Abstract
Patients with gastric variceal bleeding require a multidisciplinary team approach including hepatologists, endoscopists, diagnostic radiologists, and interventional radiologists. Upper gastrointestinal endoscopy is the first-line diagnostic and management tool for bleeding gastric varices, as it is in all upper gastrointestinal bleeding scenarios. In the United States when endoscopy fails to control gastric variceal bleeding, a transjugular intrahepatic portosystemic shunt (TIPS) traditionally is performed along the classic teachings of decompressing the portal circulation. However, TIPS has not shown the same effectiveness in controlling gastric variceal bleeding that it has with esophageal variceal bleeding. For the past 2 decades, the balloon-occluded retrograde transvenous obliteration (BRTO) procedure has become common practice in Asia for the management of gastric varices. BRTO is gaining popularity in the United States. It has been shown to be effective in controlling gastric variceal bleeding with low rebleed rates. BRTO has many advantages over TIPS in that it is less invasive and can be performed on patients with poor hepatic reserve and those with encephalopathy (and may even improve both). However, its by-product is occlusion of a spontaneous hepatofugal (TIPS equivalent) shunt, and thus it is contradictory to the traditional American doctrine of portal decompression. Indeed, BRTO causes an increase in portal hypertension, with potential aggravation of esophageal varices and ascites. This article discusses the concept, technique, and outcomes of BRTO within the broader management of gastric varices.
Keywords: BRTO, transvenous obliteration, gastric, varices, TIPS, portal hypertension
Objectives: On completion of this article, the reader will be able to describe the current indications, technique, complications, and outcomes associated with the BRTO procedure.
Accreditation: Tufts University School of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Patients with gastric variceal bleeding typically are cirrhotics and require a multidisciplinary team approach including hepatologists, endoscopists, diagnostic radiologists, and interventional radiologists.1,2 Upper gastrointestinal endoscopy is the first-line diagnostic and management tool for bleeding gastric varices, as it is in all upper gastrointestinal bleeds.1,2 Traditionally, in the United States when endoscopy fails to control gastric variceal bleeding, a transjugular intrahepatic portosystemic shunt (TIPS) is performed, which is along the classic teachings of decompressing the portal circulation.3 However, TIPS has not demonstrated the same effectiveness in controlling gastric variceal bleeding that it has shown with esophageal variceal bleeding.3,4
For the past 2 decades, the balloon-occluded retrograde transvenous obliteration (BRTO) procedure has become common practice in Asia for the management of gastric varices, and it is currently gaining popularity in the United States.5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36 BRTO has shown considerable effectiveness in controlling gastric variceal bleeding with low rebleed rates.4 Its advantages over TIPS include the fact that it is less invasive and can be performed on patients with poor hepatic reserve and encephalopathy (and may even improve both).3,4 However, its by-product is occlusion of a spontaneous hepatofugal (TIPS equivalent) shunt, and thus it is contradictory to the traditional doctrine of portal decompression.3 Indeed, BRTO may cause an increase in portal hypertension with concomitant worsening of esophageal varices and ascites.
This article is an adjunct to a prior Seminars of Interventional Radiology issue (volume 28, 2011) and discusses the concept, technique, and outcomes of BRTO for the management of gastric varices.
Concept of the BRTO Procedure
The concept of BRTO is accessing the portosystemic gastro-renal shunt via the left renal vein from a transjugular or transfemoral approach.6,7,8,37 Most authors refer to Kanagawa et al (1991–1993) as the inventor of BRTO.6 However, the first published document of an attempt at balloon-occluded sclerotherapy of the gastrorenal shunt for the management of gastric varices was authored by Olson et al in 1984.37 Olson et al used a transfemoral balloon occlusion catheter and absolute alcohol for the successful sclerosis attempt.37 Embolic coils were also placed in the outflow gastrorenal shunt.37 The term used for the procedure was transrenal-vein reflux ethanol sclerosis and not BRTO.37
Subsequently, Kanagawa and coworkers revived the “BRTO concept” and developed the BRTO procedure in the early 1990s, coining the currently used term.6 They utilized 5% ethanolamine oleate (EO) (Oldamin; Grelan Pharmaceutical, Tokyo, Japan) as an endovascular sclerosant, with which European and American interventional radiologists were not familiar.5,6 EO is an established upper endoscopy variceal sclerosant. However, when used during endovascular procedures, there is a risk for hemolysis, hemoglobinuria, and potentially hemoglobin-induced renal tubular dysfunction.7,8 Haptoglobin can be administered in response to free hemoglobin, although it is available in Japan but not commercially available in the United States for human use. The Japanese then developed, evolved, and clinically applied this procedure.6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29 They took it to the clinical level and established it as the viable and successful procedure it is today.6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29
Subsequently, in 2006–2007, American interventionalists introduced a different sclerosant: 3% sodium tetradecyl sulfate (3% STS) (Sotradecol, AngioDynamics, Inc. Queensbury, NY) to perform the BRTO procedure.5 It was developed out of necessity due to the lack of an antidote for hemolysis (haptoglobin) in the United States, as well as the unfamiliarity with EO as an endovascular sclerosant. The first two institutions to use 3% STS were Mount Sinai (New York) and the Dotter Institute (Oregon),5 subsequently and independently followed by the University of Virginia.5
Preprocedure Clinical and Endoscopic Evaluation and Management
Initial management of gastric variceal bleeding involves diagnostic and therapeutic considerations. Patients who are planning to undergo a BRTO procedure for bleeding gastric varices require preprocedural assessments that include endoscopy and clinical, laboratory, and imaging evaluation. Patients undergoing BRTO are cirrhotics that require a multidisciplinary team approach, as described earlier.2
Clinical Assessment
Patients who are potential candidates for BRTO are either stable or unstable. BRTO has been reported to be effective in controlling active bleeding from gastric varices.10,14 Obviously, stabilizing patients is a priority before full clinical and diagnostic assessment, although realistically both clinical assessment and stabilization occur simultaneously. Almost all patients with gastric varices with established gastrorenal shunts (traditional BRTO candidates) have cirrhosis that may be suspected by prior evaluation or through historical, physical examination, and laboratory findings.1,2 In patients with known or suspected portal hypertension, medical therapy is required, including antiportal hypertension medications and antibiotic prophylaxis.2
Fluid Resuscitation
Not all patients undergoing BRTO for gastric varices are unstable; in fact, most are stabilized before the actual BRTO procedure. Basic life-sustaining and resuscitative measures routine to any form of gastrointestinal bleeding are initiated. However, physicians must be cognizant of avoiding overaggressive fluid resuscitation that can exacerbate portal hypertension because multiple reports have established that volume expansion increases portal vein pressure.2,38,39,40 Variceal bleeding is predominantly portal pressure driven; thus it is apparent that minimizing portal pressure is an important goal in managing these patients. To avoid significant volume expansion and subsequent elevated portal venous pressures, systemic blood pressures lower than normal are therefore acceptable. Also to this end, a lower target hematocrit (21%) with packed red blood cell transfusions, along with optimization of platelet count and function, are commonly targeted goals.41,42 Renal function support may also be necessary for volume control and platelet function.
