Abstract
Liver biopsy is considered the gold standard for the evaluation of acute and chronic liver disorders. Transjugular liver biopsy (TJLB) was described by Dotter in 1964 and clinically performed for the first time by Hanafee in 1967. TJLB consists of obtaining liver tissue through a rigid cannula introduced into one of the hepatic veins, typically using jugular venous access. The quality of the TJLB specimens has improved so much that the samples obtained by this method are comparable with those obtained with the percutaneous technique. TJLB is indicated for patients with coagulopathy, ascites, peliosis hepatis, morbid obesity, liver transplant, or in patients undergoing a transjugular intrahepatic portosystemic shunt procedure. The technical success rate for a TJLB procedure ranges from 87 to 97%. Sample fragmentation has been reported in 14 to 25% of the TJLB samples. The complication rates are low and range between 1.3% and 6.5%. The purpose of this article is to provide a review of the fundamental aspects of the TJLB procedure, including technique, indications, contraindications, results, and complications.
Keywords: liver biopsy, transjugular, interventional, image-guided
Objectives: On completion of this article, the reader will be able to describe transjugular liver biopsy technique and outcomes, and be able to describe the potential complications arising from the procedure.
Accreditation: Tufts University School of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Liver biopsy is considered the gold standard for the evaluation of acute and chronic liver disorders. Liver biopsy provides information regarding diagnosis, disease progression, and response to therapy in patients with chronic liver diseases. Paul Elrich first described liver biopsy in 1883, and since then, several approaches have been developed to sample the liver parenchyma including open surgical, percutaneous in either a blind or image-guided (ultrasound [US] or computed tomography [CT]) fashion, laparoscopic, and transjugular. Transjugular liver biopsy (TJLB) was described by Dotter in 1964 in an experimental model1 and clinically performed for the first time by Hanafee in 1967.2
TJLB consists of obtaining liver tissue through a rigid cannula introduced into one of the hepatic veins typically using jugular venous access. This approach reduces the risk of hemorrhage after biopsy because the bleeding resulting from the biopsy needle will drain into the hepatic veins. In the past, the specimens obtained by a transjugular approach were considered suboptimal compared with the samples obtained with percutaneous needles because they were smaller and more fragmented. However, with the development of automatic cutting-type Tru-cut needles and the improvement of the device systems,3,4 the quality of the TJLB specimens has improved so much that the samples obtained by this method are comparable with those obtained with percutaneous technique.5,6,7
TJLB was initially indicated for patients who had a contraindication to percutaneous biopsy such as those with a coagulopathy or congenital clotting disorders,8,9 ascites,10,11 acute liver failure,12 large amount of adipose tissue,13 and patients after liver transplantation. The clinical role of TJLB has expanded due to the possibility of performing hemodynamic evaluation of the hepatic and portal venous systems,14 which provides useful information and may guide therapy in patients with portal hypertension.
This article reviews the fundamental aspects of the TJLB procedure, including technique, indications, contraindications, results, and complications.
Indications
TJLB is indicated for patients with diffuse liver disease who need a biopsy and have a either a contraindication to percutaneous biopsy or require hemodynamic evaluation as part of their diagnostic workup. One of the most common indications for TJLB is a coagulation disorder.15 The degree of coagulopathy at which percutaneous biopsy is contraindicated varies in each institution. In general, it is advisable not to perform a percutaneous biopsy if the international normalized ratio (INR) is >1.5 or the platelet count is <50,000 × 109/L.16 Other common indications include the presence of ascites, peliosis hepatis, morbid obesity, liver transplant, patients in whom a percutaneous biopsy has failed, or in patients undergoing a transjugular intrahepatic portosystemic shunt procedure.
Contraindications
There are no specific contraindications to perform a TJLB. Abnormal coagulation is not a contraindication, but attempts should be made to correct coagulation derangements before proceeding. The Society of Interventional Radiology (SIR) guidelines establish that additional blood products should be administered if the INR is >2.5 times the control and the platelet count is <50,000. At our institution, correction of coagulopathy is corrected if the platelet count is <10,000 × 109/L or the INR is >3.5. Other relative contraindications to TJLB include lack of suitable venous access and biopsy of a focal lesion. In general, TJLB is not indicated as a diagnostic method for focal liver lesions; however, in some cases, a combination of transjugular and US-guided techniques may be used to sample focal liver lesions in patients who are at high risk of bleeding and have the lesion in the vicinity of one of the hepatic veins.
