Abstract
Lymph nodes (LN) are one of the important sites in the body where immune responses to pathogenic antigens are initiated. This immunological function induced by cells within the LN is an extensive area of research. To clarify the general function of LN, to identify cell populations within the lymphatic system and to describe the regeneration of the lymph vessels, the experimental surgical technique of LN dissection has been established in various animal models. In this review different research areas in which LN dissection is used as an experimental tool will be highlighted. These include regeneration studies, immunological analysis and studies with clinical questions. LN were dissected in order to analyse the different cell subsets of the incoming lymph in detail. Furthermore, LN were identified as the place where the induction of an antigen-specific response occurs and, more significantly, where this immune response is regulated. During bacterial infection LN, as a filter of the lymph system, play a life-saving role. In addition, LN are essential for the induction of tolerance against harmless antigens, because tolerance could not be induced in LN-resected animals. Thus, the technique of LN dissection is an excellent and simple method to identify the important role of LN in immune responses, tolerance and infection.
Keywords: immune response, infection, oral tolerance
Introduction
The lymphoid system consists of three different types of lymphoid tissues: primary, secondary and tertiary lymphoid. The primary lymphoid organs are the bone marrow (BM) and thymus, and the secondary lymphoid organs include the spleen, Peyer's patches (PP) and lymph nodes (LN). Tertiary lymphoid tissues develop during inflammation and are therefore highly variable structures. As this review focuses on LN dissection, all other lymphoid tissue structures will not be mentioned further (for more details see [1]).
In mammals, LN are located all over the body. They all have the same architecture and are populated by the same cell types (Fig. 1). Their function is to filter the lymph coming from the draining area and to scan the lymph for antigens. Either an immune response to pathogenic antigens is initiated or, in the case of harmless antigens, tolerance [2]. In brief, antigen-loaded dendritic cells (DC), coming from the draining area via the afferent lymphatics, present their antigens to T lymphocytes in the T cell area or the paracortex. T cells which are T cell receptor-specific for the presented antigens are activated; they differentiate and proliferate. T helper cells, one class of activated T lymphocytes, migrate into the B cell area or cortex to assist B cells. These antigen-specific B cells differentiate into plasma cells for effective antibody production. All activated effector cells, such as plasma cells, CD4+ or CD8+ T cells, migrate to the medulla, where they leave the LN via efferent lymphatics or the blood system to travel to the inflamed or endangered area of their specific draining area. This precise migration is possible because of homing molecules which are up-regulated on effector cells after activation. Recently, a new cell population, the backbone-forming stromal cells, has been identified as also being involved in this process [3]. These cells are able to present antigens to lymphocytes, and play a role in the up-regulation of homing molecules such as DC [4,5].
Fig. 1.
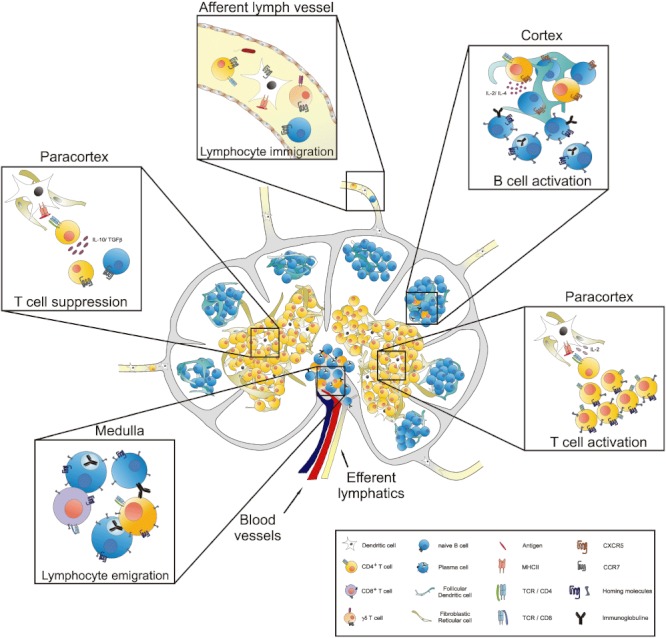
Function of the lymph nodes (LN). LN are the site where immune reactions or suppression takes place. Cells of the afferent or efferent lymphatics are collected via LN dissection and the cells can be analysed. As well as different dendritic cell (DC) populations, different lymphocyte subpopulations such as CD4+ T cells or γδ T cells are also detected. Within the paracortex of the lymph node, DC present antigens to T cells which proliferate and differentiate into effector or helper T cells after recognizing the specific antigen whereby an immune response is initiated. T helper cells migrate into the cortex to assist B cells to proliferate and differentiate into effector or plasma cells. All lymphocytes, including naive or effector cells, migrate to the medulla to leave the LN via the efferent lymphatics or the blood system. Conversely, oral tolerance is activated if regulatory T cells are activated via DC, which prevent immune response induction. Without the LN all these functions are reduced or lacking.
In contrast to immune response induction, tolerance is the unresponsiveness of the immune system via suppression of T and B cell activation by regulatory T cells, deletion or anergy.
However, there are many open questions about the function of the LN, including the migration of cells from the draining area, the role of the LN in the induction of immune responses, the control of parasites or tolerance. It is possible to use knock-out mice, e.g. lymphotoxin α or retinoic acid-related orphan receptor (Ror)-γt knock-out mice to study the function of LN. These mice have reduced or no LN, but they all have further disorders, particularly in the spleen [6,7]. To circumvent the problems of immune system dysfunction caused by these gene knock-outs, a second method of studying LN function is to remove only the LN of interest. This LN dissection technique permits identification of the role of a specific LN without affecting further organs or areas.
