Abstract
Heterotopic tendon mineralization (ossification or calcification), which may be a feature of tendinopathy or which may develop following surgical trauma (repair or graft harvest), has not received much attention. The purpose of this article is to review the prevalence, mechanisms and consequences of heterotopic tendon mineralization and to identify the gaps in our current understanding. We focus on endochondral heterotopic ossification and draw on knowledge of the mechanisms of this process in other tissues and conditions. Finally, we introduce a novel murine Achilles tendon needle injury model, which will enable us to further study the mechanisms and biomechanical consequences of tendon mineralization.
Keywords: bone morphogenetic protein, inflammation, micro-computed tomography, prostaglandin, rotator cuff, substance P
Prevalence of tendon mineralization in tendinopathy or following surgical trauma
Mineralization is one of several possible histological features of tendinopathy (Jarvinen et al. 1997) and is particularly common in rotator cuff tendons. Within the general European and European-derived population, rotator cuff tendon mineralization has a prevalence of 2.7–22% (women 30–50 years old are mostly affected) and in 10% of cases the condition is bilateral [reviewed by Oliva et al. (2011a,b)]. Although less frequently reported, many other tendons including the Achilles (twice as frequently in men), biceps brachii, extensor pollicus longus and quadriceps tendons (Kannus & Jozsa 1991; Richards et al. 2008) may also be affected by mineralization. In addition, this pathology has been observed in ligaments such as the anterior cruciate (Tsujii et al. 2012) and medial collateral (Muschol et al. 2005) of the knee.
Mineralization has been reported in 14–62% of cases following percutaneous or open repair of the Achilles tendon (Ateschrang et al. 2008). In 31 patients examined a mean of 10 years following anterior cruciate ligament reconstruction with autologous bone – patellar tendon – bone grafts, intratendinous mineralization was detected at the site of graft harvest in nine cases (Jarvela et al. 2004).
Consequences of tendon mineralization
Tendon mineralization may be a cause of pain and tendon weakness. Between 7 and 17% of chronic shoulder pain has been associated with rotator cuff tendon mineralization (Welfing et al. 1965; Hedtmann & Fett 1989), and pain may be a feature of mineralization within other tendons and ligaments also (Muschol et al. 2005; Richards et al. 2008; Tsujii et al. 2012). In an examination of 891 tendon ruptures, excluding rotator cuffs, Kannus and Jozsa (1991) found that the occurrence of mineralization was three times higher in affected tendons compared with controls and was the only abnormal histological feature in these cases. In a large cadaver survey, Cotton and Rideout (1964) found that 6.6% of shoulders had rotator cuff tendon mineralization and all of these cuffs had tendon tears.
Does tendon mineralization in tendinopathy or resulting from surgical trauma involve endochondral ossification?
Heterotopic mineralization may be due to calcification or ossification. In pathological calcification, calcium salts are deposited in normal (metastatic calcification) or damaged (dystrophic calcification) tissue, whereas the term ‘ossification’ implies bone formation (calcification in a collagen matrix) (Chan et al. 2002). The two processes are radiographically indistinguishable as pseudotrabeculation may occur with calcification (Strumia et al. 1997). Ossification can result from two distinct processes (Karsenty & Wagner 2002). Intramembranous ossification (IM) involves the condensation of mesenchymal cells which then directly differentiate into bone-forming osteoblasts. In endochondral ossification (EO), mesenchymal stem cells condense and differentiate into chondrocytes. In turn, this cartilage anlagen directs the formation of osteoblasts that form woven and then lamellar (mature) bone. Here, we use the term ‘mineralization’ to encompass ossification and calcification where the distinction is unclear.
It is likely that tendon mineralization frequently involves EO. Fenwick et al. (2002) demonstrated that mineralized deposits in the Achilles (n = 3) and patellar tendons (n = 1), isolated from human patients with chronic tendinopathy, involved EO. In a review, Richards et al. (2008) noted descriptions of calcification and both EO and IM (two cases reported both processes) in the human Achilles tendon. Furthermore, EO occurs in several animal models of tendon mineralization: mouse and rat models of Achilles tendon transection (McClure 1983; Rooney et al. 1992, 1993; Lin et al. 2009); patellar tendon window injury in rats (Lui et al. 2012) and injection of collagenase into the patellar tendon in rats (Lui et al. 2009a,b; Yee Lui et al. 2011).
However, the involvement of EO in the most common presentation of rotator cuff tendon mineralization is disputed. Uhthoff (1975) and Uhthoff and Loehr (1997) characterized mineralization at this location as a three-stage cell-mediated process resembling ‘incomplete EO’. In the precalcific stage, fibrocartilaginous metaplasia occurs: tendon fibroblasts transform into chondrocytes (a morphological characterization) that produce alkaline phosphatase and are surrounded by proteoglycan. The second (calcific) stage is subdivided into formative, resting and resorptive phases. In the formative phase, calcium crystals are deposited in matrix vesicles that coalesce. Notably, the fibrocartilage septa between the foci of calcification are generally devoid of vascular channels and these septa do not consistently stain positive for type II collagen. The deposit is chalk-like in appearance if surgery is performed at this time. In the resting phase, fibrocartilaginous tissue borders the foci of calcification. The start of the resorptive phase is indicated by the appearance of thin-walled channels followed by a cellular reaction, including macrophages and multinucleated cells (possibly osteoclasts). Grossly, the deposit appears creamy or toothpaste-like material under pressure at this time. In the postcalcific stage, granulation tissue occupies the space of the mineralized lesion and then remodels (type III collagen is replaced by type I). Differences noted by the authors between this presentation of mineralization and EO which occurs at other locations, such as growth plates, include the lack vasculature between the developing foci and a different crystal appearance to classic apatite. These authors and others (Archer et al. 1993) documented true bone ossicles in a small number of rotator cuff tendon samples concurrently with the more common lesion type. In support of Uhthoff (1975) and Uhthoff and Loehr (1997), Neuwirth et al. (2006) showed gene expression of collagen types II and X in tissue from the acromial bursa and supraspinatus tendon. Additionally, Nakase et al. (2000) identified osteoclasts in supraspinatus tendon. In contrast, several authors [Riley et al. (1996) and references therein] found no evidence of EO in rotator cuff tendons based on the absence of matrix vesicles and collagen types II and X although, as Riley et al. (1996) suggested, these differences between studies may in part be due to epitopes being obscured on tissue sections.
In a further complication, Riley et al. (1996) suggested that within supraspinatus tendons the aetiology of large radiographically detectable calcium deposits and smaller deposits detectable only histologically was different, even though both originate within fibrocartilaginous tendon regions. The basis for this hypothesis was that the matrix composition of samples from each group was different. In particular, samples from tendons with the smaller deposits showed increased type III collagen and total glycosaminoglycan content compared with normal controls unlike samples from tendons with the larger lesions which were not different in these respects from the controls. Arguably, both lesion types may represent different stages of the same pathology.
In conclusion, therefore, elements of the EO process may contribute to rotator cuff tendon mineralization, but it appears likely that in most cases the process is not the same as that which occurs during bone development. It is possible too that mineralization develops by more than one mechanism. Whether a similar spectrum of events occurs in other tendons is not known.
Interestingly, a shift towards a chondrocytic phenotype occurs in tendinopathic equine flexor tendons (Clegg et al. 2007), but although tendon injury is a very common disease in this species (Dowling et al. 2000; Williams et al. 2001), tendon mineralization is a rare event. Anecdotally, the occurrence of mineralization in equine tendons is associated with prior intratendinous medication with corticosteroids. In addition, a shift towards a chondrocyte phenotype has been identified in the rat model of rotator cuff tendon overuse injury, but tendon mineralization has not been reported (Archambault et al. 2007; Attia et al. 2012). On the other hand, Achilles tendon mineralization develops spontaneously in aged C57Bl/6J mice (O'Brien 2009) (and perhaps other strains too). These observations suggest that species differences exist in the regulation of tendon mineralization.
Fibrodysplasia ossificans progressiva (FOP) and progressive osseous heteroplasia (POH) are both rare diseases caused by single-gene mutations (of activin receptor type 1 and the GNAS gene respectively) and involve extensive and progressive formation of bone within soft tissues, including tendons, in human patients (Shore & Kaplan 2010). Bone formation in FOP occurs mostly by EO but mostly by IM in POH (Shore & Kaplan 2010). It is likely that EO is responsible for the formation of entheseophytes and syndesmophytes in ankylosing spondylitis (AS; Lencel et al. 2011). EO has also been implicated in ossification of the posterior longitudinal ligament (OPLL), a condition common amongst East Asian populations causing myeloradiculopathy as a result of chronic pressure on the spinal cord and nerve roots (Inamasu et al. 2006).