Endoscopic Management of Bleeding Gastric Varices
Once the patient is stabilized, upper gastrointestinal endoscopy is usually undertaken as a routine early measure to evaluate upper gastrointestinal bleeding. Upper gastrointestinal endoscopy is essential for diagnosing, triaging the management of, and/or treating upper gastrointestinal bleeding.1,2 The identification of which varices, if any, are bleeding is essential. Esophageal varices can be controlled effectively by endoscopic-guided banding and sclerotherapy. Bleeding esophageal varices that cannot be controlled medically and endoscopically would warrant a TIPS procedure and not a BRTO.43,44 Bleeding from gastric varices that are small and exhibit slow flow by endoscopic Doppler ultrasound (EUS) can be sclerosed (or “glued”) endoscopically and may not necessarily require a BRTO procedure. However, if there are large fundic and/or cardiac gastric varices exhibiting high flow, some endoscopists would defer to a BRTO procedure due to concerns about causing intravascular (usually systemic venous) nontarget embolization of the sclerosant. Obviously, if bleeding is from a combined gastric and esophageal varices, then the esophageal varices can be managed by endoscopy and large high-flow gastric varices could be managed by BRTO. In the presence of large high-flow gastric varices and prominent but not bleeding esophageal varices, preemptive esophageal variceal banding may be warranted because BRTO exacerbates portal hypertension and may aggravate esophageal varices.10,13,35
Preprocedure Imaging Evaluation
Quality imaging is critical to evaluate for patient candidacy for the BRTO procedure and important for the planning of the procedure.2 The main anatomical and hemodynamic features (Fig. 1) to be considered before performing the BRTO procedure include portal vein patency, presence and size of gastrorenal shunts (for conventional BRTO), presence and sizes of alternative portosystemic shunts, and leading systemic veins (for unconventional BRTO) that can be located above or below the diaphragm (Fig. 2). These peri-/transdiaphragmatic veins include the left phrenic, left pericardiac vein, ileocolic veins, and the azygo-esophago venous/variceal axis (Fig. 3A–3C).45,46,47,48,49,50,51,52,53,54,55,56,57
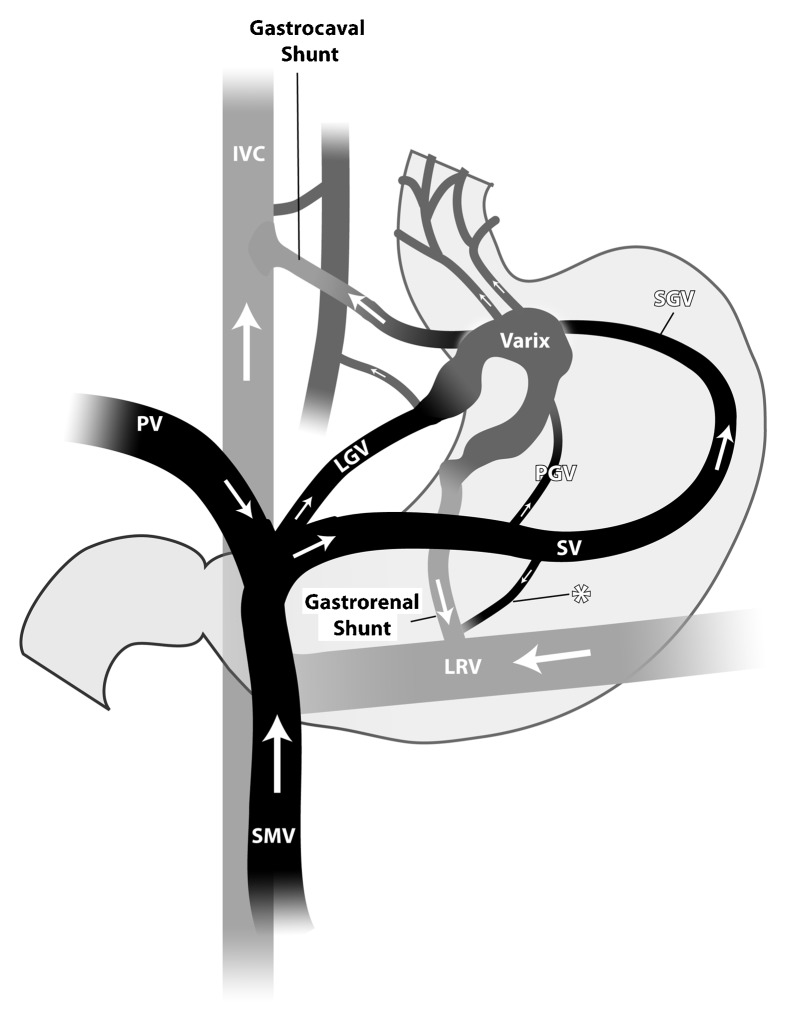
Figure 1.
The large and more common gastric varices and shunts. The two main infra-diaphragmatic portosystemic shunts: the gastrocaval and gastrorenal shunts. The common afferent (portovenous feeders) to the gastric varices are left gastric vein (LGV) (also known as the coronary vein), the posterior gastric vein(s) (PGV), and the short gastric veins (SGV). The SGV and PGV arise from the splenic vein (SV), and the LGV arises from the confluence of the splenic vein and the mesenteric vein(s) (SMV). The LGV may also arise from the proximal main portal vein (PV). The efferent limbs of the gastric varices, which drain the gastric varices into the systemic circulation, either drain directly into the inferior vena cava (IVC) (the gastrocaval shunt) or into the left renal vein (LRV) (gastrorenal shunt). The asterisk denotes a direct communication between the splenic vein and the shunt/left renal vein, demonstrating a spleno-renal shunt or a spleno-renal component of the gastrorenal shunt. (Used with permission from Figure 2 of Al-Osaimi AMS, Caldwell SH. Medical and endoscopic management of gastric varices. Semin Interv Radiol 2011;28:273–282.)
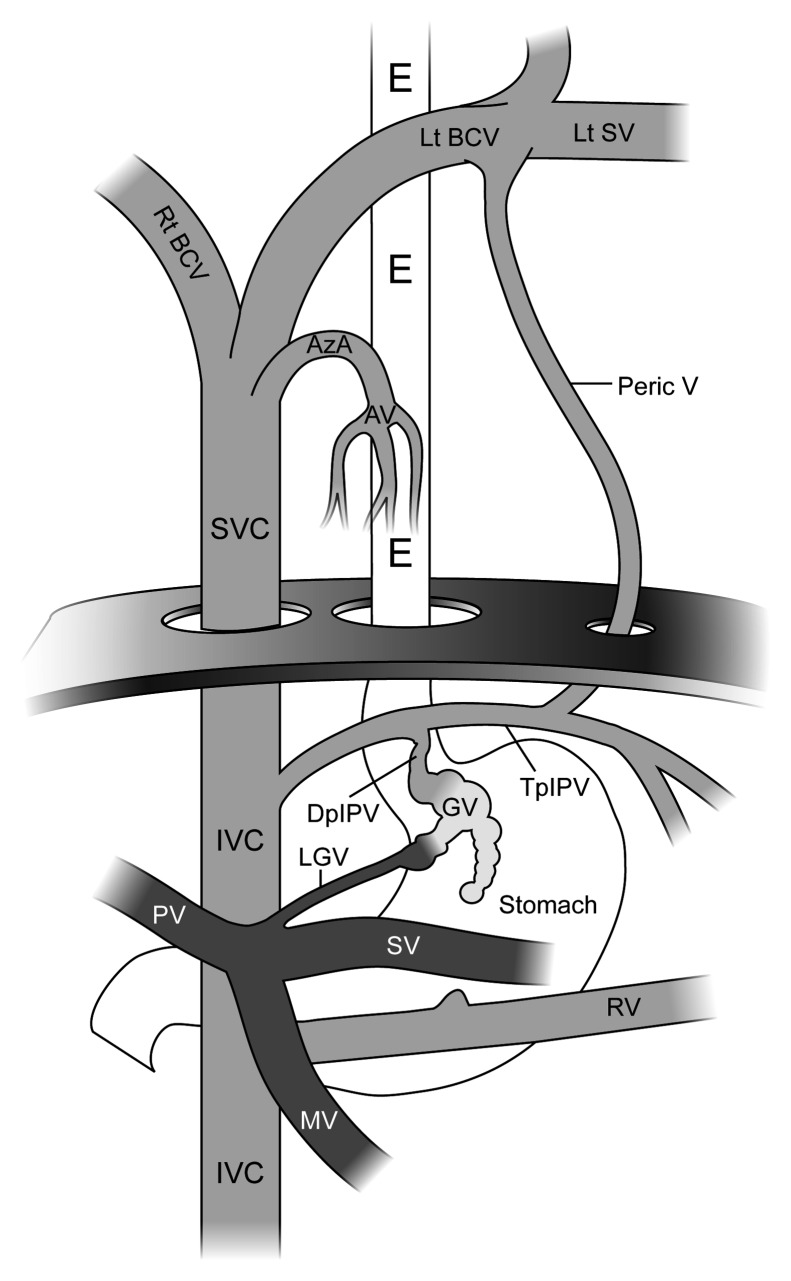
Figure 2.
Basic anatomy of a gastric varix, with the portal circulation shaded dark gray and the systemic circulation shaded light gray. On this image, the esophagus (E), stomach, the first part of the duodenum, and the left hemidiaphragm are also displayed. The figure demonstrates the para- and supradiaphragmatic portosystemic venous circulation, representing alternative access routes to the balloon-occluded retrograde transvenous obliteration (BRTO) procedure. The gastrorenal shunt in this drawing is rudimentary (compare with Fig. 1). IVC, inferior vena cava; SVC, superior vena cava; Rt BCV, right brachiocephalic vein; Lt BCV, left brachiocephalic vein; Lt SV, left subclavian vein; Peric V, Pericardiol or pericardio-phrenic vein; AzA, azygous arch; AV, azygous venous system or azygo-paraesophageal venous system; IPV, inferior phrenic vein: descending portion (DpIPV) and transverse portion (TpIPV); GV, gastric varices (varix); MV, mesenteric vein; SV, splenic vein; PV, portal vein. (Used with permission from Figure 7A of Saad WEA, Sze DY. Variations of balloon-occluded antegrade transvenous obliteration (BATO) and alternative/adjunctive routes for BRTO. Semin Interv Radiol 2011;28:314–324.)
