Abstract
Autoimmune diseases are more represented in Down syndrome (DS) individuals compared to chromosomally normal people. Natural T regulatory cells (nTreg) have been considered to be primary in the role of controlling the intensity and targets of the immune response. We have investigated the phenotypical and functional alteration of nTreg in a group of DS people. The phenotypical characteristic of Treg cells of 29 DS was analysed and compared with an age-matched healthy control group. The inhibitory potential of CD4+CD25highCD127low T regulatory cells was evaluated on autologous CD4+CD25- T cell proliferation in response to activation with a mytogenic pan-stimulus (anti-CD2, anti-CD3 and anti-CD28 antibodies). The CD4+CD25high cells in the DS and control groups were 2·692 ± 0·3808%, n = 29 and 1·246 ± 0·119, n = 29%, respectively (P = 0.0007), with a percentage of forkhead box protein 3 (FoxP3)-expressing cells of 79·21 ± 3·376%, n = 29 and 59·75 ± 4·496%, respectively (P = 0.0015). CD4+CD25+FoxP3+ cells were increased in peripheral blood from DS subjects (DS mean 5·231 ± 0·6065% n = 29, control mean 3·076 ± 0·3140% n = 29). The majority of CD4+CD25high were CD127low and expressed a high percentage of FoxP3 (natural Treg phenotype). While the proliferative capacity of DS T cells was not altered significantly compared to normal individuals, a reduced inhibitory potential of Treg compared to healthy controls was clearly observed (mean healthy control inhibition in Teff : Treg 1:1 co-culture: 58·9% ± 4·157%, n = 10 versus mean DS inhibition in Teff : Treg 1:1 co-culture: 39·8 ± 4·788%, n = 10, P = 0.0075; mean healthy control inhibition in Teff : Treg 1:0·5 co-culture: 45·10 ± 5·858%, n = 10 versus DS inhibition in Teff : Treg 1:0·5 co-culture: 24·10 ± 5·517%, n = 10, P = 0.0177). DS people present an over-expressed peripheral nTreg population with a defective inhibitory activity that may partially explain the increased frequency of autoimmune disease.
Keywords: autoimmunity, coeliac disease, Down syndrome, Hashimoto disease, regulatory T cells
Introduction
Autoimmune diseases have a high incidence and prevalence among Down syndrome individuals (DS) compared to chromosomally normal people [1–3]. Coeliac disease has a prevalence of 4·5–7%, autoimmune thyroiditis is diagnosed in 5–54% of DS subjects and type 1 diabetes (T1D) is present in 1%. DS is caused by trisomy of human chromosome 21 and occurs in approximately one of 700 newborns. DS shows various complex phenotypes, including developmental abnormalities, immune system deficiency, typical facial features, mental retardation and congenital heart and gastrointestinal malformations [4–6]. Leukaemias and testicular tumours have an abnormally high incidence in DS individuals, while solid tumours are extremely rare [7]. In the past, when DS children were commonly institutionalized, mortality from respiratory infections was particularly elevated [8]. The increased susceptibility to bacterial or viral infections and leukaemias has been attributed to the dysregulation of the immune system that is one pathological feature of the syndrome [9,10]. In 1979, Levin et al. were the first to reveal in a group of 15 infants (aged 1–15 months) that the thymic histological picture was abnormal [11]. They showed that ‘the normal thymic cortico-medullary demarcation was often missing due to marked lymphocyte depletion in the cortex’. In 1992 Murphy et al. demonstrated an interferon (IFN)-γ and tumour necrosis factor (TNF)-α over-expression [12] and in 1995 they proposed a model suggesting that in DS the over-expression of chromosome 21-encoded gene products leads to impaired interaction between immature thymocyte and thymic stromal cells [13]. The thymus has two main functions for sustaining immunological self-tolerance: clonal deletion of self-reactive T cells (negative selection) and the production of natural CD4+CD25high regulatory T cells (Treg) cells that express the transcription factor forkhead box protein 3 (FoxP3) [14]. The FoxP3-expressing cells differentiate and mature in the human and murine thymus under the influence of thymic stromal lymphopoietin (TSLP) together with IL-7 [15–18] following ligation of high-affinity T cell receptor (TCR) [19]. The TCR of thymocytes is reduced in DS patients [20]. Subsequently the natural T (nTreg), CD4+CD25high migrate into the periphery, suppress the autoreactive T cells that escape the thymic negative selection and simultaneously regulate the pathogen-induced inflammatory reactions [21–25]. nTreg play also a role in favouring tumour growth, tolerance towards transplanted organs or suppressing the graft-versus-host reaction in transplanted patients [26,27].
The higher prevalence of autoimmune disorders present in DS, along with the role of nTreg cells, led us to investigate if the frequency and the function of circulating nTreg were normal in a group of 29 children and young adults with DS. For this purpose, Treg cells, sorted as CD4+CD25highCD127low, were isolated and cultured with autologous T cells and stimulated with a pan-T stimulus (anti-CD2, anti-CD3 and anti-CD28 monoclonal antibodies-loaded beads). We expected to find a significantly reduced number of nTreg, but their frequency in the periphery was actually increased. Interestingly, when cultured in vitro, they displayed a reduced suppressive activity, as could be expected according to the increased incidence of autoimmunity in DS individuals.