Current treatments for mineralization lesions in isolated tendons
Conservative treatments for rotator cuff tendon mineralization include physical therapy, systemic non-steroidal antiinflammatory drugs (NSAIDs), subacromial corticosteroid injection and extracorporeal shock-wave therapy (Gosens & Hofstee 2009). Disruption of these lesions by needling (also known as barbotage) with or without irrigation may be a simpler and less-expensive alternative to surgery (Maugars et al. 2009). Surgery to remove mineralized foci within rotator cuff tendons is usually effective when other treatments have failed (Gosens & Hofstee 2009; Seyahi & Dermirhan 2009). Successful conservative management of Achilles tendon lesions has been described (Sasaki et al. 2005), but excision may be necessary (Richards et al. 2008).
Mechanisms of heterotopic ossification (HO) within tendons
Central role of bone morphogenetic proteins (BMPs) in normal bone formation and in tendon HO
Members of the BMP cytokine family are expressed by a variety of cell types throughout normal skeletogensis (Li & Cao 2006) and fracture repair, although less widely in repair by IM than EO (Yu et al. 2010). Binding of these cytokines [members of the transforming growth factor-beta family (TGFβ) family] to complexes of type I and type II cell surface receptors leads to transcription of a range of genes involved in the regulation of bone formation (Tsumaki & Yoshikawa 2005). Increased levels of BMPs have been identified in experimental models of tendon injury [BMP 2, 4, 7 (Lin et al. 2009) and BMP 2, 4, 7 (Yee Lui et al. 2011)]. In samples from patients with rotator cuff tendon tears, Neuwirth et al. (2006) demonstrated gene expression of BMP 2 in the supraspinatus tendon and BMP 2 and BMP 7 in the subacromial bursa. It is perhaps surprising that another study found that expression of BMP 4 and 6 genes was significantly reduced and expression of the BMP 2 gene unchanged, in patient samples of mineralized rotator cuff tissue compared with non-mineralized areas (Oliva et al. 2011a). Intratendinous injection of BMP has been shown to result in HO in rabbit Achilles tendons (Hashimoto et al. 2007), and in a murine model of mineralization following Achilles tendon transection, treatment with the BMP antagonist Noggin markedly reduced the development of HO (Hannallah et al. 2004). FOP is reported to result from a mutation of the BMP type I receptor, activin receptor type I (Shore & Kaplan 2010). This activating mutation induces BMP signalling in both BMP-independent and BMP-responsive manners (Shen et al. 2009).
Does inflammation initiate BMP production within tendons?
The efficacy of prophylactic treatment with NSAIDs to prevent HO in other tissues following trauma (an approximately 60% reduction in occurrence) provides clinical evidence that inflammation is a prerequisite for HO development (Baird & Kang 2009). Furthermore, in patients with FOP clinical observations implicate injury and associated inflammation with flare-ups of the disease (Lounev et al. 2009).
Macrophages are capable of BMP synthesis (Champagne et al. 2002; Kan et al. 2009) and are a likely source in cases of HO following surgical tendon trauma and in tendinopathy (Figure 1). In a transgenic mouse line which overexpresses BMP 4 in macrophages and neurons (and some other cells) under the control of the neuron-specific enolase promoter (Nse- 4), Kan et al. (2009) demonstrated that macrophage depletion significantly reduced the occurrence of HO following wounding. Furthermore, when the authors crossed this mouse strain with recombination activating gene 1 (RAG1) null mice (which lack mature B and T lymphocytes), HO developed without delay following injury but the rate of spreading and overall amount was much smaller. Consistent with this finding, Rifas (2006) showed that T cell cytokines could act synergistically to induce BMP 2 production by human bone marrow–derived stem cells leading to matrix mineralization. Lymphocytes and macrophages have been identified in chronically painful Achilles tendons (Schubert et al. 2005).
Figure 1.
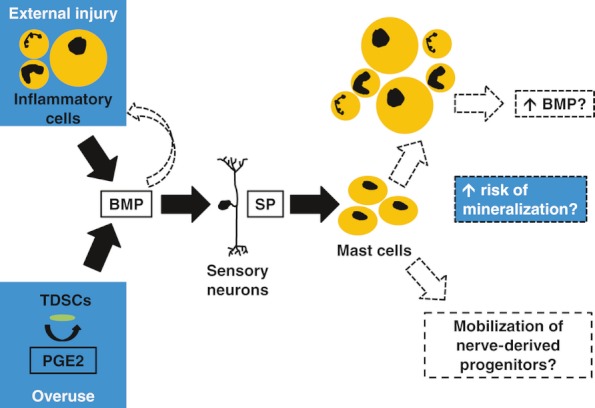
Sequence of events proposed to occur prior to heterotopic ossification within tendons. Bone morphogenetic protein (BMP)-stimulated release of substance P (SP) leading to mast cell recruitment is likely to be a key event. Following surgical trauma, bone marrow-derived inflammatory cells may be the source of BMPs required to initiate this pathway, whereas in overuse tendinopathy increased intratendinous PGE 2, which in turn promotes BMP production by tendon-derived stem cells (TDSCs), may be the initiator. Mast cells recruited by stimulated sensory neurons may cause accumulation of more inflammatory cells in addition to releasing progenitor cells from neural tissue. Both of these events may predispose the development of ossification. BMPs may also be chemotactic for mononuclear cells, further amplifying the inflammatory response to surgical trauma.
Prostaglandin production may also play a prominent role in BMP expression (Figure 1). In a murine model, intratendinous levels of prostaglandin E2 (PGE 2) were markedly increased after an episode of overuse (Zhang & Wang 2010). These authors showed how in vitro treatment of tendon-derived stem cells (TDSCs) with PGE 2 resulted in dose-dependent production of BMP 2 and osteoblast differentiation and the latter effect was nearly abolished by incubation with BMP 2 antibodies (Zhang & Wang 2012). Tendon fibroblasts (Yang et al. 2005), as well as macrophages (Kuroda & Yamashita 2003) and neutrophils (Wright et al. 2010), may be responsible for production of PGE 2.
BMP-induced neuro-amplification of inflammation
Two recent independent reports demonstrated that a pathway involving BMP-stimulated sensory neuron signalling via substance P (SP) is necessary for HO within muscles. We posit that a similar process may occur in tendons (Figure 1). Absence of sensory neurons dramatically reduced HO formation in murine models (Kan et al. 2011; Salisbury et al. 2011), and absence of the gene encoding SP prevented HO entirely (Kan et al. 2011). Stabilizing mast cells (using cromolyn) (Salisbury et al. 2011) and abrogating the activity of the SP receptor, in particular by downregulating SP receptor neurokinin 1 (NK1r) expressing mast cells, reduced HO significantly (Kan et al. 2011). Mast cells were enriched in areas surrounding early inflammation, often in close proximity to neurons and blood vessels (Kan et al. 2011; Salisbury et al. 2011). Ablating sensory neurons or blocking NK1r reduced inflammation (Kan et al. 2011), suggesting that the role of this pathway is to amplify the local inflammatory response. In turn, presumably this outcome leads to enhanced BMP production sufficient for EO development.
Given the role of neuron-derived SP in muscles, it is perhaps surprising that in tendon samples from overuse or collagenase injury models, other cell types appeared to be more prominent producers of the neuropeptide. In a rabbit model of tendon overuse, occasional nerve fascicles immunostained for SP (Backman et al. 2011), but in this study the main site of SP production appeared to be associated with blood vessels (mineralization has not been described in this model). The authors also found evidence of the neuropeptide in occasional tendon fibroblasts by in situ hybridization but not immunostaining. In further contrast, in rat patellar tendons following collagenase treatment, SP was immunostained in tendon fibroblasts, chondrocyte-like cells and very intensely within calcified deposits (Lui et al. 2010). No neural tissue was detected in histological sections in that study.
Certain BMPs may be chemotactic for mononuclear cells, further amplifying the inflammatory response (Cunningham et al. 1992).