Therefore, in this review different research areas are illustrated where LN dissection was performed to identify the function of LN or the consequences of a missing LN.
The LN dissection technique
LN dissection is an experimental surgical technique which has been used for many years not only to analyse the role of LN in the immune system and lymph fluid transport, but also in different diseases in animal models. LN were removed from many different draining sites such as the skin-draining site (for example the axilliary LN [8], the brachial LN [9], the popliteal LN [10–12] or the inguinal LN [13,14]), the head–neck region (cervical LN [15–19]) or the peritoneal area (the mesenteric LN [20–23] and the coeliac LN [24]).
For dissection of the mesenteric LN (mLN), for example, the abdomen was opened and the gut was taken out so that the mLN were visible (Fig. 2a). The mLN were excised carefully in order not to injure the superior mesenteric artery lying behind, whereas the connection of the lymph vessels and small blood vessels to the LN was disturbed. Afterwards, the gut was replaced in the abdomen and the abdomen was closed.
Fig. 2.
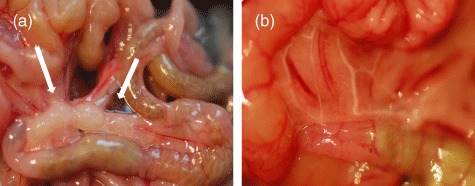
The gut system with and without mesenteric lymph nodes (mLN). Mice were anaesthetized and the abdomen was opened. The gut was taken out and the mLN were seen (a, mLN indicated by arrows). The mLN were removed carefully. Afterwards the gut was replaced in the abdomen and the abdomen was closed. After 4 weeks animals were studied by feeding oil, which is transported via the lymphatics, to check for newly connected pseudo-afferent lymphatics (b).
Monitoring lymph vessel regeneration after LN removal
LN are integrated as central organs in the lymph vessel system. The afferent lymphatics coming from the draining area, which could be the gut system or the skin, transport fluid, proteins, lipids and different cell populations of the immune system to the LN sinus. The efferent lymphatics leave the LN at the medullar site to greater LN or veins of the blood system. After LN dissection, the lymph vessel system is destroyed and the afferent and efferent system vessels are reconnected. This new regenerated lymph vessel, the so-called pseudo-afferent lymphatic vessel [25,26], develops quickly and is detectable within 7 days in mice and rats [20,27], but is regenerated fully 4 weeks after dissection [11,23].
For detection of the regeneration of the pseudo-afferent lymphatic vessels, different imaging techniques are possible: the pseudo-afferent lymphatic vessels can be strained by injecting a dye which is transported from the draining area via the lymphatics, or much more easily by applying oil by oral gavage. The oil is also transported by the lymphatic system, whereby the lymph system appears white (Fig. 2b) [20]. Lymph vessel integrity after LN dissection in other regions except the gut, for example the skin, could be shown by injecting a blue dye into the draining area which is then transported via the lymph vessels. For high-resolution analysis it is possible to employ lymphograms or lymphoscintigraphy as two-dimensional methods or single photon computed tomography–computerized tomography (SPECT-CT) magnetic resonance tomography (MRT) as a three-dimensional technique, in which contrast medium is injected and the lymphatic vessels are highlighted. These techniques allow one animal or human to be scanned several times to study the lymphangiogenesis in vivo[11,14,28,29], or in clinical use to identify sentinel lymph nodes for dissection [30]. Transmission digital microscopy is another method with which to analyse lymphatics in vivo[23]. Using this technique the cellular composition of newly developed lymph vessels has been identified, and Ikomi et al. have shown fully functional newly formed lymph vessels using X-ray lymphograms [11].
Impact of LN dissection for studying immune functions
The function of the peripheral LN at their draining sites
Different research areas using LN dissection could be identified in the field of immune function analysis. On one hand, the peripheral or skin-draining LN, and on the other hand the mesenteric LN draining the gut system, are under intensive investigation. Furthermore, various questions focus on cell migration through the lymphatic vessels to the draining LN and immune response induction after antigen administration.
Several groups have removed peripheral LN (pLN) to analyse the cell subset composition of the incoming lymph in order to identify area-specific or activated cells.
In this regard, some groups were interested in different DC populations found in the afferent lymphatics. In these studies LN were removed, the lymphatics in peripheral sites were cannulated and the DC subsets were analysed and compared to DC isolated from other tissues or other species [31,32]. One of these studies detected a similar DC subset in mice, sheep and humans, which showed not only a similar phenotype, but also a similar function [31]. Similar examinations were performed by other groups analysing the lymph of cattle. Large numbers of DC and γδ T cells were identified after removing skin-draining LN [33,34]. Furthermore, Bonneau et al. cannulated the cervical duct to analyse the lymph in sheep [35]. They identified different T cell subsets (CD4+, CD8+, γδ T cells) and B lymphocytes as well as monocytes, granulocytes and DC in the lymph [36]. To analyse monocytes, granulocytes and DC in more detail they injected a vaccine strain of Salmonella abortusovis and followed the absorbed cell types [37]. Thus, different cell populations coming from the draining area of peripheral LN were identified, and after antigen administration were analysed in more detail.
