Abstract
Aim: Preoperative fasting is an established procedure to be practiced for patients before surgery, but optimal preoperative fasting time still remains controversial. The aim of this study was to investigate the effect of “shortened preoperative fasting time” on the change in the amount of total body water (TBW) in elective surgical patients. TBW was measured by multi-frequency impedance method.
Methods: The patients, who were scheduled to undergo surgery for stomach cancer, were divided into two groups of 15 patients each. Before surgery, patients in the control group were managed with conventional preoperative fasting time, while patients in the “enhanced recovery after surgery (ERAS)” group were managed with “shortened preoperative fasting time” and “reduced laxative medication.” TBW was measured on the day before surgery and the day of surgery before entering the operating room. Defecation times and anesthesia-related vomiting and aspiration were monitored.
Results: TBW values on the day of surgery showed changes in both groups as compared with those on the day before surgery, but the rate of change was smaller in the ERAS group than in the control group (2.4±6.8% [12 patients] vs. −10.6±4.6% [14 patients], p<0.001). Defecation times were less in the ERAS group. Vomiting and aspiration were not observed in either group.
Conclusion: The results suggest that preoperative management with “shorted preoperative fasting time” and “reduced administration of laxatives” is effective in the maintenance of TBW in elective surgical patients.
Keywords: preoperative patient management, shorted preoperative fasting time, total body water (TBW), multi-frequency impedance method, enhanced recovery after surgery (ERAS)
Introduction
An approach for minimizing surgery-related stress and reducing subsequent complications during the preoperative period was introduced in 2005 by Fearon et al. as the “enhanced recovery after surgery (ERAS)” protocol, and the protocol has been considered to be a useful means for preoperative management of patients before surgery 1. The ERAS protocol recommends practices such as shortening preoperative fasting time, reducing the dose of laxatives, and intake of clear fluids containing carbohydrates in order to enhance postoperative recovery of patients.
ESPEN (European Society for Clinical Nutrition and Metabolism) guidelines in 2009 mentions that preoperative fasting from midnight is unnecessary in most patients (Grade A recommendation), and oral intake of carbohydrate-rich drink before surgery is beneficial and recommended in most patients (Grade A recommendation) 2.
Since May 2009, patients undergoing stomach surgery at our hospital have been managed according to the ERAS protocol, now essentially shifted to preoperative management with shorted fasting time and reduced laxative medication. In terms of patient satisfaction, shortening the fasting time (and taking clear fluid or appropriate food instead) is well accepted by patients scheduled for elective surgery, which helps decrease feeling of preoperative discomfort, feeling of dry mouth, and feeling of hunger. For preoperative fluid management, it is of significant to elucidate how the shortened fasting before surgery may affect total body water (TBW). In the present study, we hypothesized that the preoperative amount of TBW would be maintained appropriately by reducing preoperative fasting time and laxative medication. TBW was measured by multi-frequency impedance method.
Methods
This study was approved by the institutional review board of the institution (Kanagawa Cancer Center, Japan) and was conducted in accordance with the Declaration of Helsinki. Voluntary written informed consent was obtained from all subjects enrolled in the study.
Patients were those with physical status classification I or II of the American Society of Anesthesiologists Physical Status (ASAPS), who were scheduled to enter the operating room at 8:45 to undergo elective surgery for stomach cancer. Patients, who had to be supported by intravenous therapy due to the inability of oral intake before surgery, were excluded from the study.
The study was conducted as a nonrandomized, controlled intervention study.
Patients were divided into two groups (“the control group” managed by conventional preoperative procedure with conventional fasting time and “the ERAS group” managed by the ERAS protocol with shortened fasting time). As mentioned in the Introduction section, since May 2009, our hospital has been shifting the preoperative management from the conventional procedure to the ERAS protocol in the patients undergoing stomach surgery. With regard to the patients in this study, 15 patients, who gave their consent to the study, were allocated to the control group, while 15 patients, who gave their consent to the study, were allocated to the ERAS group.
