Abstract
The objective of this study was to investigate the hepatoprotective effect of a bark extract of Bathysa cuspidata extract (BCE) in a murine model of severe liver injury induced by carbon tetrachloride (CCl4). Forty-two Wistar rats were randomized into six groups of seven animals each: Group 1(G1): CCl4; Group 2 (G2): dimethyl sulfoxide (DMSO) + CCl4; Group 3 (G3): BCE 400 mg/kg alone; Group 4 (G4): BCE 200 mg/kg + CCl4; Group 5 (G5): BCE 400 mg/kg + CCl4; Group 6 (G6): DMSO alone. The extract was administered by gavage for 18 days beginning 6 days prior to the first application of CCl4. After completing CCl4 administration, the animals were euthanized. The animals in G1, G2, G4 and G5 experienced significant body weight loss and had an increased liver somatic index compared with G3 and G6 (P < 0.05). A significant reduction in serum aspartate and alanine transaminase and gamma-glutamyl transferase (P < 0.05) and a significant increase in the activity of the anti-oxidant enzyme superoxide dismutase were found in G5 (P < 0.05). Lower proportions of cellular necrosis and lipid droplets were found in the livers of animals in G4 and G5 compared with G1 and G2 (P < 0.05). These results confirm the marked hepatoprotective activity of the bark extract of Bathysa cuspidata in severe injuries induced by CCl4 in rats and suggest that this effect may be associated with the inhibition of oxidative damage.
Keywords: carbon tetrachloride, hepatotoxicity, oxidative stress, phytotherapy
The prevalence of liver diseases induced by chemical agents has increased progressively over recent decades in various countries (Lee 2003). The liver is involved in xenobiotic metabolism, playing an important role in detoxifying the organism. Following exposure to hepatotoxic compounds such as products originating from fungi, bacterial metabolites, heavy metals, environmental pollutants and chemotherapeutic agents, the liver becomes vulnerable to several disorders (Ranawat et al. 2010).
Carbon tetrachloride (CCl4) is an industrial solvent with known hepatotoxic activity. CCl4 induces liver lesions by producing radical species (•CCl3 and •Cl) derived from its metabolism through the mitochondrial NADPH/cytochrome P450 enzyme system (Brattin et al. 1985; Bedda et al. 2003). These radicals are highly reactive and may progressively oxidize unsaturated lipids and proteins from cell membranes, culminating in alterations to cell structure and function and eventually in cell death (Brattin et al. 1985). Owing to its low cost and good reproducibility in inducing liver injury, CCl4 is widely used in animal models of hepatotoxicity. Furthermore, this experimental model represents an important tool in the investigation of plants with a potentially hepatoprotective effect that is currently used in traditional medicine, even though their efficacy remains to be proven scientifically (Ranawat et al. 2010; Xiao-Yan et al. 2010).
Biodiversity is a great reservoir of bioactive secondary metabolites that, in conjunction with traditional knowledge, have led to the discovery of drugs for the treatment of different human pathologies such as cancer, infections, ulcers, diabetes and bronchitis. In this context, various countries have sought to introduce medicinal plants as an integrated and supplementary practice in primary health care. However, although different plants are used in popular medicine, it is estimated that <10% of plants have been investigated in sufficient depth to evaluate their therapeutic properties (Ranawat et al. 2010).
Bathysa cuspidata, which belongs to the Rubiaceae family, is a plant popularly known in South America as ‘quina-do-mato’, the bark of which is generally used for the production of tonics that are used in folk medicine for the treatment of various disorders including stomach and liver problems, as well as anti-inflammatory and healing agents (Novaes et al. 2012a,b). A previous study conducted by our research group indicated the potential pneumoprotective and hepatoprotective effect of the B. cuspidata extract (BCE) in rats exposed to paraquat (Novaes et al. 2012a,b). However, paraquat does not affect the structure and function of the liver in the same way as CCl4. Thus, as the pathogenic mechanism of CCl4 is completely different from that of paraquat and the resultant liver injury is markedly more severe, the experimental model used in this study was designed to determine whether the action spectrum of the BCE also offers protection in drastic conditions of liver injury. To clarify its relevance, efficacy and possible mechanism of action, the objective of this study was to investigate the effect of a bark extract of Bathysa cuspidata in a murine model of severe liver injury induced by CCl4.
Materials and methods
Plant collection and preparation of the extract
The species B. cuspidata was collected in a biome of the Atlantic rainforest in the state of Minas Gerais, Brazil (20°43′00″S and 42°29′10″W, at 1200 m above sea level). Samples of the bark were separated, dried at room temperature for 48 h in a dark, well-ventilated room and then pulverized in a knife mill and stored. Samples were documented and deposited at the herbarium of the Federal University of Viçosa (VIC 21559).
