Abstract
Central nervous system (CNS) infections in ruminant livestock, such as listeriosis, are of major concern for veterinary and public health. To date, no host-specific in vitro models for ruminant CNS infections are available. Here, we established and evaluated the suitability of organotypic brain-slices of ruminant origin as in vitro model to study mechanisms of Listeria monocytogenes CNS infection. Ruminants are frequently affected by fatal listeric rhombencephalitis that closely resembles the same condition occurring in humans. Better insight into host–pathogen interactions in ruminants is therefore of interest, not only from a veterinary but also from a public health perspective. Brains were obtained at the slaughterhouse, and hippocampal and cerebellar brain-slices were cultured up to 49 days. Viability as well as the composition of cell populations was assessed weekly. Viable neurons, astrocytes, microglia and oligodendrocytes were observed up to 49 days in vitro. Slice cultures were infected with L. monocytogenes, and infection kinetics were monitored. Infected brain cells were identified by double immunofluorescence, and results were compared to natural cases of listeric rhombencephalitis. Similar to the natural infection, infected brain-slices showed focal replication of L. monocytogenes and bacteria were predominantly observed in microglia, but also in astrocytes, and associated with axons. These results demonstrate that organotypic brain-slice cultures of bovine origin survive for extended periods and can be infected easily with L. monocytogenes. Therefore, they are a suitable model to study aspects of host–pathogen interaction in listeric encephalitis and potentially in other neuroinfectious diseases.
Keywords: hippocampus, in vitro model, Listeria monocytogenes, organotypic brain-slice cultures, rhombencephalitis, ruminants
Infectious diseases of the central nervous system (CNS) in cattle and other ruminant livestock may have severe economic impacts and are in some cases zoonoses. Prominent examples are bovine spongiform encephalopathy (BSE) and listeriosis (Belay & Schonberger 2005; Oevermann et al. 2008). Such diseases pose a risk to public health, therefore causing an increasing demand for efficient disease control and food-safety measures. To optimize and further develop such measures, a profound understanding of the disease pathogenesis and host–pathogen interaction is required. Currently, research into these mechanisms largely depends on in vivo bioassays. However, experiments involving large animals are time-consuming, require appropriate animal facilities and may raise animal welfare concerns. Studies conducted in rodent disease models require less space, but the translation of results from these models to the situation in the natural host often remains difficult (van der Worp et al. 2010). This situation points to the need of host-specific in vitro systems that facilitate studies of neuroinfectious CNS diseases in ruminant livestock.
To date, there are very few in vitro models of ruminant CNS origin established, most of which are based on primary neural cell cultures originating from bovine foetuses (Peruffo et al. 2008). However, the availability of foetal bovine tissue is limited, and none of these systems reflects the intricate, three-dimensional structure of the brain and the complex interplay between different cell types present in CNS tissue.
Organotypic slices cultures are thin slices of organs maintained on a semipermeable membrane at a liquid–air interface and were initially established for brain tissues by Gähwiler 1981 and later modified by Stoppini et al. 1991. The main advantage of organotypic brain-slice cultures is the conservation of a three-dimensional tissue morphology (Gähwiler et al. 1997) over a time period of several weeks in a controlled environment. Therefore, organotypic brain-slice cultures are a widely used technique to study physiological, pharmacological and infectious processes in vitro (Stoppini et al. 2000; Gianinazzi et al. 2003, 2005; Mayer et al. 2005; Scheidegger et al. 2005; Falsig et al. 2008b).
The aim of this study was to establish and assess the suitability of bovine organotypic brain-slice cultures as a host-specific in vitro model for ruminant CNS diseases. We worked with slaughterhouse-derived brain tissue from young cattle and established a clean and easy tissue sampling method that is well incorporated in slaughterhouse procedures. Viability analysis and cell type compositions of bovine brain-slices as well as infection assays using Listeria monocytogenes as a model agent are shown. L. monocytogenes was chosen because of the striking similarity between human and ruminant rhombencephalitis (Antal et al. 2005; Oevermann et al. 2010b) and its impact on veterinary and public health. The pathogenesis of listeric rhombencephalitis is poorly understood, and therefore, a better insight into host–pathogen interactions in the ruminant disease would further our understanding of CNS listeriosis, also in humans.
Materials and methods
Transport buffer and culture media
Transport buffer consisted of Gey’s balanced salt solution (GBSS) (Falsig & Aguzzi 2008a) supplemented with 33.33 mM glucose (Sigma-Aldrich Co., Buchs, Switzerland, G8769) and 1 M kynurenic acid (Sigma-Aldrich Co., Buchs, Switzerland, K3375). Low-melting-point agar (2% (w/v), Invitrogen, Carlsbad, CA, USA, 16520100) was prepared in transport buffer, boiled in a microwave and cooled to 37 °C. Culture medium was prepared according to a protocol from Falsig et al. (Falsig & Aguzzi 2008a) with slight modifications; for a total of 100 ml culture medium, 46.6 ml MEM (Invitrogen, Carlsbad, CA, USA, 21575-022), 25 ml BME (Invitrogen, Carlsbad, CA, USA, 41010-026), 25 ml horse serum (Invitrogen, Carlsbad, CA, USA, 26050-088), 1 ml glutamax (Invitrogen, Carlsbad, CA, USA, 350 50-038, final concentration 2 mM), 1 ml penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA, 15140122, final concentration 100 U penicillin/100 μg streptomycin), 1 ml HEPES (final concentration 0.01 M) and 1.37 ml glucose 45% (Sigma-Aldrich Co., Buchs, Switzerland, G8769) were mixed, adjusted to pH 7.36 and sterile filtered. Finally, neural growth factor (NGF, N0513, Sigma-Aldrich Co., Buchs, Switzerland) was added to a final concentration of 1 nM.
