Abstract
Despite modern medical breakthroughs, diabetes mellitus is a worldwide leading cause of morbidity and mortality. Definitive surgical treatment of diabetes mellitus was established with the advent and refinement of clinical pancreas transplantation in the 1960s. During the following decades, critical discoveries involving islet isolation and engraftment took place. Clinical islet cell transplantation represents the potential for reduced insulin requirements and debilitating hypoglycemic episodes without the morbidity of surgery. Unfortunately, islet cell transplantation was unable to achieve comparable results with solid organ transplantation. This was until the Edmonton protocol (steroid-free immunosuppression) was described, which demonstrated that islet cell transplantation could be a viable alternative to pancreas transplantation. Significant advances in islet purification techniques and novel immunomodulatory agents have since renewed interest in islet cell transplantation. Yet the field is still challenged by a limited supply of islet cells, inadequate engraftment, and the deleterious effects of chronic immunosuppression. This article discusses the history and the current status of clinical islet cell transplantation.
Keywords: Islet cell transplantation, Type 1 diabetes, β cells
Objectives: On completion of this article, the reader should be able to define the indications for and the process of islet transplantation.
Accreditation: Tufts University School of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Diabetes mellitus (DM) is a clinical syndrome of abnormal glucose metabolism characterized by hyperglycemia and glucosuria. Classically, type 1 DM is defined as an autoimmune disease that results in progressive and ultimately permanent destruction of the insulin-producing β cells of the pancreatic islets of Langerhans.1 Once a loss of 90% of the β-cell mass has occurred, it consigns individuals to a lifelong requirement of exogenous insulin therapy.2 Given the recent establishment of certain HLA alleles and this disease, type 1 DM patients are known to be genetically predisposed to diabetes.3 Although the etiology of this disease is unclear, it is believed that environmental factors such as infections or allergens lead to cytotoxic T-cell-mediated destruction of the β cells.4
Globally, the incidence of type 1 DM has been steadily rising, representing a significant burden to health-care systems. A recent worldwide epidemiology survey reported that the incidence of childhood-onset type 1 DM has been steadily rising, with an annual increase of 3.4% between 1995 and 1999.5 It is the leading cause of adult blindness, of 25% of cardiac surgeries, and of 40% of end-stage renal disease. In the United States alone, the economic cost to society is overwhelming, annually costing an estimated $85 billion (10%) of total health-care expenditures.6
In type 1 DM, the lack of endogenous insulin production must be balanced by exogenous insulin supplementation via multiple daily injections or by pump therapy, and directed by intensive blood glucose monitoring.7 The discovery of insulin by Banting and Best in 1922 allowed a previously fatal disease to become a chronic maintainable condition.8 Although intensive therapy has been shown to delay the progression of microvascular manifestations of DM such as retinopathy, nephropathy, and neuropathy, it is quite difficult to achieve and maintain for the long term.9 In the most difficult of cases, patients can lose their ability to appreciate the hypoglycemic prodrome symptoms such as anxiety, sweating, and tachycardia and are at risk for life-threatening syncopal episodes. Replacement of β cells is the only definitive treatment that can reestablish consistent normoglycemia.
The quest for the surgical treatment of DM began over a century ago when, in 1889, von Mering and Minkowski incidentally discovered that total pancreatectomy led to a classical syndrome of type 1 DM with hyperglycemia, glucosuria, ketoacidosis, and subsequent death.10 The first clinical pancreas transplant took place in 1894 by Williams, who implanted fragments of sheep pancreas into the subcutaneous tissue of a 13-year-old boy. There was temporary improvement in his clinical condition, but the patient ultimately died 3 days later. In 1966, the first whole organ pancreas transplantation was successfully performed by Kelley et al at the University of Minnesota.11 Today, whole organ transplantation is effective in restoring normoglycemia with rates of insulin independence of >80% beyond 1 year.12 Unfortunately, there are the inherent risks associated with major abdominal surgery in addition to the complications associated with the management of the unessential exocrine secretions of the pancreas.13
The Evolution of Clinical Islet Transplantation
Soon after the initial success of clinical pancreas transplantation, investigators conceptualized that the ideal procedure would only transplant the endocrine components of the pancreas. The visionary work of Paul Lacy at the University of Washington focused on the process of islet cell isolation and transplantation in rodent models.14,15 Ultimately, he and his colleagues were able to demonstrate that the liver is the ideal environment for islet engraftment.16 These lessons were further developed for the clinical setting by John Najarian and David Sutherland at the University of Minnesota. In 1977, Najarian et al reported the first successful clinical islet cell transplantation with concomitant administration of azathioprine and corticosteroids.17 Largiadèr et al were the first to report insulin independence following islet cell allotransplantation in a patient with type 1 DM in 1980.18 Over the next 2 decades, there were only sporadic reports of insulin independence in islet cell recipients.19,20,21 Of the 267 islet preparations transplanted since 1990, <10% were insulin independent for >1 year.22
In 2000, a landmark paper described a protocol in which seven type 1 DM patients underwent islet cell transplantation.23 First the patients received at least two different islet transplantations; thus the total transplant islet cell mass was significantly higher than previous reports. Second, the patients received a steroid-free immunosuppressive regimen with anti-interleukin (IL)-2 receptor antagonist antibody therapy (daclizumab). Over a median follow-up of 11.9 months, all patients were insulin free. Since this exciting report, clinical islet transplantation activity has dramatically increased all over the world.
Clinical Islet Cell Transplantation
Indications
Islet cell transplantation has not become the mainstay treatment for type 1 DM because of numerous factors, including clinical success rates not yet comparable with whole organ transplantation, a shortage of high-quality donors for islet isolation, and the high cost of a specialized human islet cell isolation facility. The risk-benefit ratio of islet cell transplantation should be carefully discussed with all potential candidates. The ideal candidates for islet cell transplantation are those with unstable (or brittle) type 1 DM with severe hypoglycemic unawareness who have failed conservative medical management. Islet cell transplantation has consistently been shown to improve recipients' hypoglycemic unawareness in the long term. There is growing evidence that islet cell transplantation may also improve the survival of a kidney transplant recipient.24,25 Because these patients are already subjected to the morbidity of chronic immunosuppression, the clinical decision to perform an islet transplant adds little additional risk.