Dried bark of B. cuspidata (500 g) was subjected to exhaustive extraction by percolation with 95% ethanol. The extract was concentrated under vacuum in a rotary evaporator at a temperature ≤50 °C. To completely remove the solvent, the extract was then lyophilized, producing 139 g of dry extract (BCE) (27.8% yield). Phytochemical screening of the major groups of natural products in the BCE was performed on chromatography plates coated with silica gel GF 254® (Merck, Darmstadt, Germany) using different mobile phases and detection reagents in accordance with the protocol described by Wagner and Bladts (2009).
Phytochemical characterization of the extract
To measure the total phenol and proanthocyanidin contents, powdered stem bark (1.0 g) was extracted with 200 ml of water at 100 °C under reflux for 30 min. The concentration of total phenols was determined colorimetrically (absorbance at 760 nm) using the Folin–Ciocalteau method (Verza et al. 2007).
The proanthocyanidin content was determined using the procedure of Price et al. (1980). The absorbance of the sample and standard were measured at 500 nm and the proanthocyanidin was expressed as milligrams of catechin equivalents per gram of dry matter.
The content of total flavonoids was determined using rutin as the reference compound. This method is based on the formation of a flavonoid–aluminium trichloride complex with maximum absorption at 420 nm. The absorption of standard rutin solution in methanol was measured under the same conditions. All determinations were performed in triplicate and the results were averaged.
Chemicals
The diagnostic kits used to evaluate aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP) and bilirubin were acquired from human in vitro diagnostics (Itabira, MG, Brazil). Superoxide dismutase (SOD) activity was measured using a commercial kit (Cayman Chemical Company, Ann Arbor, MI, USA).
Animals
Forty-two male Wistar rats weighing 235 ± 37.62 g were kept in an experimental animal house under controlled light (12 h light/dark cycle) and temperature (21 ± 2 °C), with relative air humidity of 60–70%. Water and standard rat chow were supplied ad libitum.
Ethical approval
The research protocol was approved by the Ethics Committee for the Care and Use of Laboratory Animals of the Federal University of Minas Gerais (UFMG) (protocol 122/2008).
Model of severe liver injury and treatment groups
The animals were randomly divided into six groups of seven animals in accordance with the treatment administered. Group 1 (G1): CCl4; Group 2 (G2): dimethyl sulfoxide (DMSO) + CCl4 (vehicle control); Group 3 (G3): BCE 400 mg/kg (control extract); Group 4 (G4): BCE 200 mg/kg + CCl4; Group 5 (G5): BCE 400 mg/kg + CCl4; Group 6 (G6): DMSO. Liver injury was induced by intraperitoneal administration of CCl4 (60% v/v, 1 ml/kg) every 48 h for 12 days (Xiao-Yan et al. 2010). Administration of BCE at 200 mg/kg (BCE 200) and 400 mg/kg (BCE 400) was initiated 6 days prior to the first application of CCl4 and continued to be administered to the animals during the 12 days of application of CCl4. The same protocol described previously to administer the extract was used for the group of animals treated with vehicle (DMSO) alone. The BCE was resuspended in 700 μl (w/v) of the vehicle DMSO and administered by gavage.
Biometric analysis and tissue collection
The animals were weighed before and after the experiment. Forty-eight hours after receiving the final treatment the animals were euthanized by deep anaesthesia (Ketamine 45 mg/kg and Xylazine, 5 mg/kg) followed by cardiac puncture. The livers were removed en bloc and weighed. The liver somatic index (LSI,%) was calculated by normalizing the liver weight by the final body weight. For histopathological analysis and biochemical measurements in liver tissue, two fragments of the liver (median lobe) from each animal were rapidly removed, one being immersed in Karnovsky's fixative solution for 24 h and the other frozen in liquid nitrogen (−196 °C) respectively.
Biochemical analysis
Aliquots of blood were centrifuged and the serum was immediately used for the biochemical analyses of AST, ALP, GGT and total bilirubin. Aliquots of 500 mg of the frozen liver (median lobe) were homogenized in phosphate buffer and centrifuged under refrigeration (5 °C) and the supernatant was used for the analysis of SOD and catalase (CAT). CAT was evaluated by measuring the rate of hydrogen peroxide decomposition (Aebi 1984). Lipid hydroperoxides were detected using the methodology standardized by Nourooz-Zadeh et al. (1994). Total protein levels were measured using the Bradford method (Bradford 1976).