Serum-free medium for infection experiments was made of 100 ml neurobasal medium (Invitrogen, Carlsbad, CA, USA, 21103-049), supplemented with 1 ml glutamax (Invitrogen, Carlsbad, CA, USA, 350 50-038, final concentration 2 mM), 1 ml HEPES (final concentration 0.01 M) and 2 ml B27 supplement (Invitrogen, Carlsbad, CA, USA, 17504-044).
Animals and CNS tissue sampling
Samples of brain stem, cerebellum and hippocampus were taken from six calves approximately half a year of age at a local slaughterhouse, where the animals were shot with a captive bolt pistol. Samples were removed as shown in Figure 1, cut into 1 × 1 cm pieces and submerged in ice cold transport buffer. Transport from the slaughterhouse to the laboratory was within 30 min.
Figure 1.
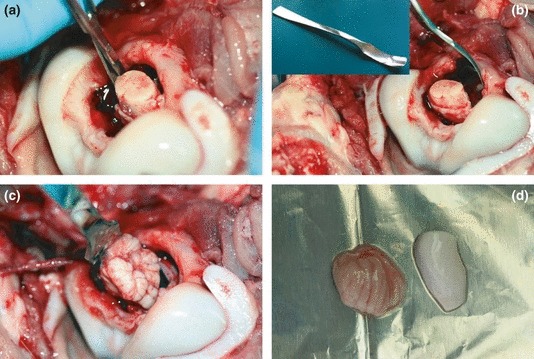
(a) First, the head was cut off from the animal directly after slaughter and cranial nerves were cut with fine sterile scissors through the foramen magnum. (b) In a multistep sampling procedure with several insertions of the spoon tool (inset) the caudal brainstem was removed. (c) Then, the cerebellum was removed. Once the whole cerebellum was extracted from the scull, access to the telencephalon including the hippocampus was possible. (d) Final samples of hippocampus (right) and cerebellum (left) are shown.
CNS tissue slicing
All tissue processing in the laboratory was carried out in a laminar-flow cabinet with previously sterilized instruments. First, samples in transport buffer were moved into a 100-mm petri dish and cut into pieces of 5 × 5 × 5 mm using two feather blades in a scissor-like manner. The tissue was then transferred to the buffer tray of a vibratome (Leica VT1000S, Wetzlar, Germany) containing fresh ice-cooled sterile transport buffer. The pia mater and meningeal blood vessels were removed using two Dumont forceps No 5 and the magnifying glass of the vibratome. Samples were then cast into low-melting-point agar blocks (Falsig & Aguzzi 2008a), which were glued to the stage top of the vibratome with cyanacrylat (UHU super-glue; UHU-GmbH, Bühl-Baden, Germany). Finally, the stage was mounted onto the vibratome, and slices of 350 μm thickness were cut. The cut brain-slices were freed of agar, transferred to 35-mm petri dishes and kept in fresh transport buffer at 4 °C for 1 h. In parallel, semipermeable Transwell membrane inserts (Corning, New York, NY, USA) were mounted on 6-well plates, which were supplied with 1 ml culture medium per well, and kept in an incubator at 37 °C and 5% (v/v) CO2 for 1 h. Slices were then plated onto the membrane inserts and cultured at 37 °C and 5% (v/v) CO2 up to 49 days, with culture medium changes three times per week.
Viability staining
Brain-slices were washed three times with PBS, and dead cells were stained with fixable-viability-stain 660 (eBioscience San Diego, CA, USA, 65-0864-14, 2 μl/ml, in transport buffer [devoid of kynurenic acid]) for 3 h at 37 °C and 5% CO2. To verify the suitability of the fixable-viability-stain 660 for dead cells in brain-slice cultures, parallel stains with PI (Noraberg et al. 1999) were performed and yielded comparable results (data not shown). Then, the whole slices were fixed in 4% paraformaldehyde (PFA) in PBS for at least 1 h. In a second stage, the slices were permeabilized with 0.5% Triton in PBS, and propidium iodide (PI, 2 μM for 3 h at 4 °C) was added to stain all nuclei. Standardized images of the whole slice culture were taken on the laser microscope using the 555 and 632 nm channels.
Immunofluorescence
Immunofluorescence was performed on 4.5-μm-thick cryosections after PFA fixation of brain-slices and mounted on glass slides according to standard procedures.