Donor Evaluation
Donor selection has been repeatedly shown to be a critical factor in successful islet cell isolation and transplantation. Donor variables consistently demonstrated with higher isolation yields include body mass index (BMI), donor age, and retrieval by a local surgical team.26 Although donors with high BMI are not routinely used in whole organ transplantation, paradoxically these organs generally provide higher islet yields compared with lean donors.26,27 The major obstacle in the isolation of islets from young donors is the inability of separating islets from the surrounding acinar tissue without injuring the islets themselves.27 Low islet yields have also been shown to correlate with donors who have experienced prolonged hypotension, longer cold ischemia time, longer duration of cardiac arrest, and elevated serum creatinine.28
Islet Preparation
Islet cell isolation from human pancreata has significantly improved over the past 3 decades. The landmark advance was the development of the semiautomatic method for controlled pancreatic digestion using a dissociation chamber (Ricordi Chamber), which has dramatically increased islet cell yield and is still the basis for current islet cell isolation technology.29 This novel technique allows for islet separation from the exocrine tissue in a digestion chamber where a mechanically enhanced continuous enzymatic process occurs. As the pancreatic tissue is progressively digested, the released material is continuously being collected in a separate large volume of solution designed to inhibit further enzyme activity.
Digestion
The enzymatic dissociation of islet cells from the surrounding acinar tissue by collagenase is the critical aspect of successful human islet transplantation.30,31 Roche Applied Science developed Liberase HI, which was a highly purified, low endotoxin enzyme blend of collagenase I and II as well as neutral protease thermolysin. This product consistently demonstrated high yields of excellent quality human islets when compared with previous crude blends of enzymes.32 Unfortunately, concerns about the potential for prion disease was raised with Liberase HI because it was from media with brain-heart infusion broth.33 Roche has developed a mammalian tissue-free Liberase MTF-S as an alternative to its original product.34 In addition, there are two additional commercially available products that have both demonstrated comparable digestive capabilities: Serva Collagenase NB1 and Vitacyte collagenase HA.35,36 Today, ongoing research is being performed to identify the ideal alternative to Liberase HI, the previous gold standard.
Purification
After complete digestion of the pancreatic tissue, it is critical to isolate the islet cells (which only represent 1 to 2% of the total tissue) through a purification step. This process allows for isolation of the islet cells and reduction of the volume of tissue that will ultimately be implanted in the patient. The semiautomated computerized COBE-2991 cell processor is the gold standard for islet cell purification because it allows for large-volume processing in a short time.37 In addition, a critical feature is the ability to collect serial fractions to select those islet cells with the highest degree of purity. Finally, the top-loading feature has the advantage of allowing the digested material to remain in physiological media and minimizes trauma from centrifugal forces.
Pretransplant Culturing
The process of human islet cell isolation generates sizable stress and trauma to the islet cells as demonstrated by the induction of apoptosis, necrosis, and proinflammatory cytokines and chemokines.38,39 Thus optimal culture conditions should allow isolated islet cells the oxygen and nutrients to recover and prevent further cell loss. Culturing islets prior to transplantation has practical advantages including time to arrange patient transportation, the logistics of coordinating islet cell infusion, patient precondition with immunosuppression, and microbiological testing of the isolate for contamination. Numerous media formulations and culture protocols are currently being used.40 Further clinical research is necessary to optimize the pretransplant culturing process to improve β-cell mass and successful clinical islet cell transplantation.
Islet Cell Infusion and Complications
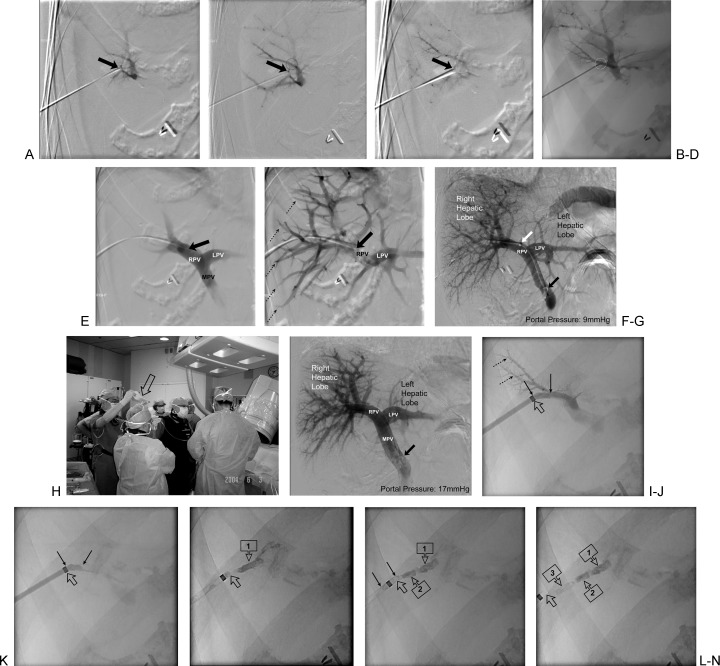
In most centers, the islet preparations are implanted into the recipient's liver through the portal vein via a percutaneous transhepatic cannulation (Fig. 1A–1E). The catheter can be placed under either initial sonographic followed by fluoroscopic guidance, or entirely by fluoroscopic guidance as depicted in Fig. 1A–F.41,42 Both techniques are described in the current issue (see “Percutaneous Portal Vein Access and Transhepatic Tract Hemostasis”).43 Once the catheter's location has been confirmed, portal pressure is measured by an indirect pressure transducer (Fig. 1G). Subsequently, islet cells are gradually infused by gravity via a closed bag system (Fig. 1H). The infusion of islets occurs over 15 to 40 minutes depending on the volume of tissue to be infused and the changes in portal pressure that occur during islet infusion. The portal pressures are measured every 5 minutes by interrupting the infusion. If the portal pressure doubles, the infusion is held until the pressure returns toward baseline (Fig. 1H). Postinfusion portography and pressures are obtained at the completion of the islet cell infusion just before establishing hemostasis (Fig. 1I). The entry tract is typically plugged with a hemostatic sealant (Fig. 1J–N). Technical details describing transhepatic sealing/closure are discussed in the current issue (see “Percutaneous Portal Vein Access and Transhepatic Tract Hemostasis”).43
Figure 1.