Numerous studies focus on the presence of pLN for immune response induction. One study concerns the impact of the cervical LN (cLN) of rats in activation of the immune system after antigen was microinfused into the cerebrospinal fluid [38]. It was shown that the cLN respond in an antibody producing manner for antigen which comes from the central nervous system, and furthermore, after removing the LN, the antigen-specific antibody titre in the serum was perceptably reduced. It was concluded that the LN is important for the induction of a humoral immune response to central nervous system antigens [38]. After recognizing the cLN as the brain-draining LN, Phillips et al. hypothesized that the LN play a role in multiple sclerosis (MS) as well as in experimental autoimmune encephalomyelitis (EAE), the animal model for MS. MS is thought to be an organ-specific autoimmune disorder and/or a chronic inflammatory disease of the central nervous system [39] (for more detail see [40]). Genetic risk factors [human leucocyte antigen (HLA) haplotypes] and also environmental factors (Epstein–Barr virus, smoking and sunlight exposure) were identified in MS development [40]. Pathological demyelination of different brain areas (cerebrum, brain stem or spinal cord) with axonal destruction was found. So far, CD4+ T cells and CD8+ T cells (adaptive immune system) have also been related to the disease, as well as natural killer (NK) cells, which belong to the innate immune system. All these cells were detected in higher numbers in the patients or specifically in the lesions [39,40]. Furthermore, anti-inflammatory therapies and immune modulation are beneficial to the disease process [39]. The deep and superficial cLN were removed, EAE was induced and a reduced enhancement of the disease was found. Different areas in the brain were analysed for EAE lesions and significant differences were found between LN-resected and LN-bearing rats [17]. It was concluded that removing the LN leads to a break in the pathway of immune cells into the brain which reduces the lesions found normally in EAE. More than 10 years later this study was repeated and expanded by van Zwam et al., who were able to show variations at different stages of the disease (acute, chronic and chronic relapsing EAE) which seem to be cLN-dependent. Furthermore, they concluded that tolerance of antigen from the brain is not induced in the cLN [27]. Thus, they believe that the brain-draining LN could be a useful target for therapeutic strategies against MS. The effect of cLN dissection on immunoglobulin (Ig) production and S. pneumoniae colonization after nasal vaccination with pneumococcal polysaccharide was also analysed. Decreased IgA levels were seen accompanied by fewer IgA+ cells in the serum and the analysed mucosa, and consequentially higher numbers of bacteria in the nasopharynx lavage [19].
Thus, using the LN dissection technique at peripheral sites, various studies were able to identify the role of the draining LN for the induction of a specific immune response.
The function of mesenteric LN at their draining sites
Several groups are interested in the role of the mLN and their function in the gut system. Besides lymph vessel cannulation, immune response activation was also performed after dissection of the mLN. MacPherson et al., for example, conducted many straightforward analyses in this field. They cannulated lymph vessels in rats after removing the mLN to analyse the phenotype, behaviour and function of DC in the intestinal lymph [41] (see also [26]). They demonstrated that only DC carry an applied antigen into the LN, where they present the antigen to T lymphocytes [42]. Following-up this question, they found that intestinal DC migrated into the LN, whereas another DC subset (plasmacytoid DC) did not [43]. After isolating them, they also looked at the function of these migrating DC. They reported that subpopulations of intestinal DC induce T cells to become a different subtype; for example, by producing cytokines such as interleukin (IL)-10 to induce regulatory T cells or IL-2 to induce a T helper type 1 (Th1) phenotype [44]. Rothkötter et al. [21] are also pioneers in the field of lymph cannulation; they examined the lymph fluid of pigs for all migrating cells and described the presence of different T cell subsets and immunoglobulin-producing cells.
In our studies, we were interested in the role of the mLN in immune responses triggered by the application of cholera toxin (CT) [20]. Administration of CT induced an increase of germinal centres and an increased number of antigen-specific IgA+ cells in the mLN. These antigen-specific cells were also found in higher numbers in the lamina propria of the gut, producing high amounts of antigen-specific IgA (Fig. 3) [20]. Thus, we hypothesized that the mLN play an important role in the induction of a specific immune response initiated in the gut. To our surprise, we found far higher numbers of antigen-specific IgA+ cells in the lamina propria of mLN-resected rats compared to mLN-bearing animals. In addition, higher amounts of antigen-specific IgA were measured in the gut lavage [20]. We concluded that the mLN plays a role not only in the induction of an antigen-specific response, but more significantly in the regulation of this immune response. Furthermore, there was an increase in the proliferation and number of germinal centres in the spleen. Activated B cells and antigen-specific IgM+ cells were detected and increased amounts of antigen-specific IgM were seen in the serum of mLN-resected rats [20]. Using this experimental setup, not only could the role of the mLN be analysed, but the importance and influence of other tissues on immune response induction could also be addressed.
Fig. 3.
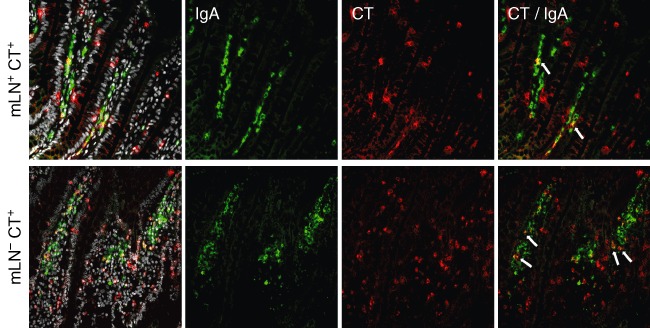
The number of antigen-specific immunoglobulin (Ig)A+ cells in the lamina propria is increased after mesenteric lymph node (mLN) resection. Immunofluorescence staining of the lamina propria of the gut in mLN-bearing and mLN-resected rats, which had been treated with cholera toxin (CT), was carried out with antibodies against IgA (green) and CT (red); 4',6-diamidino-2-phenylindole (DAPI) was used to visualize all cells. CT-specific IgA+ cells (arrows) are seen in both groups, but to a greater extent in mLN-resected animals.