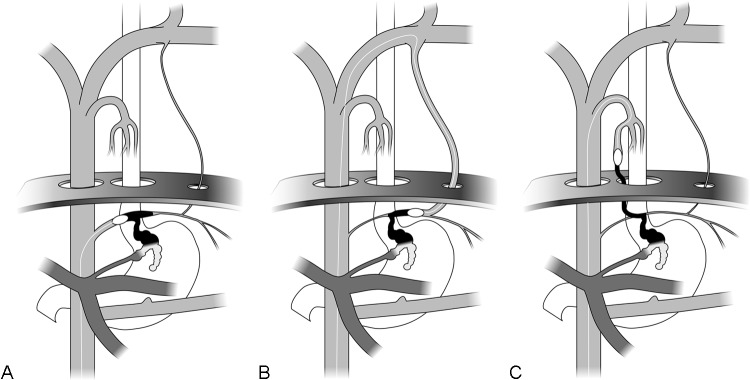
Figure 3.
Compare with Figure 2. (A) The balloon-occlusion catheter being advanced from a transfemoral approach and positioned and inflated in the transverse portion of the inferior phrenic vein (Fig. 2: TpIPV) and filling of the gastric varix (Fig. 2: GV) with contrast (black). (B) The balloon-occlusion catheter being advanced from a transfemoral approach and positioned and inflated in the transverse portion of the inferior phrenic vein (Fig. 2: TpIPV) via the left brachiocephalic (Fig. 2: Lt BCV) and pericardial veins (Fig. 2: Peric V),with ultimate filling of the gastric varix (Fig. 2: GV) with contrast (black). (C) Balloon-occlusion catheter being advanced from a transfemoral approach and positioned and inflated in the descending portion of the inferior phrenic vein (Fig. 2: DpIPV) via the azygous arch (Fig. 2: AzA) and azygous-paraesophageal (Fig. 2: AZ) venous system, ultimately filling the gastric varix (Fig. 2: GV) with contrast (black). (Used with permission from Fig. 7B, 7C, and 7D of Saad WEA, Sze DY. Variations of balloon-occluded antegrade transvenous obliteration (BATO) and alternative/adjunctive routes for BRTO. Semin Interv Radiol 2011;28:314–324.
Main Portal Vein Patency
Gastrorenal shunts and splenorenal shunts, if present, are portosystemic collaterals that naturally occur and promote hepatofugal flow.5 To what extent the “catchment veins” are affected by gastro-spleno-renal shunts is unknown but likely depends on the size of the gastrorenal shunt, the presence of a TIPS, the patency of the main portal vein, and other hemodynamic variables such as the resistance of the sinusoidal bed. However, in the presence of a completely thrombosed main portal vein, gastrorenal shunts act as the primary outflow of the splenic and mesenteric veins.2 In the presence of a thrombosed main portal vein, occlusion of the gastrorenal shunt, which is a by-product of the BRTO procedure, would potentially cause mesenteric venous hypertension, mesenteric ischemia, and possibly thrombosis of the entire splanchnic portal venous circulation. Although portal vein thrombosis is not an absolute contraindication to BRTO, it is a hemodynamic dilemma that has not been investigated fully. Chronic occlusion of the main portal vein with cavernous transformation may provide sufficient outflow for the portal venous system after occluding the portosystemic shunts, and therefore it may be acceptable to proceed with the BRTO procedure with the risks and benefits of the procedure taken into consideration.2
Partial thromboses of the main portal vein, or the presence of a diminutive but patent main portal vein, may actually improve after occluding the competing hepatofugal portosystemic shunts because the result is improved flow through the main portal vein.6,7,8,58 Conversely, a very diminutive portal vein may be a sign of a long-standing very large gastrorenal shunt, which if occluded in the process of a BRTO procedure may be overwhelmed by the increased flow. If the portal vein does not dilate and the liver cannot accept this excess hepatopetal flow, there will be no outflow to the portal vein, which will in turn lead to portal venous flow stagnation and possible portal vein thrombosis.
Presence of a Gastrorenal or Gastrocaval Shunt
For the conventional BRTO procedure, a large infradiaphragmatic “left-sided” portosystemic collateral is required.3,4,5 The most common shunt to be occluded during a conventional BRTO procedure is a gastrorenal shunt, which provides venous outflow in 90% of gastric varices cases. The remaining 10% of gastric varices drain through a gastrocaval shunt (infra-diaphragmatically), or other less common transdiaphragmatic veins.45,46,47,48,49,50,51,52,53,54,55,56,57 Preprocedural imaging is important in assessing the sites, types, and morphology of these portosystemic shunts.2
The diameter of these shunts (particularly the gastrorenal shunt) needs to be measured to plan for the BRTO procedure. The diameter of the gastrorenal shunt is typically measured at the base of the shunt, at the communication point with the left renal vein. This is typically the site at which interventionalists attempt to occlude the shunt with the balloon-occlusion catheter for the BRTO procedure.2 However, it may not necessarily be the narrowest site, and experienced interventionalists will evaluate the entire shunt to determine the narrowest points at which they can occlude the shunt safely. One common cause of technical failure of the BRTO procedure is the presence of shunts that are too large for the available balloon-occlusion catheter inventory.4 Thus knowing shunt sizes, areas of narrowing, and knowing the available balloon-occlusion catheter inventory is essential for the planning of the BRTO procedure.
Cross-Sectional Imaging Modalities Used
Three-phase contrast-enhanced computer tomography (CE-CT) or contrast-enhanced magnetic resonance angiography/magnetic resonance venography (MRV) provides optimal visualization of the main portal vein and its tributaries.2 It is important not to use oral contrast agents because these may obscure visualization of the varices. Perhaps the best CT or MR reformat plane to evaluate gastro-variceal systems for pre-BRTO candidacy evaluation and procedure planning is coronal reformats.2 Contrast-enhanced MRI is similar to three-phase CE-CT and provides optimal visualization of the portal veins and its branches as well as the gastrorenal and gastrocaval shunts, the gastric varices, and the afferent gastric veins.2 Dynamic postcontrast sequences provide the most information to plan the BRTO procedure as described later. T2-weighted sequences can also provide adequate visualization of the components of the gastric varices, as well as the inflow and outflow veins.2
Portal vein patency with or without cavernous transformation can be easily demonstrated by cross-sectional imaging. Large portosystemic shunts such as gastrorenal shunts can easily be identified, characterized, and sized. Smaller venous collaterals and portosystemic shunts can be more difficult to assess fully and require dedicated imaging techniques. The afferent gastric veins (portal venous branches leading to the gastric varices) contributing to the gastric varices can also be identified and assessed on CE-CT or MRV. This is especially true for simple gastric-variceal systems, such as type 1 gastric varices. Type I varices occur when there is afferent flow from one dominant afferent (portal branch) vein into the posterior or left gastric veins, leading to the gastric varices.2 However, it may be difficult to fully identify and assess all contributor veins in complex gastric-variceal systems with numerous, and usually smaller, portosystemic veins.
One of the rarely mentioned advantages of three-phase CE-CT or MRV is that it evaluates the whole abdomen. Secondary findings of cirrhosis (including liver morphology and ascites) would support that the patient is cirrhotic. Furthermore, ascites is a key finding that should be noted in BRTO candidates. The presence of ascites is usually as sign of relative decompensation and would be expected to increase after BRTO. For elective BRTO, this should be evaluated in the risks-versus-benefit discussion of the procedure, and possibly future TIPS placement may be contemplated and discussed with the patient prior to performing the BRTO procedure.