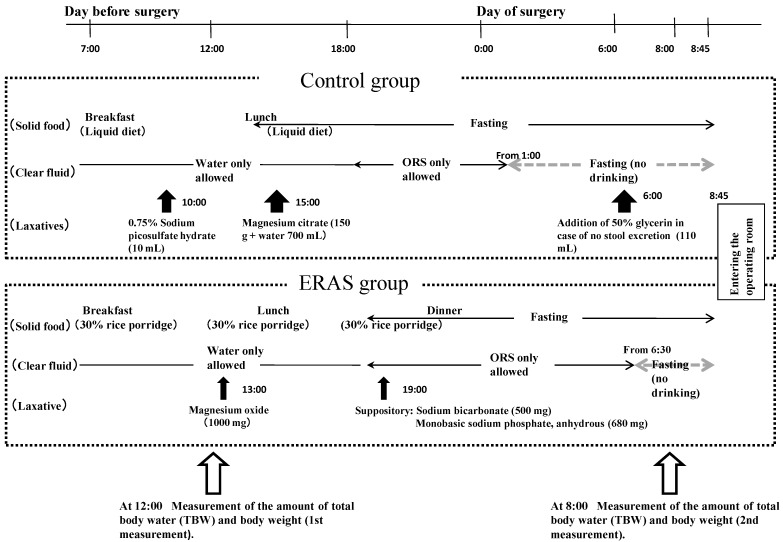
Schedule for the study (such as intake of diets, intake of clear liquids, and laxative medication) is shown in Figure 1. In the control group, on the day before surgery, patients consumed a liquid diet at 7:00 for breakfast and at 12:00 for lunch (totaling 650 kcal/day and 20 g protein/day), after which the patients fasted. With regard to fluid intake on the day before surgery, clear fluids (such as water, oral rehydration solution [ORS], coffee or tea without milk, and juice not containing dietary fibers) were permitted, not limiting the volume of intake, and after 18:00, patients were permitted to consume ORS only (limiting to the maximum intake to 1500 mL) until 1:00 on the day of surgery. Laxatives were medicated at 10:00 (10 mL of 0.75% sodium picosulfate hydrate) and 15:00 (150 g of magnesium citrate, with 700 mL of water) on the day before surgery. If there was no defecation at 6:00 on the surgery day, 110 mL of 50% glycerin enema was prescribed as an additional medication.
Figure 1.
Schedule for the study and measurements of the amount of total body water (TBW) and body weight. ORS, oral rehydration solution.
In the ERAS group, on the day before surgery, patients consumed 30%-rice porridge at 7:00 for breakfast, 12:00 for lunch, and 18:00 for dinner (totaling 1000 kcal/day and 45 g protein/day), followed by a fast thereafter. For fluid intake on the day before surgery, clear fluids were permitted until before dinner, not limiting the volume of intake, and after dinner, ORS only was permitted up to 1500 mL until 6:30 on the day of surgery. Laxatives were taken orally at 13:00 (1000 mg of magnesium oxide) and administered anally at 19:00 (500 mg sodium bicarbonate combined with 680 mg of monobasic sodium phosphate, anhydrous) on the day before surgery. Additional laxatives were not prescribed regardless of the presence or absence of defecation.
The consumed amount of solid foods (liquid diet in the control group and 30%-rice porridge in the ERAS group) was checked after each meal by ward nurses and recorded in nursing records in a 4-rating criteria (all consumed, 1/2 consumed, 1/3 consumed, and no consumption). Caloric intake in each patient was calculated based on the calorie value of each solid food and the amount that the patient consumed. The amount of fluids consumed was self-reported to nurses by patients and recorded in the nursing records. The calorie amount from fluid intake was not included in the total calorie calculation.
TBW and body weight were measured twice (at noon before lunch on the day before surgery and at 8:00 on the day of surgery). Body weight was measured by a body composition InnerScan BC-560 (Tanita Corporation, Tokyo), and values were input into a multi-frequency impedance apparatus MLT-100 (Sekisui Chemical Co., Ltd., Osaka). TBW was measured by a multi-frequency impedance apparatus MLT-100 using a frequency of 2.5 kHz to 350 kHz (140 kinds of frequency, 100 μArms)3. The apparatus was corrected according to its specification 30 min before measurement.