Technique for percutaneous transhepatic pancreatic Islet cell transplantation. (A–C) Three sequential images of a digitally subtracted portogram using a 21-gauge percutaneously placed micropuncture needle (arrow at needle tip access of a small peripheral portal vein radical) via a right intercostal approach. This is typical of the fluoroscopic-guided right-sided percutaneous transhepatic approach. The dashed circle (B) is the site of the definitive access and correlates with the dashed circle in (D). (D) Single fluoroscopic spot image of the 21-gauge micropuncture access needle (arrow at the definitive needle tip access of a small peripheral portal vein radical) via a right intercostal approach. The dashed circle is the site of the definitive access and correlates with the dashed ellipse in (B). (E–F) Two sequential images of a digitally subtracted access portogram and initial access with a 5F micropuncture transition sheath catheter (solid arrow at catheter tip in right portal vein). The 5F transition sheath enables the operator to upsize the 0.018-inch wire for a 0.035-inch wire. The dashed arrows in (F) indicate some of the right-sided peripheral portal radicals. LPV, left portal vein; MPV, main portal vein; RPV, right portal vein. (G) Single digitally subtracted angiogram of the formal percutaneous transhepatic portogram just before the infusion of the pancreatic islets. The transhepatic portogram is being performed using a 5F pigtail catheter with its formed pigtail tip (solid black arrow) in the proximal portal vein near the confluence of the splenic vein and the mesenteric vein(s). The 5F pigtail catheter has been advanced through a short (11-cm) 6F transhepatic sheath (solid white arrow at sheath tip), which is placed to secure the percutaneous transhepatic access. At this point in time, the operator measures pressures through the pigtail catheter to obtain a baseline portal pressure. In this case the portal pressure was 9 mm Hg. Most operators use a cutoff of 10 to 12 mm Hg to decide whether to proceed with the islet cell infusion (<10 to 12 mm Hg is acceptable for subsequent islet cell infusion). The infusion of islets occurs over 15 to 40 minutes, depending on the volume of tissue to be infused and the changes in portal pressure that occur during islet infusion. The portal pressures are measured every 5 minutes interrupting the infusion. If the portal pressure doubles, the infusion is held until the pressure returns toward baseline. RPV, right portal vein; LPV, left portal vein. (H) Photograph of the pancreatic islet cell infusion. The islets are gradually infused by gravity using a closed bag system (hollow arrow). The infusion catheter in the portal vein is a 4- to 5F endhole catheter. (I) Single digitally subtracted angiogram of the percutaneous transhepatic portogram just after the pancreatic islet cell infusion. The transhepatic portogram is again being performed using the 5F pigtail catheter with its formed pigtail tip (solid black arrow) in the proximal portal vein. The formal postinfusion portogram is performed to rule out portal vein thrombosis, which can occur with pancreatic islet cell transplantation via the portal vein. At this time, the portal pressure is measured through the pigtail catheter to obtain a postprocedural portal pressure. In this case the portal pressure was 17 mm Hg. LPV, left portal vein; MPV, main portal vein; RPV, right portal vein. (J) Single fluoroscopic spot image of the transhepatic track near the portal vein radical that had been accessed. The peripheral portal vein radicals are seen filled with contrast (dashed arrows). Contrast is being injected through the 6F sheath (open arrow at radio-opaque sheath tip). The transhepatic track is seen between the two solid black arrows. (K) Single fluoroscopic spot image of the transhepatic track (between the solid black arrows) after the contrast has been washed away by the portal flow. Only the contrast in the transhepatic track (between solid arrows) is now seen. The open arrow points to the radio-opaque sheath tip. (L–N) Three fluoroscopic spot images in sequence as contrast-impregnated Gelfoam torpedoes are deployed in the transhepatic track to achieve hemostasis. (L) Deployment of the first (deeper) Gelfoam torpedo (boxed arrow 1). Notice that the actual sheath tip (open arrow) is beyond the radio-opaque marker of the sheath. (M) After the deployment of the first and second Gelfoam torpedoes (boxed arrows 1 and 2, respectively). The third Gelfoam torpedo (between solid arrows) is being deployed and is just within the actual tip of the sheath (open arrow). (N) After the deployment of all three contrast-impregnated Gelfoam torpedoes (boxed arrows 1 to 3), the actual sheath tip has fallen out of the capsular orifice of the transhepatic track.
Early complications of islet cell transplantation are related to the hepatic infusion and include bleeding, portal vein thrombosis, and hepatic infarct.44,45 Theoretically, monitoring of portal venous pressure and acute elevations in these pressures should be a harbinger of potential portal venous thrombosis. Recent studies have identified risk factors for acute portal pressure rise including packed cell volume after purification, the number of transplanted cells, and the absence of purification stage (as is common in auto islet transplantation).46,47 Blockage of the portal vein flow from the transplanted islet emboli induces a local inflammatory reaction called an immediate blood-mediated inflammatory reaction, which results in loss of islet cell function.48,49 We recommend a packed cell volume <5 mL and that a portal pressure rise be limited to 5 mm Hg. In addition, this inflammatory state can activate the coagulation cascade and potentially progress to portal thrombosis. The use of heparin in the islet cell preparation and also for several days after the procedure might improve graft survival through minimizing the local hypercoagulable state.50,51
Immunosuppression
The seminal Edmonton protocol changed the immunosuppression regimens through the elimination of β-cell-toxic corticosteroids.23 Instead sirolimus, a mammalian target of rapamycin inhibitor (mTORi), was used with the additional advantage of being nonnephrotoxic. The chimeric monoclonal anti-IL-2 receptor antibody, daclizumab, also provided additional immediate immunosuppressive coverage. More aggressive immunosuppressive regimens may be necessary to assist in prolonging islet graft survival, although it has not been elucidated whether graft loss is secondary to immunologic or nonimmunologic factors, or most likely a combination of both. There is growing evidence that sirolimus may have some deleterious long-term effects on islet cell function and engraftment.52 Not surprisingly, there are numerous immunosuppression protocols describing the entire gambit of agents through which future clinical trials will be necessary to determine both the most efficacious and least morbid regimen.
