Abstract
Objective: We assessed the serum glucagon-like peptide-1 (GLP-1) levels for Chinese adults with pre-diabetes (PD) and newly-diagnosed diabetes mellitus (NDDM) during oral glucose tolerance test (OGTT). The relationships between total GLP-1 level and islet β cell function, insulin resistance (IR) and insulin sensitivity (IS) were also investigated.
Methods: A 75g glucose OGTT was given to 531 subjects. Based on the results, they were divided into groups of normal glucose tolerance (NGT), isolated impaired fasting glucose (IFG), isolated impaired glucose tolerance (IGT), IFG combined IGT (IFG+IGT) and NDDM. Total GLP-1 levels were measured at 0- and 2-hour during OGTT. Homeostasis model assessment of β cell function (HOMA-β), HOMA of insulin resistance (HOMA-IR), Gutt and Matsuda indexes were calculated. The relationships between GLP-1 level and β cell function, IR and IS were analyzed.
Results: The levels of total fasting GLP-1 (FGLP-1), 2h GLP-1 (2hGLP-1) and 2hGLP-1 increments (∆GLP-1) following OGTT reduced significantly in IFG+IGT and NDDM groups (P<0.005). HOMA-β , HOMA-IR, Gutt and Matsuda indexes demonstrated various patterns among NGT, isolated IFG, isolated IGT, IFG+IGT and NDDM groups (P<0.05). Spearman rank correlation analysis and multivariable linear regression model suggested that some levels of correlation between GLP-1 levels, ∆GLP-1 and β cell function, IR (P<0.05).
Conclusions: The total GLP-1 levels and its response to glucose load decreased significantly in IFG+IGT group, compared to isolated IFG or IGT group. They were even similar to that of NDDM group. Moreover, there were observable correlations between impaired GLP-1 secretion and β cell function, IR and IS.
Keywords: β cell function, insulin resistance, insulin sensitivity, newly-diagnosed diabetes mellitus, pre-diabetes, total glucagon-like peptide-1.
Introduction
Glucagon-like peptide-1 (GLP-1) is a potent glucose-dependent insulinotropic hormone, which assists in glucose homeostasis by increasing insulin and reducing glucagon secretions 1. It also inhibits gastric motility, enhances the satiety and stimulates glucose utilization in the peripheral tissues 1, 2. GLP-1 has important pleiotrophic actions both on mature β cells and ductal cells. It has been reported to promote β cell regeneration, suppress its apoptosis, and stimulate insulin gene transcription 1, 3, 4. Thus, GLP-1 is beneficial for the islet β cells in terms of both their quantities and functions.
Toft-Nielsen et al. 5 found that the fasting GLP-1 (FGLP-1) level in patients with type 2 diabetes mellitus (T2DM) did not decrease significantly compared to that in normal glucose tolerance (NGT) group. However, the following 4-hour mixed meal tolerance tests indicated that the postprandial GLP-1 levels, the area under the curve (AUC) and the GLP-1 increments were significantly lower as compared to that of NGT group. And for the participants with impaired glucose tolerance (IGT), those levels were in between the above mentioned two groups (the NGT and T2DM groups). Nonetheless, previous studies regarding to GLP-1 level in pre-diabetic or diabetic populations were not concordant 3, 4, 6-21. The divergence may result from the sample size, the participants with different durations of diabetes mellitus (DM) and the pre-diabetic categories (i.e. whether or not all of the pre-diabetes (PD) individuals with isolated impaired fasting glucose (IFG), isolated IGT or IFG combined IGT (IFG+IGT) were included). Otherwise, diverse hyperglycemic conditions and different durations of PD and DM may lead to the divergence in the β cell function and insulin sensitivity (IS) and may further result in the divergence of the GLP-1 levels. In the presenting study, we investigated the total FGLP-1 and total 2-hour GLP-1 (2hGLP-1) concentrations in the serum during the 75g glucose oral glucose tolerance test (OGTT) among persons with NGT, isolated IFG, isolated IGT, IFG+IGT and newly-diagnosed diabetes mellitus (NDDM). And the relationships between the total GLP-1 and β cell function, insulin resistance (IR) and IS were also evaluated.
Materials and Methods
Subjects and study protocol
This study selected 2502 out of 2950 participants with the age of 35 and above, who underwent the routine health examination in our hospital, from October 2007 to April 2011. All participants were of Chinese nationality and from the Han ethnic group. An informed consent was taken from every participant before the study. A 75g glucose OGTT was given to every participant. Based on their OGTT results, the participants with isolated IFG (n=98), isolated IGT (n=101), IFG+IGT (n=104) and NDDM (n=105) were selected. Subsequently, the normal control group (n=123) with matched age and gender was randomly selected from the remaining 2094 participants with NGT. All subjects were free from known history of DM, thyrotoxicosis, gastrointestinal diseases, malignancy, serious hepatic diseases and/or abnormal glomerular filtration rate. Those individuals who took drugs known to influence the glucose and lipids such as hypoglycemic or hypolipidemic treatment or glucocorticoids were excluded, except for those who took antihypertensive drugs. The entire study procedure was approved by the Medical Ethics Committee of West China Hospital of Sichuan University and conducted in accordance with the principles expressed in the Declaration of Helsinki (as revised in Edinburgh 2000).