For the measurement, the patients rested on the floor for 5 min, and then rested in a supine position spreading the axilla and thigh. Silver electrodes (which were developed for this measurement) were tightly placed to the designated sites of the body after wiping the skin surface well with alcohol cotton. There were two sites for electrode placement: one on the central part of the dorsal side between the styloid process of the ulna of the right upper limb and the styloid process of the radius in the right upper limb and the other on the central part of the dorsal side between the medial malleolus and the lateral malleolus of the fibula in the right lower limb. The detection electrode was placed 5 cm away from the current source electrodes on the elbow side or knee side 4, 5. The equation for calculating TBW is shown in Table 1 6.
Table 1.
Calculation of total body water (TBW).
| Equations and values | ||||||
|---|---|---|---|---|---|---|
| β=Rm/ (1/Rint-1/R0) Rint = (Rm+ (Rm2+4β)0.5)/2 Recw=1/(Rint -1 - RICW -1) LTM=A*Ht2/RECW+B*Ht2/RICW+C*Htα ECW=A*Ht2/RECW+D*LTM ICW=B*Ht2/RICW+2*D*LTM TBW=ECW+ICW |
Values for Rm, α, A, B, C, and D are given in the table below. | |||||
| Rm (Ω) | α | A (kgΩ/cm2) | B (kgΩ/cm2) | C (kgΩ/cmα) | D | |
| Male | 400 | 2.3 | 0.3993 | 0.3368 | 0.000143 | 0.0474 |
| Female | 300 | 2 | 0.3716 | 0.2360 | 0.000785 | 0.0993 |
Rm: Resistance of cell membrane (Ω). RICW: Resistance of intracellular water (Ω). RECW: Resistance of extracellular water (Ω). RW: Resistance at a wave frequency of ∞ (Ω). R0: Resistance at a wave frequency of 0 (Ω). LTM: Lean tissue mass (kg). Ht: Height (cm). Wt: Body weight (kg). A: Age (year). ECW: Extracellular water (L). ICW: Intracellular water (L).
Evaluation items were as follows. 1) Percent changes in TBW and body weight were obtained at 2 measurement points (at 12:00 before lunch on the day before surgery and 8:00 on the day of surgery); 2) defecation times were checked from 10:00 on the day before surgery up to the time before entering the operating room. Defecation, if observed at 30 min or later after the previous one, was counted as the new defecation; and 3) frequencies of vomiting and aspiration were checked at the induction of general anesthesia. If vomiting and regurgitation of gastric contents were noted in the oral cavity, it was regarded as vomiting, and then the presence and absence of aspiration into the tracheal tube were checked. Silent aspiration was not investigated.
Data for changes in TBW and body weight, calorie intake from solid food, consumption of clear fluids, age, and BMI (body mass index) were analyzed using the unpaired t-test (two-sided at α=0.05). Data for sex difference, ASAPS, surgical procedure, and occurrence of vomiting or aspiration at the induction of general anesthesia were analyzed using the χ2 test (two-sided at α=0.05). For statistical analysis, a software package SPSS15.0 (SPSS Japan, Tokyo) was used. Data were presented as mean±SD.
Results
Thirty patients were enrolled in the study (15 in the control group and 15 in the ERAS group). Patient baseline characteristics are shown in Table 2. In the control group, one patient (7%) caught a cold on the day before surgery, and three patients (30%) were prescribed laxatives other than protocoled laxatives for additional laboratory examinations. Thus, these patients were excluded from the study as dropouts. No differences were noted for sex difference, age, BMI, and ASAPS between the groups.
Table 2.
Baseline characteristics of patients.
| Control group | ERAS group | Statistical analysis (p value) | |
|---|---|---|---|
| Number of patients | 14 | 12 | |
| Sex (female/male) | 2/12 | 2/10 | 0.87 |
| Age | 61.9±9.4* | 69.9±7.4* | 0.03 |
| BMI (kg/cm2) | 23.3±3.3* | 23.5±3.8* | 0.91 |
| ASAPS (I/II) | 8/6 | 7/5 | 0.95 |
| Surgical procedure (distal gastrectomy/total gastrectomy) |
9/5 | 8/4 | 0.90 |
Data for age and BMI (body mass index) were analyzed using the unpaired t-test (two-sided at α=0.05). Data for sex difference, ASAPS, and surgical procedure were analyzed using the χ2 test (two-sided at α=0.05). * Data are shown as mean±SD. ASAPS: American Society of Anesthesiologists Physical Status.