Material
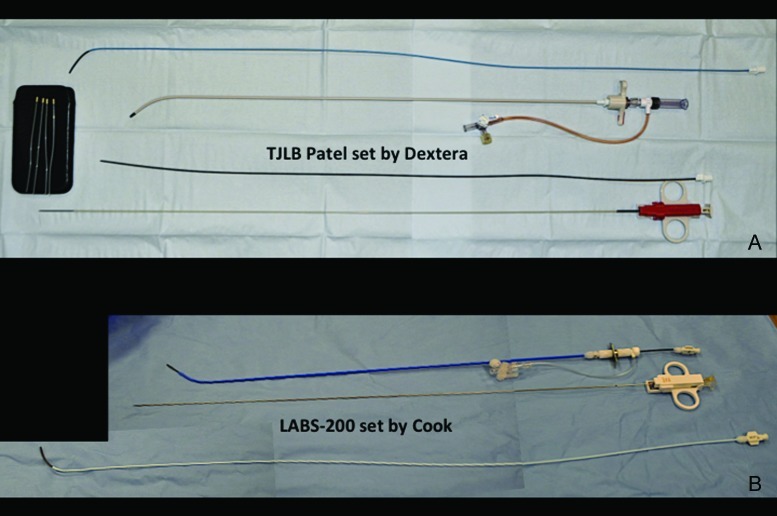
The material required for a TJLB should be available in any interventional angiography suite. It includes portable US unit with linear array probe for vascular access, multiplanar fluoroscopy unit, venous access set, 9F × 35-cm vascular sheath, 0.035-inch × 180-cm Bentson guidewire (Cook Medical, Bloomington, IN), 0.035-inch × 180-cm Rosen wire (Cook)or Amplatz superstiff wire (Boston Scientific, Natick, MA), biopsy kit system (the LABS-100 or LABS-200 set [Cook] or the TLAB system by Dextera Surgical [Franklin, IN] may be used). Table 1 shows the basic characteristics of these needle systems. These specific biopsy two sets are approved by the Food and Drug Administration for this purpose. Both sets contain the following materials: a 7F × 49-cm sheath over angled metal sheath stiffener, 5F multipurpose catheter for hepatic vein catheterization, 8F dilator, and the biopsy needle.
Table 1. Characteristics of the Approved Transjugular Liver Biopsies Sets Approved by the Food and Drug Administration.
| LABS-100 or 200* | TLAB-Dextera** | |
|---|---|---|
| Stiff cannula/sheath | 7F | 7F |
| Needle | Quick-Core | Flexcore |
| 18 or 19 gauge | 18 or 19 gauge | |
| Needle trocar length | 60 cm | 68 cm |
| Needle notch | 15 mm | 15 mm |
| Needle throw length | 20 mm | 20 mm |
Cook Medical, Inc., Bloomington, IN.
Dextera Surgical, Franklin, IN.
Patient Preparation
TJLB is usually performed as an outpatient elective procedure. The procedure is sometimes performed on inpatients and, occasionally, in patients with fulminant liver failure. In the latter instance, the procedure is performed on an emergency basis. Fasting for 6 hours before the procedure is recommended because most patients require conscious sedation. Informed consent is obtained following the SIR guidelines. All patients are evaluated for possible contrast allergy; patients with moderate reactions can be premedicated using standardized guidelines.17 Carbon dioxide is used at our institution in patients with a history of severe contrast allergy or patients with elevated serum creatinine. As mentioned previously, coagulopathy is not a contraindication to TJLB; however, in cases of severe coagulopathy (variably defined), correction of the coagulation factors is recommended.