Diagnostic criteria of NGT, PD and DM
The classification of different stages on the basis of their fasting plasma glucose (FPG) and 2-hour plasma glucose (2hPG) levels during the OGTT was in accordance to the diagnostic criteria given by American Diabetes Association (ADA) in 2006 22. NGT: FPG < 5.6mmol/l (100mg/dl ) and 2hPG < 7.8 mmol/l (140 mg/dl); IFG: 5.6 mmol/l (100mg/dl ) ≤ FPG < 7.0 mmol/l (125 mg/dl) and 2hPG < 7.8 mmol/l (140 mg/dl); IGT: FPG < 5.6mmol/l (100mg/dl ) and 7.8 mmol/l (140 mg/dl) ≤ 2hPG < 11.1 mmol/l (200 mg/dl); IFG+IGT: 5.6 mmol/l (100mg/dl ) ≤ FPG < 7.0 mmol/l (125 mg/dl) and 7.8 mmol/l (140 mg/dl) ≤ 2hPG < 11.1 mmol/l (200 mg/dl); DM: FPG ≥ 7.0 mmol/l (125 mg/dl) and/or 2hPG ≥ 11.1 mmol/l (200 mg/dl).
Methods
Anthropometric measurements, OGTT and specimen collection
Height, weight, waist circumference and blood pressure were measured in the basic ways 6, 7, 9. A standard 75g glucose OGTT was conducted for each subject after an overnight fasting (longer than twelve hours). Blood samples were collected only at the 0 and 2 hours following OGTT, as most of the subjects were unwilling to accept the blood collections at 15, 30 and 60 min during their OGTTs. The blood used for the GLP-1 determinations was collected in tubes without any aprotinin, DPP-IV inhibitor or anticoagulant. After centrifugation at 4 ℃, all serum samples were stored at -80 ℃ till they were analyzed. And the storage time was not longer than two months per batch.
Biochemical measurements
The total cholesterol (TC), triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C) concentrations (at the fasting state) and the plasma glucose (PG) levels (at each time point) were measured by a Bayer 560 automatic biochemistry analyzer with MAKER kits by the same laboratory technician. Glucose was measured by hexokinase method, while blood lipids were measured with automated enzymatic method or dextran sulfate-manganese precipitation. Insulin concentrations were detected by radioimmunoassay (RIA) with BNIBT kits (North Institute of Biological Technology, Beijing, China), of which the intra-assay coefficient of variation was below 10% and the inter-assay coefficient of variation was below 15%. The total GLP-1 levels were determined by enzyme-linked immunosorbent assay (ELISA) with USCNLIFETM kits (Uscnlife Science & Technology Company, USA) , of which the intra-assay coefficient of variation was below 8.3%, inter-assay coefficient of variation was below 6.6%, and the sensitivity was typically less than 0.78 pmol/l.
Evaluation of islet β cell function, IR and IS
As this study only included blood samples collected at 0- and 2-hour following the OGTT, the homeostasis model assessment of β cell function (HOMA-β) 23 was used to evaluate basal insulin secretion. Meanwhile, we chose the HOMA assessment of IR (HOMA-IR) 23 to estimate IR at the fasting state. Both Gutt index 24 and Matsuda index 25 were analyzed for IS state before and after the glucose load. Formulas are as follows:
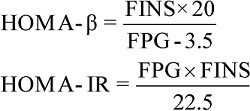 |
 |
where F means fasting, 2h means the 2-hour point during an OGTT, PG (mg/dl in Matsuda index formula, mmol/l in other formulas) means plasma glucose, INS (mU/l) means serum insulin concentrations and BW (kg) means body weight.
Statistical analysis
Data analyses were carried out with SPSS 16.0 (Chicago, IL, USA). All data were expressed as means ± SD. After the distribution tests, variables with skewed distribution were logarithmically transformed for the same test again. ANOVA analysis was applied for parametric materials. Rank sum test was used for nonparametric materials. The comparison of constituent ratio was assessed by X2 test. Spearman rank correlation analysis was used to explore the correlations between the total FGLP-1, total 2hGLP-1 levels, the incremental 2hGLP-1 (∆GLP-1) and HOMA-β, HOMA-IR, Gutt and Matsuda indexes. Multiple linear regression analysis was also performed to adjust the confounding factors for the total FGLP-1, total 2hGLP-1 and ∆GLP-1. The overall p- value among all groups was two-tailed and a p-value less than 0.05 was considered to be statistically significant. Furthermore, the Bonferroni correction and the chi-square segmentation were used to adjust for multiple comparisons.
Results
Comparisons of general characteristics of diverse hyperglycemic conditions
Table 1 demonstrated the general characteristics of the subjects involved in this study. The age and the gender showed no significant differences among groups (P>0.05).
Table 1.