Recently, a study was published in which the gut tropism of effector cells was analysed after removal of the mLN [45]. Antigen-specific T lymphocytes were injected into mLN-bearing and mLN-resected mice. Afterwards the mice were treated with ovalbumin and retinoic acid by subcutaneous injection, and the up-regulation of gut-specific homing molecules on these T lymphocytes was measured within peripheral LN, as well as the gut. It was shown that after treatment with ovalbumin and retinoic acid at the peripheral site, effector cells were generated which home to the gut region independent of the presence of the mLN [45].
Other groups working on graft-versus-host disease (GvHD), which is a major problem after transplantation, removed LN to analyse the survival of the graft as well as the host. Lück et al. studied extensively the effect of mLN resection after small bowel transplantation in the dependence of major histocompatibility complex (MHC) expression. After removing the mLN the animals survived transplantation, whereas mLN-bearing rats died within 2 weeks. Furthermore, MHC molecules were found to play a major role in GvHD and the mLN were also involved in this process by sending lymphocytes to the small bowel graft [46–48]. However, the dependence of graft survival and LN were also analysed by other groups. They all concluded that the regional LN of the graft are responsible for the GvHD, independent of the location of the transplantation [49–51]. Recently, Panoskaltsis-Mortari et al., using the bone marrow transplantation (BMT) model, showed accumulation and proliferation of T cells in the LN and spleen [52]. Further studies identified cell surface molecules such as CD103, leucocyte function-associated antigen-1 (LFA-1) or L-selectin and β7 integrin on T lymphocytes, which enables them to migrate in a molecule-dependent manner to the target organs [9,53,54]. In all these studies the absence of the molecules increased the animals' survival. Furthermore, DC, which imprinted lymphocytes, were identified as playing a major role in GvHD [55]. Thus, removing the mLN not only allowed the identification of various specific cells coming from the draining area, but also showed the impact on immune responses triggered in the LN.
The role of LN in tolerance induction
A further function of LN is the induction of mucosal tolerance. Oral tolerance is the unresponsiveness of the immune system on recognizing a harmless antigen. This phenomenon has hardly been studied and is little understood. The presence of DC and also regulatory T cells (Tregs) coming from the draining area seem to be essential for the induction of tolerance after feeding low doses of antigen [56–58]. Recently, it became clear that CD103+ DC which migrate permanently from the lamina propria to the mLN, carrying in the majority of cases microbial antigens from the commensal bacteria, produce IL-10, transforming growth factor (TGF)-β, retinoic acid and indoleamine-2, 3-dioxygenase (IDO) [57,59–62]. All these factors resulted in a tolerogenic phenotype of the DC which drive the induction of forkhead box protein 3 (FoxP3+) Tregs. These Tregs suppressed Th1 and Th2 responses. Furthermore, tolerance induced via feeding high doses of antigen resulted in anergy or depletion of antigen-specific cells [58,63]. Plasmacytoid DC seem to be responsible for this reaction [58]. To identify the role of the LN in mucosal tolerance induction, LN were removed and the lymph vessels regenerated. It was found that without the presence of the mLN oral tolerance was no longer inducible [57]. These findings are in line with a previous study, where nose-draining LN were removed and intranasal tolerance was induced. It was shown that tolerance was also prevented after removing all or two specific LN from this area [15]. Thus, LN of the draining area of the mucosal site are essential for the induction of mucosal tolerance. In future it will be interesting to study whether the LN is important as a meeting point of immune cells or whether the presence of a specific cell population within the LN is necessary.
The role of LN during infection
Other groups were interested in infection models. Different bacteria strains were injected and the development of the infection was analysed. Voedisch et al. infected control mice, CCR7-deficient mice and mice treated with a Toll-like receptor (TLR)-7/8 ligand (R848) with S. typhimurium to identify DCs as the major cell type carrying the bacteria into the mLN [22]. Compared to the control mice they found higher numbers of S. typhimurium in the mLN of R848-treated mice, which enhance the migration of DC from the gut to the mLN and reduce bacteria in CCR7-deficient mice where DC migration is disturbed. In a second step, they removed the mLN and infected the mice with S. typhimurium to identify the role of the mLN in expansion of the bacteria over the body. They detected higher numbers of bacteria in liver and spleen compared to mLN-bearing mice. Thus the mLN act as a barrier to S. typhimurium infection [22]. During Trypanosoma cruzi infection an mLN-dependent course of disease was also shown, whereby in this study the impact of T cells was more focused [64]. It was shown that T cells underwent caspase-9-dependent apoptosis after infection within the mLN, and atrophy developed for that reason. After removing the mLN the infection of T. cruzi increased compared to sham operated mice. It was concluded that mLN T cells are crucial for the control of T. cruzi infection [64]. In contrast to this study, Egan et al. found increased numbers of CD4+ T cells and also γδ T cells migrating from the skin through the afferent lymph after Lucilia cuprina infection in sheep. Furthermore, they analysed the mRNA level of these cells within the lymph and found higher levels of inflammatory cytokines such as IL-1β and IL-8 in cells cannulated after infection [65]. Other authors infected two different sheep strains with Trichostrongylus colubriformis, whereby one was resistant and the other was susceptible to the pathogen, to identify the mechanisms of the parasite during infection [66–69]. They removed the mLN of the sheep and cannulated the lymph to analyse the cells for their expression pattern. In the first study, increased levels of Th2 type and proinflammatory cytokines such as IL-5, IL-13 and tumour necrosis factor (TNF)-α were detected in the resistant sheep compared to the susceptible ones [68]. Furthermore, they showed a changed intestinal microenvironment towards Th2 response-increased specific antibody production after repeated infection [67,69] and an increase of anti-oxidant activities using the microarray technique in cannulated cells [66]. A similar life-saving role of LN was published many years ago by other groups for M. leprae and L. tropica infection. The bacteria were injected into the footpad of mice after popliteal adenectomy and a severe exacerbation of the disease was measured [13,70]. In contrast, in immune responses to diphtheria toxin or in viral infection (influenza virus PR8) no significant difference between LN-resected and LN-bearing mice was detected [18,71]. Thus, LN are involved strongly in the induction of immune responses in many different inflammatory conditions, so they play a major life-saving role in infections [19,22,64,72].