Histopathological analysis
For the histopathological analysis, the fragments fixed in Karnovsky's were dehydrated in ethanol and embedded in methacrylate (Leica, Nussloch, Germany). Serial 4-μm-thick sections were cut in a Multicut 2045® rotary microtome (Reichert-Jung, Nussloch, Germany) and stained with haematoxylin–eosin. Seventy random images were captured for each group using a BX-60® light microscope (Olympus, Tokyo, Japan) connected to a QColor-3® digital camera (Olympus, Tokyo, Japan). A test system with 200 test points was placed over each digitalized image to quantify the proportion of necrotic areas and lipid droplets in the liver tissue (% per histological area). image pro-plus 4.5® software was used (Media Cybernetics, Silver Spring, MD, USA) in these morphological analyses.
Statistical analysis
The data were expressed as means and standard deviations (mean ± SD). The normality of the data was verified using the Kolmogorov–Smirnov test. The biochemical data were submitted to unifactorial analysis of variance (one-way anova), followed by Tukey's test for multiple comparisons. The Kruskal–Wallis test was used to analyse the morphological parameters. P < 0.05 was regarded as significant. All tests were performed using the graphpad Prism 5.0® statistical software program (GraphPad, Sand Diego, CA, USA).
Results
Preliminary phytochemical analysis
The phytochemical analysis of the BCE showed positive results for alkaloids, coumarins, flavonoids, tannins and triterpenes. The total phenol and proanthocyanidin content was 58.7 mg/g of dry matter (expressed as pirogalol) and 37.9 mg/g dry matter (expressed as catechin) respectively. The total flavonoid content was 3.4 mg/g dry matter using the calibration curve of rutin.
Biometric parameters
At the beginning of the study, the animals' weight did not differ between groups (Table 1). At the end of the experiment, body weight had decreased in all groups; however, the animals in G3 and G6 were significantly heavier than the animals in the other groups (P < 0.05). The animals in G1, G2 and G4 had a significantly higher LSI than the other groups.
Table 1.
Effects of treatment with bark extract of Bathysa cuspidata extract (BCE) on biometric parameters of rats exposed to CCl4
| Groups | Initial body weight (g) | Final body weight (g) | Variation weight (g) | LSI (%) |
|---|---|---|---|---|
| G1 | 271.43 ± 20.56a | 210.05 ± 10.13a | −61.38 ± 16.41a | 5.97 ± 0.42a |
| G2 | 259.29 ± 10.97a | 211.14 ± 8.59a | −48.15 ± 15.36a | 5.85 ± 0.51a |
| G3 | 263.58 ± 20.35a | 247.43 ± 16.47b | −16.15 ± 13.09b | 4.10 ± 0.49b |
| G4 | 256.71 ± 13.67a | 215.00 ± 8.01a | −41.71 ± 10.17a | 5.71 ± 0.43a |
| G5 | 268.57 ± 14.06a | 212.14 ± 23.07a | −56.42 ± 12.10a | 4.41 ± 0.53b |
| G6 | 261.45 ± 13.49a | 250.33 ± 11.52b | −11.12 ± 8.73b | 4.18 ± 0.50b |
LSI, liver somatic index. Liver injury was induced by intraperitoneal administration of CCl4 (60% v/v, 1 ml/kg) every 48 h for 12 days. Group 1 (G1): CCl4; Group 2 (G2): dimethyl sulfoxide (DMSO) + CCl4; Group 3 (G3): BCE (400 mg/kg); Group 4 (G4): BCE 200 + CCl4; Group 5 (G5): BCE 400 + CCl4; Group 6 (G6): DMSO. (−) Negative sign indicates weight loss. Data are expressed as mean ± SD. Different letters in the columns indicate statistical difference between groups (P < 0.05), and groups with the same letter do not differ statistically, one-way anova.
Measurement of serum enzymes
Serum AST and ALT levels were significantly lower in the animals in G3 and G6 compared with the other groups and higher in G1, G2 and G4 compared with G3, G5 and G6 (P < 0.05). Higher GGT levels were found in G1 and G2 compared with the other groups (P < 0.05). The total bilirubin levels were significantly lower in the animals in G3 and G6 (P < 0.05) compared with all other groups (Table 2).
Table 2.
Effects of treatment with bark extract of Bathysa cuspidata extract (BCE) on metabolic parameters of rats exposed to CCl4
| Groups | AST (U/l) | ALT (U/l) | GGT (U/l) | T-bilirubin (mg/dl) |
|---|---|---|---|---|
| G1 | 718.71 ± 142.34a | 144.10 ± 35.47a | 12.14 ± 6.96a | 0.61 ± 0.34a |
| G2 | 620.85 ± 116.52a | 126.82 ± 30.11a | 8.57 ± 2.57a | 0.47 ± 0.16a |
| G3 | 87.85 ± 20.86c | 47.58 ± 5.19c | 3.51 ± 1.00b | 0.18 ± 0.10b |
| G4 | 482.71 ± 118.30a | 118.91 ± 28.77a | 3.71 ± 1.79b | 0.50 ± 0.24a |
| G5 | 166.14 ± 44.99b | 69.21 ± 6.35b | 3.01 ± 1.25b | 0.40 ± 0.13a |
| G6 | 52.11 ± 16.95c | 42.29 ± 4.50c | 2.57 ± 0.97b | 0.20 ± 0.10b |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyl transferase; T-bilirubin, total bilirubin. Liver injury was induced by intraperitoneal administration of CCl4 (60% v/v, 1 ml/kg) every 48 h for 12 days. Group 1 (G1): CCl4; Group 2 (G2): dimethyl sulfoxide (DMSO) + CCl4; Group 3 (G3): BCE (400 mg/kg); Group 4 (G4): BCE 200 + CCl4; Group 5 (G5): BCE 400 + CCl4; Group 6 (G6): DMSO. Data are expressed as mean ± SD. Different letters in the columns indicate statistical difference between groups (P < 0.05), and groups with the same letter do not differ statistically, one-way anova.