BRTO Procedure
The BRTO procedure is an endovascular technique that causes occlusion of outflow portosystemic shunt, such as a gastrorenal shunt, using an occlusion balloon followed by the endovascular injection of a sclerosing agent directly into the gastro-variceal system/complex.6,7,8,20,58 The gastro-variceal system/complex is a collective term for the gastro-renal shunt and the gastric or gastroesophageal varices. Balloon occlusion is used to for two technical reasons: (1) occlusion of the gastrorenal shunt so that retrograde (upstream) venography can be performed to visualize the gastric-variceal system/complex and (2) to modulate flow and cause stagnation of the sclerosant within the gastric-variceal system without reflux of the sclerosant into either the portal or systemic vasculature.3,5 Stagnation in the flow is helpful to maximize sclerosant dwell time to achieve maximal effect of the sclerosant on the gastro-variceal system endothelial lining, leading to thrombosis and subsequent scarring of the system. As can be deduced, stagnation of flow (both for diagnosis and therapy) is essential to the BRTO procedure.4
Unconventional routes have also been described;45 however, the scope of this article does not allow a detailed discussion of these routes. These alternative routes include but are not confined to transcaval, trans-phrenic, trans-pericardiac, trans-iliocolic, trans-TIPS, trans-gonadal, trans-azygous, and trans-renal capsular vein approaches (Figs. 3 and 4).45,46,47,48,49,50,51,52,53,54,55,56,57 Percutaneous transhepatic obliteration (PTO), practiced in the 1970s and predating the TIPS era, is actually the first described route used solely for the obliteration (embolization) of gastric and esophageal varices.59,60,61,62,63 With the advent of BRTO in the early 1990s, it has been used as a second choice approach or an adjunct to the traditional transrenal BRTO approach.7,8,64 PTO has also been termed by Saad and Sze as balloon-occluded anterograde (antegrade) transvenous obliteration (BATO).65
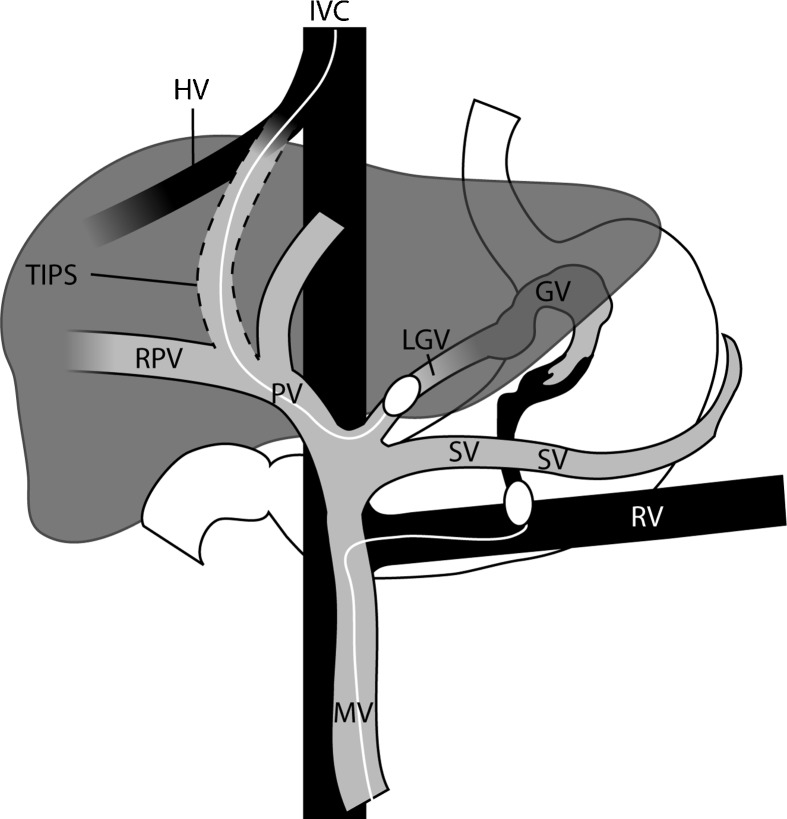
Figure 4.
The basic and surgical anatomy of a gastric varix, with the portal circulation shaded gray and the systemic circulation shaded black. A combined balloon-occluded antegrade transvenous obliteration (BATO) and balloon-occluded retrograde transvenous obliteration (BRTO) access is illustrated. The BATO access is via a transjugular intrahepatic portosystemic shunt (transjugular intrahepatic portosystemic shunt [TIPS] BATO). The balloon-occluded retrograde transvenous obliteration (BRTO) access is via the traditional transfemoro-renal access. MV, mesenteric vein; SV, splenic vein; PV, main portal vein; RPV, right portal vein; HV, hepatic vein; IVC, inferior vena cava; RV, left renal vein; LGV, left gastric vein; GV, gastric vein. (Used with permission from Fig. 5 of Saad WEA, Sze DY. Variations of balloon-occluded antegrade transvenous obliteration (BATO) and alternative/adjunctive routes for BRTO. Semin Interv Radiol 2011;28:314–324.
BRTO or BATO procedures for gastric varices are purely endovenous procedures using standard angiographic techniques with basic steps that include percutaneous venous access, endovascular approach and catheterization, retrograde balloon-aided venography, balloon-aided sclerosis, intraprocedural imaging confirmation, and balloon dwell time and removal.4,6,7
Vascular Access
Percutaneous venous access of the femoral or internal jugular vein using standard Seldinger technique is performed with placement of a 6 to 14F sheath. Most operators use a right femoral vein approach; however, certain operators have adopted the jugular vein approach exclusively.7,66 The jugular approach typically requires a longer sheath system, especially in tall patients. Careful review of preprocedure cross-sectional imaging helps decide the approach that provides the best angle for selecting the target gastrorenal shunt.
Shunt Catheterization
In Asia, catheterization of the gastrorenal shunt via the left renal vein is typically accomplished using catheters with mounted occlusion balloons that are specifically designed for the BRTO procedure.66 Reversed-shaped balloon catheters (Cobra shaped and Simmons shaped) that provide effortless and stable access into the gastrorenal shunt are available in Asia.6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30 Unfortunately, these specifically designed catheters are not available in the United States.66 For this reason, operators in the United States use reverse curve diagnostic angiographic catheters (usually Cobra shaped or Simmons shaped; Angiodynamics, Queensbury, NY) to select the gastrorenal shunt from the inferior vena cava. Subsequently, an over-the-wire exchange is made to advance the balloon-occlusion catheter into the gastrorenal shunt.
Shunt Occlusion
The balloon-occlusion catheter, with the compliant balloon mounted on the catheter, is sized to occlude the draining gastrorenal shunt. Numerous generic balloon-occlusion catheters are available in the United States. These include but are not limited to Python occlusion balloon (Applied Medical, Rancho Santa Margarita, CA), Equalizer (Boston Scientific, Natick, MA), Standard Occlusion Balloon Catheters (Boston Scientific), Flow Directed Occlusion Balloon Catheters (Cook Inc., Bloomington, IN), and the Coda (Cook). The gastrorenal shunt can be occluded at any point where there is a narrowing within the gastrorenal shunt; occlusion does not have to occur at the confluence of the shunt and the left renal vein.
Retrograde Venography with or without Cone-Beam Tomography
Balloon-occlusion venography is performed to define the type of gastric variceal system and determine the anatomy of venous drainage. The cases are classified according to the venous drainage pattern into types A, B, C, and D.7,8,66 The scope of this article does not permit description and discussion of gastric variceal types; however, in general, the operator attempts to opacify the whole gastric-variceal system with its afferent (portal venous supply) and any efferent (systemic draining veins) veins that decompress the system. Not uncommonly, draining systemic efferent veins preclude initial full opacification of the gastric variceal system and must be embolized to have a more complete evaluation of the entire system.4,66 Identification of the afferent vessels (portal venous supply) is to familiarize the operator with the “the point of no return” where sclerosant injection is stopped just before overspill occurs into the portal venous system (splenic and main portal vein).
Once the entire gastric-variceal system is opacified, a cone-beam C-arm computed tomogram (Dyna CT, Siemens Medical, Malvern, PA) can be performed. Although not necessary, the cone-beam CT can be used to ensure that the entire system is opacified, especially all the gastric varices proper.66 Such visualization can be particularly helpful for novice operators. Correlation with preprocedural axial CT and MR images using gastric, splenic, and hepatic landmarks enables the operator to identify whether he or she is filling the entire system.
Sclerosant Injection
The aim during sclerosant injection is to fill the entire gastric-variceal system so that no varices remain and no dispensable portosystemic connections are left for the system to revascularize. The embolization end point is minimal filling of the afferent portal vasculature that was previously identified during balloon retrograde venography. Injection of a sclerosing agent can be performed with or without use of a microcatheter.56 I do suggest advancing a microcatheter, if feasible, through or adjacent to the balloon-occlusion catheter as deeply as possible into the gastric-variceal system.56 This allows an even distribution of sclerosant in the system for optimal sclerosis effect.56
Numerous sclerosing agents can be used and have been described. These agents include 5 to 10% EO (Oldamin; Grelan Pharmaceutical, Tokyo, Japan), 3% STS (Sotradecol, AngioDynamics), and Polidocanol (Polidocasklerol, ZERIA Pharmaceutical, Tokyo, Japan). All agents can be used in foam, froth, or liquid form. In addition, liquid sclerosants such as N-butyl-cyanoacrylate and absolute (97 to 99%) ethanol have also been used.66,67 EO is the traditional agent used for BRTO and is still the agent of choice in Asia.6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30 EO causes hemolysis in the blood vessels; as a result, free hemoglobin is released, which may cause renal tubular disturbances and acute renal failure. To prevent renal insufficiency, 2000 to 4000 U of haptoglobin (Green Cross, Osaka, Japan) is routinely administered intravenously during the BRTO to chelate the circulating free hemoglobin to minimize its nephrotoxic effects.7,8 There are considerable limiting factors for using EO in the United States, including the lack of endovascular experience with this agent, lack of availability in the desirable concentrations, and that the antidote is not approved by the Food and Drug Administration (FDA). As a result, 3% STS is the most commonly used sclerosant in the United States.5
For sclerotherapy of superficial lower extremity varicosities and spider veins, 3% STS is used.68,69 It has also been utilized as a foam sclerosant in treating male varicoceles, pelvic congestion syndromes, and venous malformations.70,71,72,73 Its availability in the United States, familiarity with the agent by interventionalists, and the lower risk of hemolysis (no need for haptoglobin antidote) have made it the sclerosant of choice in the United States.