Comparisons of general characteristics of diverse hyperglycemic conditions.
| NGT | Isolated IFG | Isolated IGT | IFG+IGT | NDDM | Overall p value | |
|---|---|---|---|---|---|---|
| Numbers (male/female) | 123(74/49) | 98(57/41) | 101(67/34) | 104(54/50) | 105(64/41) | 0.328 |
| Age (years) | 53.6±10.1 | 55.4± 7.7 | 52.8± 10.8 | 56.1± 11.8 | 54.5± 11.2 | 0.054 |
| Height (cm) | 162.2±7.6 | 160.5±7.6 | 160.8±7.3 | 162.3±7.1 | 162.2±12.1 | 0.369 |
| Weight (kg) | 63.20±10.01 | 64.43±10.30 | 64.27±10.73 | 64.59±10.16 | 66.29±13.52 | 0.339 |
| BMI(kg/m2) | 23.93±2.89 | 24.92±2.95 | 24.75±3.15 | 24.46±3.03 | 28.28±3.88 | 0.198 |
| Waist circumference (cm) | 84.0±8.4 | 84.6± 8.4§¶ | 86.0± 9.3 | 88.0± 7.2*† | 87.7± 7.3*† | 0.000 |
| SBP(mmHg) | 125±18.7 | 136± 17.5* | 129± 17.6§¶ | 138± 13.9*‡ | 141± 14.7*‡ | 0.000 |
| DBP(mmHg) | 81±11.9 | 87± 10.4*§ | 85± 11.9 | 81± 9.7† | 84± 11.8 | 0.000 |
| TG(mmol/l) | 0.68±0.72 | 1.73±1.32* | 1.99±1.22* | 1.95±1.31* | 2.00±1.64* | 0.000 |
| TC(mmol/l) | 4.51±0.85 | 4.51±0.93§ | 4.68±0.84 | 4.90±0.94*†¶ | 4.65±0.95§ | 0.009 |
| HDL-C (mmol/l) | 1.35±0.32 | 1.40±0.33 | 1.39±0.30 | 1.38±0.32 | 1.31±0.37 | 0.172 |
BMI=weight/height2 ; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; TC, total cholesterol, HDL-C, high-density lipoprotein cholesterol; NGT, normal glucose tolerance; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; IFG+IGT, IFG combined IGT; NDDM, newly-diagnosed diabetes mellitus.
Data are expressed as means ± SD.
X2 test was applied for the gender compositions among the groups. Additionally, the chi-square segmentation was used to adjust the significance level for multiple comparisons between the groups (a'=0.005).
One-Way ANOVA analysis was used for height, weight, TC and logarithmically transformed TG among the groups; LSD analysis was used both for TC and logarithmically transformed TG in the multiple comparisons between the groups.
Kruskal-Wallis H analysis was applied for age, BMI, waist circumference, SBP, DBP and HDL-C among the groups; Mann-Whitney U analysis was used for height, weight, age, BMI, waist circumference, SBP, DBP and HDL-C between the groups, and the Bonferroni correction was performed to adjust the significance level for multiple comparisons between groups (a'=0.005).
*: vs. NGT, P < 0.005; †: vs. isolated IFG, P < 0.005; ‡: vs. isolated IGT, P < 0.005; §: vs. IFG+IGT, P < 0.005; ¶: vs. NDDM, P < 0.005.
Comparisons of plasma glucose, serum insulin, total GLP-1 concentrations (at each time point) and ∆GLP-1 in different hyperglycemic conditions
As indicated in Table 2, there was significant difference for each parameter among the groups (P<0.05). Multiple comparisons results between the groups were as follows: [1]. FPG: NGT and isolated IGT groups < isolated IFG group < IFG+IGT group < NDDM group (P<0.005); [2]. 2hPG: NGT and isolated IFG groups < isolated IGT group < IFG+IGT group < NDDM group (P<0.005); [3]. Fasting insulin (FINS) concentrations: NGT group < isolated IFG, isolated IGT, IFG+IGT and NDDM groups (P<0.005); [4]. 2-hour insulin (2hINS) concentrations during the OGTT: NGT group < isolated IFG group < isolated IGT, IFG+IGT and NDDM groups (P<0.005); [5]. Total FGLP-1 levels: NGT, isolated IFG and isolated IGT groups > NDDM group (P<0.005), while isolated IGT group > IFG+IGT group > NDDM group (P<0.005); [6]. Total 2hGLP-1 levels: NGT, isolated IFG and IGT groups > IFG+IGT and NDDM groups (P<0.005); [7]. ∆GLP-1: NGT and isolated IFG groups > IFG+IGT and NDDM groups (P<0.005), while NGT group > isolated IGT group > IFG+IGT and NDDM groups (P<0.005).
Table 2.
Comparisons of plasma glucose, serum insulin, total GLP-1 concentrations (at each time point) and ∆GLP-1 in different hyperglycemic conditions.
