tanzania. J Glob Infect Dis. 2012;4:114–119. doi: 10.4103/0974-777X.96776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen GF, Kihonda J, Lyimo E, Oketch FR, Kotas ME, Mathenge E, Schellenberg JA, Lengeler C, Smith TA, Drakeley CJ. Quantifying behavioural interactions between humans and mosquitoes: evaluating the protective efficacy of insecticidal nets against malaria transmission in rural Tanzania. BMC Infect Dis. 2006;6:161. doi: 10.1186/1471-2334-6-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrheim DN, Govere JM. Malaria outbreak control in an African village by community application of 'deet' mosquito repellent to ankles and feet. Med vetentomol. 2002;16:112–115. doi: 10.1046/j.0269-283x.2002.00349.x. [DOI] [PubMed] [Google Scholar]