Could persistent inflammation have an inhibitory role on HO within tendons?
Similar to HO following trauma (in non-tendinous tissues at least) and in patients with FOP, there appears to be a link between inflammation and the formation of syndesmophytes (bone spurs at the attachment of spinal ligaments) in AS. Maksymowych et al. (2009) showed that new syndesmophytes developed more frequently at vertebral corners with evidence of inflammation on magnetic resonance imaging (MRI) examination and more frequently where inflammation had resolved after anti-tumour necrosis factor (TNF) treatment. Recently, evidence from an in vitro study (Lencel et al. 2011) and an in vivo MRI-based report (Pedersen et al. 2011) suggests that TNF and interleukin-1β may actually function as a ‘brake’ to inhibit bone formation in AS. This putative inhibitory effect of persistent inflammation has not been examined the context of HO resulting from tendon trauma or in tendinopathy.
Cellular contribution to the development and progression of HO in muscle and tendon
Kaplan et al. (2007) examined the role of bone marrow–derived cells in the mineralized lesion in a murine model where BMP 4 was delivered intramuscularly in a matrigel carrier. Cells from the bone marrow contributed to the initial inflammatory response and later populated the bone marrow of the developing lesion but did not contribute to the fibroproliferative, chondrogenic or osteogenic stages (Figure 2). In a study using similar labelling techniques in rats, Kajikawa et al. (2007) also provided evidence that an early cellular response to tendon wounding is likely to be bone marrow–derived, suggesting that the findings of Kaplan et al. are not specific to mineralization following exogenous BMP 4 treatment. However, Suda et al. (2009) presented somewhat conflicting data to Kaplan et al. (2007). These authors found that bone marrow-derived cells [characterized as circulating osteogenic precursors (COPs)] contributed to both the inflammatory and fibroproliferative but not later preosseous stages of FOP lesions sampled from a single patient. Then, when the authors implanted immunodeficient mice with COPs from normal or FOP-affected humans, the bone which formed contained donor-derived cells, suggesting that these COPs may nucleate bone formation. Therefore in summary, it appears that both bone marrow- and locally-derived cells contribute to the development of HO in muscle and it is possible that the local cells dominate during formation of the cartilage anlagen. Lounev et al. (2009) used lineage-tracing methods to identify the cells contributing to the fibroproliferative, chondrogenic and osteogenic stages using two murine models of HO (BMP 2-matrigel implantation or injury to Nse-BMP 4 mice). These authors concluded that vascular smooth muscle cells made no contribution, and cells which expressed endothelial markers at some stage in their development (possibly from the local vasculature) contributed approximately 50%. BMP receptors are highly expressed on endothelial cells, and members of the TGF-β family, including BMPs, enable transdifferentiation of endothelial cells to mesenchymal lineages (Goumans et al. 2008). Surprisingly, satellite cells (resident stem cells of skeletal muscle) contributed minimally (<5%) leaving the lineage of almost half of the contributing cells unidentified. The recent work of Salisbury et al. (2011) suggested that nerve fibres may be an additional source of progenitors (Figure 1). In that study, cromolyn treatment of mice with induced HO led to an accumulation of cells expressing stem cell markers within the nerves. These authors suggested that this finding was an indication that nerves were a source of progenitors, because by inhibiting mast cell–induced nerve remodelling, cromolyn led to their accumulation within the neural tissue. In addition, unlike satellite cells in muscle, it seems likely that TDSCs also play a key role in formation of the endochondral anlagen in tendon HO. Bi et al. (2007) identified that biglycan and fibromodulin are critical components in the TDSC niche. Bgn−/0Fmod−/− mice showed spontaneous endochondral tendon ossification mediated by increased BMP signalling.
Figure 2.
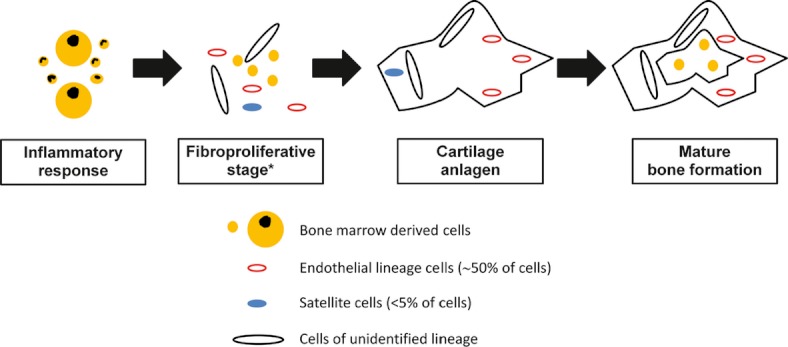
Cellular populations believed to be involved in heterotopic ossification (HO) when it occurs in muscles. *The contribution of bone marrow-derived cells to the fibroproliferative stage is disputed. The unidentified cells may include nerve-derived precursors. Within surgically traumatised tendons, similar cell populations may be involved, although tendon-derived stem cells may play a greater role than satellite cells do in affected muscles. It is unclear whether HO which occurs as a feature of tendinopathy involves bone marrow–derived inflammatory cells to the same extent.
Hypoxia in tendon HO
Hypoxia, leading to hypoxia-inducible transcription factor (HIF) signalling, is an essential feature of EO. Downstream events of HIF signalling [reviewed by Araldi and Schipani (2010)] include differentiation of chondrocytes from mesenchymal precursors; chondrocyte proliferation and survival; angiogenesis; and osteogenesis. Furthermore, activation of HIF1α signalling leads to enhanced BMP 2 production by osteoblasts (Tseng et al. 2010). Lin et al. (2009) demonstrated increased HIF1α gene expression in the rat Achilles tendon transection model of tendon HO. Later, these authors showed that treatment with small interfering RNA (siRNA) against HIF1α 48 h postoperatively reduced occurrence of HO in this model significantly compared with contralateral transected control tendons (Lin et al. 2011). Whether tissue hypoxia is a primary or secondary event following tendon injury is not known. Lin et al. (2009) reasonably suggested that vascular disruption arising following tendon injury may result in hypoxia. It is also interesting to note that the ‘critical zone’ of the supraspinatus tendon, close to its humeral insertion and where rotator cuff pathology occurs most commonly, is markedly undervascularized [reviewed by Gosens and Hofstee (2009)].
Oxidative stress in tendon HO
Reactive oxygen species (ROS) are influenced by mechanical loading of tendon (Eliasson et al. 2012), and although data are sparse, ROS are suggested to have a role in tendinopathy (Longo et al. 2008). It is therefore interesting that in vitro, in the context of vascular calcification, hydrogen peroxide treatment led to a switch by vascular smooth muscle cells from a contractile to an osteogenic phenotype via Runt-related transcription factor (Runx) signalling (Byon et al. 2008).
Influence of loading in tendon HO
Although overuse is likely to play a role in the development of HO in tendons, underuse may play a role too, presumably related to the requirement of loading to maintain tendon homoeostasis (Arnoczky et al. 2007). This is relevant as sedentary individuals may also be at risk of tendinopathy (Rolf & Movin 1997). Palmes et al. (2002) demonstrated the likely importance of mobilization in preventing HO occurrence: suture-repaired murine Achilles tendons, healing under conditions of immobilization, but not full mobilization, developed fibrocartilage. Consistent with this finding, the duration of immobilization (bed confinement) has been proposed as a key factor in the development of HO (not specifically within tendons) following burn injury (Evans & Smith 1959) and in coma patients (Mielants et al. 1975). However, it is not known whether inflammation or non-inflammatory mechanosensitive pathways, such as integrin–ion channel complexes (Mobasheri et al. 2005), play a role in HO in these cases.
Genetic factors predisposing to tendon HO
Preliminary observations suggest an increased risk of rotator cuff tendon mineralization for patients with human leucocyte antigen A1 serotype (HLA-A1) (Sengar et al. 1987). In mice, strain-specific differences in response (not specifically within tendons) to an osteogenic stimulus exist (Marusic et al. 1999), providing further evidence of a genetic component to the process of tendon mineralization.
Hormonal factors associated with tendon HO
There appears to be an association between tendon mineralization and certain endocrine disorders [reviewed by Oliva et al. (2011a,b)]. In particular, more than 30% of patients with insulin-dependent diabetes have tendon mineralization (Hurt & Baker 2003), but the mechanisms involved have not been delineated.