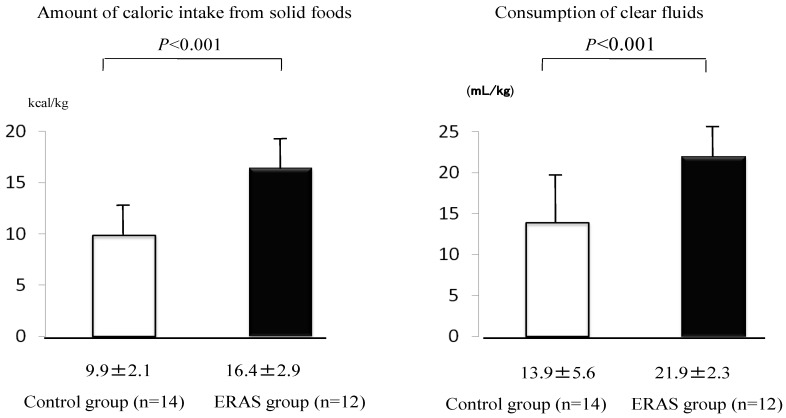
Consumption of solid food on the day before surgery was greater in the ERAS group than in the control group (16.4±2.9 kcal/kg for the ERAS group and 9.9±2.1 kcal/kg for the control, p<0.001). Consumption of clear fluids was also greater in the ERAS group than in the control group (21.9±2.3 mL/kg for the ERAS group and 13.9±5.6 mL/kg for the control group, p<0.001) (Fig. 2).
Figure 2.
Amount of caloric intake from solid foods (kcal/kg) and consumption (mL/kg) of clear fluids from 6:45 on the day before surgery to 6:45 on the day of surgery. Values were analyzed by the unpaired t-test (two-sided at α=0.05). Values are shown as mean±SD.
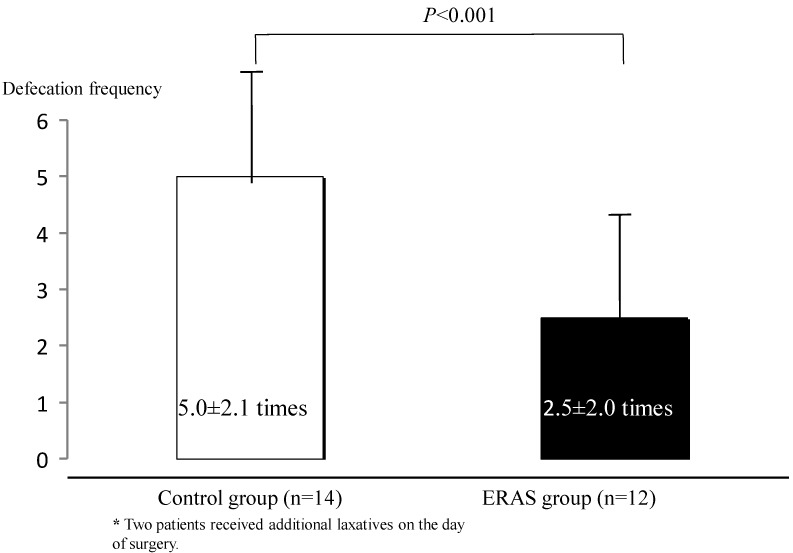
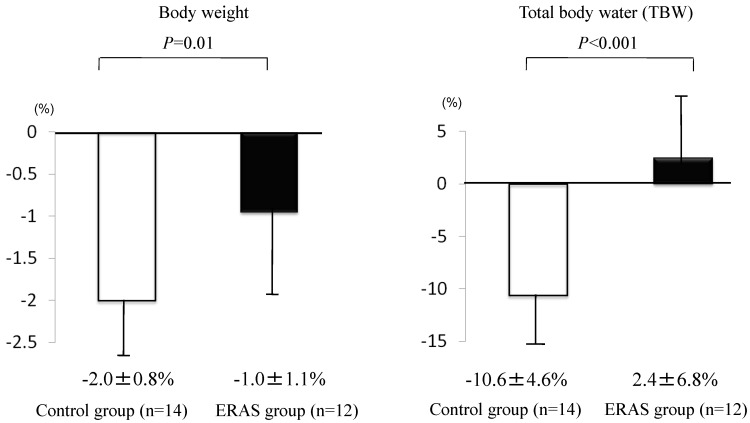
Defecation times (from 10:00 on the day before surgery up to the entry to the operation room) were less in the ERAS group than in the control group (2.5±2.0 times for the ERAS group and 5.0±2.1 times for the control group, p<0.001), and two patients in the control group were prescribed additional laxatives on the day of surgery (Fig. 3). TBW and body weight were changed in both groups, but the changes were less in the ERAS group (12 patients) than in the control group (14 patients) (TBW, −10.6±4.6% in the control group and 2.4±6.8% in the ERAS group, p<0.001; body weight, −2.0±0.8% in the control group and −1.0±1.1% in the ERAS group, p<0.01) (Fig. 4). Besides, no significant differences were noted in the TBW values between the two groups in the measurement on the day before surgery (30.6±1.4 kg in the control group and 30.1±1.7 kg in the ERAS group, p=0.89) and in the measurement on the day of surgery (27.2±1.1 kg in the control group and 31.0±2.0 kg in the ERAS group, p=0.21) .Vomiting and gastric aspiration associated with general anesthesia were not noted in both groups.