Conclusion and future prospects
There is experimental evidence to support which cell types migrate from the draining area to the LN and which function a specific cell type has in the induction of an immune response. Immune cells come together in the LN to induce a protective, directed and synchronized reaction, but many questions about the function and role of LN within the systemic organization remain to be answered. One area of research is the decision process within the LN to induce an immune response or tolerance to foreign or self-antigen. Therefore, LN dissection is an important method with which to examine all these questions (Fig. 4). Furthermore, therapeutic advantages have been found in animal models in many different diseases after LN dissection, and these also need to be determined in more detail. Understanding the mechanism of immune response or tolerance induction within the LN, and also the role of LN in systemic reaction, will lead to new insights for therapeutic studies.
Fig. 4.
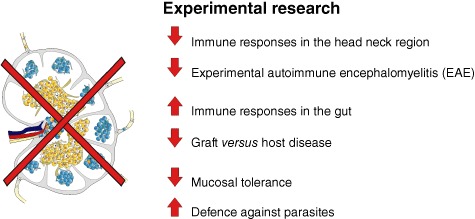
The role of lymph nodes (LN) in research. LN are unique organs distributed all over the body. They have to filter the lymph for incoming antigens from the draining area to defend the body. Removing the LN leads to various different responses in the body. For example, the immune response in the gut system is enhanced after LN dissection, whereas the immune responses in the head–neck region are diminished.
Acknowledgments
We wish to thank Melanie Bornemann for excellent technical assistance, Sheila Fryk for correction of the English and Matthias Ochs for critical reading of this manuscript. The work was supported by the Deutsche Forschungsgemeinschaft (SFB621/ A10).
Disclosure
The authors declare no conflicts of interest.
References
- 1.Pabst R. Plasticity and heterogeneity of lymphoid organs. What are the criteria to call a lymphoid organ primary, secondary or tertiary? Immunol Lett. 2007;112:1–8. doi: 10.1016/j.imlet.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Sainte-Marie G. The lymph node revisited: development, morphology, functioning, and role in triggering primary immune responses. Anat Rec (Hoboken) 2010;293:320–37. doi: 10.1002/ar.21051. [DOI] [PubMed] [Google Scholar]
- 3.Ahrendt M, Hammerschmidt SI, Pabst O, Pabst R, Bode U. Stromal cells confer lymph node-specific properties by shaping a unique microenvironment influencing local immune responses. J Immunol. 2008;181:1898–907. doi: 10.4049/jimmunol.181.3.1898. [DOI] [PubMed] [Google Scholar]
- 4.Hammerschmidt SI, Ahrendt M, Bode U, et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483–90. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magnusson FC, Liblau RS, von Boehmer H, et al. Direct presentation of antigen by lymph node stromal cells protects against CD8 T-cell-mediated intestinal autoimmunity. Gastroenterology. 2008;134:1028–37. doi: 10.1053/j.gastro.2008.01.070. [DOI] [PubMed] [Google Scholar]
- 6.Banks TA, Rouse BT, Kerley MK, et al. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155:1685–93. [PubMed] [Google Scholar]
- 7.Eberl G, Littman DR. The role of the nuclear hormone receptor RORgammat in the development of lymph nodes and Peyer's patches. Immunol Rev. 2003;195:81–90. doi: 10.1034/j.1600-065x.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 8.Tammela T, Saaristo A, Holopainen T, et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med. 2007;13:1458–66. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- 9.Zhou S, Ueta H, Xu XD, Shi C, Matsuno K. Predominant donor CD103+CD8+ T cell infiltration into the gut epithelium during acute GvHD: a role of gut lymph nodes. Int Immunol. 2008;20:385–94. doi: 10.1093/intimm/dxm153. [DOI] [PubMed] [Google Scholar]
- 10.Kim C, Li B, Papaiconomou C, Zakharov A, Johnston M. Functional impact of lymphangiogenesis on fluid transport after lymph node excision. Lymphology. 2003;36:111–9. [PubMed] [Google Scholar]
- 11.Ikomi F, Yokoyama Y, Ogiwara N, Sasaki K, Mizuno R, Ohhashi T. Recanalization of the collecting lymphatics in rabbit hind leg. Microcirculation. 2006;13:365–76. doi: 10.1080/10739680600745810. [DOI] [PubMed] [Google Scholar]
- 12.Tobbia D, Semple J, Baker A, Dumont D, Semple A, Johnston M. Lymphedema development and lymphatic function following lymph node excision in sheep. J Vasc Res. 2009;46:426–34. doi: 10.1159/000194273. [DOI] [PubMed] [Google Scholar]
- 13.Mor N, Levy L. Importance of the footpad lesion in the mouse response to local inoculation of Mycobacterium marinum. Ann Inst Pasteur Microbiol. 1985;136A:191–201. doi: 10.1016/s0769-2609(85)80058-2. [DOI] [PubMed] [Google Scholar]