Gaps in current understanding of tendon mineralization
The prevalence and relationship between different presentations of mineralization (EO, incomplete EO, IM or other) within tendons of the rotator cuff and elsewhere must be established. Mineralization within rotator cuff tendons is detected in a substantial proportion of the population but the paucity of data describing the prevalence of mineralization at other common sites of tendinopathy is interesting. Perhaps the difference relates to the more common occurrence of tendinopathy at the rotator cuff, but alternatively the explanation may relate to differential responses in changes to the loading environment (Thornton et al. 2010). Furthermore, why mineralization occurs in a subset but not all individuals affected by rotator cuff tendinopathy is not known, but may relate to differences in genetic predilection such as HLA-A1 serotype. The reported greater prevalence of rotator cuff tendon mineralization in women appears in conflict with the greater number of reports of Achilles tendon mineralization in male patients. Whether this difference is for biological reasons, such as sex-linked or hormonal factors, or simply relates to differences in activity between the sexes, needs to be determined. In addition to these considerations, it is possible that the true prevalence of mineralization associated with tendinopathy has been underestimated. Most prevalence data are based on plain radiographical examinations, but with higher resolution imaging techniques, such as computed tomography, ultrasonography or magnetic resonance imaging, detection rates may be higher (Uhthoff & Loehr 1997). Furthermore, small foci of mineralization may develop during tendinopathy but not progress to sizes that are readily detected or resolve before imaging is performed.
Several questions exist regarding the sequence of events that lead to tendon mineralization formed by EO. Studies using preclinical models have shown a clear link between tendon trauma and EO. However, the link between tendinopathy, likely to be a much more common inciting cause and a chronic condition as opposed to acute trauma, is less well understood. In particular, does tendon overuse, and perhaps underuse too, predispose to EO? Exercise-based studies using species known to develop mineralization spontaneously could be used to address this question. The recent insight that a neuro-amplification step may be essential for the formation of HO within muscle gives rise to several questions. These studies (Kan et al. 2011; Salisbury et al. 2011) employed murine models where BMPs were delivered exogenously to wild-type strains, or strains known to overexpress BMPs in certain cell types were injured. Is a similar pathway followed in tissue environments where BMP signalling may be less pronounced and where, possibly, other growth factors or matrix components play a greater role in HO? Although it would seem likely, is this BMP-sensory neuron–mast cell signalling pathway equally important in tendons as it appears to be in muscles? Perhaps most importantly, does HO, which we believe could either result from surgical trauma or occur as a feature of tendinopathy, rely on the same amplification pathway in both settings? Kan et al. (2011) showed how the BMP-sensory neuron–mast cell pathway lead to an increase in inflammatory cells (likely bone marrow–derived). Unlike tendon wounding, a prominent invasion of extrinsic inflammatory cells is not considered a feature of tendinopathy (Maffulli et al. 1998), but possibly this view will be challenged in the case of tendinopathy featuring HO.
Why certain species such as the mouse readily develop tendon mineralization with ageing but others rarely do so, is unknown. That a shift towards a chondrocytic phenotype occurs in tendinopathic equine tendons and overuse injured rat rotator cuff tendons but fails to progress to mineralization would suggest more effective regulation of the process in these species. In addition, why some mouse strains show differences in response to an osteogenic stimulus is also poorly understood, but may be a fruitful line of investigation to understand the mechanisms which regulate HO within human tendons. Better understanding of the mechanisms by which tendon mineralization develops will lead to improved treatment and prevention strategies.
Although lesions of mineralization are associated with tendon failure, little else is known about their biomechanical impact. It is reasonable to expect that such lesions may alter tendon stiffness and therefore impact the function of this tendon as an elastic energy store (Alexander 1991). Does mineralization also accelerate the process of ‘overuse’ by increasing the strain experienced by non-mineralized areas of the tissue? In turn, could this cause tendons to fail remote from sites of mineralization? Finally, although larger and more fragmented lesions are associated with rotator cuff tendon pain (Le Goff et al. #b200), the relationship between lesion characteristics (including dimensions, composition and surface appearance) and changes in tendon biomechanical properties is unknown. This information may be of great value for clinical management.
The anecdotal association between intratendinous corticosteroid injection and mineralization in equine tendons is interesting and worthy of further investigation, given the widespread use of corticosteroids to treat tendinopathy in human clinical practice (Hart 2011).
Models of tendon mineralization
Animal models are necessary to address many of the gaps in our understanding of tendon mineralization. Currently available animal models of tendon mineralization include transection of the Achilles tendon in rats and mice (McClure 1983; Rooney et al. 1992; Rooney et al. 1993; Hannallah et al. 2004; Lin et al. 2006, 2009), patellar tendon window injury in rats (Lui et al. 2009a,b, 2011, 2012), collagenase injection into the patellar or Achilles tendons in rats (Lui et al. 2009a,b; Yee Lui et al. 2011) and injection of human recombinant BMP 2 into the Achilles tendon in rabbits (Hashimoto et al. 2007). To date, mineralization has not been clearly demonstrated in preclinical models of tendon overuse or underuse.
We have developed and performed initial characterization of a novel needle injury model of Achilles tendon mineralization in mice which offers advantages over existing models of tendon mineralization. The injury is relatively simple to perform, initially only minor tendon fibre disruption occurs (Figure 3), and the biomechanical properties of murine Achilles tendons may be readily tested (Rigozzi et al. 2009). Furthermore, it will be possible to take advantage of known genetic differences between strains to explore the potential genetic components of this disease.
Figure 3.
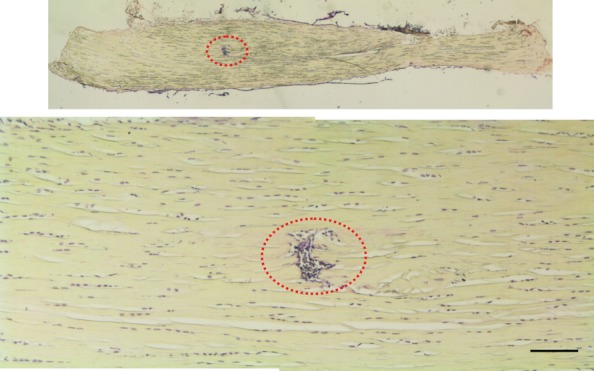
Coronal section of a C57Bl/6J murine Achilles tendon 24 h following needle injury which was performed approximately midway along the tendon length. Cells were stained with haematoxylin. Low (top; ×2.5) and high power (bottom; ×10) magnification images. Encircled area indicates an accumulation of nucleated cells at the site of needle injury. Bar = 100 µm. The disrupted fibres represent a small proportion of the tendon width so that, unlike tendon transection models, mechanical testing can be performed immediately following injury. In addition, it is likely that lesion size is fairly consistent compared with collagenase injection injury in which considerable variability has been noted (Foland et al. 1992).
Under an operating microscope, the skin overlying the Achilles tendon is incised and the tendon is isolated. A 23G hypodermic needle (635 μm external diameter) is introduced midline causing sharp transection of central and peripheral tendon fibres. Therefore, compared with suture repaired models of murine Achilles tendon injury (Palmes et al. 2002), the injury is relatively simple to perform. The procedure is performed under general anaesthesia, and perioperative buprenorphine analgesia is provided.
Based on its histological appearance (columns of chondrocyte-like cells and proteoglycan deposition) and real-time gene expression (alkaline phosphatase, collagen types II and X, fibroblast growth factor receptor 3 and osteopontin), we have confirmed that mineralization following this injury likely involves EO (O'Brien 2009).
Marusic et al. (1999) demonstrated marked differences in the responses of one outbred and seven inbred mouse strains to an osteoinductive stimulus (bone gelatin implanted in the hindlimb). Four strains (DBA/2J, RFM/Rij, C57BL/6J and AKR/J) showed induction of cartilage and/or bone consistently whereas the others (C3Hf/Bu, BALB/cJ, A/J and CD1) developed a much smaller volume or no new tissue. Furthermore, within the ‘good’ responders, the response varied: the DBA/2J, RFM/Rij and AKR/J strains showed a similar ratio (by volume) of new bone and cartilage and more bone marrow, whereas in the C57BL/6J strain, cartilage predominated. Presumably related to these strain-specific responses, Jepsen et al. (2008) demonstrated differences in the rate of EO during fracture repair. These intraspecies differences offer the possibility of quantitative trait locus (QTL) analysis.