Transjugular Biopsy Technique
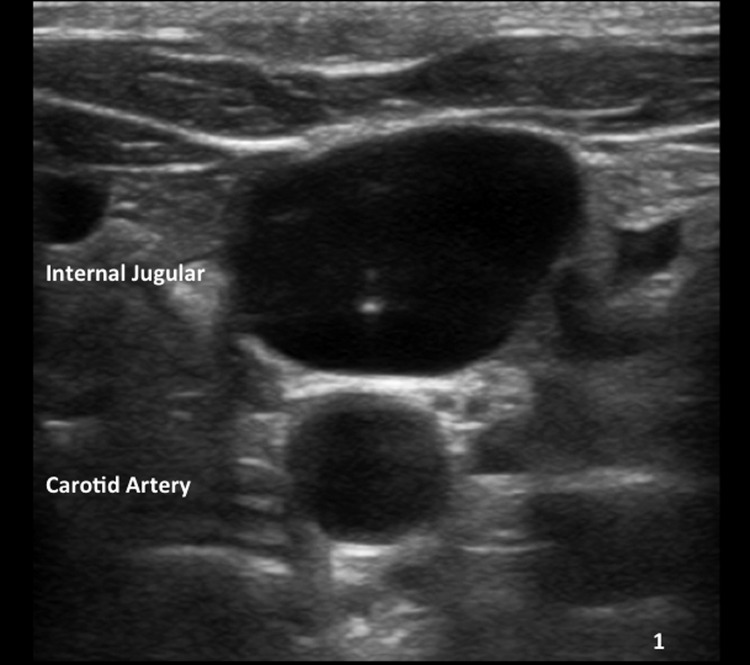
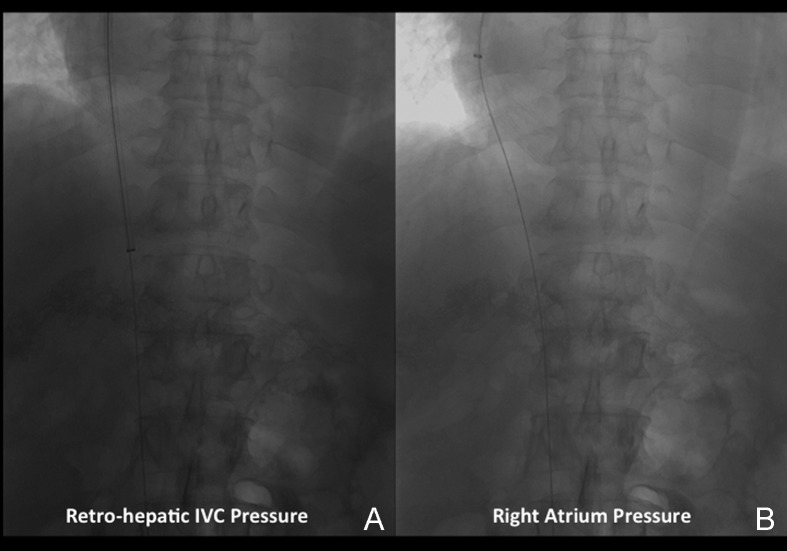
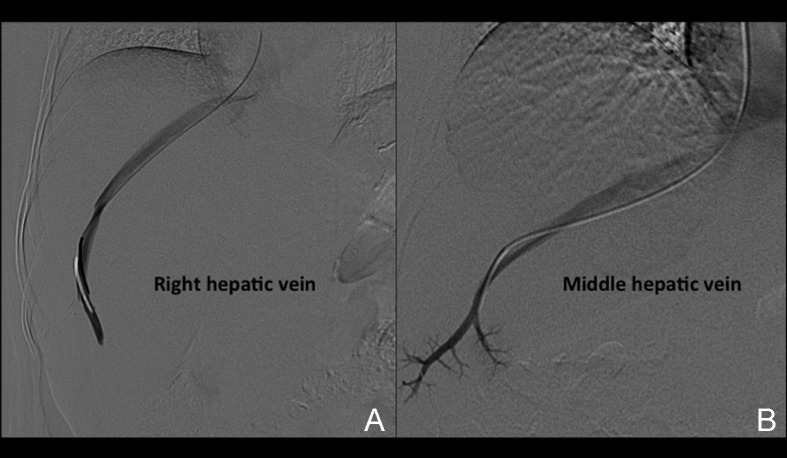
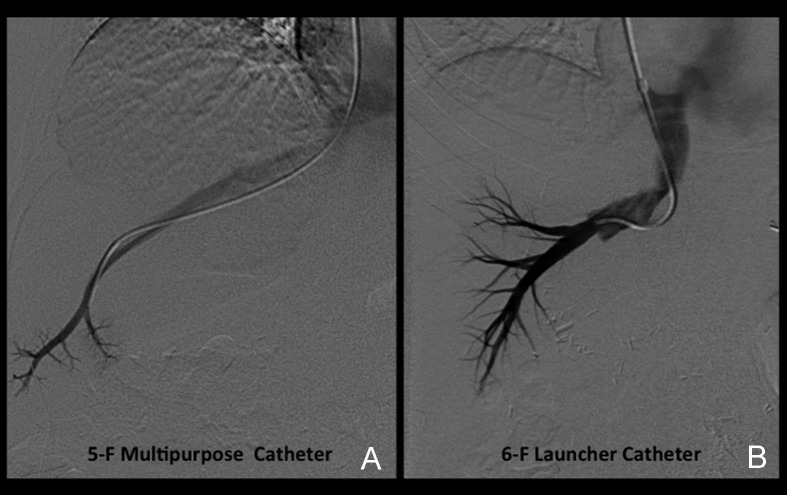
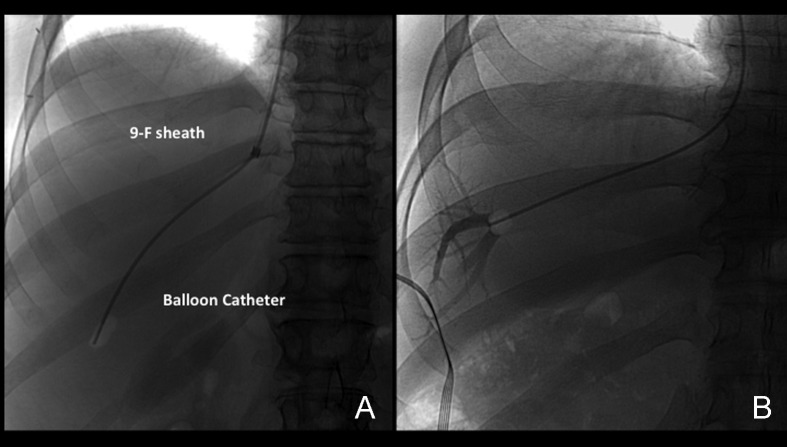
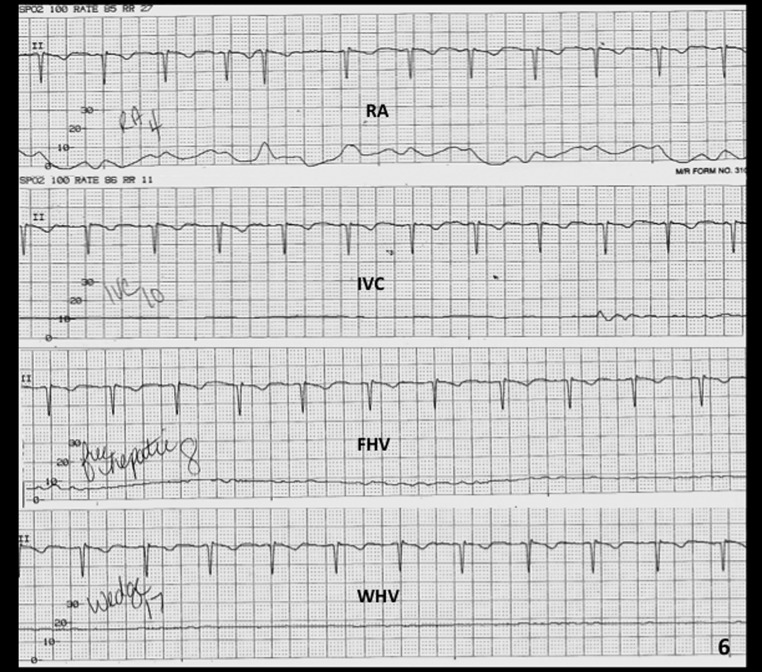
Venous access is obtained under real-time (US) guidance using a micropuncture access needle set (Cook) (Fig. 1). Once venous access is obtained, a 9F, 35-cm vascular sheath is advanced into the inferior vena cava. At this point, pressures in the inferior vena cava and right atrium may be performed (Fig. 2A, 2B). Then a 5F multipurpose catheter is used to perform selective catheterization of the right or middle hepatic vein (Figs. 3A, 3B, and 4A). If the entry angle to the hepatic veins is too acute, an alternative reverse curve catheter (such as Launcher coronary guiding catheter [Medtronic, Minneapolis, MN] can be used to obtain access into the hepatic veins (Fig. 4B). Once successful access is obtained into the hepatic vein, a hepatic venogram is performed to determine the patency of the selected vein. The diagnostic catheter is then exchanged for an occlusion balloon (Cook), which is placed within the hepatic vein in its midportion (Fig. 5A). The balloon is carefully inflated with 1 to 3 mL of diluted contrast or air, followed by a gentle injection of a small amount of contrast to ensure adequate wedge position (Fig. 5B). Next, wedge hepatic venous pressure (WHVP) and free hepatic venous pressure are obtained. We strongly recommend the use of an occlusion balloon for accurate pressure measurements. Previous studies have demonstrated that WHVP measurement with the use of an occlusion-balloon catheter is an accurate indirect method to measure the degree of portal pressure for most of the patients with cirrhosis, and that it is superior to WHVP measurement with the use of an end-hole diagnostic catheter.18 Recording the pressure tracings and measurements is strongly recommended for reference (Fig. 6).