Clinical Outcomes
Since the Edmonton protocol was published, numerous centers throughout the world have incorporated and subsequently modified the regimen, resulting in numerous published reports with varying degrees of success. In this section, we present a summary of the largest reports to provide some insights into the current status of islet cell transplantation.
In 2005, 5 years after their landmark paper, the authors from Edmonton reported on their follow-up experience with their novel approach to islet cell transplantation. In this report, there were 65 islet cell transplant recipients. Of these, 44 patients (68%) had become insulin independent at some point following their transplant, with a median duration of insulin independence of 15 months. Of those who had obtained insulin independence, 5 subjects received a single islet infusion, 33 received two infusions, and 6 received three infusions. The long-term results were mixed in terms of success. Permanent insulin independence was only present in 10% of the patients; this was despite 80% of patients having detectable c-peptide levels suggesting some basal insulin production. Importantly, the occurrence of severe hypoglycemia was essentially eradicated.53
In an effort to reproduce the results from the Edmonton center, a large international trial across the United States and Europe was sponsored by the Immune Tolerance Network (ITN). The primary endpoint of the study was insulin independence at 1 year posttransplant. There were nine centers involved; 36 patients were transplanted with the same immunosuppression as the Edmonton protocol. Sixteen of the 36 patients (44%) were insulin independent at 1 year, but only 14% remained so after 2 years. Similar to the Edmonton experience, there was some residual islet graft function (detectable c-peptide level) in most patients (70%). Perhaps the most important lesson from the ITN trial was from the highly variable results among centers. This suggests there is a need for further standardization of the isolation techniques and that restricting this complicated process to select successful centers of excellence may lead to overall higher rates of clinical success.
A recent report from the Japanese Trial of Islet Transplantation showed that only 3 of 18 recipients (17%) of islet transplantation achieved insulin independence, and independence was obtained only for a period of weeks. Once again, they were able to demonstrate islet graft function at 2 years in many patients (63%). Severe hypoglycemic unawareness also disappeared in their patients. After further review, there was one critical variable that was significantly different than the Edmonton and the U.S. colleagues: the use of non-heart-beating deceased donors. Non-heart-beating donors are patients who have suffered irreversible severe brain injury but have not fully progressed to brain death. In these circumstances, cardiopulmonary support is withdrawn and the patient progresses to cardiac arrest. After a designated period of time to assure cardiac death, the donor is then brought to the operating room and the procurement process begins. Thus non-heart-beating donor organs are associated with longer periods of warm ischemia that may lead to increased islet cell stress and potentially decreased viability, accounting for a striking difference in clinical outcomes.54
Finally, the largest registry of islet transplant data are the Collaborative Islet Transplant Registry (CITR), which represents U.S. and Canadian medical institutions as well as two European centers. In their 2010 update, considering 481 recipients of an islet cell transplant reported between 1999 and 2009, the registry reported 65% insulin independence after 1 year. The 5-year insulin independence rates of 60 to 70% were seen with combinations of T-cell depletion and tumor necrosis factor (TNF) antagonism; this was a marked improvement when compared with subjects who received IL-2 receptor antagonism (p = 0.07), and maintenance combinations of calcineurin inhibitors and antimetabolites compared with calcineurin inhibitors and mTOR inhibitors (sirolimus) (p = 0.02). As with previous reports, the prevalence of hypoglycemic unawareness decreased dramatically, and mean HgbA1c levels substantially improved. New predictors of better islet graft function included: the use of HTK (an alternative preservation solution to the standard UW solution), donor use of insulin during hospitalization, and culturing islets for a minimum of 6 hours. Recipients transplanted in 2004–2007 retained insulin independence significantly longer than those transplanted from 1999 to 2003 (p = 0.009).53
Future Developments
A progressive decline in β-cell mass during the different phases of isolation, infusion, and thereafter from immunologic and nonimmunologic mechanisms will culminate in a failure to provide long-term insulin independence. There are a multitude of strategies that are currently ongoing in various phases of conceptual and preclinical studies; all are inspired by the passion to develop the definitive cure for type 1 DM. For the purposes of this discussion, we focus on initiatives centered on strategies for single-donor islet transplantation, islet cell imaging, and stem cell therapy.
Strategies Toward Single-Donor Islet Transplantation
Islet cell transplantation will only become a feasible option for all individuals with type 1 DM once the availability of sufficient islet cells has been addressed. From the initial report from Edmonton, it was clear that islet cells from at least two to four donors was necessary to achieve insulin independence. Achieving sufficient single-donor islet engraftment would most certainly allow more patients to receive treatment. Innovations during islet culture, including additives such as insulinlike growth factor-2 and optimizing oxygen delivery with breathable membranes, may further protect islet cells from damage.55 The inflammatory process immediately following islet cell embolization into the portal system further hampers success by leading to ischemic and immunologic injury to the islets. The use of peri-transplant insulin and heparin has been shown to increase single-donor islet transplantation success from 10% to 40%.56 Hering et al incorporated TNF-α blockade therapy using etanercept in their single-donor experience to target the acute inflammatory process.55 Inhibition of the apoptosis pathway, such as through pan-caspase inhibitor, could lead to further success with marginal mass transplantation secondary to decrease islet loss during engraftment.57 The use of glucagonlike peptide-1 analogs has shown some promise in single-islet transplantation.58,59 It is unlikely that a single strategy will result in routine single-donor islet transplantation, but rather a multimodal approach to maximize the engraftment of a limited islet cell mass will be needed.