To quantify the development of mineralization following needle injury, limbs from aged non-injured and younger injured male C57Bl/6 mice (Charles River, Saint Constant, Canada) were examined using micro-computed tomography (Scanco μCT35 system; Scanco Medical, Brüttisellen, Switzerland). All animal procedures followed a protocol approved by the University of Calgary Health Sciences Animal Care Committee and complied with guidelines from the Canadian Council on Animal Care. Injury was performed at 10 weeks of age, and the mice were killed 5 (n = 3) and 20 (n = 2) weeks later. A single age-, sex- and weight-matched control animal of the same strain was killed at 20 weeks also. The location of the injury was standardized by using a pair of customized forceps to elevate the Achilles tendon and enable the needle to be introduced 1–2 mm proximal to the enthesis. The following settings were employed to yield a 10-μm isotropic voxel size: 20.5 mm field of view; 45 kVP; 177 μA; 1000 projections/180°; 400-ms integration time and reconstruction on a 2048 × 2048 matrix. Hindlimb scans included the fusion of the tibia and distal fibula proximally and at least 1.5 mm of the calcaneus distally.
Mineralization was present in the region of the Achilles tendon in single hindlimbs from two aged (65 and 92 weeks old) non-injured individuals (Figure 4). In two mice killed 5 weeks following injury, small foci of mineralization were present close to the enthesis of the Achilles tendon in injured limbs (Figure 5a,b; mean total volume = 0.0017 mm3). In the third animal, a lesion of similar size was noted in the same location on the greyscale reconstruction but its density was too low for extraction using the global thresholding procedure (Figure 5c). There was no evidence of mineralization within the contralateral limb in any of these individuals. In animals killed 20 weeks following injury, large multifocal lesions (mean total volume = 0.3499 mm3) were present in the injured limbs, and much smaller lesions (mean total volume = 0.00935 mm3) detected in the contralateral non-injured limbs (Figure 6a,b). In a single age-, sex-, weight- and strain-matched control animal, there was no evidence of mineralization associated with the Achilles tendon in either limb (Figure 6c).
Figure 4.
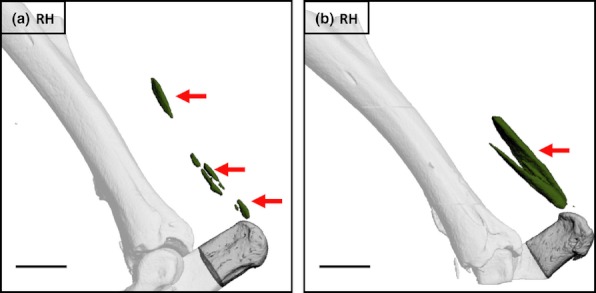
Mineralized lesions (arrows) detected in the region of the Achilles tendon within single hindlimbs from two aged non-injured C57Bl/6 male mice. a = 65 weeks old, b = 92 weeks old. Bars = 1 mm.
Figure 5.
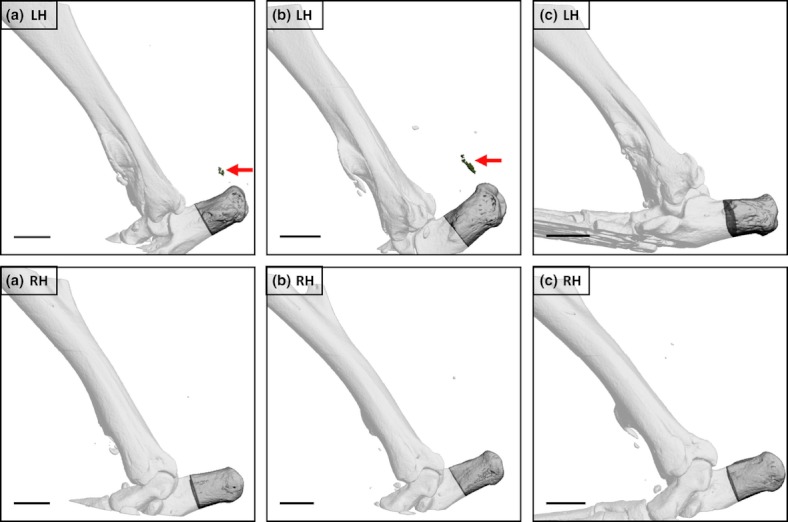
Left (LHs) and right (RHs) hindlimbs from three male C57Bl/6 mice 5 weeks following LH needle injury (performed when 10 weeks old). Arrows indicate mineralization close to the enthesis of the Achilles tendon within the LH in two of three individuals (a, b). A mineralized lesion was present in the same location in the third animal (c) but the density was too low for extraction using global thresholding protocols. No Achilles tendon-associated mineralization was detectable in the RH in any animal. Additional opacities are visible close to the Achilles tendon in some images but were positioned outside the skin or, in one case, had a far higher density than bone in that hindlimb and likely represented metallic debris deposited at the time of surgery. Bars = 1mm.
Figure 6.
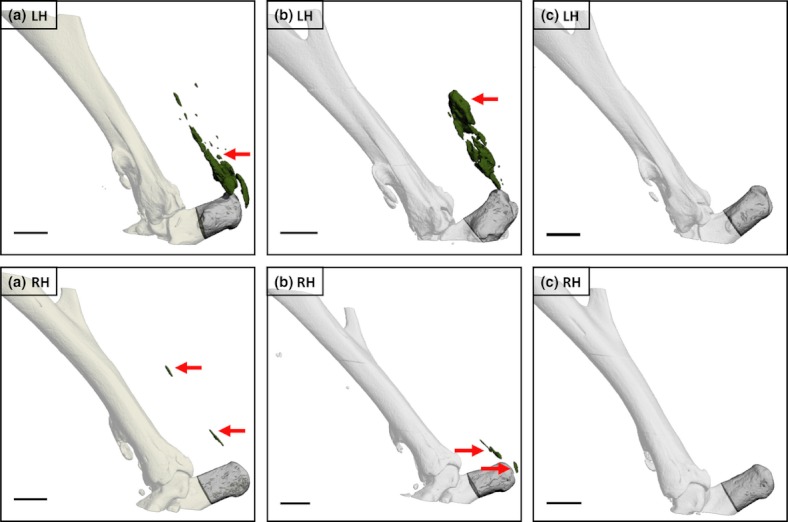
Left (LHs) and right (RHs) hindlimbs from two male C57Bl/6 mice killed 20 weeks following LH needle injury (a, b) and from a third non-injured age-, sex- and weight-matched control animal (c). Injury was performed when 10 weeks old. Arrows indicate large mineralized lesions associated with the Achilles tendon within the LHs of injured animals and smaller lesions in the contralateral limbs. No mineralized lesions were detectable in either hindlimb of the non-operated control. Bars = 1 mm.
This is the first report to describe use of μCT to study tendon mineralization and confirms that it is a sensitive tool for quantification of these lesions using our model. Mineralization was detectable as early as 5 weeks following needle injury and injury accelerated mineralization, which occurs naturally with age in this mouse strain, in both injured and contralateral non-injured limbs. Given the large size of the lesions, it should also be possible to detect mineralization using less sensitive scanners, and in particular equipment suitable for longitudinal in vivo studies. However, in view of the known inhibitory effect of radiation on heterotopic mineralization (Board et al. 2007; Baird & Kang 2009), initial longitudinal studies using this injury model will require additional no-radiation controls. Using this experimental system, we will be able to study both the biomechanical impact and biological mechanisms of HO in tendons.
Summary
Mineralization is a common feature of rotator cuff tendinopathy and may also occur at other sites of tendinopathy and following surgical tendon trauma. Affected tendons may be painful and biomechanically abnormal. Frequently, lesions are likely to develop by EO or a mechanism involving elements of the EO process, with osteogenic BMPs playing a central role. Inflammation is likely to initiate BMP production by inflammatory cells or tendon cells. However, amplification of the inflammatory response could be a critical event. BMPs acting on sensory neurons may result in the release of SP that acts on mast cells. The mast cells may in turn lead to an enhanced inflammatory response, leading subsequently to more BMP production. Bone marrow- and locally-derived cells likely contribute to the development of lesions. Locally-derived progenitors may predominate during formation of the cartilage anlagen. Although there may be tissue differences, these locally-derived cells could arise from the endothelium, neural tissue or the tendon stem cell niche. We have presented a novel model of partial Achilles tendon injury which also results in HO by EO. Coupled with μCT examination, this model will enable the biomechanical impact and biological mechanisms of tendon mineralization to be studied.