Figure 1.
Ultrasound-guided access. Ultrasound image shows the tip of the needle within a patent jugular vein.
Figure 2.
Pressure measurement. (A) Image shows the 9F sheath with radiopaque tip in the infrahepatic inferior vena cava (IVC). Mean venous pressures are measured at this site. (B) Image demonstrates the sheath withdrawn to the right atrium. Mean pressures are measured in this location.
Figure 3.
Hepatic vein catheterization. (A) Anteroposterior projection (AP) digital subtraction venogram demonstrates a patent right hepatic vein. (B) AP digital subtraction venogram demonstrates a patent middle hepatic vein. Note the more central and caudal course of this vein.
Figure 4.
Catheterization techniques. (A) Anteroposterior (AP) digital subtraction image demonstrates use of the multipurpose catheter to perform catheterization of the middle hepatic vein. (B) AP digital subtraction venogram demonstrates the use of a reverse curve catheter (Launcher) to gain access in a middle hepatic vein. Catheterization of this vein had been attempted with a multipurpose catheter but had failed.
Figure 5.
Wedge position using an occlusion balloon. (A) Anteroposterior (AP) spot film demonstrates the occlusion-balloon catheter inflated with a small amount of air. The catheter is positioned in a peripheral location within the hepatic vein. (B) AP spot film obtained during injection of a small amount of contrast with the occlusion balloon inflated with a small amount of air. Note opacification of the peripheral hepatic vein branches. No contrast leak is noted around the balloon, indicating a good wedge position.
Figure 6.
Tracing during pressure measurements. The image demonstrates hemodynamic tracings obtained during pressure measurements during a transjugular liver biopsy (TJLB) procedure. (right atrium [RA], 4 mm Hg; inferior vena cava {IVC], 10 mm Hg; free hepatic vein [FHV], 8 mm Hg; wedge hepatic vein [WHV], 17 mm Hg). In this case, the hepatic vein gradient is 9 mm Hg (WHV pressure–FHV pressure), indicating moderate portal hypertension.