Antibodies specific for neurons (monoclonal anti-NeuN, MAB377, Millipore, Billerica, MA, USA, 1:200 or monoclonal anti-Neurofilament, M0762, Dako, Glostrup, Denmark, 1:100), astrocytes (polyclonal anti-GFAP Z0334, Dako, Glostrup, Denmark 1:1000 or monoclonal anti-GFAP Ab4648, Abcam, Cambridge, UK, 1:1000), oligodendrocytes (monoclonal anti-CNP, Ma1-22149, Thermo Scientific, Erembodegem, Belgium, 1:500), microglia (polyclonal anti-Iba-1, 019-19741, Osaka, Japan, 1:500 or monoclonal anti-CD68, M0718, Dako, Glostrup, Denmark 1:100) and L. monocytogenes (polyclonal anti-Listeria O serotypes 1 and 4, No223021, Difco, Sparks, MD, USA) were used. Cryosections were thawed at room temperature, washed three times with PBST (0.1% [v/v] Tween in PBS) and blocked with 5% (v/v) normal goat serum in PBST for 30 min. Primary antibodies were incubated for 1 h at room temperature except CD68, which was incubated for 24 h at 4 °C. Secondary antibodies (Alexa Fluor, Invitrogen, Carlsbad, CA, USA, A11001, A11008, A21422, A21428, 1:100) were incubated for 1 h at room temperature. Nuclei were stained with TOTO-3 (Invitrogen, Carlsbad, CA, USA, T3604, 1:700) for 30 min. The same antibodies and dilutions were used on paraffin sections of cases of natural listeric encephalitis. Protocols were modified to take into account the need for specific antigen retrieval methods (Listeria and CD 68 or Neurofilament: 0.5% [wt/vol] trypsin [Merck, Zug, Switzerland, F606567] and chymotrypsin [Merck, Zug, Switzerland] in TBS at 37 °C for 15 min; Listeria and CNP or GFAP: Target Retrieval solution [S2031, Dako, Glostrup, Denmark] at 99 °C for 20 min in a laboratory microwave).
Imaging
Confocal images were acquired on an Olympus Fluoview FV1000 confocal microscope (Olympus, Tokyo, Japan), equipped with 488, 555 and 632-nm laser channels. For viability analysis in whole organotypic brain-slices, inserts were removed from the wells and staged on top of a glass slide, which allowed for 20× magnifications in confocal images.
Cell viability analysis
For each experiment, the slices originated from the same animal and were analysed at nine different time points in culture: days 0, 3, 7, 14, 21, 28, 35, 42 and 49. One well per time point was stained according to our viability staining protocol. Per slice, five images of defined areas (a total of 0.6 mm2) were analysed in a standardized manner. The total number of nuclei (PI positive) and the number of dead cells (fixable-viability-stain 660 positive) were counted. Based on this, the percentage of viable cells was calculated. Statistical analysis was performed using the prism Software (version 5.03, GraphPad Software Inc., La Jolla, CA, USA).
From all time points, an additional haematoxylin eosin (HE) stained cryosection was prepared for morphological assessment.
Listeria infection assays
Infection of the brain-slice cultures was performed with a 4b serotype L. monocytogenes strain (L114) isolated from bovine rhombencephalitis (Balandyte et al. 2011) and non-invasive Listeria innocua (type strain ATCC33090T [CCUG15531T purchased from the Culture Collection University of Göteborg, internal collection Nr JF5051]) as control, on day 7–13 in vitro. For this purpose, wells were washed with PBS and the culture medium was replaced with serum-free culture medium in the absence of antibiotics. Slices were infected by dripping 10 μl PBS containing approximately 100 colony-forming units (CFU) of L. monocytogenes or L. innocua on the surface. Infectious dose was determined by McFarland optical density standard and subsequently confirmed by CFU count. As negative control, three wells were mock infected with 10 μl PBS containing L. monocytogenes previously killed with gentamicin (Sigma-Aldrich Co., Buchs, Switzerland, G1272, final concentration 0.01 mg/ml). The serum-free culture medium was removed 3 h postinfection and replaced by culture medium containing gentamicin (Sigma-Aldrich Co., Buchs, Switzerland, G1272, final concentration 0.01 mg/ml), which kills extracellular bacteria. Slices were incubated for 6, 24 and 48 h post infection, respectively, at 37 °C. At each time point, one organotypic brain-slice was lysed in 1 ml PBS containing 55 μl Wampole isolator (Oxoid, Hampshire, UK), and a second brain-slice was fixed in 4% PFA to obtain cryosections. To determine the number of CFU in the slices, serial dilutions of lysates were plated on trypticase soy agar (TSA). In addition, the culture medium was plated. The TS-agar was then incubated for 24 h, and CFU were counted. In parallel, cryosections of the tissue slices were prepared and analysed by double immunofluorescence for Listeria and either neurons, astrocytes, oligodendrocytes or microglia. For comparison, double immunofluorescence was performed on paraffin-embedded sections from natural L. monocytogenes infections in sheep, goats and cattle.
Results
Viable organotypic brain-slices can be obtained at the slaughterhouse
By adopting a spoon sampling technique that had originally been developed for prion disease surveillance (World organisation for animal health (OIE) 2008), we were able to collect brain stem (obex region), cerebellar cortex and hippocampus tissues from calves through the foramen magnum within 10 min after death (Figure 1). Critical points for successful slice cultures were gentle handling of the tissue at all times, complete removal of meninges before cutting and fast transfer into culture. Owing to its rather small size, the hippocampus was the limiting factor determining the number of slices in culture. We were able to obtain about 18 hippocampal slices from one animal, thus allowing parallel or serial experiments on tissue from the same animal.