Acknowledgments
This research was supported by a Seed Grant (Hart/F1031018121) from the University of Calgary Research Grants Committee and the Alberta Innovates-Health Solutions Interdisciplinary Team Grant in Osteoarthritis (AIHS ITG OA). Dr EJO O'Brien is currently in receipt of a postdoctoral research fellowship from the AIHS ITG OA. We are grateful to Dr H Buie (University of Calgary) for guidance in the execution and analysis of murine hindlimb μCT scans. Early work leading to the development of this preclinical model was undertaken by Dr O'Brien while a PhD candidate under the supervision of Profs. CM Kielty and DA McGrouther (University of Manchester, UK). During this period, Dr O'Brien was generously supported by a Veterinary Research Training Scholarship from the Horserace Betting Levy Board (UK).
Declaration of interest
The authors have no conflicts of interest.
References
- Alexander RM. Energy-saving mechanisms in walking and running. J. Exp. Biol. 1991;160:55–69. doi: 10.1242/jeb.160.1.55. [DOI] [PubMed] [Google Scholar]
- Araldi E, Schipani E. Hypoxia, HIFs and bone development. Bone. 2010;47:190–196. doi: 10.1016/j.bone.2010.04.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault JM, Jelinsky SA, Lake SP, Hill AA, Glaser DL, Soslowsky LJ. Rat supraspinatus tendon expresses cartilage markers with overuse. J. Orthop. Res. 2007;25:617–624. doi: 10.1002/jor.20347. [DOI] [PubMed] [Google Scholar]
- Archer RS, Bayley JI, Archer CW, Ali SY. Cell and matrix changes associated with pathological calcification of the human rotator cuff tendons. J. Anat. 1993;182(Pt 1):1–11. [PMC free article] [PubMed] [Google Scholar]
- Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int. J. Exp. Pathol. 2007;88:217–226. doi: 10.1111/j.1365-2613.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ateschrang A, Gratzer C, Weise K. Incidence and effect of calcifications after open-augmented Achilles tendon repair. Arch. Orthop. Trauma Surg. 2008;128:1087–1092. doi: 10.1007/s00402-007-0441-5. [DOI] [PubMed] [Google Scholar]
- Attia M, Scott A, Duchesnay A, et al. Alterations of overused supraspinatus tendon: a possible role of glycosaminoglycans and HARP/pleiotrophin in early tendon pathology. J. Orthop. Res. 2012;30:61–71. doi: 10.1002/jor.21479. [DOI] [PubMed] [Google Scholar]
- Backman LJ, Andersson G, Wennstig G, Forsgren S, Danielson P. Endogenous substance P production in the Achilles tendon increases with loading in an in vivo model of tendinopathy-peptidergic elevation preceding tendinosis-like tissue changes. J. Musculoskelet. Neuronal Interact. 2011;11:133–140. [PubMed] [Google Scholar]
- Baird EO, Kang QK. Prophylaxis of heterotopic ossification – an updated review. J. Orthop. Surg. Res. 2009;4:12. doi: 10.1186/1749-799X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- Board TN, Karva A, Board RE, Gambhir AK, Porter ML. The prophylaxis and treatment of heterotopic ossification following lower limb arthroplasty. J. Bone Joint Surg. Br. 2007;89:434–440. doi: 10.1302/0301-620X.89B4.18845. [DOI] [PubMed] [Google Scholar]
- Byon CH, Javed A, Dai Q, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J. Biol. Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne CM, Takebe J, Offenbacher S, Cooper LF. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone. 2002;30:26–31. doi: 10.1016/s8756-3282(01)00638-x. [DOI] [PubMed] [Google Scholar]
- Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am. J. Respir. Crit. Care Med. 2002;165:1654–1669. doi: 10.1164/rccm.2108054. [DOI] [PubMed] [Google Scholar]
- Clegg PD, Strassburg S, Smith RK. Cell phenotypic variation in normal and damaged tendons. Int. J. Exp. Pathol. 2007;88:227–235. doi: 10.1111/j.1365-2613.2007.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton RE, Rideout DF. Tears of the humeral rotator cuff; a radiological and pathological necropsy survey. J. Bone Joint Surg. Br. 1964;46:314–328. [PubMed] [Google Scholar]
- Cunningham NS, Paralkar V, Reddi AH. Osteogenin and recombinant bone morphogenetic protein 2B are chemotactic for human monocytes and stimulate transforming growth factor beta 1 mRNA expression. Proc. Natl. Acad. Sci. U.S.A. 1992;89:11740–11744. doi: 10.1073/pnas.89.24.11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling BA, Dart AJ, Hodgson DR, Smith RK. Superficial digital flexor tendonitis in the horse. Equine Vet. J. 2000;32:369–378. doi: 10.2746/042516400777591138. [DOI] [PubMed] [Google Scholar]
- Eliasson P, Andersson T, Aspenberg P. Influence of a single loading episode on gene expression in healing rat Achilles tendons. J. Appl. Physiol. 2012;112:279–288. doi: 10.1152/japplphysiol.00858.2011. [DOI] [PubMed] [Google Scholar]
- Evans EB, Smith JR. Bone and joint changes following burns; a roentgenographic study; preliminary report. J. Bone Joint Surg. Am. 1959;41-A:785–799. [PubMed] [Google Scholar]
- Fenwick S, Harrall R, Hackney R, et al. Endochondral ossification in Achilles and patella tendinopathy. Rheumatology (Oxford) 2002;41:474–476. doi: 10.1093/rheumatology/41.4.474. [DOI] [PubMed] [Google Scholar]
- Foland JW, Trotter GW, Powers BE, Wrigley RH, Smith FW. Effect of sodium hyaluronate in collagenase-induced superficial digital flexor tendinitis in horses. Am. J. Vet. Res. 1992;53:2371–2376. [PubMed] [Google Scholar]
- Gosens T, Hofstee DJ. Calcifying tendinitis of the shoulder: advances in imaging and management. Curr. Rheumatol. Rep. 2009;11:129–134. doi: 10.1007/s11926-009-0018-0. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, van Zonneveld AJ, ten Dijke P. Transforming growth factor beta-induced endothelial-to-mesenchymal transition: a switch to cardiac fibrosis? Trends Cardiovasc. Med. 2008;18:293–298. doi: 10.1016/j.tcm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Hannallah D, Peng H, Young B, Usas A, Gearhart B, Huard J. Retroviral delivery of Noggin inhibits the formation of heterotopic ossification induced by BMP-4, demineralized bone matrix, and trauma in an animal model. J. Bone Joint Surg. Am. 2004;86-A:80–91. doi: 10.2106/00004623-200401000-00013. [DOI] [PubMed] [Google Scholar]
- Hart L. Corticosteroid and other injections in the management of tendinopathies: a review. Clin. J. Sport Med. 2011;21:540–541. doi: 10.1097/01.jsm.0000407929.35973.b9. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Yoshida G, Toyoda H, Takaoka K. Generation of tendon-to-bone interface “enthesis” with use of recombinant BMP-2 in a rabbit model. J. Orthop. Res. 2007;25:1415–1424. doi: 10.1002/jor.20447. [DOI] [PubMed] [Google Scholar]
- Hedtmann A, Fett H. [So-called humero-scapular periarthropathy–classification and analysis based on 1,266 cases] Z. Orthop. Ihre Grenzgeb. 1989;127:643–649. doi: 10.1055/s-2008-1040306. [DOI] [PubMed] [Google Scholar]
- Hurt G, Baker CL., Jr Calcific tendinitis of the shoulder. Orthop. Clin. North Am. 2003;34:567–575. doi: 10.1016/s0030-5898(03)00089-0. [DOI] [PubMed] [Google Scholar]
- Inamasu J, Guiot BH, Sachs DC. Ossification of the posterior longitudinal ligament: an update on its biology, epidemiology, and natural history. Neurosurgery. 2006;58:1027–1039. doi: 10.1227/01.NEU.0000215867.87770.73. discussion 1027–1039. [DOI] [PubMed] [Google Scholar]
- Jarvela T, Paakkala T, Kannus P, Toivanen J, Jarvinen M. Ultrasonographic and power Doppler evaluation of the patellar tendon ten years after harvesting its central third for reconstruction of the anterior cruciate ligament: comparison of patients without or with anterior knee pain. Am. J. Sports Med. 2004;32:39–46. doi: 10.1177/0095399703258619. [DOI] [PubMed] [Google Scholar]
