Abstract
Hepatitis E is an acute and self-limiting hepatitis, and the causative agent, hepatitis E virus, is excreted in feces and orally transmitted. The disease is common in Asia and Africa, causing outbreaks or sporadic cases. In Europe, the infection is generally observed after a history of travel in an area of endemicity. We report on an autochthonous case in southwestern France in which the diagnosis was based on molecular tools rather than serological testing.
CASE REPORT
In summer 2002, a 41-year-old woman without any medical or surgical history was admitted to the Toulouse University Hospital having suffered jaundice and fever for 2 days. On examination, the patient was subfebrile (37.8°C) and icteric. Her abdomen was pliant and painless. The liver was normal, there was a little splenomegaly, and no symptom of hepatic insufficiency was noted. Biochemical investigations revealed a marked hepatic cytolysis: serum alanine aminotransferase, bilirubin, and gamma-glutamyltransferase levels were 5,126 IU/liter (170 N), 40 μmol/liter (2.3 N), and 320 IU/liter (9.1 N), respectively. Hematological investigations revealed a hemoglobin level of 12.3 g/dl, a platelet count of 89 × 109/liter, and a white cell count of 5.8 × 109/liter. The prothrombin index was 76%, and the C-reactive protein level was 46 mg/liter. The abdominal ultrasonograph was normal. Toxin or drug causality was ruled out after questioning. The patient lived in an urban setting in the Midi Pyrénées area (southwest France) and was a teacher. Her hobby was gardening. She had not traveled outside France during the previous 6 months, and the only livestock near her house were poultry. In view of the fever, blood and urine cultures were taken and remained sterile. The serum was negative for hepatitis B virus antigen and DNA, hepatitis B virus core antibodies, hepatitis C virus RNA and antibodies, immunoglobulin M antibodies to hepatitis A virus, cytomegalovirus, and Epstein-Barr virus.
The assay for hepatitis E virus (HEV) immunoglobulin G (IgG) antibodies was negative with a ratio of 0.57 (optical density/cutoff ratio, positive if >1) according to the only serological test commercially available in France (HEV enzyme immunoassay; Abbott Laboratories, Rungis, France). Nevertheless, HEV infection was diagnosed using molecular tools (real-time PCR with Taqman detection amplifying a 189-bp product located in the ORF2 region) on icteric-phase serum and stools.
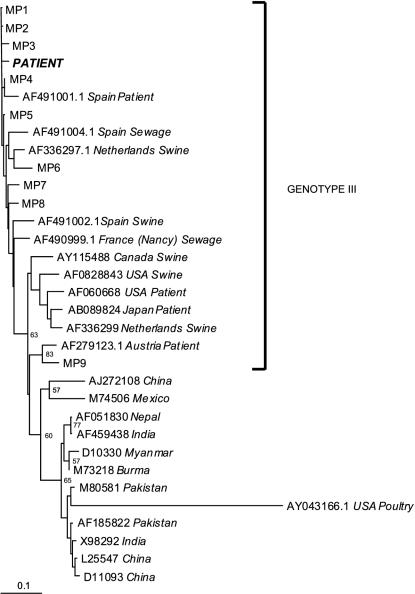
The strain was sequenced and compared with reference HEV strains (GenBank) and local controls. The resulting phylogenetic analysis (Fig. 1) demonstrated the propinquity between our strain and other HEV strains previously identified in patients with acute hepatitis living in the Midi Pyrénées area. All of the strains from Midi Pyrénées belonged to genotype III.
FIG. 1.
Phylogenetic analysis of the 189-bp ORF2 region product (“PATIENT”). The designations MP1 to MP9 represent locations of strains previously found in patients from the Midi Pyrénées region.
The patient recovered completely after a short period of hospital care, and her serum alanine aminotransferase, bilirubin, and gamma-glutamyltransferase levels returned to normal within a month. The HEV IgG assay remained negative 4 months later (ratio = 0.36).
Most cases of HEV infection in France are contracted in areas of endemicity, and autochthonous cases are scarce. Nicand et al. (4) described family cases with HEV viremia and no HEV antibodies. But these differed from our case in that they had no clinical or laboratory symptoms, suggesting that there was a human HEV reservoir. Our patient had not recently traveled outside France, even to Spain, where autochthonous cases have been recently reported (5).
To the best of our knowledge, this is the first reported case of acute HEV infection diagnosed using molecular tools alone. The possibility of a serological window was discarded because there was no HEV IgG in a serum sample collected 4 months later. The insensitivity of certain enzyme immunoassays has also been noted by others (1), making the design of more sensitive immunological tests mandatory.
The mode of transmission remains unclear. HEV has been found in poultry in the United States (2) and in swine in the United States (2), Spain (5), and The Netherlands (7). Various other domestic (3) or wild (6) animals harbor the virus. This indicates that the virus could be more widespread than was previously thought.
We suggest that HEV infection be routinely considered in cases of acute non-A, -B, -C, and -D hepatitis, even in patients who have not traveled in areas of endemicity. Molecular tools could be very useful complements to conventional serological tests.
Larger studies are now required to evaluate the incidence of human HEV infection in areas that are nonendemic and to identify any animal reservoirs of the virus.
REFERENCES
- 1.Christensen, P. B., R. E. Engle, S. E. Jacobsen, H. B. Krarup, J. Georgsen, and R. H. Purcell. 2002. High prevalence of hepatitis E antibodies among Danish prisoners and drug users. J. Med. Virol. 66:49-55. [DOI] [PubMed] [Google Scholar]
- 2.Haqshenas, G., H. L. Shivaprasad, P. R. Woolcock, D. H. Read, and X. J. Meng. 2001. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J. Gen. Virol. 82:2449-2462. [DOI] [PubMed] [Google Scholar]
- 3.Kuno, A., K. Ido, N. Isoda, et al. 2003. Sporadic acute hepatitis E of a 47-year-old man whose pet cat was positive for antibody to hepatitis E virus. Hepatol. Res. 26:237-242. [DOI] [PubMed] [Google Scholar]
- 4.Nicand, E., M. Grandadam, R. Teyssou, J. L. Rey, and Y. Buisson. 2001. Viraemia and faecal shedding of HEV in symptom-free carriers. Lancet 357:68-69. [DOI] [PubMed] [Google Scholar]
- 5.Pina, S., M. Buti, M. Cotrina, J. Piella, and R. Girones. 2000. HEV identified in serum from humans with acute hepatitis and in sewage of animal origin in Spain. J. Hepatol. 33:826-833. [DOI] [PubMed] [Google Scholar]
- 6.Tei, S., N. Kitajima, K. Takahashi, and S. Mishiro. 2003. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 362:371-373. [DOI] [PubMed] [Google Scholar]
- 7.van der Poel, W. H., F. Verschoor, R. van der Heide, et al. 2001. Hepatitis E virus sequences in swine related to sequences in humans, The Netherlands. Emerg. Infect. Dis. 7:970-976. [DOI] [PMC free article] [PubMed] [Google Scholar]