Brain-slices preserve organotypic morphology up to day 49 in vitro
Double immunofluorescence and quantitative assessment of the proportion of dead cells counts vs. total cell counts revealed a large proportion of viable cells in hippocampal (approximately 60%) and smaller numbers of viable cells in cerebellar cortex (approximately 40%) tissue slices up to day in vitro 49 (Figures 2 and 3). Notably, in the hippocampal dentate gyrus, which predominantly contains neurons, a high proportion of viable cells were observed at days 7 and 49. However, there was a marked decrease in viable cells on day in vitro 3, whereas at day in vitro 7 viable cell numbers increased again and at subsequent time points remained constant. Total cell numbers in the analysed area of 0.6 mm2 remained constant over the entire experiment (Figures 2 and 3b). Brain stem slices showed a much lower viability and were discontinued. All experiments were performed in parallel on cerebellar and hippocampal brain-slices. Culture medium yielded better results than the serum-free medium; therefore, the serum-free medium was discontinued except for infection assays. After plating the slices on the membranes, swelling of the tissue was observed macroscopically for the first 5–7 days. Then, slices grew flatter and became more transparent and from day in vitro 6 on, cells started to grow out from the periphery of the slices onto the membrane. These cells were identified as astrocytes and microglia by immunofluorescence. Cell type analysis within hippocampal and cerebellar slices identified neurons, astrocytes, oligodendrocytes and microglia up to day in vitro 49 (Figure 4). Specifically, neurons in the hippocampal dentate gyrus (Figure 4a) were preserved up to day in vitro 49. In contrast, pyramidal and Purkinje cells were completely depleted very early on. In line with the findings of the viability assay (Figures 2 and 3), the assessment of the nuclear morphology revealed many necrotic neurons on day in vitro 3. Thereafter, the proportion of healthy-appearing neuronal nuclei increased and remained constant over the next 3 weeks. On day in vitro 49, many of them revealed an altered morphology, indicating increased cell death. Still, there were clusters of neurons with normal nuclear morphology.
Figure 2.
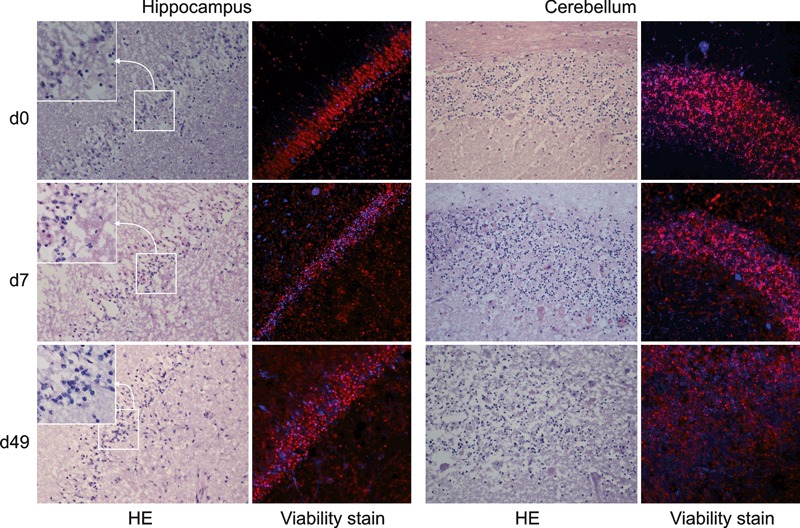
HE-stained cryosections from hippocampal and cerebellar brain-slices are compared to fluorescent images from the viability-staining. In HE-stained sections, hippocampal slices show normal architecture of the dentate gyrus (20×, from top right to bottom left within each image) and granule neurons with normal nuclear morphology (insets). Cerebellar slices show normal architecture of the granule cell layer throughout the experiment, although at day 49 a marked decrease in cell density and nuclear fragmentation can be observed. At days 7 and 49 necrotic Purkinje cells are present. Fluorescent images (20×) were taken on whole slices and represent the same region as the corresponding HE stains. Total number of nuclei is stained in red (propidium iodide after permeabilization) and nuclei of dead cells are shown in blue (eBioscience fixable-viability-stain 660). At days 7 and 49, a significant number of viable cells are present in the dentate gyrus. In the cerebellar granule cell layer, cell density is decreased at day 49 and the proportion of dead cells is higher as in the dentate gyrus. Fluorescent images were digitally enhanced and gamma settings were adjusted using the Fluoview software (Olympus FV10-ASW Version 01.07.01.00). d, day.
Figure 3.
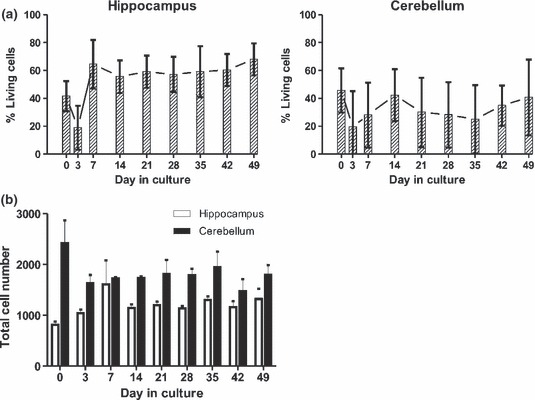
(a) The average proportion of viable cells as estimated from dead cell and total cell counts are indicated at different time points for hippocampal and cerebellar tissue-slice cultures from six different calves (error bars represent standard deviations). (b) Total cell numbers in 0.6 mm2 of the same hippocampal and cerebellar tissue-slices as in (a) at different time points.
