Abstract
An important feature of abdominal aortic aneurysm (AAA) is the destruction of vessel wall, especially elastin and collagen. Besides matrix metalloproteinases, cathepsins are the most potent elastolytic enzymes. The expression of cathepsins with known elastolytic and collagenolytic activities in the individual cells within AAA has not yet been determined. The vessel wall of 32 AAA patients and 10 organ donors was analysed by immunohistochemistry for expression of cathepsins B, D, K, L and S, and cystatin C in all cells localized within AAA. Luminal endothelial cells (ECs) of AAA were positive for cathepsin D and partially for cathepsins B, K and S. Endothelial cells of the neovessels and smooth muscle cells in the media were positive for all cathepsins tested, especially for cathepsin B. In the inflammatory infiltrate all cathepsins were expressed in the following pattern: B > D = S > K = L. Macrophages showed the highest staining intensity for all cathepsins. Furthermore, weak overall expression of cystatin C was observed in all the cells localized in the AAA with the exception of the ECs. There is markedly increased expression of the various cathepsins within the AAA wall compared to healthy aorta. Our data are broadly consistent with a role for cathepsins in AAA; and demonstrate expression of cathepsins D, B and S in phagocytic cells in the inflammatory infiltrate; and also may reveal a role for cathepsin B in lymphocytes.
Keywords: abdominal aortic aneurysm, cathepsins, cystatin C, proteolytic degradation
Abdominal aortic aneurysm (AAA) with its high prevalence (5–8%), and mortality of more than 90% in the event of rupture, is a common disease burden for both men and women over 65 years of age (Kniemeyer et al. 2000; Lindholt & Norman 2011). The risk of rupture of individual AAAs depends upon the stability of the vessel wall, defined by the structural integrity of extracellular matrix (ECM), especially elastin and collagen. During the development of AAA substantial remodelling of connective tissue within the aortic wall takes place and this contributes to the pathogenesis (Michel et al. 2011). Expression of a plethora of proteases, in particular matrix metalloproteinases (MMPs), has already been associated with the degradation of structural proteins and consequently the instability of the aortic vessel wall (Galis et al. 1994; Thompson 2003; Keeling et al. 2005).
High elastolytic activity is a well-known feature of the pathogenesis of AAA leading to a continuous loss of elasticity, dilation and development of aneurysm. Apart from MMPs able to degrade elastin, cathepsins, such as cysteine proteases B, K, L and S, and aspartate protease D, are described as the most potent elastolytic enzymes (Chapman et al. 1997; Sukhova & Shi 2006). In addition, cathepsins K, L and S possess collagenolytic activity against type I collagen (Sukhova & Shi 2006; Zavasnik-Bergant & Turk 2006). The cathepsin family of proteases is typically localized within lysosomes and endosomes degrading undesired intracellular and endocytosed proteins. Recently, however, evidence has been provided to suggest that cathepsins can also act extracellularly and are involved in the remodelling in ECM (Punturieri et al. 2000; Sukhova & Shi 2006; Zavasnik-Bergant & Turk 2006). Participation of cathepsins has been assumed in the progression of atherosclerosis and neovascularization (Liu et al. 2004). Furthermore, some cathepsins were also linked with the development and pathogenesis of AAA (Sukhova & Shi 2006; Abisi et al. 2007).
However, thus far expression of cathepsins and their contribution to the elastolytic and collagenolytic activities in the individual cells within AAA has not yet been analysed. Recently, we performed a detailed histological analysis of the expression of MMPs within AAA in association with neovascularization and inflammatory infiltrates (Reeps et al. 2009). The aim of the present study was to document a similar detailed histopathological analysis of cathepsins B, K, L, S and D, and cystatin C in specimens of AAA retrieved during operations.
Materials and methods
Patients and tissue collection
Tissue samples were obtained from 32 patients (27 men, five women) with non-ruptured AAA during conventional surgical repair. All tissue specimens were collected in a standardized manner from the anterior sac of the infrarenal abdominal aorta. Average age was 68.2 ± 10.1 years (range, 41–88 years), and average maximum AAA diameter size determined by CT scan was 6.7 ± 1.8 cm. Aortic tissue of 10 healthy subjects was received from the Institute of Forensic Medicine (eight men; two women; average age, 52.9 ± 12.4 years). All tissue samples were fixed in formalin followed by paraffin embedding and stored appropriately for further histological and immunohistochemical analyses. The study was conducted with the approval of our institutional review board, and informed consent was obtained from all patients prior to their participation in the study. The investigation conforms with the principles outlined in the Declaration of Helsinki for the use of human tissue or subjects (World Medical Association Declaration of Helsinki 1997).