- 14.Blum KS, Radtke C, Knapp WH, Pabst R, Gratz KF. SPECT-CT: a valuable method to document the regeneration of lymphatics and autotransplanted lymph node fragments. Eur J Nucl Med Mol Imaging. 2007;34:1861–7. doi: 10.1007/s00259-007-0458-6. [DOI] [PubMed] [Google Scholar]
- 15.Wolvers DAW, Coenen-de Roo CJJ, Mebius RE, et al. Intranasally induced immunological tolerance is determined by characteristics of the draining lymph nodes: studies with OVA and human cartilage gp-39. J Immunol. 1999;162:1994–8. [PubMed] [Google Scholar]
- 16.Okamoto Y, Yamashita J, Hasegawa M, et al. Cervical lymph nodes play the role of regional lymph nodes in brain tumour immunity in rats. Neuropathol Appl Neurobiol. 1999;25:113–22. doi: 10.1046/j.1365-2990.1999.00165.x. [DOI] [PubMed] [Google Scholar]
- 17.Phillips MJ, Needham M, Weller RO. Role of cervical lymph nodes in autoimmune encephalomyelitis in the Lewis rat. J Pathol. 1997;182:457–64. doi: 10.1002/(SICI)1096-9896(199708)182:4<457::AID-PATH870>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 18.Wiley JA, Tighe MP, Harmsen AG. Upper respiratory tract resistance to influenza infection is not prevented by the absence of either nasal-associated lymphoid tissue or cervical lymph nodes. J Immunol. 2005;175:3186–96. doi: 10.4049/jimmunol.175.5.3186. [DOI] [PubMed] [Google Scholar]
- 19.Sabirov A, Metzger DW. Intranasal vaccination of infant mice induces protective immunity in the absence of nasal-associated lymphoid tissue. Vaccine. 2008;26:1566–76. doi: 10.1016/j.vaccine.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahn A, Thiessen N, Pabst R, Buettner M, Bode U. Mesenteric lymph nodes are not required for an intestinal immunoglobulin A response to oral cholera toxin. Immunology. 2010;129:427–36. doi: 10.1111/j.1365-2567.2009.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothkotter HJ, Huber T, Barman NN, Pabst R. Lymphoid cells in afferent and efferent intestinal lymph: lymphocyte subpopulations and cell migration. Clin Exp Immunol. 1993;92:317–22. doi: 10.1111/j.1365-2249.1993.tb03398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voedisch S, Koenecke C, David S, et al. Mesenteric lymph nodes confine dendritic cell-mediated dissemination of Salmonella enterica serovar Typhimurium and limit systemic disease in mice. Infect Immun. 2009;77:3170–80. doi: 10.1128/IAI.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galanzha EI, Tuchin VV, Zharov VP. Optical monitoring of microlymphatic disturbances during experimental lymphedema. Lymphat Res Biol. 2007;5:11–27. doi: 10.1089/lrb.2007.5103. [DOI] [PubMed] [Google Scholar]
- 24.Yrlid U, Milling SW, Miller JL, Cartland S, Jenkins CD, MacPherson GG. Regulation of intestinal dendritic cell migration and activation by plasmacytoid dendritic cells, TNF-alpha and type 1 IFNs after feeding a TLR7/8 ligand. J Immunol. 2006;176:5205–12. doi: 10.4049/jimmunol.176.9.5205. [DOI] [PubMed] [Google Scholar]
- 25.Pabst R, Rothkotter HJ. Regeneration of autotransplanted lymph node fragments. Cell Tissue Res. 1988;251:597–601. doi: 10.1007/BF00214008. [DOI] [PubMed] [Google Scholar]
- 26.Milling S, Macpherson G. Isolation of rat intestinal lymph DC. Methods Mol Biol. 2010;595:281–97. doi: 10.1007/978-1-60761-421-0_19. [DOI] [PubMed] [Google Scholar]
- 27.van Zwam M, Huizinga R, Heijmans N, et al. Surgical excision of CNS-draining lymph nodes reduces relapse severity in chronic-relapsing experimental autoimmune encephalomyelitis. J Pathol. 2009;217:543–51. doi: 10.1002/path.2476. [DOI] [PubMed] [Google Scholar]
- 28.O'Mahony S, Rose SL, Chilvers AJ, et al. Finding an optimal method for imaging lymphatic vessels of the upper limb. Eur J Nucl Med Mol Imaging. 2004;31:555–63. doi: 10.1007/s00259-003-1399-3. [DOI] [PubMed] [Google Scholar]
- 29.Haberkorn U, Schoenberg SO. Imaging of lung cancer with CT, MRT and PET. Lung Cancer. 2001;34(Suppl. 3):S13–S23. doi: 10.1016/s0169-5002(01)00369-5. [DOI] [PubMed] [Google Scholar]
- 30.Stephan KH, Sandro JS. SPECT/CT for lymphatic mapping of sentinel nodes in early squamous cell carcinoma of the oral cavity and oropharynx. Int J Mol Imaging. 2011;2011:106068. doi: 10.1155/2011/106068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Contreras V, Urien C, Guiton R, et al. Existence of CD8alpha-like dendritic cells with a conserved functional specialization and a common molecular signature in distant mammalian species. J Immunol. 2010;185:3313–25. doi: 10.4049/jimmunol.1000824. [DOI] [PubMed] [Google Scholar]