Figure 4.
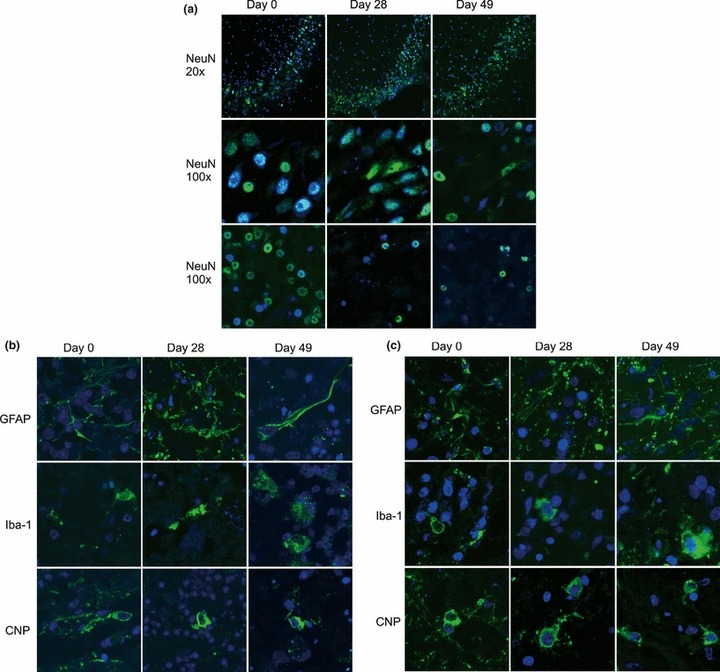
(a) Double-immunofluorescence with antibodies specific for neurons (NeuN, green) on cryosections of hippocampal and cerebellar brain-slices of the same experiment as in Figure 3 at days 0, 28 and 49 in vitro. Nuclei were stained with TOTO-3 (blue). Top row: 20× magnification of the dentate gyrus of the hippocampus. Middle row: 100× magnification of the dentate gyrus of the hippocampus. Bottom row: 100× magnification of the granule cell layer in the cerebellar cortex. (b) Cryosections of hippocampal slices from the same experiment as in Figure 3 were stained (green) with antibodies specific for astrocytes (GFAP), microglia (Iba-1) or oligodendrocytes (CNP). Nuclei were stained with TOTO-3 (blue). Images are taken from the dentate gyrus. Magnification: 100×. (c) Cryosections of cerebellar brain-slice cultures from the same experiment as in Figure 3 were stained with the same antibodies as the hippocampal slices (days 0, 28 and 49 in vitro, 100× magnification). Images were digitally enhanced and gamma settings were adjusted using the FluoView software (Olympus FV10-ASW Version 01.07.01.00).
Microglia proliferated and were increasingly activated over time. Astrocyte numbers increased during the culture period. In some cultures, astrocytes had proliferated around vessels by day in vitro 49. Oligodendrocytes were present at all time points and did not change in numbers or morphologically throughout the culture time.
HE-stained cryosections confirmed that the organotypic architecture within cerebellar and hippocampal brain-slices was clearly preserved. The morphology on the first day in vitro indicated no obvious cell necrosis, whereas on day in vitro 7 and 49, the results paralleled those of our viability experiments. At both time points, increased cytoplasmic eosinophilia, karyopyknosis and karyorrhexis indicated neuronal necrosis, in particular at day in vitro 49. Additionally, white-matter oedema, myelin loss and proliferation of astrocytes and microglia could be observed. Many microglia had a large cytoplasm with phagocytic vacuoles. Nevertheless, multifocal clusters of neurons with normal nuclear morphology were present in the dentate gyrus of hippocampal and the granule cell layer of cerebellar brain-slices (Figure 2).
Brain-slice cultures are permissive for L. monocytogenes infection
To allow for the explantation trauma to subside, hippocampal and cerebellar brain-slices were kept in culture for about 7–13 days before infection. After inoculation, L. monocytogenes replicated in the brain-slices at an exponential rate (Figure 5a). By contrast, in control brain-slices infected with L. innocua, there was no replication except for one single CFU in a total of 14 infected slices. No bacteria were detected in the medium, indicating that gentamicin efficiently prevented extracellular bacterial growth (Figure 5b).
Figure 5.