Islet Cell Imaging
In the absence of a reliable clinical assay for the detection of acute rejection of the engrafted islet cells, noninvasive monitoring may provide a critical role in tracking the progression of islet cells. Possible causes of attrition of engrafted islets include allogenic rejection; recurrence of autoimmunity; islet cell toxicity from immunosuppression; and graft “exhaustion.” Two modalities are currently being assessed for the imaging of engrafted islets: positron emission tomography (PET) and magnetic resonance (MR).60 For these techniques to be feasible, the ex vivo islet cells must be labeled before engraftment with fluorine 18 fluorodeoxyglucose (FDG) for PET or superparamagnetic iron oxide (SPIO) for MR imaging. Experimental studies have shown that engrafted islets can be successfully detected with either technique.61,62 Unfortunately, PET has substantial limitations: the short half-life of FDG, poor resolution of labeled islets, and low signal-to-noise ratio. With T2 weighted MR imaging, SPIO-labeled cells appear as low-signal dots in the liver secondary to the strong paramagnetic effects of iron. A recent report suggests that SPIO remains stable in engrafted islets and can be detected as far as 6 months posttransplant.63 Finally, preclinical models have shown that the signal intensity of SPIO-labeled islets wane in the setting of rejection, implying that MR imaging has the potential to monitor islet function or mass.62,64 Although initial results are promising, further technical refinement is necessary before these novel techniques become clinically applicable.
Stem Cell Therapy
In addition to the beneficial role of stem cells in the immunomodulatory response and in promoting vasculogenesis following transplantation, they may also play an important role in regeneration of β cells. Theoretically, one could regenerate β cells from a self-renewing, expandable stock of pluripotent embryonic stem cells (ESCs), pancreas-derived multipotent progenitor/stem cells, extrapancreatic adult stem cells, or induced pluripotent stem cells (iPSCs). These cell lines could theoretically deliver large quantities of insulin-producing cells, representing an attractive solution to the current limited supply of pancreata and islets.65,66 Through sequential exposure of human ESCs to epigenetic signals that mimic in vivo pancreatic development, insulin-producing cells can be generated.67,68 These modulated cells share many of the key features of islet cells, including synthesizing insulin and glucagon, reversing hyperglycemia in diabetic mice, and responding to glucose tolerance tests. Unfortunately, the risk of teratoma and other tumor formation is a significant drawback to these cells. Bone marrow derived mesenchymal stem cells can be easily isolated and expanded in culture into a variety of cell lines.69 In recent animal models, these cells can generate insulin-producing cells while at the same time retain their immunomodulatory properties (abrogating immune injury).70 Most human iPSC lines can be induced into Pdx-1-positive progenitor cells and then subsequently differentiated into pancreatic lineage cells in a stepwise fashion.71 Although the potential for stem cells to serve an endless supply of insulin-producing cells seems immense, it must be balanced by the unknown risk of mutagenesis and tumor formation.
Islet Autotransplantation
Chronic pancreatitis is a progressive inflammatory disease causing irreversible structural damage to the pancreatic parenchyma. In severe cases, the endocrine function is also impaired. Surgical resection of the pancreas is considered the final option in the treatment of chronic pancreatitis. Extensive pancreatic resection of >70% of the pancreas may cause diabetes.72 The addition of an islet autotransplant offers the possibility of postoperative glucose control. The first total pancreatectomy in combination with islet autotransplant was performed >30 years ago at the University of Minnesota.73 Since then, >300 islet autotransplants have been reported, most of them at the University of Minnesota. As in allogenic islet cell transplantation, intraportal access is the preferred implantation site. However, the islet preparation is often administered intraoperatively via a portal cannula. In a recent report, the outcomes of islet cell function over time were compared between islet autotransplants at the University of Minnesota and diabetic islet allograft transplant as reported by CITR. Specifically for insulin independence, 74% of the islet autograft patients were insulin independent at 2 years versus only 45% of the CITR allograft patients. There are three potential explanations for this difference: brain death donors for allografts, shorter ischemia times for autografts, and the need for chronic immunosuppression in allografts. In the latter case, many of the commonly used agents have been shown to be directly β-cell toxic74 Interestingly, for the islet cell autotransplant patients, only 46% were insulin independent at 5 years, and only 28% at 10 years.75 Due to the extensive fibrosis in chronic pancreatitis, the digestion process is often incomplete, leading to potentially lower islet mass. Yet unlike in the allograft setting, it is clear that islet graft function and efficacy following auto islet transplantation are greater, particularly given the lower infused β-cell mass.76 Narcotic independence due to pain relief after total pancreatectomy with auto islet transplantation was achieved in 58 to 81% of the patients.77,78
In a retrospective survey, >95% of the patients stated they would recommend total pancreatectomy in combination with islet autotransplantation.77 Finally, this same procedure has been shown to be effective in pediatric patients as well.79,80 They identified, as in the adult population, that it is best to perform this procedure early in the disease course to best preserve islet cell mass.76,81
Conclusions
Islet cell transplantation has raised hope for a cure of diabetes for the past 3 decades. The field of islet cell transplantation has evolved and has witnessed significant progress both scientifically and clinically. Through new innovative tools such as stem cell therapy, gene therapy, and immunomodulation, there is a tremendous opportunity for translating bench work into the clinical arena such that the goal of successful long-term functional islet graft survival is within reach.