Effects of Bathysa cuspidata extract on hydroperoxide levels
The effect of the BCE on hepatic hydroperoxide levels is presented in Figure 1. Intoxication with CCl4 resulted in a significant increase in the hydroperoxide levels in G1 and G2 compared with the other groups (P < 0.05). Additionally, this parameter was higher in G4 compared with G3, G5 and G6 (P < 0.05). Lower hydroperoxide levels were detected in G3 and G6 compared with the other groups (P < 0.05).
Figure 1.
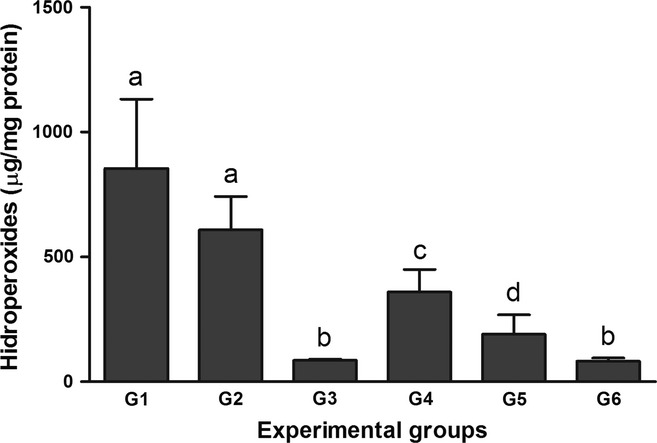
Effects of treatment with bark extract of B. cuspidata extract (BCE 200 and 400 mg/kg) on hepatic levels of lipid hydroperoxides in Wistar rats exposed to CCl4 (60% v/v, 1 ml/kg) every 48 h for 12 days. Group 1 (G1): CCl4; Group 2 (G2): dimethyl sulfoxide(DMSO) + CCl4; Group 3 (G3): BCE (400 mg/kg); Group 4 (G4): BCE 200 + CCl4; Group 5 (G5): BCE 400 + CCl4; Group 6 (G6): DMSO. The data are expressed as means ± SD. a,b,c Different letters in columns indicate statistical significance between the groups (P < 0.05), and groups with the same letter do not differ statistically, anova followed by Tukey's test.
Histopathology
Administration of CCl4 led to a high degree of tissue disorganization and lesions in the livers of animals in G1 and G2, in which intense areas of necrosis and lipid degeneration were found (Figure 2). Figure 3 shows a significantly higher proportion of lipid droplets and necrosis in the animals of G1 and G2 compared with those in the groups that received the extract or DMSO alone (P < 0.05). There was a significant reduction in lipid droplets and necrosis in the liver of animals in G4 and G5 compared with G1 and G2 (P < 0.05). The smallest quantity of lipid droplets and necrosis was found in animals in G3 and G6, because these groups were not treated with CCl4. The data described previously were confirmed by histological analysis of the groups, as shown in Figure 2.
Figure 2.
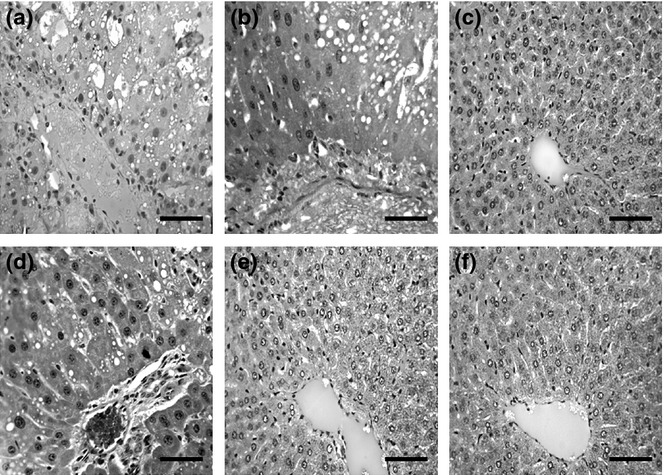
Representative photomicrographs of the liver tissue from Wistar rats observed under light microscope (hematoxylin and eosin, bar = 150 μm). Note Hepatic injury was induced by intraperitoneal administration of CCl4 (60% v/v, 1 ml/kg) every 48 h for 12 days. Group 1 (G1): CCl4; Group 2 (G2): dimethyl sulfoxide(DMSO) + CCl4; Group 3 (G3): B. cuspidata extract (BCE) (400 mg/kg); Group 4 (G4): BCE 200 + CCl4; Group 5 (G5): BCE 400 + CCl4; Group 6 (G6): DMSO.