- Jarvinen M, Jozsa L, Kannus P, Jarvinen TL, Kvist M, Leadbetter W. Histopathological findings in chronic tendon disorders. Scand. J. Med. Sci. Sports. 1997;7:86–95. doi: 10.1111/j.1600-0838.1997.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Jepsen KJ, Price C, Silkman LJ, et al. Genetic variation in the patterns of skeletal progenitor cell differentiation and progression during endochondral bone formation affects the rate of fracture healing. J. Bone Miner. Res. 2008;23:1204–1216. doi: 10.1359/JBMR.080317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa Y, Morihara T, Watanabe N, et al. GFP chimeric models exhibited a biphasic pattern of mesenchymal cell invasion in tendon healing. J. Cell. Physiol. 2007;210:684–691. doi: 10.1002/jcp.20876. [DOI] [PubMed] [Google Scholar]
- Kan L, Liu Y, McGuire TL, et al. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem cells (Dayton, Ohio) 2009;27:150–156. doi: 10.1634/stemcells.2008-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan L, Lounev VY, Pignolo RJ, et al. Substance P signaling mediates BMP dependent heterotopic ossification. J. Cell. Biochem. 2011;112:2759–2772. doi: 10.1002/jcb.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J. Bone Joint Surg. Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- Kaplan FS, Glaser DL, Shore EM, et al. Hematopoietic stem-cell contribution to ectopic skeletogenesis. J. Bone Joint Surg. Am. 2007;89:347–357. doi: 10.2106/JBJS.F.00472. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Kuroda E, Yamashita U. Mechanisms of enhanced macrophage-mediated prostaglandin E2 production and its suppressive role in Th1 activation in Th2-dominant BALB/c mice. J. Immunol. 2003;170:757–764. doi: 10.4049/jimmunol.170.2.757. [DOI] [PubMed] [Google Scholar]
- Le GoffB, Berthelot JM, Guillot P, Glémarec J, Maugars Y. Assessment of calcific tendonitis of rotator cuff by ultrasonography: comparison between symptomatic and asymptomatic shoulders. Joint Bone Spine. 2010;77:258–263. doi: 10.1016/j.jbspin.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Lencel P, Delplace S, Pilet P, et al. Cell-specific effects of TNF-alpha and IL-1beta on alkaline phosphatase: implication for syndesmophyte formation and vascular calcification. Lab. Invest. 2011;91:1434–1442. doi: 10.1038/labinvest.2011.83. [DOI] [PubMed] [Google Scholar]
- Li X, Cao X. BMP signaling and skeletogenesis. Ann. N. Y. Acad. Sci. 2006;1068:26–40. doi: 10.1196/annals.1346.006. [DOI] [PubMed] [Google Scholar]
- Lin L, Chen L, Wang H, et al. Adenovirus-mediated transfer of siRNA against Runx2/Cbfa1 inhibits the formation of heterotopic ossification in animal model. Biochem. Biophys. Res. Commun. 2006;349:564–572. doi: 10.1016/j.bbrc.2006.08.089. [DOI] [PubMed] [Google Scholar]
- Lin L, Shen Q, Xue T, Yu C. Heterotopic ossification induced by Achilles tenotomy via endochondral bone formation: expression of bone and cartilage related genes. Bone. 2009;46:425–431. doi: 10.1016/j.bone.2009.08.057. [DOI] [PubMed] [Google Scholar]
- Lin L, Shen Q, Leng H, Duan X, Fu X, Yu C. Synergistic inhibition of endochondral bone formation by silencing Hif1alpha and Runx2 in trauma-induced heterotopic ossification. Mol. Ther. 2011;19:1426–1432. doi: 10.1038/mt.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo UG, Olivia F, Denaro V, Maffulli N. Oxygen species and overuse tendinopathy in athletes. Disabil. Rehabil. 2008;30:1563–1571. doi: 10.1080/09638280701785643. [DOI] [PubMed] [Google Scholar]
- Lounev VY, Ramachandran R, Wosczyna MN, et al. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J. Bone Joint Surg. Am. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui PP, Chan LS, Cheuk YC, Lee YW, Chan KM. Expression of bone morphogenetic protein-2 in the chondrogenic and ossifying sites of calcific tendinopathy and traumatic tendon injury rat models. J. Orthop. Surg. Res. 2009a;4:27. doi: 10.1186/1749-799X-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui PP, Fu SC, Chan LS, Hung LK, Chan KM. Chondrocyte phenotype and ectopic ossification in collagenase-induced tendon degeneration. J. Histochem. Cytochem. 2009b;57:91–100. doi: 10.1369/jhc.2008.952143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui PP, Chan LS, Fu SC, Chan KM. Expression of sensory neuropeptides in tendon is associated with failed healing and activity-related tendon pain in collagenase-induced tendon injury. Am. J. Sports Med. 2010;38:757–764. doi: 10.1177/0363546509355402. [DOI] [PubMed] [Google Scholar]
- Lui PP, Cheuk YC, Lee YW, Chan KM. Ectopic chondro-ossification and erroneous extracellular matrix deposition in a tendon window injury model. J. Orthop. Res. 2012;30:37–46. doi: 10.1002/jor.21495. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840–843. doi: 10.1016/s0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- Maksymowych WP, Chiowchanwisawakit P, Clare T, Pedersen SJ, Ostergaard M, Lambert RG. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum. 2009;60:93–102. doi: 10.1002/art.24132. [DOI] [PubMed] [Google Scholar]
- Marusic A, Katavic V, Grcevic D, Lukic IK. Genetic variability of new bone induction in mice. Bone. 1999;25:25–32. doi: 10.1016/s8756-3282(99)00095-2. [DOI] [PubMed] [Google Scholar]
- Maugars Y, Varin S, Gouin F, et al. Treatment of shoulder calcifications of the cuff: a controlled study. Joint Bone Spine. 2009;76:369–377. doi: 10.1016/j.jbspin.2008.10.016. [DOI] [PubMed] [Google Scholar]
- McClure J. The effect of diphosphonates on heterotopic ossification in regenerating Achilles tendon of the mouse. J. Pathol. 1983;139:419–430. doi: 10.1002/path.1711390403. [DOI] [PubMed] [Google Scholar]
- Mielants H, Vanhove E, de Neels J, Veys E. Clinical survey of and pathogenic approach to para-articular ossifications in long-term coma. Acta Orthop. Scand. 1975;46:190–198. doi: 10.3109/17453677508989207. [DOI] [PubMed] [Google Scholar]
- Mobasheri A, Barrett-Jolley R, Carter SD, Martin-Vasallo P, Schulze-Tanzil G, Shakibaei M. Functional roles of mechanosensitive ion channels, ß1 integrins and kinase cascades in chondrocyte mechanotransduction. In: Kamkin A, Kiseleva I, editors. Moscow: Academia; 2005. Mechanosensitivity in Cells and Tissues. [PubMed] [Google Scholar]
- Muschol M, Muller I, Petersen W, Hassenpflug J. Symptomatic calcification of the medial collateral ligament of the knee joint: a report about five cases. Knee Surg. Sports Traumatol. Arthrosc. 2005;13:598–602. doi: 10.1007/s00167-004-0598-1. [DOI] [PubMed] [Google Scholar]
- Nakase T, Takeuchi E, Sugamoto K, et al. Involvement of multinucleated giant cells synthesizing cathepsin K in calcified tendinitis of the rotator cuff tendons. Rheumatology (Oxford) 2000;39:1074–1077. doi: 10.1093/rheumatology/39.10.1074. [DOI] [PubMed] [Google Scholar]
- Neuwirth J, Fuhrmann RA, Veit A, et al. Expression of bioactive bone morphogenetic proteins in the subacromial bursa of patients with chronic degeneration of the rotator cuff. Arthritis. Res. Ther. 2006;8:R92. doi: 10.1186/ar1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien EJO. United Kingdom: The University of Manchester; 2009. An evaluation of whether multipotent mesenchymal stromal cells may be used to improve the quality of tendon repair. Faculty of Life Sciences. [Google Scholar]