- 32.Marquet F, Bonneau M, Pascale F, et al. Characterization of dendritic cells subpopulations in skin and afferent lymph in the swine model. PLoS ONE. 2011;6:e16320. doi: 10.1371/journal.pone.0016320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Rhijn I, Rutten VP, Charleston B, Smits M, van Eden W, Koets AP. Massive, sustained gammadelta T cell migration from the bovine skin in vivo. J Leukoc Biol. 2007;81:968–73. doi: 10.1189/jlb.0506331. [DOI] [PubMed] [Google Scholar]
- 34.Hope JC, Howard CJ, Prentice H, Charleston B. Isolation and purification of afferent lymph dendritic cells that drain the skin of cattle. Nat Protoc. 2006;1:982–7. doi: 10.1038/nprot.2006.125. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz-Cornil I, Epardaud M, Bonneau M. Cervical duct cannulation in sheep for collection of afferent lymph dendritic cells from head tissues. Nat Protoc. 2006;1:874–9. doi: 10.1038/nprot.2006.147. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz-Cornil I, Epardaud M, Albert JP, et al. Probing leukocyte traffic in lymph from oro-nasal mucosae by cervical catheterization in a sheep model. J Immunol Methods. 2005;305:152–61. doi: 10.1016/j.jim.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Bonneau M, Epardaud M, Payot F, et al. Migratory monocytes and granulocytes are major lymphatic carriers of Salmonella from tissue to draining lymph node. J Leukoc Biol. 2006;79:268–76. doi: 10.1189/jlb.0605288. [DOI] [PubMed] [Google Scholar]
- 38.Harling-Berg C, Knopf PM, Merriam J, Cserr HF. Role of cervical lymph nodes in the systemic humoral immune response to human serum albumin microinfused into rat cerebrospinal fluid. J Neuroimmunol. 1989;25:185–93. doi: 10.1016/0165-5728(89)90136-7. [DOI] [PubMed] [Google Scholar]
- 39.Bradl M, Lassmann H. Progressive multiple sclerosis. Semin Immunopathol. 2009;31:455–65. doi: 10.1007/s00281-009-0182-3. [DOI] [PubMed] [Google Scholar]
- 40.Ramagopalan SV, Dobson R, Meier UC, Giovannoni G. Multiple sclerosis: risk factors, prodromes, and potential causal pathways. Lancet Neurol. 2010;9:727–39. doi: 10.1016/S1474-4422(10)70094-6. [DOI] [PubMed] [Google Scholar]
- 41.Pugh CW, MacPherson GG, Steer HW. Characterization of nonlymphoid cells derived from rat peripheral lymph. J Exp Med. 1983;157:1758–79. doi: 10.1084/jem.157.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu LM, MacPherson GG. Antigen acquisition by dendritic cells: intestinal dendritic cells acquire antigen administered orally and can prime naive T cells in vivo. J Exp Med. 1993;177:1299–307. doi: 10.1084/jem.177.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yrlid U, Cerovic V, Milling S, et al. Plasmacytoid dendritic cells do not migrate in intestinal or hepatic lymph. J Immunol. 2006;177:6115–21. doi: 10.4049/jimmunol.177.9.6115. [DOI] [PubMed] [Google Scholar]
- 44.Milling SW, Jenkins CD, Yrlid U, et al. Steady-state migrating intestinal dendritic cells induce potent inflammatory responses in naive CD4+ T cells. Mucosal Immunol. 2009;2:156–65. doi: 10.1038/mi.2008.71. [DOI] [PubMed] [Google Scholar]
- 45.Hammerschmidt SI, Friedrichsen M, Boelter J, et al. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J Clin Invest. 2011;121:3051–61. doi: 10.1172/JCI44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luck R, Klempnauer J, Steiniger B. Abrogation of lethal graft-versus-host disease in MHC disparate small-bowel transplantation in the rat by mesenteric lymphadenectomy. Transplant Proc. 1990;22:2471. [PubMed] [Google Scholar]
- 47.Luck R, Klempnauer J, Steiniger B. Genetic aspects of graft-vs-host reaction after small bowel transplantation with and without mesenteric lymphadenectomy. Transplant Proc. 1992;24:1151. [PubMed] [Google Scholar]
- 48.Luck R, Klempnauer J, Steiniger B. Immunogenetic investigations of graft-versus-host reactions after small bowel transplantation with mesenteric lymphadenectomy. Transplant Proc. 1993;25:2869. [PubMed] [Google Scholar]
- 49.Wotherspoon JS, Dorsch SE. The mechanism of prolonged graft survival following removal of the regional lymph node. Transplantation. 1986;42:532–7. doi: 10.1097/00007890-198611000-00019. [DOI] [PubMed] [Google Scholar]
- 50.Plskova J, Holan V, Filipec M, Forrester JV. Lymph node removal enhances corneal graft survival in mice at high risk of rejection. BMC Ophthalmol. 2004;4:3. doi: 10.1186/1471-2415-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brouha PC, Perez-Abadia G, Francois CG, et al. Lymphadenectomy prior to rat hind limb allotransplantation prevents graft-versus-host disease in chimeric hosts. Transpl Int. 2004;17:341–50. doi: 10.1007/s00147-004-0724-5. [DOI] [PubMed] [Google Scholar]