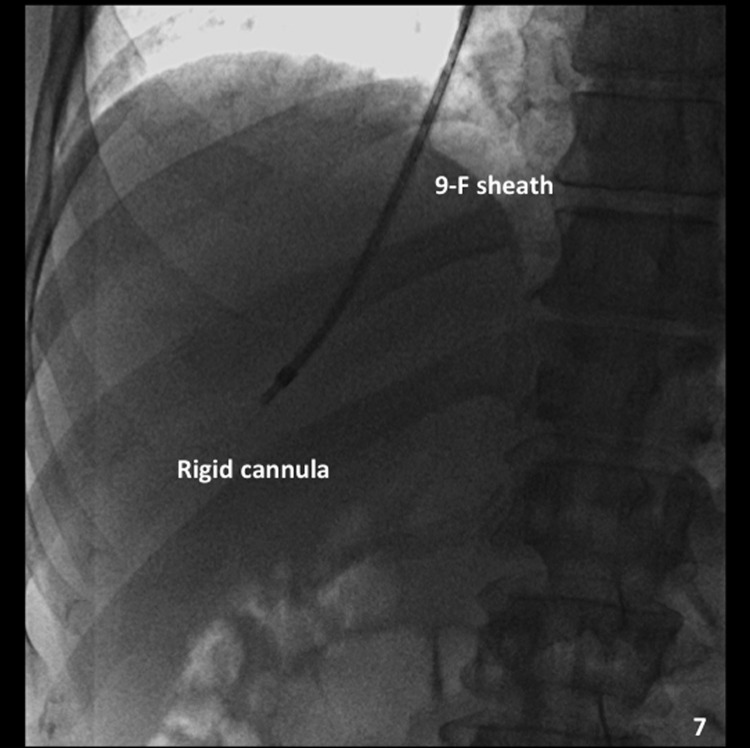
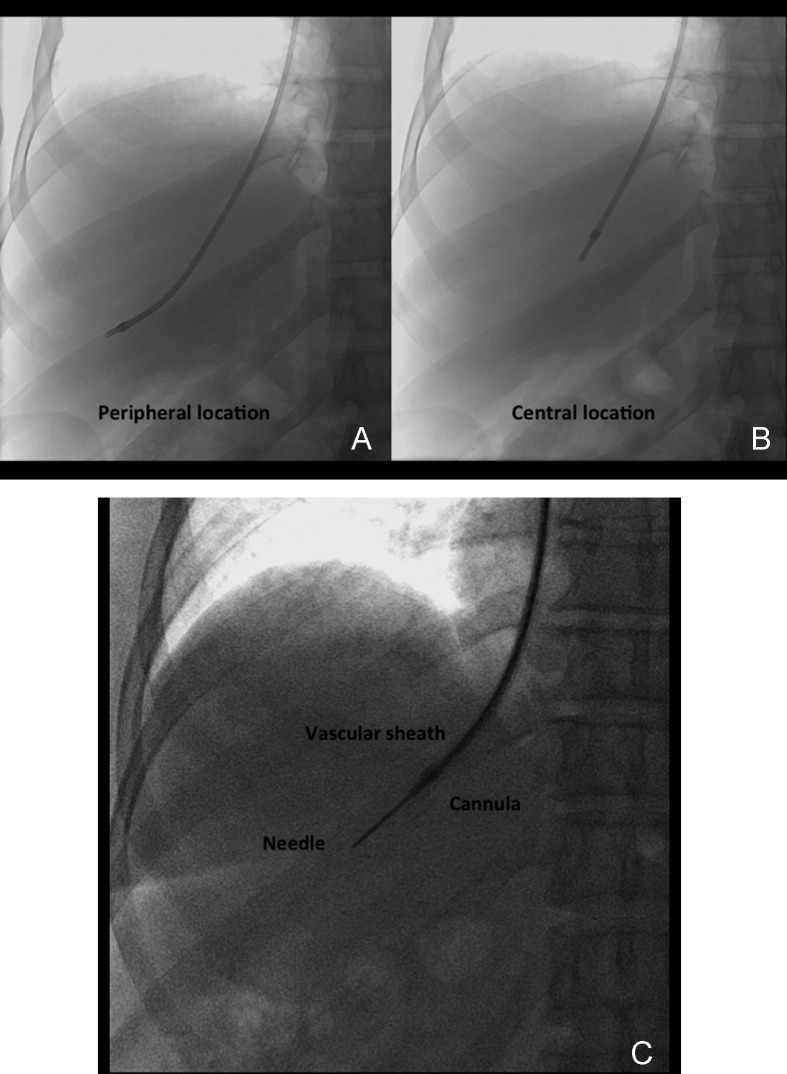
After pressure measurements are properly obtained, the occlusion balloon is removed over a wire, and the TJLB guiding cannula is advanced over the wire distally into the selected hepatic vein (Fig. 7). If the entry angle is favorable, a Rosen wire (Cook) should have enough stiffness to advance the cannula without any problems, and the tight 1.5-mm “J” curve on the Rosen wire precludes inadvertent selection of side branches. However, in some cases when the angle of entry of the hepatic vein is too acute, the use of a stiffer wire such as an Amplatz superstiff wire (Boston Scientific) is necessary to provide enough support to advance the cannula. The liver samples should be obtained using an automatic cutting-type Tru-cut TJLB needle system (Fig. 8A, 8B). A minimum of three samples should be obtained per procedure. Ideally, two samples should be obtained from the peripheral aspect of the liver and two samples from a more central position (Fig. 9A–C). This allows the pathologist to obtain an overview of the status of the liver in different anatomical areas. Standish et al have emphasized the importance of submitting multiple liver specimens to improve the diagnostic and prognostic yield of liver biopsies.19 Previous studies support the concept that a TJLB can yield sufficient information if at least three individual nonfragmented specimens are submitted for evaluation.19,20,21 The mean duration of the procedure is typically 40 minutes, the reported average fluoroscopy time is 4 to 6 minutes, and the radiation dose ranges from 0.5 to 1 mSv.
Figure 7.
Placement of the guiding cannula. Anteroposterior (AP) spot film demonstrates the rigid cannula within the 9F sheath, placed into the hepatic vein. This is a good position to obtain a sample.
Figure 8.
Transjugular liver biopsy (TJLB) needle systems. (A) Photograph demonstrates the components of the set by Dextera. The set includes four tissue-retrieving sticks (left) that assist in retrieving the sample from the needle, decreasing the risk of fragmentation. (B) Photograph demonstrates the LABORATORIES-200 set by Cook. See Table 1 for details on the components of the sets.
Figure 9.