Figure 3.
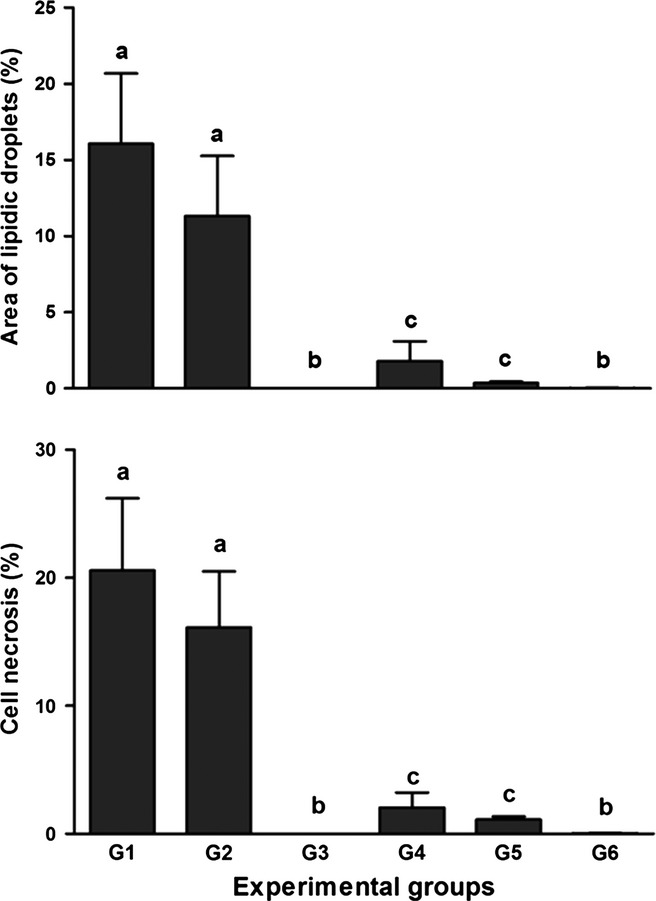
Effects of treatment with bark extract of B. cuspidata extract (BCE 200 and 400 mg/kg) on the proportion of lipid droplets and cell necrosis in the liver tissue of Wistar rats exposed to CCl4 (60% v/v, 1 ml/kg) every 48 h for 12 days. Group 1 (G1): CCl4; Group 2 (G2): dimethyl sulfoxide (DMSO) + CCl4; Group 3 (G3): BCE (400 mg/kg); Group 4 (G4): BCE 200 + CCl4; Group 5 (G5): BCE 400 + CCl4; Group 6 (G6): DMSO. The data are expressed as means ± SD. a,b,c Different letters indicate statistical significance between the groups (P < 0.05), and groups with the same letter do not differ statistically, anova followed by Tukey's test.
Determination of superoxide dismutase and catalase activities in liver tissue
Figure 4 presents the CAT and SOD activity in the liver tissue. There was a reduction in the activity of CAT in G1 and G2 compared with the other groups (P < 0.05), with the exception of G3 and G6. There were no significant differences between G3, G4 and G5.
Figure 4.