Immunohistochemistry
Histological analyses and immunohistochemistry (IHC) were performed on representative sections (2–3 μm) of aortic tissue samples as described previously (Reeps et al. 2009). Samples were routinely stained with haematoxylin–eosin (HE) and Elastica van Gieson (EvG) to assess tissue morphology, cellular composition, degree of infiltration of inflammatory cells and the content of elastin and collagen fibres. The following antibodies (Abs) were used: vascular smooth muscle cells (SMCs) (anti-smooth muscle actin, mouse monoclonal, clone HHF35, dilution 1:200; Dako, Glostrup, Denmark); endothelial cells [anti-von Willebrand factor (vWF), mouse monoclonal, clone F8/86, dilution 1:500; Dako]; macrophages/monocytes (anti-CD68, mouse monoclonal, clone KP1, dilution 1:2000; Dako); T lymphocytes (anti-CD3, rabbit polyclonal, dilution 1:400; Dako) and B lymphocytes (anti-CD20, mouse monoclonal, clone L26, dilution 1:500; Dako). For the detection of the expression of cathepsins and cystatin C, the following Abs were applied: cathepsin B [mouse monoclonal (CB131) to cathepsin B, dilution 1:50; Abcam, Cambridge, UK]; cathepsin D [mouse monoclonal (CTD-19), dilution 1:16000; Abcam]; cathepsin K [mouse monoclonal (3F9), dilution 1:200; Abcam]; cathepsin L [mouse monoclonal (13C2), dilution 1:30; Abcam]; cathepsin S (goat polyclonal, dilution 1:25; Abcam) and cystatin C (rabbit polyclonal, dilution 1:200; Abcam). Following primary antibody incubation, vWF and smooth muscle actin were visualized using the APAAP ChemMate Detection Kit (rabbit anti-mouse secondary Ab; Dako) according to the manufacturer’s instructions. All other primary Abs were detected and visualized by peroxidase/3,3′-diaminobenzidine ChemMate Detection Kit (biotinylated goat anti-mouse/anti-rabbit secondary Ab; Dako).
Consecutive staining
For the determination of cathepsin expression in the individual cells within the AAA wall, consecutive slides from each specimen were prepared and incubated with the appropriate antibodies. In all cases, one slide was stained with an antibody to detect the specific cell type (vWF for ECs, smooth muscle actin for SMCs, CD68 for monocytes/macrophages, CD3 for T lymphocytes and CD20 for B lymphocytes), and a consecutive slide was stained with antibody against the various cathepsins and cystatin C.
Histological evaluation
The semi-quantitative analysis was performed as described previously (Reeps et al. 2009). Briefly, the intensity of staining was first evaluated in all specimens to validate the overall staining intensities. Semi-quantitative graduation distinguished between various intensities from (−) no staining to (+++) highly intensive staining; (−/+) means variation of the intensity of staining between different samples and also within the same specimen.
Results
Structural composition of abdominal aortic aneurysm
In accordance with the former findings (Reeps et al. 2009), most aneurysmal specimens contained atherosclerotic areas in the tunica intima with surrounding macrophages and foam cells. Substantial neovessels were observed mainly in the tunica media and partially in the intimal layer and were predominantly associated with inflammatory infiltrates. In contrast, in control aortic tissue samples, individual microvessels were found only at the border between media and adventitia and within adventitia.
Expression of cathepsins in luminal endothelial cells and medial smooth muscle cells in a healthy aorta
Immunohistochemical staining of cathepsins and cystatin C in a healthy aortic tissue is summarized in Figure 1. For each cathepsin and cystatin C, the upper part shows the staining of ECs, and the lower part, the staining of SMCs. All cathepsins tested in our study were expressed in luminal ECs of the aortic wall. The staining was positive for all five cathepsins without any apparent differences (see Table 1). However, not all ECs of the entire AAA samples were positive. The staining of cystatin C was markedly weaker and some ECs were negative.
Figure 1.