Central and peripheral location of biopsy needle. (A) Anteroposterior (AP) spot film demonstrates the guiding cannula in a peripheral location to obtain a core sample. (B) AP spot film demonstrates the guiding cannula in a more central location to obtain a core sample. (C) AP spot film obtained during biopsy demonstrates the needle within the liver parenchyma. The position of the guiding cannula in this case is central.
Handling of Liver Specimens
The liver tissue obtained during the TJLB should be handled very carefully to avoid sample fragmentation during tissue transfers. A useful maneuver is to place the needle within the formalin cup or small amount of saline and allow the tissue to “float” into the fluid.
Postbiopsy Patient Care
The sitting or semirecumbent position is ideal for postprocedure recovery to prevent complications at the puncture site. Patients are usually in a recovery area for 2 hours. Attention is focused on vital signs and abdominal pain. Usually patients who have undergone a TJLB report a dull mild aching in the right upper quadrant or right shoulder. Severe abdominal pain after a TJLB is abnormal and should prompt further workup to search for a postprocedural complication. At our institution, patients with severe abdominal pain immediately after TJLB undergo a noncontrast CT scan to assess for subcapsular or intraperitoneal hemorrhage.
Results
The reported technical success rate for a TJLB procedure ranges from 87 to 97%.22,23 The most common cause for technical failure is the inability to catheterize a suitable hepatic vein. This is more often encountered in patients with a liver transplant where the normal anatomy is distorted and the weight of the unattached liver can rotate the drainage vasculature. Lack of transvenous access is another reason for technical failure; however, the use of alternative accesses such as the left internal jugular,24 external jugular,25 subclavian, or even femoral26 vein is a possibility and may solve this issue.
A liver specimen of 15 mm in length containing 6 to 10 complete portal tracts is regarded sufficient for the histological diagnosis of diffuse liver disease.10,27 This standard is achieved during TJLB in 89 to 96% of cases.4,21 The minimum requirement for a sample to be useful for grading and staging in chronic hepatitis is to obtain a specimen of 20 mm length and 11 complete portal tracts.4 Data previously reported by Behrens et al demonstrated that it is possible to obtain a total sample of 28 to 29 mm in length containing a mean of 10 to 12 complete portal tracts during a TJLB. It is important to emphasize the fact that to obtain such samples, it is necessary to provide at least three good tissue specimens to the pathologist.4 Sample fragmentation rate is another parameter evaluated in the literature. This is most often seen in cirrhotic patients and has been reported in 14 to 25% of the TJLB samples. Fragmentation of the sample may interfere with the diagnostic quality of the specimen.
Complications
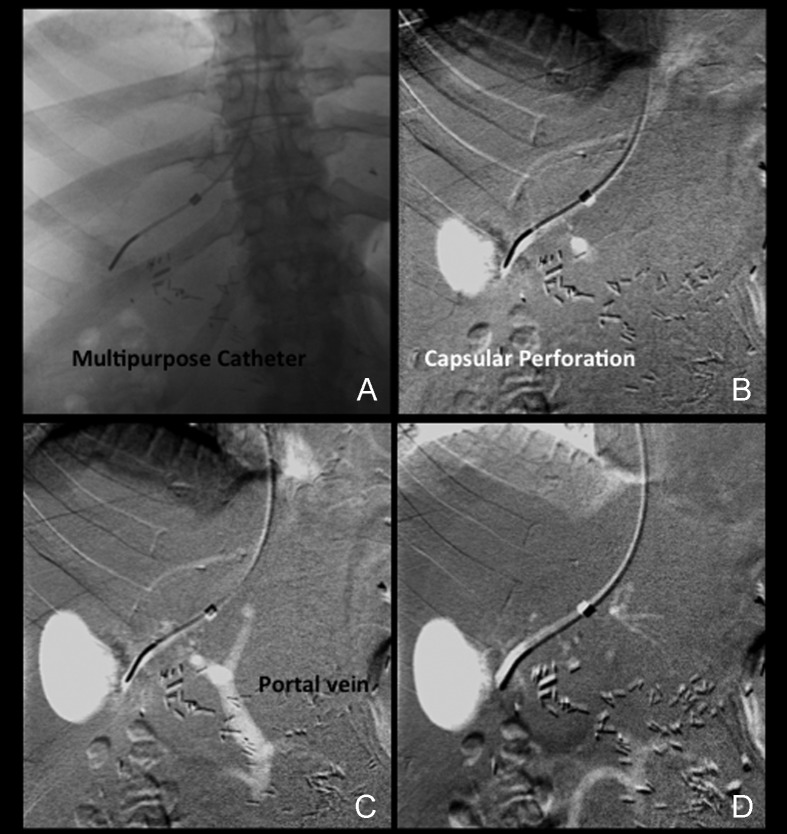
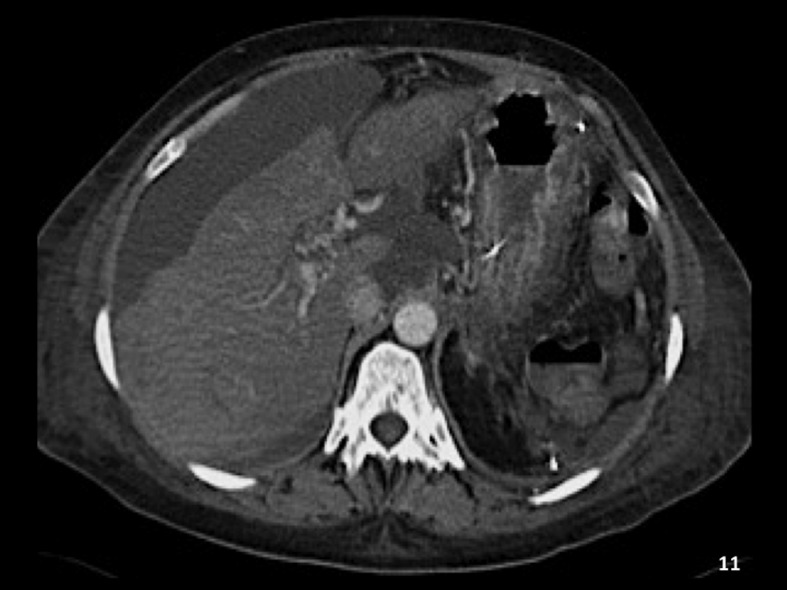
The complication rates are low and range between 1.3% and 6.5%.13 Most complications are minor and related to bleeding at the puncture site or abdominal pain related to the presence of a small hematoma distending the liver capsule. Major complications by the published SIR standards have been reported in 0.6% of patients.13 The reported mortality rate is <0.1% for adults and 0.1% for children. Mortality may be related to hemorrhage from an extracapsular liver puncture, capsular perforation after a wedge injection (Figs. 10A–D and 11), perforation of the hepatic artery, or ventricular dysrhythmia. Other complications include hemobilia and pseudoaneurysm. In general, reports in the literature agree that complication rates are lower during TJLB when compared with the percutaneous or mini-laparoscopy approach.28
Figure 10.