Once the entire gastric-variceal system is filled with sclerosant, I recommend performing a cone-beam C-arm computed tomogram to ensure that the entire system is filled with sclerosant, especially all the gastric varices proper. Correlation with preprocedural axial CT and MR images using gastric, splenic, and hepatic landmarks enables the operator to identify whether he or she is filling the entire system.66,67
Sclerosant Dwell Time (Balloon Dwell Time)
The balloon-occlusion catheters are left inflated for 4 to 24 hours. Operators at my institution start with overnight inflation (12 to 24 hours), which actually inadvertently led to some sclerosant dwell time of up to 36 hours. Over time, the dwell time has been reduced safely to 4 to 6 hours.66,67 This protocol has been adopted for logistical reasons, such as cost concerns (patients stay overnight in monitored beds), patient comfort, and reducing the infection risk of indwelling catheters tethered at skin sites.66
Technical and Hemodynamic Results of BRTO
Technical Success of the BRTO Procedure
The technical success of BRTO for patients with gastrorenal shunts (without adjunctive endoscopic sclerotherapy and/or BATO rescue) ranges from 79% to 100%.10,11,12,13,14,15,16,17,18,19,22,24,27,28,29,32,33,34,35 In 10 studies that intended to treat gastric varices from one session, a total of 457 BRTO procedures with and without BATO rescue were evaluated. In these studies, 419 procedures (91.7%) were technically successful.10,11,12,13,16,17,18,19,33,35 The range of technical success in each individual study was 84 to 100%.10,11,12,13,16,17,18,19,33,35 In two studies that specifically identified primary treatment of gastric varices with BRTO (reserving BATO via a percutaneous transhepatic route as a rescue), the technical success rates were 84 to 98% (BRTO only) and 100% (BATO only or combined BRTO and BATO).11,14
A different technical approach is to sclerose the gastric varices in multiple sessions, limiting the amount of sclerosant used. For these multisession administrations, 20 to 30 mL of EO is used for each session in an attempt to reduce the dose-related complication of hemolysis with its potential hemoglobinuria-induced renal dysfunction. In these reports, patients who did not have obliteration of all gastric varices from the prior session returned for a subsequent session.7,14,15,22,29,36 With a total of 210 BRTO procedures in five studies adopting this approach, the first, second, and third BRTO sessions were technically successful with complete obliteration of the gastric varices in 56 to 87%, 79 to 100%, and 81 to 100% of patients, respectively.7,14,15,22,29,36 Cumulatively, the first, second, and third session technical success rate of the 210 BRTO cases in these five studies is 71% (N = 150 of 210), 88% (N = 185 of 210), and 91% (N = 191 of 210), respectively.7,14,22,29,36
Causes of technical failure are classified by the current authors as types I to IV. Type I failures occur because of the inability to cannulate the gastrorenal shunt or sclerosant extravasation; type II failures occur because of an inability to occlude the shunt due to an undersized balloon (this is a type V shunt as defined by Fukuda et al).14 Type III failures are subcategorized into two types and due to the inability to opacify the shunt (despite a well-inflated and appropriate size balloon) due to a complex multicollateral gastrorenal/gastric variceal system (IIIa) or extravasation of contrast/sclerosant into the retroperitoneum (IIIb). Type IV failures are due to early (usually within 1 to 2 hours) occlusive-balloon rupture requiring a repeat BRTO. Such balloon ruptures are known to occur in 2.3 to 8.7% of BRTO cases.27,31 However, not all occur early and lead to technical failure (~50% of balloon ruptures do so).31
Unfortunately, in published reports not all technical failures are disclosed in detail, and reporting standards often are not used. Having said that, the most common cause of technical failure is likely a complex multicollateral gastrorenal system with the resultant inability to fully opacify, “trap,” and sclerose the gastric varix (type III failure).10,12,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,36,74,75,76,77,78,79 In three studies evaluating 160 BRTO procedures with 14 technical failures (91.3% technical success), type I failures (cannulation/extravasation) represented 14% of failures (N = 2 of 14) and 1.3% of BRTO cases (N = 2 of 160); type II failures (small for size balloons) represented 36% of failures (N = 5 of 14) of failures and 3.1% of BRTO cases (N = 5 of 160); type III failures (unable to opacify and “trap”) represented 43% of failures (N = 6 of 14) and 3.8% of BRTO cases (N = 6 of 160); and type IV failures (early balloon rupture) represented 7.1% of failures (N = 1 of 14) and 0.6% of BRTO cases (N = 1 of 160).11,13,25 Table 1 lists the procedural and postprocedural (including long-term) complications.10,12,14,15,16,18,19,22,23,24,27,31,32,33,34,79
Table 1. Procedural Complications of Balloon-Occluded Retrograde Transvenous Obliteration Using Ethanolamine Oleate.
| Complication Type | Incidence (%) |
|---|---|
| Procedural Complications | |
| Gross hematuria | 15–100* |
| All pulmonary embolism | 1.5–4.1 |
| Symptomatic pulmonary embolism | 1.4–2.5 |
| Cardiac arrhythmia | 1.5 |
| Anaphylaxis | 2.2–5.0 |
| Rapid/Fulminant hepatic failure | 4.8–7.0 |
| Death within 30 days from fulminant hepatic failure | 0.0–4.1 |
| Renal failure | 4.8 |
| Long-Term Complications | |
| Encephalopathy | 17.6** |
| Portal Hypertensive Gastropathy | 5.3–13.2 |
| Post-BRTO gastropathy (not to extent of portal hypertensive gastropathy) | 56.5 |
| Aggravation of esophageal varices | 14–68*** |
| Bleeding from esophageal varices | 17–24*** |
| Duodenal varices | Up to 3.2 |
| Bleeding duodenal varices | Up to 2.3 |
| Ascites | 0–43.5 |
| Spontaneous bacterial peritonitis | Up to 8.2 |
| Pleural effusion (hydrothorax) | 5.3–7.9 |
| Portal vein thrombosis | Up to 4.7 |
| Renal vein thrombosis (no clinical consequences) | Up to 5.0 |
This is a common complication and is usually without clinical sequelae.
This is thought to be part of the disease process/nature of the disease and not a complication of BRTO.
Esophageal variceal aggravation and bleeding probably vary drastically based on the vigilance and aggressiveness of endoscopists in managing esophageal varices.
Hemodynamic Outcome of the BRTO Procedure
Many studies report the success rate of BRTO based on the obliteration rate of the gastric varices by follow-up imaging, such as CT venography, MRV, and/or EUS. In 14 studies evaluating post-BRTO gastric variceal obliteration in a total of 580 BRTO procedures, obliteration rates in the intent-to-treat (including technically failed BRTO procedures) population and in technically successful BRTO procedures were 73 to 100% and 75 to 100%, respectively.10,12,14,15,16,17,22,24,27,28,29,32 Cumulatively for the same 14 studies, the technical success rate, the intent-to-treat obliteration rate, and the obliteration rate of gastric varices of technically successful BRTO procedures were 91% (N = 529 of 580), 86% (N = 496 of 580), and 94% (N = 496 of 529), respectively.10,12,14,15,16,17,22,24,27,28,29,32
Clinical Outcomes of BRTO
The primary indications for BRTO are gastric variceal bleeding (or potential bleeding) and refractory encephalopathy in the presence of a gastrorenal shunt.10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,74,75,76,77,78,79 In two studies evaluating a total of 20 patients, the effectiveness of BRTO in controlling actively bleeding gastric varices was 91 to 100%.10,14 The gastric variceal and global variceal rebleed rate are discussed later.