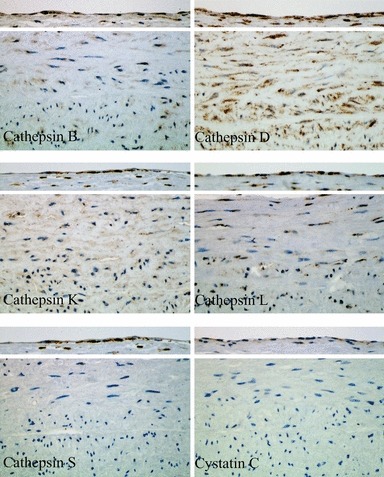
Immunohistochemical staining of cathepsins and cystatin C in luminal endothelial cells and smooth muscle cells of the media within human healthy aorta.
Table 1.
Semi-quantitative analysis of cathepsins and cystatin C in different cells located within AAA and healthy aorta
| Healthy aorta | AAA tissue specimens | Neovessels | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mature | |||||||||
| ECs of lumen | SMCs of media | ECs of lumen | SMCs of media | Macrophages | Infiltrates | Immature EC | EC | SMC | |
| Cathepsin B | + | +/− | +/− | + | ++/+++ | ++/+++ | ++ | ++ | + |
| Cathepsin D | + | ++ | + | +/++ | +++ | +/++ | +/++ | +/++ | + |
| Cathepsin K | + | +/− | +/− | + | ++ | +/− | − to +/− | − to +/− | − to +/− |
| Cathepsin L | + | +/− | − | + | +/++ | +/− | + | + | − to +/− |
| Cathepsin S | + | − | +/− | + | ++/+++ | +/++ | + | + | − to +/− |
| Cystatin C | +/− | − | − | + | ++ | − to +/− | + | + | − to +/− |
AAA, abdominal aortic aneurysm; ECs, endothelial cells; SMCs, smooth muscle cells.
Semi-quantitative evaluation was performed as follows: (−) no staining to (+++) highly intensive staining; (−/+) means variation of the intensity of staining between different samples within the same specimen.
In contrast, SMCs of the media showed low expression of most cathepsins. The only intense positive staining was observed for cathepsin D. The expression of cathepsins B, K and L was evident; however, all SMCs were not positive. No expression/staining was observed for cathepsin S and cystatin C in SMCs of the healthy aorta.
Expression of cathepsins in smooth muscle cells within abdominal aortic aneurysm
The results of immunohistochemical staining of cathepsins and cystatin C in SMCs are shown in Figure 2. Smooth muscle cells showed constant expression/intensity of staining of all cathepsins analysed and also cystatin C. Only the staining of cathepsin D was increased when compared with the other cathepsins. It is noted that we have not performed EC staining in AAA, because most of our specimens did not contain any endothelial layer, and the ECs were sporadic and seemed often to be damaged.
Figure 2.
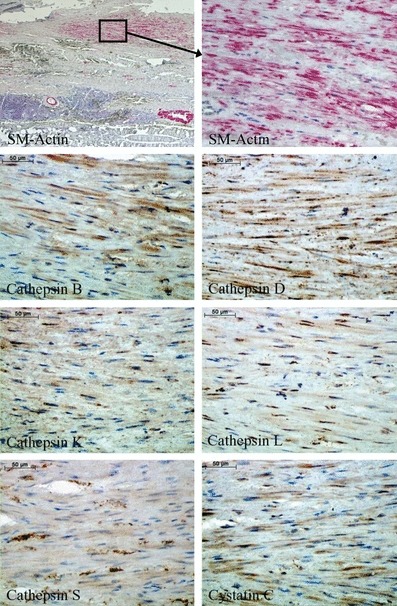
Immunohistochemical staining of cathepsins and cystatin C in smooth muscle cells within human abdominal aortic aneurysm specimens.
Expression of cathepsins in macrophages and cells with phagocytic activity within abdominal aortic aneurysm
Expression analysis of cathepsins and cystatin C on the basis of immunohistochemistry in CD68-positive cells is demonstrated in Figure 3. These cells within AAA were strongly positive for all cathepsins and cystatin C tested in our study. They demonstrated the highest intensity of staining of all cells investigated, especially for cathepsins B, D and S.
Figure 3.