Capsular perforation during carbon dioxide (CO2) wedge injection. (A) A 5F multipurpose catheter is visualized in a distal location within the hepatic vein. (B) A small amount of CO2 was injected through the catheter. Extravasation forming a relatively oval white shadow lateral to the catheter is immediately identified. (C) Faint opacification of the hepatic vein and reflux into the portal vein is identified. The oval area of extravasation has increased in size. (D) Late frame shows dissipation of CO2 into low mesenteric vein branches and further extravasation of CO2.
Figure 11.
Computed tomography (CT) scan after carbon dioxide (CO2) wedge injection and capsular perforation. Axial image obtained during a contrast-enhanced CT scan of the liver of the patient shown in Figure 10 demonstrates a large subcapsular hematoma lateral to the right edge of the liver. The patient was sent to the intensive care unit for observation.
Conclusion
Liver biopsy is still the gold standard for the evaluation of chronic liver disease. The transjugular method to obtain liver tissue has not been accepted as a standard of care, and many clinicians still believe that percutaneous liver biopsy is the best method to obtain enough liver tissue for diagnostic and staging purposes. However, there are enough data to support the utility of TJLB as a safe procedure through which liver samples of diagnostic and staging quality can be obtained. In this regard, it is important to emphasize the following factors: Operator skill is essential for an optimal result; the procedure has to be performed with careful technique and should only be performed by physicians with certified training in endovascular techniques; and the use of state-of-the-art cutting-type Tru-cut needles to obtain the specimens is essential. Multiple papers have criticized the transjugular method, arguing that the specimens obtained were suboptimal for the evaluation of chronic liver diseases. In this regard, it is very important to emphasize the fact that TJLB procedures were performed in the past using aspiration techniques and that this technique truly interferes with the quality of the specimen. Specimens should be handled with care to avoid fragmentation. Finally, measurement and monitoring of portal pressure and the portal perfusion pressure gradient has become an important diagnostic and prognostic tool that should be used in daily clinical practice.
References
- 1.Dotter C T. Catheter biopsy. Experimental technic for transvenous liver biopsy. Radiology. 1964;82:312–314. doi: 10.1148/82.2.312. [DOI] [PubMed] [Google Scholar]
- 2.Hanafee W, Weiner M. Transjugular percutaneous cholangiography. Radiology. 1967;88(1):35–39. doi: 10.1148/88.1.35. [DOI] [PubMed] [Google Scholar]
- 3.Gorriz E, Reyes R, Lobrano M B. et al. Transjugular liver biopsy: a review of 77 biopsies using a spring-propelled cutting needle (biopsy gun) Cardiovasc Intervent Radiol. 1996;19(6):442–445. doi: 10.1007/BF02577636. [DOI] [PubMed] [Google Scholar]
- 4.Behrens G, Ferral H, Giusto D, Patel J, Thiel D H Van. Transjugular liver biopsy: comparison of sample adequacy with the use of two automated needle systems. J Vasc Interv Radiol. 2011;22(3):341–345. doi: 10.1016/j.jvir.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Bruzzi J F, O'Connell M J, Thakore H, O'Keane C, Crowe J, Murray J G. Transjugular liver biopsy: assessment of safety and efficacy of the Quick-Core biopsy needle. Abdom Imaging. 2002;27(6):711–715. doi: 10.1007/s00261-002-0020-8. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa T, Kamimura H, Tsuchiya A. et al. Comparison of a new aspiration needle device and the Quick-Core biopsy needle for transjugular liver biopsy. World J Gastroenterol. 2006;12(39):6339–6342. doi: 10.3748/wjg.v12.i39.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bañares R, Alonso S, Catalina M V. et al. Randomized controlled trial of aspiration needle versus automated biopsy device for transjugular liver biopsy. J Vasc Interv Radiol. 2001;12(5):583–587. doi: 10.1016/s1051-0443(07)61479-1. [DOI] [PubMed] [Google Scholar]
- 8.Saab S, Cho D, Quon D V. et al. Same day outpatient transjugular liver biopsies in haemophilia. Haemophilia. 2004;10(6):727–731. doi: 10.1111/j.1365-2516.2004.01043.x. [DOI] [PubMed] [Google Scholar]