- Oliva F, Barisani D, Grasso A, Maffulli N. Gene expression analysis in calcific tendinopathy of the rotator cuff. Eur. Cell. Mater. 2011a;21:548–557. doi: 10.22203/ecm.v021a41. [DOI] [PubMed] [Google Scholar]
- Oliva F, Via AG, Maffulli N. Calcific tendinopathy of the rotator cuff tendons. Sports Med. Arthrosc. 2011b;19:237–243. doi: 10.1097/JSA.0b013e318225bc5f. [DOI] [PubMed] [Google Scholar]
- Palmes D, Spiegel HU, Schneider TO, et al. Achilles tendon healing: long-term biomechanical effects of postoperative mobilization and immobilization in a new mouse model. J. Orthop. Res. 2002;20:939–946. doi: 10.1016/S0736-0266(02)00032-3. [DOI] [PubMed] [Google Scholar]
- Pedersen SJ, Chiowchanwisawakit P, Lambert RG, Ostergaard M, Maksymowych WP. Resolution of inflammation following treatment of ankylosing spondylitis is associated with new bone formation. J. Rheumatol. 2011;38:1349–1354. doi: 10.3899/jrheum.100925. [DOI] [PubMed] [Google Scholar]
- Richards PJ, Braid JC, Carmont MR, Maffulli N. Achilles tendon ossification: pathology, imaging and aetiology. Disabil. Rehabil. 2008;30:1651–1665. doi: 10.1080/09638280701785866. [DOI] [PubMed] [Google Scholar]
- Rifas L. T-cell cytokine induction of BMP-2 regulates human mesenchymal stromal cell differentiation and mineralization. J. Cell. Biochem. 2006;98:706–714. doi: 10.1002/jcb.20933. [DOI] [PubMed] [Google Scholar]
- Rigozzi S, Muller R, Snedeker JG. Local strain measurement reveals a varied regional dependence of tensile tendon mechanics on glycosaminoglycan content. J. Biomech. 2009;42:1547–1552. doi: 10.1016/j.jbiomech.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Riley GP, Harrall RL, Constant CR, Cawston TE, Hazleman BL. Prevalence and possible pathological significance of calcium phosphate salt accumulation in tendon matrix degeneration. Ann. Rheum. Dis. 1996;55:109–115. doi: 10.1136/ard.55.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolf C, Movin T. Etiology, histopathology, and outcome of surgery in achillodynia. Foot Ankle Int. 1997;18:565–569. doi: 10.1177/107110079701800906. [DOI] [PubMed] [Google Scholar]
- Rooney P, Grant ME, McClure J. Endochondral ossification and de novo collagen synthesis during repair of the rat Achilles tendon. Matrix. 1992;12:274–281. doi: 10.1016/s0934-8832(11)80079-x. [DOI] [PubMed] [Google Scholar]
- Rooney P, Walker D, Grant ME, McClure J. Cartilage and bone formation in repairing Achilles tendons within diffusion chambers: evidence for tendon-cartilage and cartilage-bone conversion in vivo. J. Pathol. 1993;169:375–381. doi: 10.1002/path.1711690315. [DOI] [PubMed] [Google Scholar]
- Salisbury E, Rodenberg E, Sonnet C, et al. Sensory nerve induced inflammation contributes to heterotopic ossification. J. Cell. Biochem. 2011;112:2748–2758. doi: 10.1002/jcb.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki D, Hatori M, Kotajima S, Tanaka K, Kokubun S. Ossification of the Achilles tendon–a case report. Scott. Med. J. 2005;50:174–175. doi: 10.1177/003693300505000412. [DOI] [PubMed] [Google Scholar]
- Schubert TE, Weidler C, Lerch K, Hofstadter F, Straub RH. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann. Rheum. Dis. 2005;64:1083–1086. doi: 10.1136/ard.2004.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengar DP, McKendry RJ, Uhthoff HK. Increased frequency of HLA-A1 in calcifying tendinitis. Tissue Antigens. 1987;29:173–174. doi: 10.1111/j.1399-0039.1987.tb01571.x. [DOI] [PubMed] [Google Scholar]
- Seyahi A, Demirhan M. Arthroscopic removal of intraosseous and intratendinous deposits in calcifying tendinitis of the rotator cuff. Arthroscopy. 2009;25:590–596. doi: 10.1016/j.arthro.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Shen Q, Little SC, Xu M, et al. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J. Clin. Invest. 2009;119:3462–3472. doi: 10.1172/JCI37412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat. Rev. Rheumatol. 2010;6:518–527. doi: 10.1038/nrrheum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumia R, Lombardi AR, Altieri E. The petrified ear-a manifestation of dystrophic calcification. Dermatology. 1997;194:371–373. [PubMed] [Google Scholar]
- Suda RK, Billings PC, Egan KP, et al. Circulating osteogenic precursor cells in heterotopic bone formation. Stem cells (Dayton, Ohio) 2009;27:2209–2219. doi: 10.1002/stem.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton GM, Shao X, Chung M, et al. Changes in mechanical loading lead to tendonspecific alterations in MMP and TIMP expression: influence of stress deprivation and intermittent cyclic hydrostatic compression on rat supraspinatus and Achilles tendons. Br. J. Sports Med. 2010;44:698–703. doi: 10.1136/bjsm.2008.050575. [DOI] [PubMed] [Google Scholar]
- Tseng WP, Yang SN, Lai CH, Tang CH. Hypoxia induces BMP-2 expression via ILK, Akt, mTOR, and HIF-1 pathways in osteoblasts. J. Cell. Physiol. 2010;223:810–818. doi: 10.1002/jcp.22104. [DOI] [PubMed] [Google Scholar]
- Tsujii A, Tanaka Y, Yonetani Y, Iuchi R, Shiozaki Y, Horibe S. Symptomatic calcification of the anterior cruciate ligament: a case report. Knee. 2012;19:223–225. doi: 10.1016/j.knee.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Tsumaki N, Yoshikawa H. The role of bone morphogenetic proteins in endochondral bone formation. Cytokine Growth Factor Rev. 2005;16:279–285. doi: 10.1016/j.cytogfr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Uhthoff HK. Calcifying tendinitis, an active cell-mediated calcification. Virchows Arch. A Pathol. Anat. Histol. 1975;366:51–58. doi: 10.1007/BF00438677. [DOI] [PubMed] [Google Scholar]
- Uhthoff HK, Loehr JW. Calcific tendinopathy of the rotator cuff: pathogenesis, diagnosis, and management. J. Am. Acad. Orthop. Surg. 1997;5:183–191. doi: 10.5435/00124635-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Welfing J, Kahn MF, Desroy M, Paollaggi JB, DeSeze S. Les calcifications de l'epaule. Il La maladie des calcifications tendineuses multiples. Rev. Rhum. 1965;32:325–334. [PubMed] [Google Scholar]
- Williams RB, Harkins LS, Hammond CJ, Wood JL. Racehorse injuries, clinical problems and fatalities recorded on British racecourses from flat racing and National Hunt racing during 1996, 1997 and 1998. Equine Vet. J. 2001;33:478–486. doi: 10.2746/042516401776254808. [DOI] [PubMed] [Google Scholar]
- Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 2010;49:1618–1631. doi: 10.1093/rheumatology/keq045. [DOI] [PubMed] [Google Scholar]
- Yang G, Im HJ, Wang JH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. 2005;363:166–172. doi: 10.1016/j.gene.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee Lui PP, Wong YM, Rui YF, Lee YW, Chan LS, Chan KM. Expression of chondro-osteogenic BMPs in ossified failed tendon healing model of tendinopathy. J. Orthop. Res. 2011;29:816–821. doi: 10.1002/jor.21313. [DOI] [PubMed] [Google Scholar]
- Yu YY, Lieu S, Lu C, Miclau T, Marcucio RS, Colnot C. Immunolocalization of BMPs, BMP antagonists, receptors, and effectors during fracture repair. Bone. 2010;46:841–851. doi: 10.1016/j.bone.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang JH. Production of PGE(2) increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J. Orthop. Res. 2010;28:198–203. doi: 10.1002/jor.20962. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang JH. BMP-2 mediates PGE(2) -induced reduction of proliferation and osteogenic differentiation of human tendon stem cells. J. Orthop. Res. 2012;30:47–52. doi: 10.1002/jor.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]