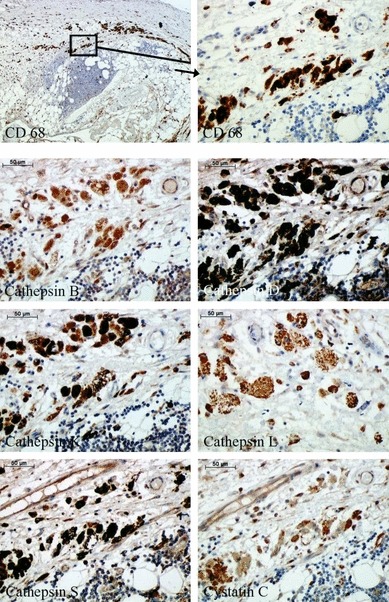
Immunohistochemical staining of cathepsins and cystatin C in macrophages and macrophage-derived foam cells within human abdominal aortic aneurysm specimens.
Expression of cathepsins in inflammatory cells within abdominal aortic aneurysm
Immunohistochemical staining of cathepsins and cystatin C in inflammatory cells is shown in Figure 4. The intensity of staining in the inflammatory cells (CD3 and CD20 positive) was the strongest for cathepsin B followed by cathepsins D and S. Staining of cathepsins K and L was markedly weaker. Expression of cystatin C was not detected. No differences in the intensity of staining were observed between B- (CD20) and T lymphocytes (CD3).
Figure 4.
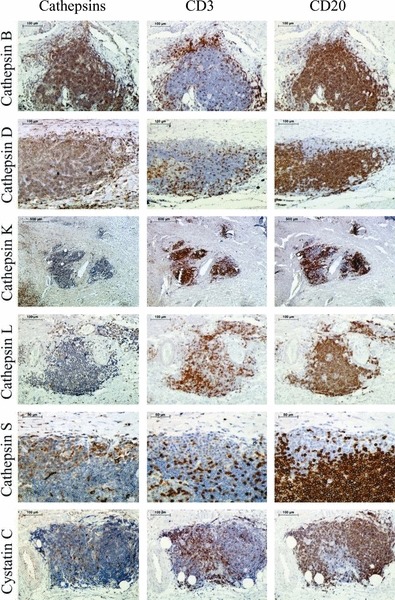
Immunohistochemical staining of cathepsins and cystatin C in inflammatory cells within abdominal aortic aneurysm. The first column shows the cathepsin staining; the second, the staining of T lymphocytes (CD3); and the third, the staining of B lymphocytes (CD20).
Expression of cathepsins in neovessels within abdominal aortic aneurysm
Finally, expression of cathepsins and cystatin C was analysed in different kinds of neovessels containing either only endothelial layer (Figure 5) or surrounded also by SMCs (Figure 6) within the aneurysmal lesions (see Table 1). Endothelial cells of the neovessels showed strong positive staining for cathepsins B and D, weak staining for cathepsins L and S, and cystatin C, and negligible reaction for cathepsin K. Regarding SMC staining surrounding neovessels, relevant expression was observed only for cathepsins B and D.
Figure 5.
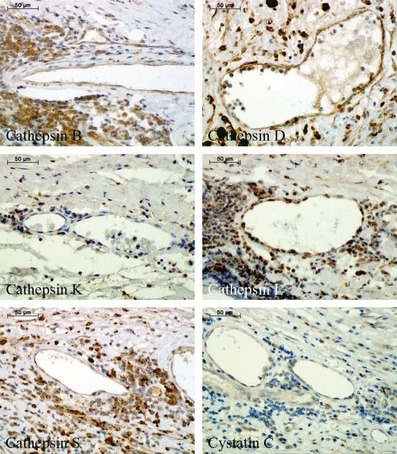
Immunohistochemical staining of cathepsins and cystatin C in the immature neovessels (containing only endothelial cells without the stabilizing layer of smooth muscle cells) within abdominal aortic aneurysm specimens.
Figure 6.
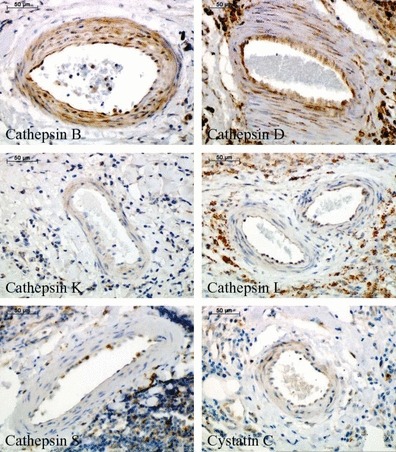
Immunohistochemical staining of cathepsins and cystatin C in the mature neovessels (containing both endothelial cells and smooth muscle cells) within abdominal aortic aneurysm specimens.