| NGT (n=123) | Isolated IFG (n=98) | Isolated IGT (n=101) | IFG+IGT (n=104) | NDDM (n=105) | Overall p value | |
|---|---|---|---|---|---|---|
| FPG (mmol/l) | 4.63± 0.49 | 6.00± 0.34*‡§¶ | 4.68± 0.54†§¶ | 6.26± 0.36*†‡¶ | 7.40± 2.63*†‡§ | 0.000 |
| FINS (mU/l) | 7.01± 2.91 | 9.70± 4.33* | 9.16± 4.47* | 11.05± 6.65* | 11.22± 9.26* | 0.000 |
| Total FGLP-1(pmol/l) | 23.59± 11.62 | 23.81± 11.73¶ | 26.34± 8.35§¶ | 20.11± 12.67‡¶ | 10.60± 8.24*†‡§ | 0.000 |
| 2hPG (mmol/l) | 5.34± 1.33 | 5.58± 1.33‡§¶ | 8.74± 0.78*†§¶ | 9.29± 0.94*†‡¶ | 14.39± 4.70*†‡§ | 0.000 |
| 2hINS(mU/l) | 32.86±26.27 | 44.85±38.70*‡§¶ | 72.76±40.56*† | 68.18±45.35*† | 69.42±60.25*† | 0.000 |
| Total 2hGLP-1(pmol/l) | 35.39±15.40 | 34.18±15.93§¶ | 35.86±13.66§¶ | 22.24±16.72*†‡ | 16.49±14.11*†‡ | 0.000 |
| ∆GLP-1(pmol/l) | 12.37±10.05 | 10.37±9.82§¶ | 9.79±10.50*§¶ | 2.07±6.31 *†‡ | 4.62±6.27 *†‡ | 0.000 |
F, fasting; PG, plasma glucose; INS, serum insulin concentrations; ∆GLP-1, the increments of 2hGLP-1; NGT, normal glucose tolerance; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; IFG+IGT, IFG combined IGT; NDDM, newly-diagnosed diabetes mellitus.
Data are expressed as means ± SD.
Kruskal-Wallis H analysis was assessed for the comparisons of all above indicators among the groups; Mann-Whitney U analysis was used in the comparisons between the groups. And the significance level had been adjusted (a'=0.005).
*: vs. NGT, P < 0.005; †: vs. isolated IFG, P < 0.005; ‡: vs. isolated IGT, P < 0.005; §: vs. IFG+IGT, P < 0.005; ¶: vs. NDDM, P < 0.005.
Although the total FGLP-1 levels were not significantly different among NGT, isolated IFG and IGT groups, they reduced obviously in the IFG+IGT and NDDM groups (P<0.005), especially in the NDDM group. After the 75g glucose load, the total 2hGLP-1 concentrations were increased in all groups. No statistical significance was found among the NGT, isolated IFG and IGT groups (P>0.005). However, the 2hGLP-1 levels in the IFG+IGT and NDDM groups were lower than that in the previous three groups (P<0.005). There was no significant disparity of ∆GLP-1 between the NGT and isolated IFG groups (P>0.005). But compared to them, the GLP-1 responses to the OGTT decreased significantly in the isolated IGT, IFG+IGT and NDDM groups (P<0.005). Moreover, there was no significant difference for ∆GLP-1 in the IFG+IGT and NDDM groups (P>0.005), however, it was manifestly lower than that in isolated IGT group (P<0.005).
Comparisons of islet β cell function, IR and IS in different hyperglycemic conditions
The multiple comparison outcomes between the groups are showed in Table 3. There were significant differences among all the indicators in different hyperglycemic conditions (P<0.05). HOMA-β, which reflects the basal insulin secretion at the fasting state, revealed the following relationship: NGT and isolated IGT groups > IFG+IGT group > isolated IFG group > NDDM group (P<0.005). HOMA-IR, which reflects the IR at the fasting state, revealed the following relationship: NGT group < isolated IGT group < isolated IFG group < IFG+IGT group < NDDM group (P<0.005). Gutt index, indicated that the IS both before and after the glucose loads were as follows: NGT group > isolated IFG group > isolated IGT group > IFG+IGT and NDDM groups (P<0.005). And Matsuda index, with the same meaning as Gutt index, revealed the following relationship: NGT group > isolated IFG group > isolated IGT group > IFG+IGT group > NDDM group (P<0.005).
Table 3.
Comparisons of islet β cell function, IR and IS in different hyperglycemic conditions.
| NGT (n=123) | Isolated IFG (n=98) | Isolated IGT (n=101) | IFG+IGT (n=104) | NDDM (n=105) | Overall p value | |
|---|---|---|---|---|---|---|
| HOMA-β | 148.10±136.55 | 79.48±39.67*‡§¶ | 176.24±209.45†§¶ | 81.25±51.25*†‡¶ | 73.80±59.48*†‡§ | 0.000 |
| HOMA-IR | 1.45±0.00 | 2.58±1.12*‡§¶ | 1.91±1.00*†§¶ | 3.07±1.83*†‡¶ | 3.62±3.18*†‡§ | 0.000 |
| Gutt | 114.94±30.19 | 85.93±26.21*‡§¶ | 61.00±10.20*†§¶ | 54.39±10.47*†‡ | 43.15±15.68*†‡ | 0.000 |
| Matsuda | 10.03±4.79 | 6.53±3.87*‡§¶ | 4.04±1.55*†§¶ | 3.61±2.03*†‡¶ | 3.21±2.37*†‡§ | 0.000 |
NGT, normal glucose tolerance; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; IFG+IGT, IFG combined IGT; NDDM, newly-diagnosed diabetes mellitus; HOMA-β, the homeostasis model assessment of β cells function; HOMA-IR, the homeostasis model assessment of insulin resistance; Gutt, an insulin sensitivity index; Matsuda, a composite index of whole-body insulin sensitivity during the OGTT.
Data are expressed as means ± SD.