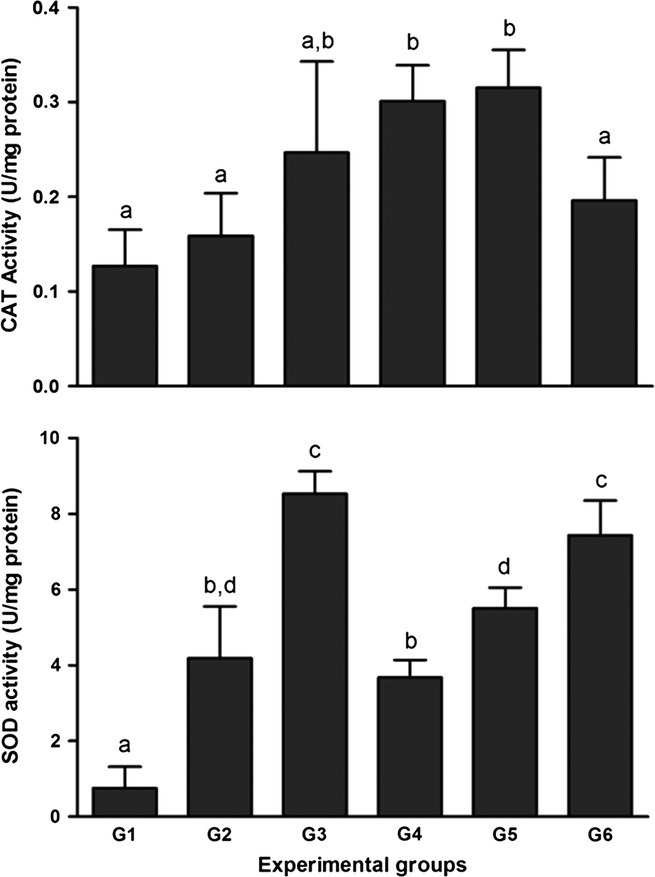
Effects of treatment with bark extract of B. cuspidata extract (BCE 200 and 400 mg/kg) on hepatic activity of Catalase (CAT) and superoxide dismutase (SOD) in Wistar rats exposed to CCl4 (60% v/v, 1 ml/kg) every 48 h for 12 days. Group 1 (G1): CCl4; Group 2 (G2): dimethyl sulfoxide (DMSO) + CCl4; Group 3 (G3): BCE (400 mg/kg); Group 4 (G4): BCE 200 + CCl4; Group 5 (G5): BCE 400 + CCl4; Group 6 (G6): DMSO. The data are expressed as means ± SD. a,b,c Different letters indicate statistical significance between the groups (P < 0.05), and groups with the same letter do not differ statistically, anova followed by Tukey's test.
A significant reduction in SOD activity was found in G1 compared with the other groups (P < 0.05). The activity of SOD was significantly higher in G3 and G6 compared with the other groups (P < 0.05). There were no statistically significant differences when G2 was compared to G4 and G5, although SOD activity was higher in G5 compared with G4 (P < 0.05), indicating a possible dose–effect relationship between the doses of BCE evaluated (Figure 4).
Discussion
It is known that CCl4 intoxication leads to systemic changes commonly manifested in clinical signs such as lethargy, anorexia and weight loss (Faremi et al. 2008). In the present study, weight loss was apparent in all animals at the end of the experiment compared with baseline measurements. Additionally, the LSI was higher in all groups intoxicated with CCl4, except in G5. These findings indicate that the metabolic dysfunction caused by CCl4 exerts a significant effect on the body and liver weight (Ranawat et al. 2010; Xiao-Yan et al. 2010) and that in this study, the BCE was not enough to maintain the animal's weight. However, the highest dose of the extract prevented the increase in the LSI, suggesting a dose-dependent effect on liver weight, possibly related to the inhibition of hepatic oedema. These findings can be related to the characteristics of the model used, which was designed to induce severe liver injury and hepatic morphological reorganization.
There were high serum levels of AST, ALT and GGT in the groups exposed to CCl4, which indicates pathological modification of membrane integrity and hepatocyte function (Drotman & Lawhorn 1978; Dufour et al. 2001; Ozer et al. 2008). The animals treated with BCE in G5 had reduced serum levels of AST, ALT and GGT, suggesting a possible dose-dependent effect of the extract. The hepatoprotective index of a drug can be evaluated by its ability to reduce harmful effects and preserve liver morphophysiology. Therefore, AST, ALT and GGT are often used as indirect markers of hepatic injury and are considered sensitive parameters to evaluate functional status (Drotman & Lawhorn 1978; Dufour et al. 2001; Ozer et al. 2008).
Lipid peroxidation is a typical metabolic process related to the pathogenesis of morphofunctional liver injuries (Brattin et al. 1985; Novaes et al. 2012a,b). Although lipid peroxidation also occurs under physiological conditions, external factors may amplify this process, leading to intense membrane lipid oxidation and eventually cell death (Brattin et al. 1985). During severe toxic liver injuries induced by CCl4, the tissue levels of oxidative markers such as hydroperoxides are frequently high, especially in the liver (Ranawat et al. 2010). This marker reflects the level of stress caused by the release of free radicals in vivo, because hydroperoxides are one of the main products of the decomposition of polyunsaturated fatty acids from the plasma membrane during oxidative events (Girotti 1998). In the present study, hydroperoxide levels were highest in G1 and G2. Therefore, it is clear that the extract protected the animals in G4 and G5 from possible lesions caused by CCl4, because the levels of this marker remained low in these groups, indicating a dose-dependent effect.