Discussion
Previous studies have shown that cathepsins may play a decisive role in the pathogenesis of AAA (Patel et al. 1996; Chapman et al. 1997; Liu et al. 2004; Sukhova & Shi 2006; Zavasnik-Bergant & Turk 2006; Abdul-Hussien et al. 2007). The expression and cellular sources of cathepsin release in AAA specimens had, however, not yet been analysed. So far, only cathepsins B and L and their inhibitor cystatin C have been examined using immunohistological methods (Gacko et al. 1999; Shi et al. 1999; Liu et al. 2006). These previous studies focussed on macrophages, SMCs and ECs. Our study here explored a range of cathepsins with known elastolytic and collagenolytic activities and their endogenous inhibitor cystatin C in all the cells found in the AAA vessel wall including lumenal ECs, SMCs in the media, cells with phagocytic activity [monocytes, macrophages, and macrophage-derived foam cells, lymphocytes (T- and B cells)] as inflammatory infiltrates, as well as the ECs and SMCs of mature and immature neovessels. Moreover, cathepsin expression in AAA was compared to their expression in ECs and SMCs of healthy aortas.
In our immunohistological examination CD68-positive cells demonstrated the highest expression of all cathepsins analysed in our study. The expression of cystatin C was also the most abundant in these cells. It has been reported previously that macrophages express intracellular cathepsins B, K, L and S (Reddy et al. 1995; Punturieri et al. 2000). However, recent data showed that cathepsins can also be secreted in the pericellular space and contribute to the degradation of ECM (Reddy et al. 1995). Hence inhibition of cathepsins L and S resulted in almost complete deregulation of the elastolytic activity of macrophages. Thus, it can be hypothesized that the high expression indicated by the high staining intensity of cathepsins in CD68-positive cells, such as macrophages, monocytes, macrophage-derived foam cells and other phagocytic cells, which are common in AAA, may contribute considerably to the destabilization of the aortic wall because of their high collagenolytic and elastolytic activities.
Inflammatory cells such as T- and B lymphocytes are also involved in the pathogenesis of atherosclerosis and AAA (Shimizu et al. 2006; Reeps et al. 2009). However, their active role in the progression of AAA remains unclear. Our group showed recently that inflammatory cells can express a plethora of MMPs, thus directly contributing to the destabilization of the vessel wall (Reeps et al. 2009). Furthermore, T lymphocytes are able to induce SMC apoptosis by the secretion of various cytokines (Henderson et al. 1999). Our current results show that lymphocytes within AAA are able to express cathepsins as well, especially cathepsins B, D and S. Furthermore, cathepsins might be involved in lymphocyte transmigration into the vascular wall by ECM degradation increasing the state of inflammation in AAA.
Apart from elastic and collagen fibres, SMCs are essential for the integrity and stability of the aortic wall. During progression of AAA, a continuous loss of SMC is observed (López-Candales et al. 1997). However, these cells also produce proteases like MMPs or cathepsins (Abdul-Hussien et al. 2007; Reeps et al. 2009). Our immunohistochemical analysis revealed that SMCs within AAA also express cathepsins as well their inhibitor cystatin C. In contrast with the exception of cathepsin D, no expression of other cathepsins tested in our study was detected in SMCs of the healthy aortas. This observation supports the upregulation of the cathepsin expression in SMCs during AAA pathogenesis. Cathepsins expressed by SMCs could play different roles in the development of AAA. First, release of cathepsins into the pericellular space might contribute to the further degradation of ECM components (Sukhova et al. 1998). Second, increased expression of cathepsins may lead to the apoptosis of SMCs, thus further destabilizing the aortic vessel wall (Chwieralski et al. 2006).
Advanced neovascularization is another essential factor characterizing AAA. The invasion of new vessels requires degradation of the perivascular ECM, where both MMPs and cathepsins play an important role (Ingber & Folkman 1989; Sukhova et al. 1998; Carmeliet 2000; Reeps et al. 2009). The cathepsins tested in our immunohistological study were expressed by ECs of mature and immature neovessels (Reeps et al. 2009). Thus our data confirmed the results of former studies assuming a crucial role of some cathepsins during wound healing and different neoplasms (Shi et al. 2003; Kruszewski et al. 2004; van Hinsbergh et al. 2006). Although cathepsin expression was markedly increased in neovessels, the ECs of the lumen were also positive for cathepsin staining. These results were observed in AAA as well as in non-aneurysmatic control aortas. Interestingly, the expression of cathepsins in the healthy aorta seemed to be more intensive than in AAA. This phenomenon could be explained by the fact that in AAA the endothelial cells are frequently affected and do not perform their normal function. These effects were also observed earlier in the context of MMPs (Reeps et al. 2009).