The aggravation of nongastric (esophageal or duodenal) varices appears to be a major problem following BRTO and reflects postprocedural increased portal hypertension.10,12,13,14,18,23,24,31,32,34,35 This effect varies widely, probably depending on the degree of vigilance, documentation, and thoroughness of follow-up endoscopy. However, in four major studies evaluating 160 patients who had undergone BRTO and who underwent continuous endoscopic follow-up post-BRTO, the esophageal variceal aggravation rate at 1, 2, and 3 years was 27 to 35%, 45 to 66%, and 45 to 91%, respectively.12,18,22,30 In another two studies evaluating 117 patients following BRTO, the percentage of patients with aggravated esophageal varices was 30 to 68%, and 36 to 57% of patients with aggravated esophageal varices went on to bleeding.13,32 Again, one can speculate that the rate of esophageal variceal bleeding may be significantly reduced by a higher vigilance of endoscopic follow-up and more aggressive endoscopic therapy. Other reported complications reflective of increased portal hypertension following BRTO are the development of portal hypertensive gastropathy (occurs in 5 to 13%), ascites (occurs in 0 to 44%), and hydrothorax/pleural effusion (occurs in 0 to 8%).15,18,19,22,27,33
The rebleeding rate following BRTO depends on the definition used. Most studies display a gastric variceal rebleed rate in patients who had undergone a successful BRTO procedure that ranges between 0% and 10%.10,11,12,13,14,15,16,17,18,19,24,32,33,35,74 However, when factoring in an intent-to-treat basis (including technical failures) for the results, the gastric variceal rebleed rate is 0 to 31.6%.10,11,12,13,14,15,16,17,18,19,24,32,33,35,74 Many studies do not clearly state what source, if any, the global rebleed rate is from: gastric, esophageal, or duodenal varices as well as portal hypertensive gastropathy.10,11,12,13,14,15,16,17,18,19,24,32,33,35,74 In three clearly reported studies evaluating a total of 141 patients who had undergone BRTO, the gastric variceal rebleed rate of successful BRTO procedures, the intent-to-treat gastric variceal rebleed rate and the global (all types of varices) variceal rebleed rates were 3.2 to 8.7%, 10 to 20%, and 19 to 31%, respectively.10,13,35
The greatest advantages of BRTO are its preservation of hepatic function and its reduction in the risk of hepatic encephalopathy.3,4 In fact, one of the indications for BRTO is encephalopathy with the presence of a gastrorenal or gastro-splenorenal shunt.14,20,21,24,28,33 In five studies evaluating a total of 35 patients with encephalopathy, there was resolution or significant reduction in encephalopathy in all patients (100% success).14,20,22,24,33 An interesting study by Kumamoto et al had three branches.19 In one group were patients with no gastrorenal shunt (the control group), the second cohort had a gastrorenal shunt that was not treated (with BRTO or by any other means), and the third group were patients with gastrorenal shunts that were treated with BRTO.19 Those with untreated gastrorenal shunts had progressively deteriorating hepatic function (by the Child-Pugh score), whereas those that had BRTO had transient improvement in hepatic function for 6 to 12 months and then a return to baseline hepatic function up to 3 years. The patients without gastrorenal shunts (control group) had stable hepatic function similar to those patients with BRTO. This suggested that BRTO has a protective long-term role in preserving hepatic function and protecting the liver from “portosystemic shunt syndrome.”19
Survival following BRTO is generally favorable. The Kaplan-Meier survival rate after BRTO at 1, 2, 3, and 5 years ranges from 83 to 98%, 76 to 79%, 66 to 85%, and 39 to 69%, respectively.11,18,24,25,30,33,36 Obviously, the greatest determinate of survival is the patient's hepatic reserve (determined by Child-Pugh score and/or model for end-stage liver disease score).13,14,28,29 However, hepatocellular carcinoma is also a significant determinate of survival22,28,29 to the extent that prior authors have considered an intrahepatic hepatocellular carcinoma >5 cm to serve as a contraindication to BRTO.22
Conclusion
BRTO has shown considerable effectiveness in controlling gastric variceal bleeding with low rebleed rates. It has many advantages versus TIPS in that it is less invasive and can be performed on patients with poor hepatic reserve and encephalopathy (and may even improve both). However, its by-product is occlusion of a spontaneous hepatofugal (TIPS equivalent) shunt and thus is somewhat contradictory to the traditional American doctrine of the need for portal decompression. Indeed, BRTO causes an increase in portal hypertension with aggravation of esophageal varices and ascites. Despite these controversies, the BRTO procedure is gaining popularity in the United States. With the increasing experience with the management of gastric varices (endoscopy, TIPS, and BRTO), one is hopeful that management of gastric varices will be tailored to patient anatomy, clinical features of portal hypertension, and hepatic reserve.
References
- 1.Al-Osaimi A MS, Caldwell S H. Medical and endoscopic management of gastric varices. Semin Intervent Radiol. 2011;28:273–282. doi: 10.1055/s-0031-1284453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Osaimi A MS, Sabri S S, Caldwell S H. Balloon-occluded retrograde transvenous obliteration (BRTO): preprocedural evaluation and imaging. Semin Intervent Radiol. 2011;28:288–295. doi: 10.1055/s-0031-1284455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saad W EA, Darcy M D. Transjugular intrahepatic portosystemic shunt (TIPS) versus balloon-occluded retrograde transvenous obliteration (BRTO) for the management of gastric varices. Semin Intervent Radiol. 2011;28:339–349. doi: 10.1055/s-0031-1284461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saad W EA, Sabri S S. Balloon-occluded retrograde transvenous obliteration (BRTO): technical results and outcomes. Semin Intervent Radiol. 2011;28:333–338. doi: 10.1055/s-0031-1284460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saad W EA. The history and evolution of balloon-occluded retrograde transvenous obliteration (BRTO): from the United States to Japan and back. Semin Intervent Radiol. 2011;28:283–287. doi: 10.1055/s-0031-1284454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanagawa H, Mima S, Kouyama H, Gotoh K, Uchida T, Okuda K. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol. 1996;11(1):51–58. doi: 10.1111/j.1440-1746.1996.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 7.Kiyosue H, Mori H, Matsumoto S, Yamada Y, Hori Y, Okino Y. Transcatheter obliteration of gastric varices. Part 1. Anatomic classification. Radiographics. 2003;23(4):911–920. doi: 10.1148/rg.234025044. [DOI] [PubMed] [Google Scholar]
- 8.Kiyosue H Mori H Matsumoto S Yamada Y Hori Y Okino Y Transcatheter obliteration of gastric varices: Part 2. Strategy and techniques based on hemodynamic features Radiographics 2003234921–937.; discussion 937 [DOI] [PubMed] [Google Scholar]
- 9.Yamagami T, Kato T, Hirota T, Yoshimatsu R, Matsumoto T, Nishimura T. Infusion of 50% glucose solution before injection of ethanolamine oleate during balloon-occluded retrograde transvenous obliteration. Australas Radiol. 2007;51(4):334–338. doi: 10.1111/j.1440-1673.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- 10.Kitamoto M, Imamura M, Kamada K. et al. Balloon-occluded retrograde transvenous obliteration of gastric fundal varices with hemorrhage. AJR Am J Roentgenol. 2002;178(5):1167–1174. doi: 10.2214/ajr.178.5.1781167. [DOI] [PubMed] [Google Scholar]
- 11.Ninoi T, Nakamura K, Kaminou T. et al. TIPS versus transcatheter sclerotherapy for gastric varices. AJR Am J Roentgenol. 2004;183(2):369–376. doi: 10.2214/ajr.183.2.1830369. [DOI] [PubMed] [Google Scholar]
- 12.Ninoi T, Nishida N, Kaminou T. et al. Balloon-occluded retrograde transvenous obliteration of gastric varices with gastrorenal shunt: long-term follow-up in 78 patients. AJR Am J Roentgenol. 2005;184(4):1340–1346. doi: 10.2214/ajr.184.4.01841340. [DOI] [PubMed] [Google Scholar]
- 13.Akahoshi T, Hashizume M, Tomikawa M. et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding and risky gastric varices: a 10-year experience. J Gastroenterol Hepatol. 2008;23(11):1702–1709. doi: 10.1111/j.1440-1746.2008.05549.x. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol. 2001;12(3):327–336. doi: 10.1016/s1051-0443(07)61912-5. [DOI] [PubMed] [Google Scholar]