In the processes of biological oxidation, CAT and SOD play an important role in protecting the liver against the toxic effects of many xenobiotics (Novaes et al. 2012b), representing a cellular defence mechanism against reactive oxygen species (Parola et al. 1999). During intense tissue oxidation, the levels of antioxidant enzymes are frequently reduced because the high levels of production and accumulation of 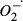 induced by CCl4 increase enzymatic consumption in the intoxicated tissues (Zhang et al. 2005; Novaes et al. 2012a,b). This feature was also apparent in the present study, in which the levels of CAT and SOD were lower in G1. Interestingly, in G4 and G5 treatment with the BCE was able to increase the activity of these enzymes, indicating a possible dose-dependent effect related to SOD activity. In addition, the groups treated with DMSO (G2 and G6) clearly showed an increase in SOD activity compared with G1. The potential influence of DMSO on hepatic metabolism has previously been reported (Sahin et al. 2004). Although DMSO interfered with enzymatic activity, it seems that this effect was specific for SOD. However, little is known about the metabolic pathways influenced by DMSO, which modulate the activity of antioxidant enzymes, and this issue requires further study.
induced by CCl4 increase enzymatic consumption in the intoxicated tissues (Zhang et al. 2005; Novaes et al. 2012a,b). This feature was also apparent in the present study, in which the levels of CAT and SOD were lower in G1. Interestingly, in G4 and G5 treatment with the BCE was able to increase the activity of these enzymes, indicating a possible dose-dependent effect related to SOD activity. In addition, the groups treated with DMSO (G2 and G6) clearly showed an increase in SOD activity compared with G1. The potential influence of DMSO on hepatic metabolism has previously been reported (Sahin et al. 2004). Although DMSO interfered with enzymatic activity, it seems that this effect was specific for SOD. However, little is known about the metabolic pathways influenced by DMSO, which modulate the activity of antioxidant enzymes, and this issue requires further study.
Histopathological analysis represents a reproducible and reliable method used to evaluate the hepatoprotective effect of a drug, especially in cases in which the chemical characteristics and dose of a xenobiotic provides sufficient toxicity to induce morphological tissue disorganization (De la Iglesia & McGuire 1981; Novaes et al. 2012b). Lipid degeneration is a morphophysiological alteration in hepatocytes that occurs as a result of various metabolic disorders, including CCl4 poisoning (Brattin et al. 1985). In the present study, the BCE effectively minimized tissue injury, including the cellular accumulation of fat induced by CCl4. In addition to the accumulation of lipids, CCl4 also promotes the production of the trichloromethyl peroxy radical, which binds covalently to macromolecules and causes degradation of the cell membrane, leading to liver necrosis (Brattin et al. 1985). Less necrosis of hepatocytes was observed in groups exposed to CCl4 and treated with the BCE, corroborating the protective effect of this extract against tissue necrosis. Therefore, it is reasonable to infer that the BCE minimizes the toxic mechanisms mentioned earlier, protecting cells from the damage induced by CCl4. The anti-oxidant action of the BCE may be associated with the presence of secondary metabolites with anti-oxidant effects, such as polyphenol compounds, tannins and flavonoids, as shown in a preliminary phytochemical investigation (Novaes et al. 2012a,b). High levels of phenol compounds, mainly flavonoids and proanthocyanidins, may be responsible for the anti-oxidant activity of the BCE, because there are several reports attributing this property to these groups of secondary metabolites (Abdel-Hameed 2009; Novaes et al. 2012a,b). In fact, the BCE contained high levels of total phenol compounds, substances with recognized anti-oxidant activity that are found in many plant extracts (Rice-Evans et al. 1997; Kähkönen et al. 1999; Fukumoto & Mazza 2000). In this context, this study supports the hypothesis that the phytochemicals identified in the BCE could have free radical scavenger activity that is able to act together with CAT and SOD in the antioxidant defence process, reducing the depletion of these enzymes and the hepatotoxicity induced by CCl4. These results make the species B. cuspidata a potential source for the discovery of new bioactive natural products that could be used to prevent and/or treat diseases related to oxidative stress, either in the form of extracts, fractions or purified compounds.
Author contributions
All listed authors meet ICMJE authorship criteria and that nobody who qualifies for authorship has been excluded. Authors contributed to research design, acquisition, analysis and interpretation of data; drafting the article or revising it critically; approval of the submitted and final versions.
Funding source
Financial support from “Fundação de Amparo à Pesquisa do Estado de Minas Gerais” (FAPEMIG) and “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” CAPES.
Conflict of interest
The Authors declare that there is no conflict of interest.