Figure 3.
Frequency of defecation from 10:00 on the day before surgery until before entering the operating room. Values were analyzed by the unpaired t-test (two-sided at α=0.05). Values are shown as mean±SD.
Figure 4.
Changes in body weight and total body water (TBW). Changes were investigated by measuring before lunch (12:00) on the day before surgery and in the morning (8:00) on the day of surgery. Values were analyzed by the unpaired t-test (two-sided at α=0.05). Values are shown as mean±SD.
Discussion
“ERAS” protocol was first proposed in the late 1990s in the northern European countries, specifically intended for “enhanced recovery after surgery,” and based on evidences from more than 800 scientific literatures 1. Other than ERAS, similar protocols such as “fast track surgery,” “multimodal analgesia,” “multimodal surgery,” and “multimodal rehabilitation” are available, but these have essentially the same purpose 7.
ERAS protocol was initially applied to laparotomic colorectal surgery and has now been applied to cases of other surgeries. Conventionally, mechanical bowel preparation (MBP) using lengthy preoperative fasting and excessive laxative medication have been the standard practice so that feces and dietary residue may not remain in the gastrointestinal tract.
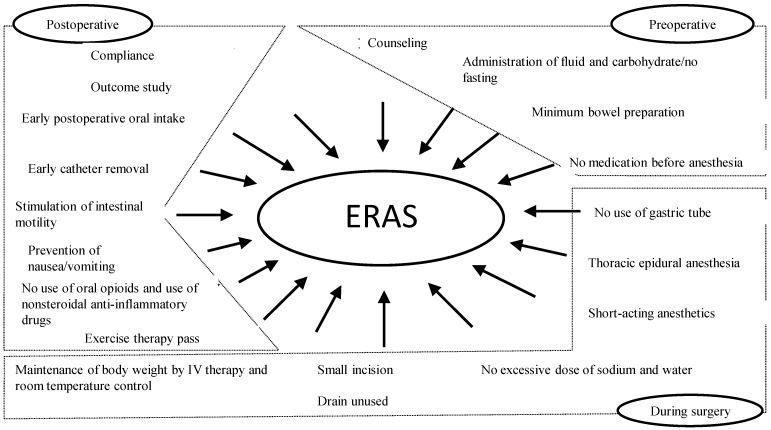
However, ERAS protocol recommends that the MBP should be used as less as possible 1, 7. In addition, preoperative carbohydrate loading and positive postoperative management such as pain control, early ambulation, and early oral feeding help alleviate the preoperative stress response. Specifically, ERAS protocol helps to strengthen postoperative recovery ability (Fig. 5) by improving insulin resistance and normalizing gastrointestinal function. As the effectiveness of ERAS, reduced postoperative morbidity, safety, shortened hospitalization, and cost reduction have been reported 8.
Figure 5.
Outline of ERAS protocol. ERAS, enhanced recovery after surgery.
In the present study, we hypothesized that preoperative total body water would be maintained appropriately by shortening the fasting time and reducing the laxative medication. Total body water was measured by multi-frequency impedance method. The results show that the amount of water in the body was favorably maintained in the ERAS group compared with the control group, encouraging the introduction of the two procedures, “shortened fasting time” and “reduced laxative medication” as the preoperative patient management. Beneficial effects and safety of ERAS protocol have been reported in many studies 9-11.