Kruskal-Wallis H analysis was assessed for the comparisons of all above indexes among the groups; Mann-Whitney U analysis was used in the comparisons between the groups. And the significance level had been adjusted (a'=0.005).
*: vs. NGT, P < 0.005; †: vs. isolated IFG, P < 0.005; ‡: vs. isolated IGT, P < 0.005; §: vs. IFG+IGT, P < 0.005; ¶: vs. NDDM, P < 0.005.
These results showed that HOMA-β could not predict the different stages of hyperglycemic states between those groups. On the contrary, HOMA-IR can be representative of IR at the fasting state. And Matsuda index was better than Gutt index to evaluate the IS in participants with various glucose metabolic states.
Spearman rank correlation analysis
Table 4 indicated that there were some levels of correlation between the three GLP-1 measurements and the four evaluating indexes of all subjects (n=531). These results revealed that the total FGLP-1, 2hGLP-1 levels and ∆GLP-1 were directly proportional with the HOMA-β, Gutt and Matsuda indexes (P<0.05), while they were inversely proportional with the HOMA-IR (P<0.05). Thus, we could deduce that a higher level of total FGLP-1 resembles to a better β cell function, less IR and an increase in IS along with the total 2hGLP-1 level and ∆GLP-1 during the OGTT. But it is noteworthy that post oral glucose load, the relation between the total GLP-1 level and β cell function was not assessed in our research.
Table 4.
Spearman rank correlation analysis of total GLP-1 concentrations at each time point, ∆GLP-1, HOMA-β, HOMA-IR, Gutt and Matsuda indexes.
| Total FGLP-1 (n=531) | Total 2hGLP-1 (n=531) | ∆GLP-1 (n=531) | ||
|---|---|---|---|---|
| HOMA-β | r | 0.151 | 0.186 | 0.203 |
| P | 0.001 | 0.000 | 0.000 | |
| HOMA-IR | r | -0.259 | -0.326 | -0.304 |
| P | 0.000 | 0.000 | 0.000 | |
| Gutt | r | 0.442 | 0.408 | 0.091 |
| P | 0.000 | 0.002 | 0.035 | |
| Matsuda | r | 0.260 | 0.368 | 0.371 |
| P | 0.000 | 0.000 | 0.000 |
F, fasting; 2h, at the 2-hour point during an OGTT; ∆GLP-1, the 2hGLP-1 increments; HOMA-β, the homeostasis model assessment of β cells function; HOMA-IR, the homeostasis model assessment of insulin resistance; Gutt, an insulin sensitivity index; Matsuda, a composite index of whole-body insulin sensitivity during the OGTT ; r, correlation coefficient.
Multiple linear regression analysis
Multiple linear regression model analysis for the total GLP-1 was applied to adjust for the possible confounding factors. For the regression analysis, we selected FGLP-1 and 2hGLP-1 as dependent variables respectively and gender, age, weight, height, BMI, waist circumference, SBP, DBP, TC, HDL-C, TG, PG and INS (at each time point) as independent variables simultaneously. Then a stepwise selection was adopted to obtain an equation that was fit for the statistical conditions and had statistical significance (p<0.05). In the first equation (adjust r2 = 0.219, p<0.001), the total FGLP-1 levels was positively correlated with the HDL-C (p=0.037), but negatively correlated with the weight (p<0.001). And it showed that the female had a higher FGLP-1 concentrations than the male (p<0.001). In the second equation (adjust r2 = 0.241, p<0.001), there was similar relevance between the total 2hGLP-1 levels and the HDL-C (p=0.002), weight (p<0.001) and gender (p<0.001) to the above one. Additionally, the 2hGLP-1 formula displayed a negative correlation between the GLP-1 level and SBP (p=0.015).
After that, the ∆GLP-1 was considered as the dependent variable, while FGLP-1 and 2hGLP-1 indicators were added into the previous independent variables group. Consequently, a new equation (adjust r2 = 0.841, p<0.001) was obtained through the same selection pattern as before. It showed that there was a negative correlation between the ∆GLP-1 and TG (p=0.012).
The results of multiple linear regression analysis suggested that if subjects had the risk factors which increases IR such as abnormal lipid concentrations, heavy weight and the boosted SBP etc, the total FGLP-1, 2hGLP-1 and ∆GLP-1 levels after the oral glucose load may decrease significantly.
Discussion
The release of GLP-1following oral administration of nutrients (carbohydrates and lipids in particular) has two overlapping phases. The early (first) phase takes place within a few minutes after a meal and lasts for 30~60 min; while the delayed (second) phase continues for 60~180 min 1, 26. Until now, there have been several controversies about the mechanisms of the early phase release. Some suggested that just after administration although nutrients are far away from the duodenum, GLP-1 as an intestinal signal material has a prior stimulatory effect on insulin secretion 1, 26. Other studies suggested that, since the majority of the L cells lie in both of the distal jejunum and the entire ileum, this phase of GLP-1 release is likely to be mediated by hormones (e.g. glucose-dependent insulinotropic peptide (GIP) or gastrin-releasing peptide (GRP)) or neural signals rather than being directly contacted with nutrients and the intestinal tracts 26. The second phase of prolonged secretion may be concerned with the direct actions on the luminal contents by the L cells in distal jejunum and ileum 1, 10, 26.