- 15.Sonomura T, Sato M, Kishi K. et al. Balloon-occluded retrograde transvenous obliteration for gastric varices: a feasibility study. Cardiovasc Intervent Radiol. 1998;21(1):27–30. doi: 10.1007/s002709900206. [DOI] [PubMed] [Google Scholar]
- 16.Kiyosue H, Matsumoto S, Onishi R. et al. Balloon-occluded retrograde transvenous obliteration (B-RTO) for gastric varices: therapeutic results and problems [in Japanese] Nippon Igaku Hoshasen Gakkai Zasshi. 1999;59(1):12–19. [PubMed] [Google Scholar]
- 17.Koito K, Namieno T, Nagakawa T, Morita K. Balloon-occluded retrograde transvenous obliteration for gastric varices with gastrorenal or gastrocaval collaterals. AJR Am J Roentgenol. 1996;167(5):1317–1320. doi: 10.2214/ajr.167.5.8911204. [DOI] [PubMed] [Google Scholar]
- 18.Chikamori F, Kuniyoshi N, Kawashima T, Takase Y. Gastric varices with gastrorenal shunt: combined therapy using transjugular retrograde obliteration and partial splenic embolization. AJR Am J Roentgenol. 2008;191(2):555–559. doi: 10.2214/AJR.07.3356. [DOI] [PubMed] [Google Scholar]
- 19.Kumamoto M, Toyonaga A, Inoue H. et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric fundal varices: hepatic deterioration links to portosystemic shunt syndrome. J Gastroenterol Hepatol. 2010;25(6):1129–1135. doi: 10.1111/j.1440-1746.2010.06262.x. [DOI] [PubMed] [Google Scholar]
- 20.Chikamori F, Kuniyoshi N, Shibuya S, Takase Y. Transjugular retrograde obliteration for chronic portosystemic encephalopathy. Abdom Imaging. 2000;25(6):567–571. doi: 10.1007/s002610000046. [DOI] [PubMed] [Google Scholar]
- 21.Chikamori F, Kuniyoshi N, Shibuya S, Takase Y. Combination treatment of transjugular retrograde obliteration and endoscopic embolization for portosystemic encephalopathy with esophageal varices. Hepatogastroenterology. 2004;51(59):1379–1381. [PubMed] [Google Scholar]
- 22.Chikamori F, Kuniyoshi N, Shibuya S, Takase Y. Eight years of experience with transjugular retrograde obliteration for gastric varices with gastrorenal shunts. Surgery. 2001;129(4):414–420. doi: 10.1067/msy.2001.112000. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto Y, Oho K, Kumamoto M, Toyonaga A, Sata M. Balloon-occluded retrograde transvenous obliteration improves liver function in patients with cirrhosis and portal hypertension. J Gastroenterol Hepatol. 2003;18(8):934–942. doi: 10.1046/j.1440-1746.2003.03087.x. [DOI] [PubMed] [Google Scholar]
- 24.Hirota S, Matsumoto S, Tomita M, Sako M, Kono M. Retrograde transvenous obliteration of gastric varices. Radiology. 1999;211(2):349–356. doi: 10.1148/radiology.211.2.r99ma25349. [DOI] [PubMed] [Google Scholar]
- 25.Sugimori K, Morimoto M, Shirato K, Kokawa A, Tomita N, Numata K. et al. Retrograde transvenous obliteration of gastric varices associated with large collateral veins or a large gastrorenal shunt. J Vasc Intervent Radiol. 2005;16:113–118. doi: 10.1097/01.RVI.0000143765.38128.23. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda T, Hirota S, Matsumoto S. et al. Application of balloon-occluded retrograde transvenous obliteration to gastric varices complicating refractory ascites. Cardiovasc Intervent Radiol. 2004;27(1):64–67. doi: 10.1007/s00270-003-2715-9. [DOI] [PubMed] [Google Scholar]
- 27.Shimoda R, Horiuchi K, Hagiwara S. et al. Short-term complications of retrograde transvenous obliteration of gastric varices in patients with portal hypertension: effects of obliteration of major portosystemic shunts. Abdom Imaging. 2005;30(3):306–313. doi: 10.1007/s00261-004-0270-8. [DOI] [PubMed] [Google Scholar]
- 28.Takuma Y, Nouso K, Makino Y, Saito S, Shiratori Y. Prophylactic balloon-occluded retrograde transvenous obliteration for gastric varices in compensated cirrhosis. Clin Gastroenterol Hepatol. 2005;3(12):1245–1252. doi: 10.1016/s1542-3565(05)00744-5. [DOI] [PubMed] [Google Scholar]
- 29.Arai H, Abe T, Takagi H, Mori M. Efficacy of balloon-occluded retrograde transvenous obliteration, percutaneous transhepatic obliteration and combined techniques for the management of gastric fundal varices. World J Gastroenterol. 2006;12(24):3866–3873. doi: 10.3748/wjg.v12.i24.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arai H, Abe T, Shimoda R, Takagi H, Yamada T, Mori M. Emergency balloon-occluded retrograde transvenous obliteration for gastric varices. J Gastroenterol. 2005;40(10):964–971. doi: 10.1007/s00535-005-1654-4. [DOI] [PubMed] [Google Scholar]
- 31.Park S J, Chung J W, Kim H-C, Jae H J, Park J H. The prevalence, risk factors, and clinical outcome of balloon rupture in balloon-occluded retrograde transvenous obliteration of gastric varices. J Vasc Interv Radiol. 2010;21(4):503–507. doi: 10.1016/j.jvir.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 32.Hong C H, Kim H J, Park J H. et al. Treatment of patients with gastric variceal hemorrhage: endoscopic N-butyl-2cyanoacrylate injection versus balloon-occluded retrograde transvenous obliteration. Hepatology. 2009;24:372–378. doi: 10.1111/j.1440-1746.2008.05651.x. [DOI] [PubMed] [Google Scholar]
- 33.Park K S, Kim Y H, Choi J S. et al. Therapeutic efficacy of balloon-occluded retrograde transvenous obliteration in patients with gastric variceal bleeding [in Korean] Korean J Gastroenterol. 2006;47(5):370–378. [PubMed] [Google Scholar]
- 34.Kim E S, Park S Y, Kwon K T. et al. The clinical usefulness of balloon occluded retrograde transvenous obliteration in gastric variceal bleeding [in Korean] Taehan Kan Hakhoe Chi. 2003;9(4):315–323. [PubMed] [Google Scholar]
- 35.Cho S K, Shin S W, Lee I H. et al. Balloon-occluded retrograde transvenous obliteration of gastric varices: outcomes and complications in 49 patients. AJR Am J Roentgenol. 2007;189(6):W365-72. doi: 10.2214/AJR.07.2266. [DOI] [PubMed] [Google Scholar]
- 36.Choi Y H, Yoon C J, Park J H, Chung J W, Kwon J W, Choi G M. Balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding: its feasibility compared with transjugular intrahepatic portosystemic shunt. Korean J Radiol. 2003;4(2):109–116. doi: 10.3348/kjr.2003.4.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olson E, Yune H Y, Klatte E C. Transrenal-vein reflux ethanol sclerosis of gastroesophageal varices. AJR Am J Roentgenol. 1984;143(3):627–628. doi: 10.2214/ajr.143.3.627. [DOI] [PubMed] [Google Scholar]
- 38.Kravetz D, Bosch J, Arderiu M, Pilar Pizcueta M, Rodés J. Hemodynamic effects of blood volume restitution following a hemorrhage in rats with portal hypertension due to cirrhosis of the liver: influence of the extent of portal-systemic shunting. Hepatology. 1989;9(6):808–814. doi: 10.1002/hep.1840090603. [DOI] [PubMed] [Google Scholar]
- 39.Castañeda B, Morales J, Lionetti R. et al. Effects of blood volume restitution following a portal hypertensive-related bleeding in anesthetized cirrhotic rats. Hepatology. 2001;33(4):821–825. doi: 10.1053/jhep.2001.23437. [DOI] [PubMed] [Google Scholar]
- 40.Villanueva C, Ortiz J, Miñana J. et al. Somatostatin treatment and risk stratification by continuous portal pressure monitoring during acute variceal bleeding. Gastroenterology. 2001;121(1):110–117. doi: 10.1053/gast.2001.25536. [DOI] [PubMed] [Google Scholar]
- 41.de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43(1):167–176. doi: 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Gabriel D A, Li X, Monroe D MIII III, Roberts H R. Recombinant human factor VIIa (rFVIIa) can activate factor FIX on activated platelets. J Thromb Haemost. 2004;2(10):1816–1822. doi: 10.1111/j.1538-7836.2004.01015.x. [DOI] [PubMed] [Google Scholar]
- 43.García-Pagán J C, Caca K, Bureau C. et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362(25):2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362(9):823–832. doi: 10.1056/NEJMra0901512. [DOI] [PubMed] [Google Scholar]
- 45.Araki T, Hori M, Motosugi U. et al. Can balloon-occluded retrograde transvenous obliteration be performed for gastric varices without gastrorenal shunts? J Vasc Interv Radiol. 2010;21(5):663–670. doi: 10.1016/j.jvir.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Ota K, Okazaki M, Higashihara H. et al. Combination of transileocolic vein obliteration and balloon-occluded retrograde transvenous obliteration is effective for ruptured duodenal varices. J Gastroenterol. 1999;34(6):694–699. doi: 10.1007/s005350050321. [DOI] [PubMed] [Google Scholar]