References
- 1.Gepts W. Islet morphology in type I diabetes. Behring Inst Mitt. 1984;(75):39–41. [PubMed] [Google Scholar]
- 2.Atkinson M A, Eisenbarth G S. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358(9277):221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 3.Larsen C E, Alper C A. The genetics of HLA-associated disease. Curr Opin Immunol. 2004;16(5):660–667. doi: 10.1016/j.coi.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Knip M. Environmental triggers and determinants of beta-cell autoimmunity and type 1 diabetes. Rev Endocr Metab Disord. 2003;4(3):213–223. doi: 10.1023/a:1025121510678. [DOI] [PubMed] [Google Scholar]
- 5.Bruno G, Landi A. Epidemiology and costs of diabetes. Transplant Proc. 2011;43(1):327–329. doi: 10.1016/j.transproceed.2010.09.098. [DOI] [PubMed] [Google Scholar]
- 6.Rubin R J, Altman W M, Mendelson D N. Health care expenditures for people with diabetes mellitus, 1992. J Clin Endocrinol Metab. 1994;78(4):809A–809F. doi: 10.1210/jcem.78.4.8157701. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association Task Force for Writing Nutrition Principles and Recommendations for the Management of Diabetes and Related Complications . American Diabetes Association position statement: evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. J Am Diet Assoc. 2002;102(1):109–118. doi: 10.1016/s0002-8223(02)90031-3. [DOI] [PubMed] [Google Scholar]
- 8.Banting F G, Best C H, Collip J B, Campbell W R, Fletcher A A. Pancreatic extracts in the treatment of diabetes mellitus. Can Med Assoc J. 1922;12(3):141–146. [PMC free article] [PubMed] [Google Scholar]
- 9.The Diabetes Control and Complications Trial Research Group . Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46(2):271–286. [PubMed] [Google Scholar]
- 10.von Mering J, Minkowski O. Diabetes mellitus after pancreas extirpation. Arch Exp Pathol Pharmakol. 1889;26:111. [Google Scholar]
- 11.Kelly W D, Lillehei R C, Merkel F K, Idezuki Y, Goetz F C. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery. 1967;61(6):827–837. [PubMed] [Google Scholar]
- 12.Gruessner A C, Sutherland D E. Pancreas transplant outcomes for United States (US) and non-US cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR) as of June 2004. Clin Transplant. 2005;19(4):433–455. doi: 10.1111/j.1399-0012.2005.00378.x. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland D E, Gruessner R W, Dunn D L. et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001;233(4):463–501. doi: 10.1097/00000658-200104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacy P E, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- 15.Kemp C B, Knight M J, Scharp D W, Ballinger W F, Lacy P E. Effect of transplantation site on the results of pancreatic islet isografts in diabetic rats. Diabetologia. 1973;9(6):486–491. doi: 10.1007/BF00461694. [DOI] [PubMed] [Google Scholar]
- 16.Scharp D W, Kemp C B, Knight M J, Ballinger W F, Lacy P E. The use of ficoll in the preparation of viable islets of Langerhans from the rat pancreas. Transplantation. 1973;16(6):686–689. doi: 10.1097/00007890-197312000-00028. [DOI] [PubMed] [Google Scholar]
- 17.Najarian J S, Sutherland D E, Matas A J, Steffes M W, Simmons R L, Goetz F C. Human islet transplantation: a preliminary report. Transplant Proc. 1977;9(1):233–236. [PubMed] [Google Scholar]
- 18.Largiadèr F, Kolb E, Binswanger U. A long-term functioning human pancreatic islet allotransplant. Transplantation. 1980;29(1):76–77. doi: 10.1097/00007890-198001000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Tzakis A G, Ricordi C, Alejandro R. et al. Pancreatic islet transplantation after upper abdominal exenteration and liver replacement. Lancet. 1990;336(8712):402–405. doi: 10.1016/0140-6736(90)91946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oberholzer J, Triponez F, Mage R. et al. Human islet transplantation: lessons from 13 autologous and 13 allogeneic transplantations. Transplantation. 2000;69(6):1115–1123. doi: 10.1097/00007890-200003270-00016. [DOI] [PubMed] [Google Scholar]
- 21.Socci C, Falqui L, Davalli A M. et al. Allotransplantation of fresh islets in four type I diabetic patients. Transplant Proc. 1992;24(3):965–966. [PubMed] [Google Scholar]
- 22.Brendel M, Hering B, Schulz A, Bretzel R. Glessen, Germany: University of Glessen; 1999. International Islet Transplant Registry report; pp. 1–20. [Google Scholar]
- 23.Shapiro A J, Lakey J T. et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 24.Cure P, Pileggi A, Froud T. et al. Improved metabolic control and quality of life in seven patients with type 1 diabetes following islet after kidney transplantation. Transplantation. 2008;85(6):801–812. doi: 10.1097/TP.0b013e318166a27b. [DOI] [PubMed] [Google Scholar]
- 25.Fiorina P, Venturini M, Folli F. et al. Natural history of kidney graft survival, hypertrophy, and vascular function in end-stage renal disease type 1 diabetic kidney-transplanted patients: beneficial impact of pancreas and successful islet cotransplantation. Diabetes Care. 2005;28(6):1303–1310. doi: 10.2337/diacare.28.6.1303. [DOI] [PubMed] [Google Scholar]
- 26.Zeng Y, Torre M A, Karrison T, Thistlethwaite J R. The correlation between donor characteristics and the success of human islet isolation. Transplantation. 1994;57(6):954–958. doi: 10.1097/00007890-199403270-00031. [DOI] [PubMed] [Google Scholar]
- 27.Ricordi C. Quantitative and qualitative standards for islet isolation assessment in humans and large mammals. Pancreas. 1991;6(2):242–244. doi: 10.1097/00006676-199103000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Brandhorst H, Brandhorst D, Hering B J, Federlin K, Bretzel R G. Body mass index of pancreatic donors: a decisive factor for human islet isolation. Exp Clin Endocrinol Diabetes. 1995;103 02:23–26. doi: 10.1055/s-0029-1211388. [DOI] [PubMed] [Google Scholar]