References
- Abdel-Hameed ESS. Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chem. 2009;114:1271–1277. [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Bedda S, Laurent A, Conti F, et al. Mangafodipir prevents liver injury induced by acetaminophen in the mouse. J. Hepatol. 2003;39:765–772. doi: 10.1016/s0168-8278(03)00325-8. [DOI] [PubMed] [Google Scholar]
- Bradford MA. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal. Biochem. 1976;82:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brattin WJ, Glende EA, Jr, Recknagel RO. Pathological mechanisms in carbon tetrachloride hepatotoxicity. J. Free Radic. Biol. Med. 1985;1:27–38. doi: 10.1016/0748-5514(85)90026-1. [DOI] [PubMed] [Google Scholar]
- De la Iglesia FA, McGuire EJ. Quantitative stereology: toxicologic pathology applications. Toxicol. Pathol. 1981;9:21–28. [Google Scholar]
- Drotman RB, Lawhorn GT. Serum enzymes as indicators of chemical induced liver damage. Drug Chem. Toxicol. 1978;1:163–171. doi: 10.3109/01480547809034433. [DOI] [PubMed] [Google Scholar]
- Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin. Chem. 2001;47:1133–1135. doi: 10.1093/clinchem/46.12.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faremi TY, Suru SM, Fafunso MA, Obioha EU. Hepatoprotective potentials of Phyllanthus amarus against ethanol-induced oxidative stress in rats. Food Chem. Toxicol. 2008;8:2658–2664. doi: 10.1016/j.fct.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Fukumoto LR, Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000;48:3597–3604. doi: 10.1021/jf000220w. [DOI] [PubMed] [Google Scholar]
- Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- Kähkönen MP, Hopia AI, Vuorela HJ, et al. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Lee WM. Drug-induced hepatotoxicity. N. Engl. J. Med. 2003;349:474–481. doi: 10.1056/NEJMra021844. [DOI] [PubMed] [Google Scholar]
- Nourooz-Zadeh J, Tajaddini-Sarmadi J, Wolf SP. Measurement of plasma hydroperoxide concentrations by the ferrous oxidation xylenol orange assay in conjunction with thiphenylphosphine. Anal. Biochem. 1994;220:403–409. doi: 10.1006/abio.1994.1357. [DOI] [PubMed] [Google Scholar]
- Novaes RD, Gonçalves RV, Cupertino MC, et al. Bark extract of Bathysa cuspidata attenuates extra-pulmonary acute lung injury induced by paraquat and reduces mortality in rats. Int. J. Exp. Pathol. 2012a;93:225–233. doi: 10.1111/j.1365-2613.2012.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novaes RD, Gonçalves RV, Marques DC, et al. Effect of bark extract of Bathysa cuspidata on hepatic oxidative damage and blood glucose kinetics in rats exposed to paraquat. Toxicol. Pathol. 2012b;40:62–70. doi: 10.1177/0192623311425059. [DOI] [PubMed] [Google Scholar]
- Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245:194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Parola M, Bellomo G, Robino G, Barrera G, Dianzani MU. 4- Hydroxynonental as a biological signal: molecular basis and pathophysiological implications. Antioxid. Redox Signal. 1999;1:255–284. doi: 10.1089/ars.1999.1.3-255. [DOI] [PubMed] [Google Scholar]
- Price ML, Hagerman AE, Butler LG. Tannin content of cowpeas, chickpeas, pigeon peas and mung beans. J. Agric. Food Chem. 1980;28:459–461. doi: 10.1021/jf60228a047. [DOI] [PubMed] [Google Scholar]
- Ranawat L, Bhatt J, Patel J. Hepatoprotective activity of ethanolic extracts of bark of Zanthoxylum armatum DC in CCl4 induced hepatic damage in rats. J. Ethnopharmacol. 2010;127:777–780. doi: 10.1016/j.jep.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- Sahin M, Avsar FM, Ozel H, et al. The effects of dimethyl sulfoxide on liver damage caused by ischemia-reperfusion. Transplant. Proc. 2004;36:2590–2592. doi: 10.1016/j.transproceed.2004.09.057. [DOI] [PubMed] [Google Scholar]
- Verza SG, Kreinecker MT, Reis V, Henriques AT, Ortega GG. Evaluation of analytical variables of the Folin-Ciocalteu method for the quantitation of the total tannins content using a Psidium guajava L. leaves aqueous extract as a model. Quim. Nova. 2007;30:815–820. [Google Scholar]
- Wagner H, Bladts S. Plant Drug Analysis. In: Wagner H, Bladts S, editors. A Thin Layer Chromatography Atlas. Berlin: Springer; 2009. pp. 62–73. [Google Scholar]
- Xiao-Yan J, Qing-An Z, Zhi-Qi Z, et al. Hepatoprotective effects of almond oil against carbon tetrachloride induced liver injury in rats. Food Chem. Toxicol. 2010;125:673–678. [Google Scholar]
- Zhang D, Wu J, Zhang S, Huang J. Oleanane triterpenes from Aegiceras corniculatum. Phytother. Res. 2005;76:131–133. doi: 10.1016/j.fitote.2004.10.017. [DOI] [PubMed] [Google Scholar]