- 52.Panoskaltsis-Mortari A, Price A, Hermanson JR, et al. In vivo imaging of graft-versus-host-disease in mice. Blood. 2004;103:3590–8. doi: 10.1182/blood-2003-08-2827. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Li D, Jones D, et al. Blocking LFA-1 activation with lovastatin prevents graft-versus-host disease in mouse bone marrow transplantation. Biol Blood Marrow Transplant. 2009;15:1513–22. doi: 10.1016/j.bbmt.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dutt S, Ermann J, Tseng D, et al. L-selectin and beta7 integrin on donor CD4 T cells are required for the early migration to host mesenteric lymph nodes and acute colitis of graft-versus-host disease. Blood. 2005;106:4009–15. doi: 10.1182/blood-2005-06-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim TD, Terwey TH, Zakrzewski JL, et al. Organ-derived dendritic cells have differential effects on alloreactive T cells. Blood. 2008;111:2929–40. doi: 10.1182/blood-2007-06-096602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garside P, Steel M, Liew FY, Mowat AM. CD4+ but not CD8+ T cells are required for the induction of oral tolerance. Int Immunol. 1995;7:501–4. doi: 10.1093/intimm/7.3.501. [DOI] [PubMed] [Google Scholar]
- 57.Worbs T, Bode U, Yan S, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–27. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dubois B, Goubier A, Joubert G, Kaiserlian D. Oral tolerance and regulation of mucosal immunity. Cell Mol Life Sci. 2005;62:1322–32. doi: 10.1007/s00018-005-5036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schulz O, Jaensson E, Persson EK, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–14. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shiokawa A, Tanabe K, Tsuji NM, Sato R, Hachimura S. IL-10 and IL-27 producing dendritic cells capable of enhancing IL-10 production of T cells are induced in oral tolerance. Immunol Lett. 2009;125:7–14. doi: 10.1016/j.imlet.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Matteoli G, Mazzini E, Iliev ID, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 63.Melamed D, Friedman A. Direct evidence for anergy in T lymphocytes tolerized by oral administration of ovalbumin. Eur J Immunol. 1993;23:935–42. doi: 10.1002/eji.1830230426. [DOI] [PubMed] [Google Scholar]
- 64.de Meis J, Ferreira LM, Guillermo LV, Silva EM, DosReis GA, Lopes MF. Apoptosis differentially regulates mesenteric and subcutaneous lymph node immune responses to Trypanosoma cruzi. Eur J Immunol. 2008;38:139–46. doi: 10.1002/eji.200737582. [DOI] [PubMed] [Google Scholar]
- 65.Egan PJ, Kimpton W, Seow HF, Bowles VM, Brandon MR, Nash AD. Inflammation-induced changes in the phenotype and cytokine profile of cells migrating through skin and afferent lymph. Immunology. 1996;89:539–46. doi: 10.1046/j.1365-2567.1996.d01-776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Knight JS, Baird DB, Hein WR, Pernthaner A. The gastrointestinal nematode Trichostrongylus colubriformis down-regulates immune gene expression in migratory cells in afferent lymph. BMC Immunol. 2010;11:51. doi: 10.1186/1471-2172-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pernthaner A, Cole SA, Morrison L, Green R, Shaw RJ, Hein WR. Cytokine and antibody subclass responses in the intestinal lymph of sheep during repeated experimental infections with the nematode parasite Trichostrongylus colubriformis. Vet Immunol Immunopathol. 2006;114:135–48. doi: 10.1016/j.vetimm.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 68.Pernthaner A, Cole SA, Morrison L, Hein WR. Increased expression of interleukin-5 (IL-5), IL-13, and tumor necrosis factor alpha genes in intestinal lymph cells of sheep selected for enhanced resistance to nematodes during infection with Trichostrongylus colubriformis. Infect Immun. 2005;73:2175–83. doi: 10.1128/IAI.73.4.2175-2183.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pernthaner A, Shaw RJ, McNeill MM, Morrison L, Hein WR. Total and nematode-specific IgE responses in intestinal lymph of genetically resistant and susceptible sheep during infection with Trichostrongylus colubriformis. Vet Immunol Immunopathol. 2005;104:69–80. doi: 10.1016/j.vetimm.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 70.Poulter LW, Pandolph CR. Mechanisms of immunity to leishmaniasis. IV. Significance of lymphatic drainage from the site of infection. Clin Exp Immunol. 1982;48:396–402. [PMC free article] [PubMed] [Google Scholar]
- 71.Heatley RV, Stark JM, Whitehead RH, Locke J, O'Donovan T. The effects of splenectomy, mesenteric lymphadenectomy and portacaval shunt on antibody responses to antigens within the small intestine. Immunology. 1977;32:573–9. [PMC free article] [PubMed] [Google Scholar]
- 72.Tokyay R, Zeigler ST, Loick HM, et al. Mesenteric lymphadenectomy prevents postburn systemic spread of translocated bacteria. Arch Surg. 1992;127:384–8. doi: 10.1001/archsurg.1992.01420040026003. [DOI] [PubMed] [Google Scholar]