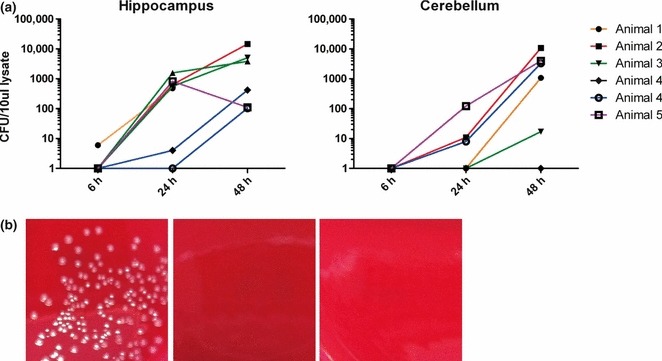
(a) Quantification of colony forming units (CFU) in seven hippocampal slices and six cerebellar slices per time-point. Hippocampal and cerebellar brain-slices from five different animals were infected with Listeria monocytogenes on days 7–13 in vitro (animal 1: day 12; animal 2: day 7, animal 3; day 13, animal 4: day 12; animal 5: day 7). Brain-slices were lysed at different time-points (6, 24, 48 h) after infection. (b) CFU’s were determined by plating lysates and medium on blood agar plates. Photographs of the agar plates are shown: lysate (left) and culture medium (middle) of hippocampal brain-slices infected with L. monocytogenes. Lysate from a Listeri innocua-infected brain-slice culture served as control (right).
Immunofluorescence for L. monocytogenes on cryosections of infected brain-slices identified single bacteria at 6 h post infection. After 24 h, multiple foci with groups of bacteria were detected. These foci were larger at 48 h post infection and were frequently located on the surface and the edge of the slice.
Most bacteria were found inside microglia, some could be observed associated with axons or astrocytes and on rare occasions inside oligodendrocytes. Immunofluorescence on paraffin sections from naturally occurring cases of listeric encephalitis in ruminants showed similar multifocal growth of bacteria within predominantly microglia, macrophages and neutrophils. Additionally, some bacteria were associated with axons (Figure 6).
Figure 6.
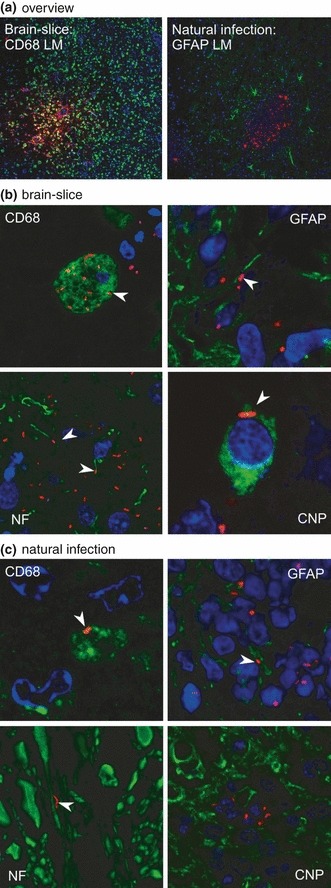
Comparison of infected brain-slices with natural infections. In all images, Listeria monocytogenes are stained in red (LM). Microglia (CD68), neuronal processes (NF), astrocytes (GFAP) and oligodendrocytes (CNP) are stained in green. Nuclei (TOTO-3) are blue. Images were digitally enhanced and gamma settings were adjusted using the FluoView software (Olympus FV10-ASW Version 01.07.01.00). (a) Overview: focal growth of L. monocytogenes (red) in brain-slices and natural cases of listeriosis. On the left microglia are stained in green (10× magnification). On the right astrocytes are stained in green (20× magnification). (b) Immunofluorescence on cryosections of infected brain-slices. CD68: L. monocytogenes are present in microglial cells (arrowhead, 300× magnification). Neurofilament (NF): L. monocytogenes are closely associated with axons (arrowheads, 200× magnification). GFAP: L. monocytogenes are closely associated with astrocytic processes (arrowhead, 200× magnification). CNP: in brain-slices, L. monocytogenes are only rarely found inside oligodendrocytes (arrowhead, 600× magnification). (c) Immunofluorescence on paraffin-sections of a natural case of listeric encephalitis. CD68: L. monocytogenes are present in microglial cells (arrowhead, 300× magnification). Neurofilament (NF): L. monocytogenes are closely associated with axons (arrowhead, 300× magnification). GFAP: L. monocytogenes are closely associated with astrocytic processes (arrowhead, 400× magnification). CNP: in natural infections no L. monocytogenes were found within oligodendrocytes (400× magnification).
Discussion
In this study, we investigated the potential of bovine organotypic brain-slice cultures as a host-specific in vitro model for CNS diseases. We worked with slaughterhouse-derived brain tissue from young cattle to profit from the vast availability of these tissues. Our self-made spoon tool allowed for fast and easy tissue sampling in line with normal slaughterhouse procedures while avoiding the contamination risk of opening the skull. In these cultures, we found all pertinent cell types up to day in vitro 49. Also, we successfully infected organotypic brain-slices with L. monocytogenes.