With regard to the preoperative fasting time, there are now much evidence to advocate the shorter fasting duration 12, 13, and societies of anesthesiology in the United States and most European countries have changed the practice guidelines for preoperative fasting 14, so that oral intake of solids is permissible up to 6 to 8 hr before surgery and clear fluids are permissible up to 2 to 3 hr before surgery (Table 3).
Table 3.
Practice guidelines for preoperative fasting in North America and Europe.
| Guidelines | Exclusion conditions | |
|---|---|---|
| UK | • Clear fluid permissible up to 3 hr before surgery • Solid food discontinued from 6-8 hr before surgery |
Emergency and digestive tract disease |
| Canada | • Clear fluid permissible up to 2 hr before surgery • Solid food discontinued from 6-8 hr before surgery |
|
| USA | • Clear fluid permissible up to 2 hr before surgery • Solid food discontinued from 6 hr before surgery |
Emergency, pregnancy, and digestive tract disease |
| Norway | • Clear fluid permissible up to 2 hr before surgery • Solid food discontinued from 6 hr before surgery |
Emergency and gastrointestinal disease |
| Sweden | • Clear fluid permissible up to 2-3 hr before surgery • Solid food discontinued after midnight on the day before surgery |
Emergency and gastrointestinal disease |
| Germany | • Clear fluid permissible up to 2 hr before surgery • Solid food discontinued from 6 hr before surgery |
In this study, solid foods were consumed in the ERAS group up to 6 hr later than in the control group, and clear fluids were consumed in the ERAS group up to 5 hr and 30 min later than in the control group. With regard to safety associated with the induction of general anesthesia, vomiting did not occur in any patients, although the number of patients enrolled in this study was small. Although there are no practice guidelines for preoperative fasting management in Japan authorized by medical societies, our previous studies have confirmed the safety of the preoperative consumption of ORS in more than 1000 patients 15, 16. In this study, only ORS was used as the clear fluid to be consumed before surgery, because its safety is already confirmed in previous studies.
Next, with regard to the preoperative management with the use of laxatives in lower intestinal tract surgery such as colon, negative results have been reported in multicenter cooperative studies, suggesting that laxative medication would rather not be encouraged 10. The reason lies in that the structure of intestinal mucosa membrane wall may be disturbed or infection may occur during surgery due to liquefaction of feces 17. However, with regard to the upper intestinal tract surgery investigated in the present study, there is still no evidence.
TBW and body weight were changed in both groups, but the changes were less in the ERAS group than in the control group. Two interventions (shortened fasting and reduced laxatives) practiced in the ERAS group are considered to have contributed to such results. First, the amounts of consumed solid foods and fluids were large in the ERAS group, because the duration of fasting was shortened. Secondly, defecation times were decreased, because laxative medication was reduced. Clinically, maintenance of preoperative TBW may help prevent a decrease of blood pressure associated with the use of anesthesia-induction agents or local epidural anesthetics, which would likely minimize the necessity of acute administration of parenteral solution for the maintenance of circulating blood volume.
If TBW is decreased, a decrease in blood pressure may be induced, so that acute infusion of parenteral solution or administration of pressor agent becomes necessary. Excessive administration of parenteral solutions in perioperative periods may contribute to increasing perioperative complications such as suture failure, cardiopulmonary complications, etc. and would prolong the duration of hospitalization 18. In this regard, ERAS clinical protocol recommends that excessive loading of water and sodium should be avoided 8.
On the other hand, earlier postoperative recovery has been reported in patients who were treated with a relatively large amount of parenteral solutions, not restricting the dose of parenteral solutions during surgery, in laparoscopic cholecystectomy 19. Yet, there are not definite answers concerning the doses of parenteral solutions during surgery. Further studies are necessary to investigate the preoperative anesthetic management in association with the maintenance of preoperative TBW, and also the effect on long-term prognosis should be clarified.