- 29.Ricordi C, Lacy P E, Finke E H, Olack B J, Scharp D W. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 30.Kin T, Johnson P R, Shapiro A M, Lakey J R. Factors influencing the collagenase digestion phase of human islet isolation. Transplantation. 2007;83(1):7–12. doi: 10.1097/01.tp.0000243169.09644.e6. [DOI] [PubMed] [Google Scholar]
- 31.Kin T, Zhai X, Murdoch T B, Salam A, Shapiro A M, Lakey J R. Enhancing the success of human islet isolation through optimization and characterization of pancreas dissociation enzyme. Am J Transplant. 2007;7(5):1233–1241. doi: 10.1111/j.1600-6143.2007.01760.x. [DOI] [PubMed] [Google Scholar]
- 32.Olack B J, Swanson C J, Howard T K, Mohanakumar T. Improved method for the isolation and purification of human islets of Langerhans using Liberase enzyme blend. Hum Immunol. 1999;60(12):1303–1309. doi: 10.1016/s0198-8859(99)00118-4. [DOI] [PubMed] [Google Scholar]
- 33.Alejandro R Barton F B Hering B J Wease S; Collaborative Islet Transplant Registry Investigators. 2008 Update from the Collaborative Islet Transplant Registry Transplantation 200886121783–1788. [DOI] [PubMed] [Google Scholar]
- 34.Caballero-Corbalan J, Brandhorst H, Asif S, Korsgren O, Engelse M, de Koning E. et al. Mammalian tissue-free liberase: a new GMP-graded enzyme blend for human islet isolation. Transplantation. 2010;90(3):332–333. doi: 10.1097/TP.0b013e3181e117e3. [DOI] [PubMed] [Google Scholar]
- 35.Caballero-Corbalán J, Friberg A S, Brandhorst H. et al. Vitacyte collagenase HA: a novel enzyme blend for efficient human islet isolation. Transplantation. 2009;88(12):1400–1402. doi: 10.1097/TP.0b013e3181bd1441. [DOI] [PubMed] [Google Scholar]
- 36.Szot G L, Lee M R, Tavakol M M. et al. Successful clinical islet isolation using a GMP-manufactured collagenase and neutral protease. Transplantation. 2009;88(6):753–756. doi: 10.1097/TP.0b013e3181b443ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goto M, Eich T M, Felldin M. et al. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation. 2004;78(9):1367–1375. doi: 10.1097/01.tp.0000140882.53773.dc. [DOI] [PubMed] [Google Scholar]
- 38.Abdelli S, Ansite J, Roduit R. et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes. 2004;53(11):2815–2823. doi: 10.2337/diabetes.53.11.2815. [DOI] [PubMed] [Google Scholar]
- 39.Bottino R, Balamurugan A N, Tse H. et al. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53(10):2559–2568. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- 40.Murdoch T B, McGhee-Wilson D, Shapiro A M, Lakey J R. Methods of human islet culture for transplantation. Cell Transplant. 2004;13(6):605–617. [PubMed] [Google Scholar]
- 41.Venturini M, Angeli E, Maffi P. et al. Technique, complications, and therapeutic efficacy of percutaneous transplantation of human pancreatic islet cells in type 1 diabetes: the role of US. Radiology. 2005;234(2):617–624. doi: 10.1148/radiol.2342031356. [DOI] [PubMed] [Google Scholar]
- 42.Baidal D A, Froud T, Ferreira J V, Khan A, Alejandro R, Ricordi C. The bag method for islet cell infusion. Cell Transplant. 2003;12(7):809–813. doi: 10.3727/000000003108747280. [DOI] [PubMed] [Google Scholar]
- 43.Saad W, Maddoff D. Percutaneous portal vein access and transhepatic tract hemostasis. Semin Intervent Radiol. 2012;29(2):71–80. doi: 10.1055/s-0032-1312567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casey J J, Lakey J R, Ryan E A. et al. Portal venous pressure changes after sequential clinical islet transplantation. Transplantation. 2002;74(7):913–915. doi: 10.1097/00007890-200210150-00002. [DOI] [PubMed] [Google Scholar]
- 45.Ryan E A, Paty B W, Senior P A, Shapiro A M. Risks and side effects of islet transplantation. Curr Diab Rep. 2004;4(4):304–309. doi: 10.1007/s11892-004-0083-8. [DOI] [PubMed] [Google Scholar]
- 46.Moberg L, Johansson H, Lukinius A. et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360(9350):2039–2045. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 47.Johansson H, Lukinius A, Moberg L. et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54(6):1755–1762. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 48.Kawahara T, Kin T, Kashkoush S, Gala-Lopez B, Bigam D L, Kneteman N M. et al. Portal vein thrombosis is a potentially preventable complication in clinical islet transplantation. Am J Transplant. 2011;11(12):2700–2707. doi: 10.1111/j.1600-6143.2011.03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawahara T, Kin T, Shapiro A M. A comparison of islet autotransplantation with allotransplantation and factors elevating acute portal pressure in clinical islet transplantation. J Hepatobiliary Pancreat Sci. 2012;19(3):281–288. doi: 10.1007/s00534-011-0441-2. [DOI] [PubMed] [Google Scholar]
- 50.Johansson H, Goto M, Dufrane D. et al. Low molecular weight dextran sulfate: a strong candidate drug to block IBMIR in clinical islet transplantation. Am J Transplant. 2006;6(2):305–312. doi: 10.1111/j.1600-6143.2005.01186.x. [DOI] [PubMed] [Google Scholar]
- 51.Cabric S, Sanchez J, Lundgren T. et al. Islet surface heparinization prevents the instant blood-mediated inflammatory reaction in islet transplantation. Diabetes. 2007;56(8):2008–2015. doi: 10.2337/db07-0358. [DOI] [PubMed] [Google Scholar]
- 52.Berney T, Secchi A. Rapamycin in islet transplantation: friend or foe? Transpl Int. 2009;22(2):153–161. doi: 10.1111/j.1432-2277.2008.00743.x. [DOI] [PubMed] [Google Scholar]
- 53.Ryan E A, Paty B W, Senior P A. et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 54.Kenmochi T, Asano T, Maruyama M. et al. Clinical islet transplantation in Japan. J Hepatobiliary Pancreat Surg. 2009;16(2):124–130. doi: 10.1007/s00534-008-0020-3. [DOI] [PubMed] [Google Scholar]