In recent years, a few investigators have illustrated that there are numerous high-affinity GLP-1 binding receptors expressing on the insulin sensitive peripheral tissues (e.g. liver, skeletal muscle and fat cells) 1. Several evidences also indicated that GLP-1 in those tissues may enhance their glucose utilizations for the inductions of lipid and glycogen synthesis. And the anabolic actions of GLP-1 are independent of insulin 1, 2.
In the presenting study, the levels of total FGLP-1 and 2hGLP-1 during the 75g oral glucose loads did not change significantly among the subjects with NGT, isolated IFG and IGT. However, they decreased significantly in individuals with IFG+IGT and NDDM, who were accompanied with the more severe stages of glucose metabolism disorders. The ∆GLP-1 did not change in individuals with isolated IFG, when compared to the participants with NGT. But there were some significant reductions in the participants with isolated IGT, IFG+IGT and NDDM, especially in the latter two kinds. These findings were comparable to some previous research 5-7, 10-15, 19, 20. The impaired total GLP-1 concentration and its response to glucose load may indicate that the GLP-1 defects are already present in PD patients, particularly in those with IFG+IGT. But its specific role in process to type 2 diabetes is still unknown.
However, a few scholars pointed out recently that the GLP-1 levels were not reduced in patients with IGT or type 2 diabetes 16-18, 21. Though the cause and the mechanism for this discrepancy are not clear now, the possible major factors for it may be: [1]. Categories of PD. According to the diagnostic criteria of ADA in 2006 22, in some previous studies, the “IGT” individuals were actually consisted of the patients both with isolated IGT and IFG+IGT 16, 18. And none of them 16-18, 21 included all the categories of PD (especially the isolated IFG and IFG+IGT). However, there was a complete classification of PD in this study. [2]. Course of disease. Various durations of DM would lead to different stages of glucose metabolism disorders and complicated situations of GLP-1 secretion. In the presenting study, the major focus was on the total GLP-1 release situation in the preliminary and initial stage of Chinese type 2 diabetes. So far, there is much less little information in this field. [3]. Sample size. The sample sizes of some previous research 16-18, 21 were small (n = 14 ~ 48), and none of them brought all three subgroups of PD or NDDM into consideration. However, the presenting study was based on a large cohort of 303 PD and 105 NDDM (n=531). Moreover, in some other large-sampled research, i.e. Laakso et al.'s 6, there was a consistent conclusion as ours, which also revealed that the total GLP-1 release reduced in subjects with IFG, IGT or IFG+IGT. [4]. Treatments influence. In most of the previous studies, the subjects had taken the hypoglycemic therapies 5, 17-19. However, many kinds of glucose-lowering medications have a positive effect on the GLP-1 release, such as metformin, thiazolidinedione and insulin etc 17, 18, 27. In the presenting study, individuals with any anti-diabetic drug were excluded. Therefore, the data could reflect the natural alterations of total GLP-1 from NGT to PD, then to NDDM more properly.
In addition, there may be some other factors that lead to the divergent results: [1]. Sampling time. In some of the previous studies 5, 6, 17, with postprandial or glucose stimulations, there were notable differences in terms of the release duration at the second phase (120~180 min). And the data in our study just reflected the secretion situation at this period. [2]. Detection methods. The total GLP-1 (all forms including intact GLP-1 and the degraded, non-insulinotropic form GLP-1) concentrations in serum during OGTT were detected in this study. The impaired GLP-1 release phenomenon was in accordance with most previous published reports, which were similarly measured total GLP-1 levels after OGTT 6, 20.
The results of this study also suggested that the total GLP-1 levels (at each time point) and the 2hGLP-1 increments after OGTT were correlated to β cell function, IR degree and IS state. Furthermore, there was correlation between impaired GLP-1 release and risk factors of IR aggravation (or IS decrement), such as decreased HDL-C, increased TG, boosted SBP and heavy weight. The subjects with IFG+IGT and NDDM obviously had impaired insulin secretion, reduced IS and aggravated IR. Previous research suggested that the decrement of GLP-1 secretion might be related to IR or its risk factors, too 18, 21. It is assumed that the earlier IR occurs, the more severe impaired GLP-1 secretion may appear. Meanwhile, the decreased GLP-1 release may induce the dysfunction of β cell, exacerbation of IR and decrease in IS. Subsequently, this may form a vicious circle which could constantly aggravate the glucose metabolism disorder. And the consequence, which demonstrated that the GLP-1 receptor agonists treatment could ameliorate both β cell function 28, 29 and IS 30, 31, was consistent with our findings.
There were no detectable associations between the GLP-1 levels and ages, BMI or waist circumference as previous studies 17, 18. It is possible that these general characteristics in our patients did not reach the sufficient ranges to influence GLP-1 secretion. And the selections of various statistical methods may lead to the diversities, too. But the relevance between GLP-1 secretion and the genders was in conformity with the previous reports 17, 18.
The presenting study was lack of the information that could directly reflect the early phase of GLP-1 release since most subjects rejected to get blood collection at 15, 30 and 60 min during their OGTTs. This was one limitation of this study. Another limitation is that the mixed meal tolerance tests for the evaluation of GLP-1 were not chosen for the study.