- 47.Ono S, Irie T, Kuramochi M, Kamoshida T, Hirai S, Oka Y. Successful treatment of mesenteric varices with balloon-occluded retrograde transvenous obliteration via an abdominal wall vein. J Vasc Interv Radiol. 2007;18(8):1033–1035. doi: 10.1016/j.jvir.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 48.Ibukuro K, Sugihara T, Tanaka R. et al. Balloon-occluded retrograde transvenous obliteration (BRTO) for a direct shunt between the inferior mesenteric vein and the inferior vena cava in a patient with hepatic encephalopathy. J Vasc Interv Radiol. 2007;18(1 Pt 1):121–125. doi: 10.1016/j.jvir.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Kimura T Haruta I Isobe Y et al. A novel therapeutic approach for rectal varices: a case report of rectal varices treated with double balloon-occluded embolotherapy Am J Gastroenterol 1997925883–886. S [PubMed] [Google Scholar]
- 50.Akazawa Y, Murata I, Yamao T. et al. Successful management of bleeding duodenal varices by endoscopic variceal ligation and balloon-occluded retrograde transvenous obliteration. Gastrointest Endosc. 2003;58(5):794–797. doi: 10.1016/s0016-5107(03)02008-x. [DOI] [PubMed] [Google Scholar]
- 51.Tominaga K, Montani A, Kuga T. et al. Combined balloon-occluded embolization for treatment of concurrent duodenal, gastric, and esophageal varices: a case report. Gastrointest Endosc. 2001;53(6):665–668. doi: 10.1067/mge.2001.113279. [DOI] [PubMed] [Google Scholar]
- 52.Onozato Y, Kakizaki S, Iizuka H. et al. Ectopic varices rupture in the gastroduodenal anastomosis successfully treated with N-butyl-2-cyanoacrylate injection. Acta Med Okayama. 2007;61(6):361–365. doi: 10.18926/AMO/32880. [DOI] [PubMed] [Google Scholar]
- 53.Zamora C A, Sugimoto K, Tsurusaki M. et al. Endovascular obliteration of bleeding duodenal varices in patients with liver cirrhosis. Eur Radiol. 2006;16(1):73–79. doi: 10.1007/s00330-005-2781-2. [DOI] [PubMed] [Google Scholar]
- 54.Haruta I, Isobe Y, Ueno E. et al. Balloon-occluded retrograde transvenous obliteration (BRTO), a promising nonsurgical therapy for ectopic varices: a case report of successful treatment of duodenal varices by BRTO. Am J Gastroenterol. 1996;91(12):2594–2597. [PubMed] [Google Scholar]
- 55.Ohta M, Yasumori K, Saku M, Saitsu H, Muranaka T, Yoshida K. Successful treatment of bleeding duodenal varices by balloon-occluded retrograde transvenous obliteration: a transjugular venous approach. Surgery. 1999;126(3):581–583. [PubMed] [Google Scholar]
- 56.Sonomura T, Horihata K, Yamahara K. et al. Ruptured duodenal varices successfully treated with balloon-occluded retrograde transvenous obliteration: usefulness of microcatheters. AJR Am J Roentgenol. 2003;181(3):725–727. doi: 10.2214/ajr.181.3.1810725. [DOI] [PubMed] [Google Scholar]
- 57.Tsurusaki M, Sugimoto K, Matsumoto S. et al. Bleeding duodenal varices successfully treated with balloon-occluded retrograde transvenous obliteration (B-RTO) assisted by CT during arterial portography. Cardiovasc Intervent Radiol. 2006;29(6):1148–1151. doi: 10.1007/s00270-004-0189-z. [DOI] [PubMed] [Google Scholar]
- 58.Hiraga N, Aikata H, Takaki S. et al. The long-term outcome of patients with bleeding gastric varices after balloon-occluded retrograde transvenous obliteration. J Gastroenterol. 2007;42(8):663–672. doi: 10.1007/s00535-007-2077-1. [DOI] [PubMed] [Google Scholar]
- 59.Funaro A H, Ring E J, Freiman D B, Oleaga J A, Gordon R L. Transhepatic obliteration of esophageal varices using the stainless steel coil. AJR Am J Roentgenol. 1979;133(6):1123–1125. doi: 10.2214/ajr.133.6.1123. [DOI] [PubMed] [Google Scholar]
- 60.Scott J, Dick R, Long R G, Sherlock S. Percutaneous transhepatic obliteration of gastro-osephageal varices. Lancet. 1976;10:53–55. doi: 10.1016/s0140-6736(76)92281-9. [DOI] [PubMed] [Google Scholar]
- 61.Lunderquist A, Simert G, Tylén U, Vang J. Follow-up of patients with portal hypertension and esophageal varices treated with percutaneous obliteration of gastric coronary vein. Radiology. 1977;122(1):59–63. doi: 10.1148/122.1.59. [DOI] [PubMed] [Google Scholar]
- 62.Lunderquist A, Vang J. Transhepatic catheterization and obliteration of the coronary vein in patients with portal hypertension and esophageal varices. N Engl J Med. 1974;291(13):646–649. doi: 10.1056/NEJM197409262911303. [DOI] [PubMed] [Google Scholar]
- 63.Lunderquist A, Vang J. Sclerosing injection of esophageal varices through transhepatic selective catheterization of the gastric coronary vein. A preliminary report. Acta Radiol Diagn (Stockh) 1974;15(5):546–550. doi: 10.1177/028418517401500509. [DOI] [PubMed] [Google Scholar]
- 64.Fukuda T, Hirota S, Sugimoto K, Matsumoto S, Zamora C A, Sugimura K. “Downgrading” of gastric varices with multiple collateral veins in balloon-occluded retrograde transvenous obliteration. J Vasc Interv Radiol. 2005;16(10):1379–1383. doi: 10.1097/01.RVI.0000175336.05823.eb. [DOI] [PubMed] [Google Scholar]
- 65.Saad W EA, Sze D. Variations of balloon-occluded retrograde transvenous obliteration (BRTO): balloon-occluded antegrade transvenous obliteration (BATO) and alternative/adjunctive routes for BRTO. Semin Intervent Radiol. 2011;28:314–324. doi: 10.1055/s-0031-1284458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sabri S S, Saad W EA. Balloon-occluded retrograde transvenous obliteration (BRTO): technique and intraprocedural imaging. Semin Intervent Radiol. 2011;28:303–313. doi: 10.1055/s-0031-1284457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sabri S, Swee W, Turba U C, Saad W E, Park A W, Al-Osaimi A M. et al. Bleeding gastric varices obliteration with balloon-occluded retrograde transvenous obliteration (BRTO) utilizing sodium tetradecyl sulfate foam. J Vasc Interv Radiol. 2011;22:309–316. doi: 10.1016/j.jvir.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 68.Demagny A. Comparative study into the efficacy of a sclerosant product in the form of liquid or foam in echo-guided of arches of the long and short saphenous veins. Phlebologie. 2002;55:133–137. [Google Scholar]
- 69.Coleridge Smith P. Sclerotherapy and foam sclerotherapy for varicose veins. Phlebology. 2009;24(6):260–269. doi: 10.1258/phleb.2009.009050. [DOI] [PubMed] [Google Scholar]
- 70.Gandini R, Chiocchi M, Konda D, Pampana E, Fabiano S, Simonetti G. Transcatheter foam sclerotherapy of symptomatic female varicocele with sodium-tetradecyl-sulfate foam. Cardiovasc Intervent Radiol. 2008;31(4):778–784. doi: 10.1007/s00270-007-9264-6. [DOI] [PubMed] [Google Scholar]
- 71.Gandini R, Konda D, Reale C A. et al. Male varicocele: transcatheter foam sclerotherapy with sodium tetradecyl sulfate–outcome in 244 patients. Radiology. 2008;246:612–618. doi: 10.1148/radiol.2462061295. [DOI] [PubMed] [Google Scholar]
- 72.Tan K T, Kirby J, Rajan D K, Hayeems E, Beecroft J R, Simons M E. Percutaneous sodium tetradecyl sulfate sclerotherapy for peripheral venous vascular malformations: a single-center experience. J Vasc Interv Radiol. 2007;18(3):343–351. doi: 10.1016/j.jvir.2006.12.735. [DOI] [PubMed] [Google Scholar]
- 73.Yamaki T, Nozaki M, Sakurai H, Takeuchi M, Soejima K, Kono T. Prospective randomized efficacy of ultrasound-guided foam sclerotherapy compared with ultrasound-guided liquid sclerotherapy in the treatment of symptomatic venous malformations. J Vasc Surg. 2008;47(3):578–584. doi: 10.1016/j.jvs.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 74.Matsumoto A, Hamamoto N, Nomura T. et al. Balloon-occluded retrograde transvenous obliteration of high risk gastric fundal varices. Am J Gastroenterol. 1999;94(3):643–649. doi: 10.1111/j.1572-0241.1999.00928.x. [DOI] [PubMed] [Google Scholar]
- 75.Hayashi S, Saeki S, Hosoi H. et al. A clinical and portal hemodynamic analysis for obliteration of gastric-renal shunt communicated with gastric fundic varice. s [in Japanese] Nippon Shokakibyo Gakkai Zasshi. 1998;95(7):755–763. [PubMed] [Google Scholar]
- 76.Tanihata H, Minamiguchi H, Sato M. et al. Changes in portal systemic pressure gradient after balloon-occluded retrograde transvenous obliteration of gastric varices and aggravation of esophageal varices. Cardiovasc Intervent Radiol. 2009;32(6):1209–1216. doi: 10.1007/s00270-009-9679-3. [DOI] [PubMed] [Google Scholar]
- 77.Choi Y S, Lee J H, Sinn D H. et al. Effect of balloon-occluded retrograde transvenous obliteration on the natural history of coexisting esophageal varices. J Clin Gastroenterol. 2008;42(9):974–979. doi: 10.1097/MCG.0b013e318126c154. [DOI] [PubMed] [Google Scholar]
- 78.Nakamura S, Torii N, Yatsuji S. et al. Long-term follow up of esophageal varices after balloon-occluded retrograde transvenous obliteration for gastric varices. Hepatol Res. 2008;38(4):340–347. doi: 10.1111/j.1872-034X.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- 79.Yamagami T, Kato T, Hirota T, Yoshimatsu R, Matsumoto T, Nishimura T. Infusion of 50% glucose solution before injection of ethanolamine oleate during balloon-occluded retrograde transvenous obliteration. Australas Radiol. 2007;51(4):334–338. doi: 10.1111/j.1440-1673.2007.01746.x. [DOI] [PubMed] [Google Scholar]