With regard to the appropriateness of multi-frequency impedance analysis used for the measurement of TBW in this study, there are a number of studies 3, 4. The ESPEN guidelines (2004) for “Bioelectrical impedance analysis” describe the principles and methods of multi-frequency impedance analysis 3, 4. We performed the measurements according to the particulars of the guidelines. The ESPEN guidelines mention that accuracy of measurements may be decreased in patients with serious fluid and electrolyte abnormalities. In the present study, although electrolyte abnormality was not observed in any subjects, the data of TBW include those of the subjects who had about 10% dehydration.
In Japan, only a few institutions are practicing the ERAS protocol at present. The underlying reasons may be that “guidelines for preoperative fasting” are not yet established by scientific organizations such as medical societies, and thus anesthesiologists just hesitate to shorten the duration of preoperative fasting 20. Besides favorable effects of shortened fasting on TBW, intake of carbohydrates helps decrease preoperative discomfort, feeling of dry mouth, and feeling of hunger 21. Furthermore, the duration of fasting time may relate to the reduction of insulin resistance 22, prevention of postoperative nausea and vomiting 23, and maintenance of immunocompetence 24. Therefore, like USA and EU countries, “guidelines for preoperative fasting time” should be established in Japan so that anesthesiologists may not be unnecessarily concerned about the duration of preoperative fasting. We would like to recommend “preoperative management with shorted fasting period,” and anticipate timely establishment of the “perioperative guidelines” for early postoperative recovery of surgical patients.
In conclusion, the results suggest that preoperative management with “shorted fasting time” and “reduced administration of laxatives” is effective in the maintenance of the amount of TBW before surgery. We believe that such management may help enhance postoperative recovery of patients.”
Acknowledgments
This report is based on our previous report published in the Journal of Japan Society for Clinical Anesthesia 2010;30:383-92 in Japanese language 25.
References
- 1.Fearon KC, Ljungqvist O, Von Meyenfeldt M. et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24:466–77. doi: 10.1016/j.clnu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Braga M, Ljungqvist O, Soeters P. et al. ESPEN Guidelines on Parenteral Nutrition: Surgery. Clin Nutr. 2009;28:378–86. doi: 10.1016/j.clnu.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka k, Kim H, Nakanishi T, Amagai H. Multi-frequency bioelectrical impedance method for the assessment of body composition in Japanese adults. Nihon Undoseirigaku Zasshi (Journal of Exercise and Sports Physiology) 1999;6:37–45. (In Japanese with English abstract) [Google Scholar]
- 4.Kyle UG, Bosaeus I, De Lorenzo AD. et al. Bioelectrical impedance analysis part I: review of principles and methods. Clin Nutr. 2004;23:1226–43. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Kyle UG, Gosaeus I, De Lorenzo AD. et al. Bioelectrical impedance analysis part II: utilization in clinical practice. Clin Nutr. 2004;23:1430–53. doi: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka k, Kim H, Nakanishi T, Amagai H. Multi-frequency bioelectrical impedance method for the assessment of body composition n Japanese adults. Nihon Undoseirigaku Zasshi (Journal of Exercise and Sports Physiology) 1999;6:37–45. (in Japanese with English abstract) [Google Scholar]
- 7.Serclová Z, Dyrych P, Marvan J. et al. Fast-track in open intestinal surgery: Prospective randomized study. Clin Nutr. 2009;28:618–24. doi: 10.1016/j.clnu.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Hendry PO, Hausel J, Nygren J. et al. Determinants of outcome after colorectal resection within an enhanced recovery programme. Br J Surg. 2009;96:197–205. doi: 10.1002/bjs.6445. [DOI] [PubMed] [Google Scholar]
- 9.Brady MC, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. In Cochrane Database Syst Rev. 2003;(4):CD004423. doi: 10.1002/14651858.CD004423. [DOI] [PubMed] [Google Scholar]