In summary, the total FGLP-1 levels, which may be concerned with the basal insulin secretion, were similar in participants with isolated IFG and isolated IGT. These were also observed in the total 2hGLP-1 levels during a 75g oral glucose load. However, on the basis of the large cohort of NGT, PD (including all categories) and NDDM without any hypoglycemic therapy, this study suggested that the individuals with IFG+IGT and NDDM had the significant GLP-1 secretion impairment, who were in more severe hyperglycemic conditions than those with isolated IFG or IGT. Furthermore, the increment of 2hGLP-1 (after the oral glucose stimulation) was reduced in isolated IGT subjects, and which were reduced even more obviously in individuals with IFG+IGT and NDDM. Therefore, our results provide valuable information on the preventions and treatments options for Chinese patients with type 2 diabetes mellitus.
Acknowledgments
This work was supported by West China Hospital of Sichuan University. The authors gratefully acknowledge the guidance by Professor Zhang Juying and Vice-professor Zhang Qiang in the Department of Health Statistics, West China Public Health College of Sichuan University. And finally, we appreciated the Fifth People's Hospital of Chengdu, too.
Abbreviations
- GLP-1
glucagon-like peptide-1
- OGTT(s)
oral glucose tolerance test(s)
- ∆GLP-1
the increment in 2hGLP-1
- PG
plasma glucose
- INS
insulin
- F
fasting
- AUC
area under the curve
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- TG
triglyceride
- TC
total cholesterol
- HDL-C
high-density lipoprotein cholesterol
- PD
pre-diabetes
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- IFG+IGT
IFG combined IGT
- NDDM
newly-diagnosed diabetes mellitus
- DM
diabetes mellitus
- T2DM
type 2 diabetes mellitus
- NGT
normal glucose tolerance
- IR
insulin resistance
- IS
insulin sensitivity
- HOMA-β
the homeostasis model assessment of β cell function
- HOMA-IR
the homeostasis model assessment of IR
- ADA
American Diabetes Association
- RIA
radioimmunoassay
- ELISA
enzyme-linked immunosorbent assay
- GRP
gastrin-releasing peptide
- GIP
glucose-dependent insulinotropic peptide.
Biographies
Dr. Nanwei Tong is a professor of endocrinology at West China Hospital of Sichuan University. He has coauthored many publications in lots of magazines and monographs. The current research interests in Professor Tong's group includes: (1) Mechanism of diabetes mellitus; (2) The current situation of metabolic syndrome in West China; (3) Multiple effects of PPAR-δ.
Fang Zhang obtained her bachelor's degree from Chongqing Medical University in 2009. She is currently a MD student under the supervision of Prof. Nanwei Tong. Her research is centered on the mechanism of diabetes mellitus.
References
- 1.Kieffer JT, Habener FJ. The Glucagon-Like Peptides. Endocr Rev. 1999;20(6):876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 2.Yang H, Egan MJ, Wang Y. et al. GLP-1 action in L6 myotubes is via a receptor different from the pancreatic GLP-1 receptor. Am J Physiol-Cell Ph. 1998;275:C675– C683. doi: 10.1152/ajpcell.1998.275.3.C675. [DOI] [PubMed] [Google Scholar]
- 3.Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol-Endoc M. 2004;287:E199– E206. doi: 10.1152/ajpendo.00545.2003. [DOI] [PubMed] [Google Scholar]
- 4.Vahl T, Alessio DD. Enteroinsular signaling: perspectives on the role of the gastrointestinal hormones glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide in normal and abnormal glucose metabolism. Curr Opin Clinl Nutr. 2003;6:461–468. doi: 10.1097/01.mco.0000078991.96795.84. [DOI] [PubMed] [Google Scholar]
- 5.Toft-Nielsen BM, Damholt BM, Madsbad S. et al. Determinants of the Impaired Secretion of Glucagon-Like Peptide-1 in Type 2 Diabetic patients. J Clin Endocr Metab. 2001;86:717–723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 6.Laakso M, Zilinskaite J, Hansen T. et al. Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia. 2008;1:502–511. doi: 10.1007/s00125-007-0899-2. [DOI] [PubMed] [Google Scholar]
- 7.Vaag AA, Holst JJ, Volund A. et al. Gut incretin hormones in identical twins discordant for non-insulin-dependent diabetes mellitus (NIDDM)—evidence for decreased glucagon-like peptide 1 secretion during oral glucose ingestion in NIDDM twins. Eur J Endocrinol. 1996;135:425–432. doi: 10.1530/eje.0.1350425. [DOI] [PubMed] [Google Scholar]
- 8.Tripathy D, Carlsson M, Almgren P. et al. Insulin Secretion and Insulin Sensitivity in Relation to Glucose Tolerance Lessons from the Botnia Study. Diabetes. 2000;49:975–980. doi: 10.2337/diabetes.49.6.975. [DOI] [PubMed] [Google Scholar]
- 9.Hanefeld M, Koehler C, Fuecker K. et al. Insulin Secretion and Insulin Sensitivity Pattern Is Different in Isolated Impaired Glucose Tolerance and Impaired Fasting Glucose. The Risk Factor in Impaired Glucose Tolerance for atherosclerosis and Diabetes Study. Diabetes Care. 2003;26:868–874. doi: 10.2337/diacare.26.3.868. [DOI] [PubMed] [Google Scholar]