Hippocampal brain-slices had the best survival rates of about 60% living cells between day in vitro 7 and 49. In comparison, cerebellar and brain stem brain-slices had a significantly lower survival rate. In line with other studies, there was a transient drop in viability on day 3 in vitro, which is likely due to the explantation trauma (Lossi et al. 2009; Staal et al. 2011). Most dead cells were found in areas where many neuronal bodies are located. Especially large neurons with long axons (e.g. Purkinje cells of the cerebellum) did not survive more than a few days in culture. This may be due to the cutting procedure that severs processes of cells resulting in cell death (Lossi et al. 2009). Likewise, the poor survival rate we found in brain stem slices could be attributed to this effect, considering the anatomy of this region with long projections to remote nuclei. In turn, hippocampal granule neurons of the dentate gyrus remained partially viable as shown with the viability stain. Hippocampal granule neurons have relatively short connections to neighbouring neurons and therefore are less likely to be harmed by tissue cutting. Also, the presence of neuronal progenitor cells in the subgranular zone of the hippocampus may contribute to the increased viability of neurons in hippocampal brain-slices in comparison with cerebellar brain-slices (Ming & Song 2011). As opposed to primary cultures of brain cells, in slice cultures original cell–cell interactions are not disrupted and therefore may mirror the situation in vivo more closely. To what extent these interactions are altered by the in vitro conditions and the presence of dead cells in the brain-slice remains to be determined. We demonstrated 60% of living cells in brain-slices and the presence of all relevant cell types up to day in vitro 49. Also, organotypic morphology was retained throughout the experiment without substantial overgrowth of one cell population.
We suspected that the captive bolt shot at the slaughterhouse would negatively influence cell viability in the brain-slices. Surprisingly, data from a few experiments with tissue derived from euthanized calves did not suggest a negative influence of the captive bolt shot (data not shown). Also, we are aware of the superior ability of foetal tissue to adapt to culture conditions. In some cases, we were able to obtain bovine foetal tissue and survival analysis suggests between 20 and 30% more living cells compared to slaughterhouse-derived material (data not shown), although there was a considerable variation between experiments. The latter may be due to the different gestational age of the foetuses, because the fast foetal development of the brain is associated with major morphological and physiological changes (Sanes et al. 2012). Standardization of foetal brain-slice cultures would require foetal tissue within a narrow gestational age, which is impossible to obtain without aborting cows on purpose.
We could show that L. monocytogenes replicates in brain-slices and grows exponentially over a course of 48 h. Significant variations in absolute CFU numbers between some experiments (Figure 5a) could be due to differences in slice viability, slice size and/or the inoculation technique. Variations in slice viability of 20% as observed in our study are unlikely to cause differences in CFU numbers of this magnitude. We cannot rule out an impact of slice size on CFU numbers of LM. Therefore, normalization of bacterial load to tissue amount should be considered when performing infection assays in this model. The impact of slice size might have been strongly enhanced by the use of an inoculation technique, where the bacterial suspension was applied on the surface of the slice and may have leaked from the slice causing variations in LM numbers in contact with the slice. Ongoing experiments in our laboratory show that microinjections of bacteria into a defined area of the slice cause focal LM replication and considerably decreased variability in CFU between experiments (data not shown). After infection, intracellular bacterial growth was enforced by the addition of gentamicin. The culture medium at the end point after 48 h was always sterile and L. innocua never established infection. Taken together, these findings indicate that all extracellular bacteria were successfully killed and L. monocytogenes replication occurred in the intracellular compartment of viable host cells.
In comparison with naturally occurring cases of listeric encephalitis, we found many similarities. In both slices and natural cases, L. monocytogenes grew focally and similar cell populations were affected. L. monocytogenes grew mainly in microglia, but they were also found associated with axons and in some cases astrocytes and oligodendrocytes. However, the lack of a functional vascular system and immigrating leucocytes in brain-slices accounts for disparities between natural infections and brain-slices. Consequently, in natural infections, many bacteria could be observed within neutrophils that were lacking in brain-slices. In turn, within brain-slices, microglial cells were activated owing to explantation trauma and cell death before the infection occurred. Another shortcoming of brain-slices is the mode of L. monocytogenes inoculation that bypasses the natural CNS invasion route.
Within these limitations, brain-slices have great potential for the study of host–pathogen interactions and agent spread within the CNS in a controlled in vitro environment as they show many features similar to the situation in vivo.
Specifically, brain-slices offer the possibility to investigate dynamics and mechanisms of axonal spread as suggested by a previous study of natural listeriosis (Oevermann et al. 2010a). Also, agent-related determinants involved in neurovirulence and axonal spread of L. monocytogenes are largely unknown. Previous work from our group showed that L. monocytogenes strains from clinical cases of ruminant rhombencephalitis were clustered in a distinct genetic complex. In combination with epidemiological and clinical data, this suggests that particularly neurovirulent strains of L. monocytogenes exist (Balandyte et al. 2011). To further explore this hypothesis, an in vitro system that allows standardizing host factors is highly desirable. Inversely, the same bacterial strain could be applied to brain-slices originating from different animals to explore putative host factors involved in the neuropathogenesis of listeriosis.
In conclusion, slaughterhouse-derived brain-slices showed sufficient viability parameters and could successfully be infected with L. monocytogenes. The suitability of the system for long culture times lends itself to the investigation of slow-growing agents like prions (Falsig & Aguzzi 2008a). In the future, organotypic brain-slice cultures of ruminant origin will support research into CNS listeriosis, advancing the understanding of pathomechanisms in human listeriosis as well. Their use might be extended to the investigation of other, even yet unknown, neuroinfectious diseases in ruminants.
Acknowledgments
We thank Michaela Gsponer, Yousif Ahmed, Evelyne Rohrer, Manuela Bozzo, Erika Garchi and Amandine Ruffieux for their excellent technical support.
Funding
This work was financed by the 3R Research Foundation Switzerland (grant number 116/09).