- 9.Dawson M A, McCarthy P H, Walsh M E. et al. Transjugular liver biopsy is a safe and effective intervention to guide management for patients with a congenital bleeding disorder infected with hepatitis C. Intern Med J. 2005;35(9):556–559. doi: 10.1111/j.1445-5994.2005.00877.x. [DOI] [PubMed] [Google Scholar]
- 10.Bravo A A, Sheth S G, Chopra S. Liver biopsy. N Engl J Med. 2001;344(7):495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 11.Lebrec D. Various approaches to obtaining liver tissue—choosing the biopsy technique. J Hepatol. 1996;25 01:20–24. [PubMed] [Google Scholar]
- 12.Miraglia R, Luca A, Gruttadauria S. et al. Contribution of transjugular liver biopsy in patients with the clinical presentation of acute liver failure. Cardiovasc Intervent Radiol. 2006;29(6):1008–1010. doi: 10.1007/s00270-006-0052-5. [DOI] [PubMed] [Google Scholar]
- 13.Kalambokis G, Manousou P, Vibhakorn S. et al. Transjugular liver biopsy—indications, adequacy, quality of specimens, and complications—a systematic review. J Hepatol. 2007;47(2):284–294. doi: 10.1016/j.jhep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Azoulay D, Raccuia J S, Roche B, Reynes M, Bismuth H. The value of early transjugular liver biopsy after liver transplantation. Transplantation. 1996;61(3):406–409. doi: 10.1097/00007890-199602150-00015. [DOI] [PubMed] [Google Scholar]
- 15.Strassburg C P, Manns M P. Approaches to liver biopsy techniques—revisited. Semin Liver Dis. 2006;26(4):318–327. doi: 10.1055/s-2006-951599. [DOI] [PubMed] [Google Scholar]
- 16.Malloy P C, Grassi C J, Kundu S. et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2009;20(7, Suppl):S240–S249. doi: 10.1016/j.jvir.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 17.Greenberger P A, Patterson R, Radin R C. Two pretreatment regimens for high-risk patients receiving radiographic contrast media. J Allergy Clin Immunol. 1984;74(4 Pt 1):540–543. doi: 10.1016/0091-6749(84)90391-9. [DOI] [PubMed] [Google Scholar]
- 18.Maleux G, Willems E, Fieuws S. et al. Prospective study comparing different indirect methods to measure portal pressure. J Vasc Interv Radiol. 2011;22(11):1553–1558. doi: 10.1016/j.jvir.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Standish R A, Cholongitas E, Dhillon A, Burroughs A K, Dhillon A P. An appraisal of the histopathological assessment of liver fibrosis. Gut. 2006;55(4):569–578. doi: 10.1136/gut.2005.084475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cholongitas E, Quaglia A, Samonakis D. et al. Transjugular liver biopsy in patients with diffuse liver disease: comparison of three cores with one or two cores for accurate histological interpretation. Liver Int. 2007;27(5):646–653. doi: 10.1111/j.1478-3231.2007.01496.x. [DOI] [PubMed] [Google Scholar]
- 21.Cholongitas E, Quaglia A, Samonakis D. et al. Transjugular liver biopsy: how good is it for accurate histological interpretation? Gut. 2006;55(12):1789–1794. doi: 10.1136/gut.2005.090415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebrec D, Goldfarb G, Degott C, Rueff B, Benhamou J P. Transvenous liver biopsy: an experience based on 1000 hepatic tissue samplings with this procedure. Gastroenterology. 1982;83(2):338–340. [PubMed] [Google Scholar]
- 23.Gamble P, Colapinto R F, Stronell R D, Colman J C, Blendis L. Transjugular liver biopsy: a review of 461 biopsies. Radiology. 1985;157(3):589–593. doi: 10.1148/radiology.157.3.4059543. [DOI] [PubMed] [Google Scholar]
- 24.Yavuz K, Geyik S, Barton R E. et al. Transjugular liver biopsy via the left internal jugular vein. J Vasc Interv Radiol. 2007;18(2):237–241. doi: 10.1016/j.jvir.2006.12.730. [DOI] [PubMed] [Google Scholar]
- 25.Siegel E L, Caresio J, Eckard D A. Use of the external jugular vein approach for transvenous liver biopsy. J Vasc Interv Radiol. 1992;3(2):371–374. doi: 10.1016/s1051-0443(92)72046-6. [DOI] [PubMed] [Google Scholar]
- 26.Khosa F, McNulty J G, Hickey N. et al. Transvenous liver biopsy via the femoral vein. Clin Radiol. 2003;58(6):487–491. doi: 10.1016/s0009-9260(02)00576-7. [DOI] [PubMed] [Google Scholar]
- 27.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39(2):239–244. doi: 10.1016/s0168-8278(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 28.Beckmann M G, Bahr M J, Hadem J. et al. Clinical relevance of transjugular liver biopsy in comparison with percutaneous and laparoscopic liver biopsy. Gastroenterol Res Pract. 2009:947014. doi: 10.1155/2009/947014. [DOI] [PMC free article] [PubMed] [Google Scholar]