- 10.Vilsbøll T, Krarup T, Deacon FC. et al. Reduced Postprandial Concentrations of Intact Biologically Active Glucagon-Like Peptide 1 in Type 2 Diabetic Patients. Diabetes. 2001;50:609–613. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 11.Rask E, Olsson T, So¨ derberg S. et al. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care. 2001;4(9):1640–1645. doi: 10.2337/diacare.24.9.1640. [DOI] [PubMed] [Google Scholar]
- 12.Rask E, Olsson T, So¨ derberg S. et al. Insulin Secretion and Incretin Hormones After Oral Glucose in Non-obese Subjects With Impaired Glucose Tolerance. Metabolism. 2004;53(5):624–631. doi: 10.1016/j.metabol.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Muscelli E, Mari A, Natali A. et al. Impact of incretin hormones on b-cell function in subjects with normal or impaired glucose tolerance. Am J Physiol-Endoc M. 2006;91:E1144– E1150. doi: 10.1152/ajpendo.00571.2005. [DOI] [PubMed] [Google Scholar]
- 14.Lugari R, Dei CA, Ugolotti D. et al. Evidence for Early Impairment of Glucagon-Like Peptide 1-Induced Insulin Secretion in Human Type 2 (Non Insulin-Dependent) Diabetes. Horm Metab Res. 2002;34:150–154. doi: 10.1055/s-2002-23199. [DOI] [PubMed] [Google Scholar]
- 15.Vilsbøll T, Agersø H, Krarup T. et al. Similar Elimination Rates of Glucagon-Like Peptide-1 in Obese Type 2 Diabetic Patients and Healthy Subjects. J Clin Endocr Metab. 2003;8:220–224. doi: 10.1210/jc.2002-021053. [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Yabe D, Nohtomi K. et al. Intact glucagon-like peptide-1 levels are not decreased in Japanese patients with type 2 diabetes. Endocr J. 2010;7(2):119–126. doi: 10.1507/endocrj.k09e-269. [DOI] [PubMed] [Google Scholar]
- 17.Vollmer K, Holst JJ, Baller B. et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678–687. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]
- 18.Nauck AM, Vardarli I, Deacon FC. et al. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia. 2011;54:10–18. doi: 10.1007/s00125-010-1896-4. [DOI] [PubMed] [Google Scholar]
- 19.Nathanson D, Zethelius B, Berne C. et al. Reduced plasma levels of glucagon-like peptide-1 in elderly men are associated with impaired glucose tolerance but not with coronary heart disease. Diabetologia. 2010;53:277–280. doi: 10.1007/s00125-009-1596-0. [DOI] [PubMed] [Google Scholar]
- 20.Yabe D, Kuroe A, Lee S. et al. Little enhancement of meal-induced glucagon-like peptide 1 secretion in Japanese: Comparison of type 2 diabetes patients and healthy controls. Journal of Diabetes Investigation. 2010;1:56–59. doi: 10.1111/j.2040-1124.2010.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozawa J, Okita K, Imagawa A. et al. Similar incretin secretion in obese and non-obese Japanese subjects with type 2 diabetes. Biochem Bioph Res Co. 2010;393:410–413. doi: 10.1016/j.bbrc.2010.01.134. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes care. 2006;29(1):543–548. [Google Scholar]
- 23.Matthews RD, Hosker RJ, Rudenski SA. et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;8:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Gutt M, Davis LC, Spitzer BS. et al. Validation of the insulin sensitivity index (ISI0,120): comparison with other measures. Diabetes Res Clin Pr. 2000;7:177–184. doi: 10.1016/s0168-8227(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda M, Defronzo AR. Insulin Sensitivity Indices Obtained From Oral Glucose Tolerance Testing. Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;2:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 26.Reimann F, Gribble MF. Glucose-Sensing in Glucagon-Like Peptide-1-Secreting Cells. Diabetes. 2002;51:2757–2763. doi: 10.2337/diabetes.51.9.2757. [DOI] [PubMed] [Google Scholar]
- 27.Zinman B, Buse BJ, Lewin A. et al. Efficacy and safety of the human GLP-1 analog liraglutide in combination with metformin and TZD in patients with type 2 diabetes mellitus (LEAD-4 Met+TZD) Diabetes care. 2009;32:1224–1230. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van-Raalte DH, van-Genugten RE, Linssen MM. et al. Glucagon-like peptide-1 receptor agonist treatment prevents glucocorticoid-induced glucose intolerance and islet-cell dysfunction in humans. Diabetes Care. 2011;4(2):412–417. doi: 10.2337/dc10-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wajcberg E, Amarah A. Liraglutide in the management of type 2 diabetes. Drug Design, Development and Therapy. 2010;4:279–290. doi: 10.2147/DDDT.S10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang AM, Jakobsen G, Sturis J. et al. The GLP-1 derivative NN2211 restores beta-cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes. 2003;52(7):1786–1791. doi: 10.2337/diabetes.52.7.1786. [DOI] [PubMed] [Google Scholar]
- 31.Kjems LL, Holst JJ, Vølund A. et al. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52(2):380–386. doi: 10.2337/diabetes.52.2.380. [DOI] [PubMed] [Google Scholar]