- 10.Walter CJ, Collin J, Dumville JC, Drew PJ, Monson JR. Enhanced recovery in colorectal resections: a systematic review and meta-analysis. Colorectal Dis. 2009;11:344–53. doi: 10.1111/j.1463-1318.2009.01789.x. [DOI] [PubMed] [Google Scholar]
- 11.Guenaga K, Matos D, Wille J. Mechanical bowel preparation for elective colorectal surgery. In Cochrane Database Syst Rev. 2009 Jan 21;(1):CD001544. doi: 10.1002/14651858.CD001544.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Philips S, Hutchinson S, Davidson T. Preoperative drinking does not affect gastric contents. Br J Anaesth. 1993;70:6–9. doi: 10.1093/bja/70.1.6. [DOI] [PubMed] [Google Scholar]
- 13.Søreide E, Strømskag K, Steen P. Statistical aspects in study of preoperative fluid intake and gastric content. Acta Anaesthesiol Scand. 1995;39:738–43. doi: 10.1111/j.1399-6576.1995.tb04162.x. [DOI] [PubMed] [Google Scholar]
- 14.Søreide E, Eriksson LI, Hirlekar G. et al. Pre-operative fasting guidelines: an update. Acta Anaesthesiol Scand. 2005;49:1041–7. doi: 10.1111/j.1399-6576.2005.00781.x. [DOI] [PubMed] [Google Scholar]
- 15.Taniguchi H, Sasaki T, Fujita H. et al. Preoperative fluid and electrolyte management with oral rehydration therapy. J Anesth. 2009;23:222–9. doi: 10.1007/s00540-009-0743-6. [DOI] [PubMed] [Google Scholar]
- 16.Taniguchi H, Sasaki T, Fujita H. The use of oral rehydration therapy for preoperative fluid and electrolyte management. Nihon Rinsho Masui Gakaishi. 2009;29:815–23. (In Japanese) [Google Scholar]
- 17.Harris LJ, Moudgill N, Hager E, Abdollahi H, Goldstein S. Incidence of anastomotic leak in patients undergoing elective colon resection without mechanical bowel preparation: our updated experience and two-year review. Am Surg. 2009;75:828–33. [PubMed] [Google Scholar]
- 18.Joshi GP. Intraoperative fluid restriction improves outcome after major elective gastrointestinal surgery. Anesth Analg. 2005;101:601–5. doi: 10.1213/01.ANE.0000159171.26521.31. [DOI] [PubMed] [Google Scholar]
- 19.Holte K, Klarskov B, Christensen DS. et al. Liberal versus restrictive fluid administration to improve recovery after laparoscopic cholecystectomy: a randomized, double-blind study. Ann Surg. 2004;240:892–9. doi: 10.1097/01.sla.0000143269.96649.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shime N, Ono A, Chihara E, Tanaka Y. Current practice of preoperative fasting: a nationwide survey in Japanese anesthesia-teaching hospitals. J Anesth. 2005;19:187–92. doi: 10.1007/s00540-005-0319-z. [DOI] [PubMed] [Google Scholar]
- 21.Hausel J, Nygren J, Lagerkranser M. et al. A Carbohydrate-Rich Drink Reduces Preoperative Discomfort in Elective Surgery Patients. Anesth Analg. 2001;93:1344–50. doi: 10.1097/00000539-200111000-00063. [DOI] [PubMed] [Google Scholar]
- 22.Faria MS, de Aguilar-Nascimento JE, Pimenta OS, Dock-Nascimento DB, Slhessarenko N. Preoperative Fasting of 2 Hours Minimizes Insulin Resistance and Organic Response to Trauma after Video-Cholecystectomy: a randomized controlled clinical trial. World J Surg. 2009;33:1158–64. doi: 10.1007/s00268-009-0010-x. [DOI] [PubMed] [Google Scholar]
- 23.Hausel J, Nygren J, Thorell A, Lagerkranser M, Ljungqvist O. Randomized clinical trial of the effects of oral preoperative carbohydrates on postoperative nausea and vomiting after Laparoscopic cholecystectomy. Br J Surgery. 2005;92:415–21. doi: 10.1002/bjs.4901. [DOI] [PubMed] [Google Scholar]
- 24.Melis G, van Leeuwen P, von Blomberg-van der Flier BME. et al. A carbohydrate-rich beverage prior to surgery prevents surgery induced immunodepression: a randomized, controlled, clinical trial. J Parenter Enteral Nutr. 2006;30:21–6. doi: 10.1177/014860710603000121. [DOI] [PubMed] [Google Scholar]
- 25.Taniguchi H, Sasaki T, Makise K, Fujita H. Proposal for preoperative management with a shortened fasting period: an investigation of the amount of water in the body using a multi-frequency impedance method. The Journal of Japan Society for Clinical Anesthesia. 2010;30:383–92. (in Japanese with English abstract) [Google Scholar]