- 55.Papas K K, Avgoustiniatos E S, Tempelman L A. et al. High-density culture of human islets on top of silicone rubber membranes. Transplant Proc. 2005;37(8):3412–3414. doi: 10.1016/j.transproceed.2005.09.086. [DOI] [PubMed] [Google Scholar]
- 56.Koh A, Senior P, Salam A, Kin T, Imes S, Dinyari P. et al. Insulin-heparin infusions peritransplant substantially improve single-donor clinical islet transplant success. Transplantation. 2010;89(4):465–471. doi: 10.1097/TP.0b013e3181c478fd. [DOI] [PubMed] [Google Scholar]
- 57.McCall M, Toso C, Emamaullee J, Pawlick R, Edgar R, Davis J. et al. The caspase inhibitor IDN-6556 (PF3491390) improves marginal mass engraftment after islet transplantation in mice. Surgery. 2011;150(1):48–55. doi: 10.1016/j.surg.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 58.Faradji R N, Tharavanij T, Messinger S. et al. Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation. 2008;86(12):1658–1665. doi: 10.1097/TP.0b013e31818fe448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gangemi A, Salehi P, Hatipoglu B. et al. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant. 2008;8(6):1250–1261. doi: 10.1111/j.1600-6143.2008.02234.x. [DOI] [PubMed] [Google Scholar]
- 60.Low G, Hussein N, Owen R J, Toso C, Patel V H, Bhargava R. et al. Role of imaging in clinical islet transplantation. Radiographics. 2010;30(2):353–366. doi: 10.1148/rg.302095741. [DOI] [PubMed] [Google Scholar]
- 61.Eich T Eriksson O Lundgren T; Nordic Network for Clinical Islet Transplantation Visualization of early engraftment in clinical islet transplantation by positron-emission tomography N Engl J Med 2007356262754–2755. [DOI] [PubMed] [Google Scholar]
- 62.Tai J H, Foster P, Rosales A. et al. Imaging islets labeled with magnetic nanoparticles at 1.5 Tesla. Diabetes. 2006;55(11):2931–2938. doi: 10.2337/db06-0393. [DOI] [PubMed] [Google Scholar]
- 63.Toso C, Vallee J P, Morel P. et al. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am J Transplant. 2008;8(3):701–706. doi: 10.1111/j.1600-6143.2007.02120.x. [DOI] [PubMed] [Google Scholar]
- 64.Jirák D, Kríz J, Herynek V. et al. MRI of transplanted pancreatic islets. Magn Reson Med. 2004;52(6):1228–1233. doi: 10.1002/mrm.20282. [DOI] [PubMed] [Google Scholar]
- 65.Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6(3):139–148. doi: 10.1038/nrendo.2009.274. [DOI] [PubMed] [Google Scholar]
- 66.Furth M E, Atala A. Stem cell sources to treat diabetes. J Cell Biochem. 2009;106(4):507–511. doi: 10.1002/jcb.22000. [DOI] [PubMed] [Google Scholar]
- 67.D'Amour K A, Bang A G, Eliazer S. et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24(11):1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 68.Kroon E, Martinson L A, Kadoya K. et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 69.Volarevic V, Arsenijevic N, Lukic M L, Stojkovic M. Concise review: Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 2011;29(1):5–10. doi: 10.1002/stem.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boumaza I, Srinivasan S, Witt W T. et al. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun. 2009;32(1):33–42. doi: 10.1016/j.jaut.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 71.Zhang D, Jiang W, Liu M. et al. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19(4):429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- 72.Slezak L A, Andersen D K. Pancreatic resection: effects on glucose metabolism. World J Surg. 2001;25(4):452–460. doi: 10.1007/s002680020337. [DOI] [PubMed] [Google Scholar]
- 73.Sutherland D E, Matas A J, Najarian J S. Pancreatic islet cell transplantation. Surg Clin North Am. 1978;58(2):365–382. doi: 10.1016/s0039-6109(16)41489-1. [DOI] [PubMed] [Google Scholar]
- 74.Ruggenenti P, Remuzzi A, Remuzzi G. Decision time for pancreatic islet-cell transplantation. Lancet. 2008;371(9616):883–884. doi: 10.1016/S0140-6736(08)60395-5. [DOI] [PubMed] [Google Scholar]
- 75.Sutherland D E, Gruessner A C, Carlson A M. et al. Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation. 2008;86(12):1799–1802. doi: 10.1097/TP.0b013e31819143ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kobayashi T, Manivel J C, Carlson A M. et al. Correlation of histopathology, islet yield, and islet graft function after islet autotransplantation in chronic pancreatitis. Pancreas. 2011;40(2):193–199. doi: 10.1097/mpa.0b013e3181fa4916. [DOI] [PubMed] [Google Scholar]
- 77.Blondet J J Carlson A M Kobayashi T et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis Surg Clin North Am 20078761477–1501., x [DOI] [PubMed] [Google Scholar]
- 78.Ahmad S A, Lowy A M, Wray C J. et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg. 2005;201(5):680–687. doi: 10.1016/j.jamcollsurg.2005.06.268. [DOI] [PubMed] [Google Scholar]
- 79.Bellin M D, Carlson A M, Kobayashi T. et al. Outcome after pancreatectomy and islet autotransplantation in a pediatric population. J Pediatr Gastroenterol Nutr. 2008;47(1):37–44. doi: 10.1097/MPG.0b013e31815cbaf9. [DOI] [PubMed] [Google Scholar]
- 80.Bellin M D, Blondet J J, Beilman G J. et al. Predicting islet yield in pediatric patients undergoing pancreatectomy and autoislet transplantation for chronic pancreatitis. Pediatr Diabetes. 2010;11(4):227–234. doi: 10.1111/j.1399-5448.2009.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takita M, Naziruddin B, Matsumoto S. et al. Variables associated with islet yield in autologous islet cell transplantation for chronic pancreatitis. Proc (Bayl Univ Med Cent) 2010;23(2):115–120. doi: 10.1080/08998280.2010.11928597. [DOI] [PMC free article] [PubMed] [Google Scholar]