Study limitations. Despite using the carefully optimized antibodies for cathepsin staining, our statements regarding the different expression patterns of cathepsins are limited by the quality of the individual histological specimens and the sensitivity of the immunohistochemistry. Furthermore, immunohistochemistry cannot differentiate between cathepsins expressed by cells or cathepsins endocytosed from the extracellular space (Cavallo-Medved et al. 2009). In addition, cathepsins are often localized on cell membranes or secreted and localized in endosomal or lysosomal vesicles, suggesting that their enzymatic substrates and functions might change with their localization. Recently, co-localisation of active cathepsins with integrin ανβ3 (as a receptor on the vascular SMC surface) has been demonstrated suggesting that they may play an important role in SMC-mediated ECM degradation (Cheng et al. 2006).
In summary, this study provides a detailed histological evaluation of cysteine cathepsins B, K, L and S, their endogenous inhibitor cystatin C and the aspartic protease cathepsin D. This was the first comprehensive analysis of cathepsin expression in all cells of AAA wall, including luminal ECs, SMCs of the media, macrophages, lymphocytes and ECs, and SMCs of the mature and immature neovessels. Our results demonstrate markedly increased expression of various cathepsins within the AAA wall compared to healthy aorta. Thus, our data are broadly consistent with a possible role of cathepsins in AAA, favouring the expression of cathepsins D, B and S in phagocytic cells, and may reveal a new role for lymphocyte cathepsin B. It is noteworthy that cathepsin expression is not confined to the phagocytic cells since lymphocytes within AAA appear not only to stimulate the expression of cathepsins by cytokine release, but also express a variety of cathepsins autonomously themselves, has been described previously for macrophages and SMCs.
Acknowledgments
The authors would like to thank in particular Renate Hegenloh for her excellent technical support in the histological and immunohistochemical staining.
Funding
There was no special funding. This work was supported by clinic internal funds.
Declaration of interest
The authors declare there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Abdul-Hussien H, Soekhoe RG, Weber E, et al. Collagen degradation in the abdominal aneurysm: a conspiracy of matrix metalloproteinase and cysteine collagenases. Am. J. Pathol. 2007;170:809–817. doi: 10.2353/ajpath.2007.060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abisi S, Burnand KG, Waltham M, et al. Cysteine protease activity in the wall of abdominal aortic aneurysms. J. Vasc. Surg. 2007;46:1260–1266. doi: 10.1016/j.jvs.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Cavallo-Medved D, Rudy D, Blum G, et al. Live-cell imaging demonstrates extracellular matrix degradation in association with active cathepsin B in caveolae of endothelial cells during tube formation. Exp. Cell. Res. 2009;315:1234–1246. doi: 10.1016/j.yexcr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman HA, Riese RJ, Shi GP. Emerging roles for cysteine proteases in human biology. Annu. Rev. Physiol. 1997;59:63–88. doi: 10.1146/annurev.physiol.59.1.63. [DOI] [PubMed] [Google Scholar]
- Cheng XW, Kuzuya M, Nakamura K, et al. Localization of cysteine protease, cathepsin S, to the surface of vascular smooth muscle cells by association with integrin αnuβ3. Am. J. Pathol. 2006;168:685–694. doi: 10.2353/ajpath.2006.050295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwieralski CE, Welte T, Bühling F. Cathepsin-regulated apoptosis. Apoptosis. 2006;11:143–149. doi: 10.1007/s10495-006-3486-y. [DOI] [PubMed] [Google Scholar]
- Gacko M, Chyczewski L, Chrostek L. Distribution, activity and concentration of cathepsin B and cystatin C in the wall of aortic aneurysm. Pol. J. Pathol. 1999;50:83–86. [PubMed] [Google Scholar]