References
- Antal EA, Loberg EM, Dietrichs E, Maehlen J. Neuropathological findings in 9 cases of Listeria monocytogenes brain stem encephalitis. Brain Pathol. 2005;15:187–191. doi: 10.1111/j.1750-3639.2005.tb00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balandyte L, Brodard I, Frey J, Oevermann A, Abril C. Ruminant Rhombencephalitis-Associated Listeria monocytogenes Alleles Linked to a Multilocus Variable-Number Tandem-Repeat Analysis Complex. Appl. Environ. Microbiol. 2011;77:8325–8335. doi: 10.1128/AEM.06507-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belay ED, Schonberger LB. The public health impact of prion diseases. Annu. Rev. Public Health. 2005;26:191–212. doi: 10.1146/annurev.publhealth.26.021304.144536. [DOI] [PubMed] [Google Scholar]
- Falsig J, Aguzzi A. The prion organotypic slice culture assay – POSCA. Nat. Protoc. 2008a;3:555–562. doi: 10.1038/nprot.2008.13. [DOI] [PubMed] [Google Scholar]
- Falsig J, Julius C, Margalith I, Schwarz P, Heppner FL, Aguzzi A. A versatile prion replication assay in organotypic brain slices. Nat. Neurosci. 2008b;11:109–117. doi: 10.1038/nn2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gähwiler BH. Organotypic monolayer cultures of nervous tissue. J. Neurosci. Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Gianinazzi C, Grandgirard D, Imboden H, et al. Caspase-3 mediates hippocampal apoptosis in pneumococcal meningitis. Acta Neuropathol. 2003;105:499–507. doi: 10.1007/s00401-003-0672-7. [DOI] [PubMed] [Google Scholar]
- Gianinazzi C, Schild M, Müller N, et al. Organotypic slice cultures from rat brain tissue: a new approach for Naegleria fowleri CNS infection in vitro. Parasitology. 2005;131:797–804. doi: 10.1017/S0031182005008619. [DOI] [PubMed] [Google Scholar]
- Lossi L, Alasia S, Salio C, Merighi A. Cell death and proliferation in acute slices and organotypic cultures of mammalian CNS. Prog. Neurobiol. 2009;88:221–245. doi: 10.1016/j.pneurobio.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Mayer D, Fischer H, Schneider U, Heimrich B, Schwemmle M. Borna disease virus replication in organotypic hippocampal slice cultures from rats results in selective damage of dentate granule cells. J. Virol. 2005;79:11716–11723. doi: 10.1128/JVI.79.18.11716-11723.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noraberg J, Kristensen BW, Zimmer J. Markers for neuronal degeneration in organotypic slice cultures. Brain Res. Brain Res. Protoc. 1999;3:278–290. doi: 10.1016/s1385-299x(98)00050-6. [DOI] [PubMed] [Google Scholar]
- Oevermann A, Botteron C, Seuberlich T, et al. Neuropathological survey of fallen stock: active surveillance reveals high prevalence of encephalitic listeriosis in small ruminants. Vet. Microbiol. 2008;130:320–329. doi: 10.1016/j.vetmic.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Oevermann A, Di PS, Doherr MG, Abril C, Zurbriggen A, Vandevelde M. Neuropathogenesis of naturally occurring encephalitis caused by Listeria monocytogenes in ruminants. Brain Pathol. 2010a;20:378–390. doi: 10.1111/j.1750-3639.2009.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oevermann A, Zurbriggen A, Vandevelde M. Rhombencephalitis caused by Listeria monocytogenes in humans and ruminants: a zoonosis on the rise? Interdiscip. Perspect. Infect Dis. 2010b;2010:Article ID 632513. doi: 10.1155/2010/632513. doi: 10.1155/2010/632513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruffo A, Cozzi B, Ballarin C. Ontogenesis of brain aromatase P450 expression in the bovine hypothalamus. Brain Res. Bull. 2008;75:60–65. doi: 10.1016/j.brainresbull.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Sanes D, Reh T, Harris W. Genesis and Migration. Development of the Nervous System. 3rd edn. Oxford: Academic Press; 2012. pp. 49–77. [Google Scholar]
- Scheidegger A, Vonlaufen N, Naguleswaran A, et al. Differential effects of interferon-gamma and tumor necrosis factor-alpha on Toxoplasma gondii proliferation in organotypic rat brain slice cultures. J. Parasitol. 2005;91:307–315. doi: 10.1645/GE-379R. [DOI] [PubMed] [Google Scholar]
- Staal JA, Alexander SR, Liu Y, Dickson TD, Vickers JC. Characterization of cortical neuronal and glial alterations during culture of organotypic whole brain slices from neonatal and mature mice. PLoS ONE. 2011;6:e22040. doi: 10.1371/journal.pone.0022040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Brun R, et al. Infection of organotypic slice cultures from rat central nervous tissue with Trypanosoma brucei brucei. Int. J. Med. Microbiol. 2000;290:105–113. doi: 10.1016/S1438-4221(00)80113-7. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Howells DW, Sena ES, et al. Can animal models of disease reliably inform human studies? PLoS. Med. 2010;7:e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World organisation for animal health, OIE. 6th edn. Paris: OIE; 2008. Bovine Spongiform Encephalitis, Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; pp. 671–682. [Google Scholar]