- Galis ZS, Sukhova GK, Lark MW, et al. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson EL, Geng YJ, Sukhova GK, et al. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99:96–104. doi: 10.1161/01.cir.99.1.96. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh VWM, Engelse MA, Quax PHA. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2006;26:716–728. doi: 10.1161/01.ATV.0000209518.58252.17. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Folkman J. How does extracellular matrix control capillary morphogenesis? Cell. 1989;58:803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- Keeling WB, Armstrong PA, Stone PA, et al. An overview of matrix metalloproteinases in the pathogenesis and treatment of abdominal aortic aneurysms. Vasc. Endovascular Surg. 2005;39:457–464. doi: 10.1177/153857440503900601. [DOI] [PubMed] [Google Scholar]
- Kniemeyer HW, Kessler T, Reber PU, et al. Treatment of ruptured abdominal aortic aneurysm, a permanent challenge or a waste of resources? Prediction of outcome using a multi-organ-dysfunction score. Eur. J. Vasc. Endovasc. Surg. 2000;19:190–196. doi: 10.1053/ejvs.1999.0980. [DOI] [PubMed] [Google Scholar]
- Kruszewski WJ, Rzepko R, Wojtacki J, et al. Overexpression of cathepsin B correlates with angiogenesis in colon adenocarcinoma. Neoplasma. 2004;51:38–43. [PubMed] [Google Scholar]
- Lindholt JS, Norman PE. Meta-analysis of postoperative mortality after elective repair of abdominal aortic aneurysms detected by screening. Br. J. Surg. 2011;98:619–622. doi: 10.1002/bjs.7464. [DOI] [PubMed] [Google Scholar]
- Liu J, Sukhova GK, Sun JS, et al. Lysosomal cysteine proteases in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004;4:1359–2366. doi: 10.1161/01.ATV.0000134530.27208.41. [DOI] [PubMed] [Google Scholar]
- Liu J, Sukhova GK, Yang JT, et al. Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis. 2006;184:302–311. doi: 10.1016/j.atherosclerosis.2005.05.012. [DOI] [PubMed] [Google Scholar]
- López-Candales A, Holmes DR, Liao S, et al. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am. J. Pathol. 1997;150:993–1007. [PMC free article] [PubMed] [Google Scholar]
- Michel JB, Martin-Ventura JL, Egido J, et al. FAD EU consortium: novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc. Res. 2011;90:18–27. doi: 10.1093/cvr/cvq337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MI, Melrose J, Ghosh P, et al. Increased synthesis of matrix metalloproteinases by aortic smooth muscle cells is implicated in the etiopathogenesis of abdominal aortic aneurysms. J. Vasc. Surg. 1996;24:82–92. doi: 10.1016/s0741-5214(96)70148-9. [DOI] [PubMed] [Google Scholar]
- Punturieri A, Filippov S, Allen E, et al. Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K-deficient human macrophages. J. Exp. Med. 2000;192:789–799. doi: 10.1084/jem.192.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy VY, Zhang QY, Weiss SJ. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc. Natl Acad. Sci. USA. 1995;92:3849–3853. doi: 10.1073/pnas.92.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeps C, Pelisek J, Seidl S, et al. Inflammatory infiltrates and neovessels are relevant sources of MMPs in abdominal aortic aneurysm wall. Pathobiology. 2009;76:243–252. doi: 10.1159/000228900. [DOI] [PubMed] [Google Scholar]
- Shi GP, Sukhova GK, Grubb A, et al. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J. Clin. Invest. 1999;104:1191–1197. doi: 10.1172/JCI7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G, Sukhova GK, Kuzuya M, et al. Deficiency of the cysteine protease cathepsin S impairs microvessel growth. Circ. Res. 2003;92:493–500. doi: 10.1161/01.RES.0000060485.20318.96. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- Sukhova GK, Shi GP. Do cathepsins play a role in abdominal aortic aneurysm pathogenesis? Ann. N. Y. Acad. Sci. 2006;1085:161–169. doi: 10.1196/annals.1383.028. [DOI] [PubMed] [Google Scholar]
- Sukhova GK, Shi GP, Simon DI, et al. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J. Clin. Invest. 1998;102:576–583. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MM. Controlling the expansion of abdominal aortic aneurysms. Br. J. Surg. 2003;90:897–898. doi: 10.1002/bjs.4280. [DOI] [PubMed] [Google Scholar]
- World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. Cardiovasc. Res. 1997;35:2–3. [PubMed] [Google Scholar]
- Zavasnik-Bergant T, Turk B. Cysteine cathepsins in the immune response. Tissue Antigens. 2006;67:349–355. doi: 10.1111/j.1399-0039.2006.00585.x. [DOI] [PubMed] [Google Scholar]