Materials and methods
Subjects
Eligible patients were selected from a group of subjects with Down syndrome connected to a specific follow-up programme based on AAP (American Academy of Pediatrics) guidelines [3] at our Institution. Flow cytometry data were obtained from 29 DS subjects and 29 healthy age- and sex-matched donors' control group (HD); the proliferation assays were carried out on 10 DS and 10 age- and sex-matched control subjects. DS group included 15 males and 14 females (mean age 11·4 years, range: 1·4–22·8 years). The HD group included 15 males and 14 females (mean age 9·3 years, range: 1·2–23 years); clinical data are summarized in Table 1. In the DS group, five patients (17·2%) had positive antibodies against thyroid peroxidase (TPO) and specific ultrasound scan characteristics for Hashimoto's disease. One patient had positive antibodies against thyrotrophin (TSH) receptor. Positive anti-gliadin antibodies of IgG class were found in 14 patients (45%). Three patients (10·5% overall) presented positive anti-endomysial, anti-tissue transglutaminase immunoglobulin (Ig)A antibodies and a positive duodenal biopsy. One patient was diagnosed with chronic inflammatory demyelinating polyneuropathy (3·5%) and one had vitiligo (3·5%). In our group no patients had type 1 diabetes (T1D). The study protocol was approved by the Ethics Committee of the Insubria University. All parents provided written informed consent prior to participation in the study.
Table 1.
Clinical characteristics of Down syndrome (DS) and healthy donor (HD) subjects
| Group | DS | HD |
|---|---|---|
| Number (n) | 29 | 29 |
| Gender (male/female) | 15/14 | 15/14 |
| Mean age/range (years) | 11·4/1·4–22·8 | 9·3/1·2–23 |
| Hashimoto thyroiditis | 5 | – |
| Grave's disease | 1 | – |
| Coeliac disease | 3 | – |
| Atopy | 2 | – |
| Vitiligo | 1 | – |
| Type 1 diabetes mellitus | 0 | – |
| Chronic inflammatory demyelinating polyneuropathy | 1 | – |
Flow cytometry analysis of peripheral T cells and assessment of the Treg phenotype
Human peripheral blood mononuclear cells were freshly separated by Ficoll-Paque (BioWhittaker 17-829E; BioWhittaker, Milan, Italy) from 10–20 ml blood of 29 DS subjects and 29 healthy controls. Immunophenotypic analysis was performed by flow cytometry (BD FACSAria II™ apparatus) and immunofluorescence using the following antibodies: phycoerythrin (PE) mouse anti-human CD25 (clone M-A251; BD Pharmingen, Milan, Italy), PE-cyanin 7 (Cy7) mouse anti-human CD4 (clone: SK3; BD Pharmingen), PE-Cy5 mouse anti-human CD8a (clone: RPA-T8; eBioscience, Milan, Italy) and AlexaFluor® 647 mouse anti-human CD127 (clone HIL-7R-M21; BD Pharmingen). After surface staining, cells were stained intracellularly for FoxP3 according to the manufacturer's recommendations (FoxP3 staining buffer set; eBioscience), and treated with fluorescein isothiocyanate (FITC) rat anti-human FoxP3 antibody (clone: PCH101; eBioscience). Flow cytometry analysis was made with the BD FACSAria II™ apparatus.
In-vitro suppression assay
CD4+CD25highCD127low Treg cells [28] and CD4+CD25- responder T cells (Teff) from patients with DS and HD were isolated from peripheral blood mononuclear cells (PBMCs) by fluorescence-activated cell sorting. The functional characteristic of Treg cells was tested with the Suppression Inspector human kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). It is based on anti-biotin MACSiBead™ particles that are loaded with biotinylated anti-CD2, anti-CD3 and anti-CD28 monoclonal antibodies. One MACSiBead particle per cell (bead-to-cell ratio 1:1) is used for stimulation. Treg were analysed functionally in vitro by a co-culture assay system with Teff at different ratios (Teff : Treg, 1:0, 1:0·5 and 1:1) in the presence of MACSiBead™ polyclonal stimulus. Treg alone show a hypoproliferative response (anergy), whereas Teff alone show a proliferative response. Briefly, 5 × 104 Teff cells were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE) (5µM CFSE staining solution) and incubated alone or in the presence of 2·5 × 104 or 5 × 104 purified Treg for 4 days at 37°C in RPMI-1640 culture medium. Because CFSE is incorporated into living cells, its intensity decreases as a function of cell proliferation. The suppressive capacity of Treg cells towards responder cells in co-culture (Teff : Treg ratio 1:0·5 or 1:1) was expressed as the ratio between the percentage of cells proliferating in the presence or absence of Treg according to the formula [100 × (1 – % CFSE low CD4+CD25-T cells in co-culture/% CFSE low CD4+CD25- T cells alone)].
Statistical analysis
The normality of variable distribution was assessed, and once the hypothesis of normality was accepted (P < 0·05) comparisons were performed by Student's paired or unpaired t-tests, as appropriate. Results were expressed as the mean ± standard error of the mean. Grubbs' test was performed on reference interval data to detect outliers. All statistical analyses were performed using Prism version 5·0 software (GraphPad Software, San Diego, CA, USA; QuickCalcs, http://www.graphpad.com/quickcalcs/index.cfm). P-values less than 0·05 were considered significant.
Results
Circulating T cells
To assess the proportion of distinct subpopulations of T cells in DS patients, a comparative study of CD4+ and CD8+ T cells was performed. In DS subjects CD4+ cells were decreased compared to HD; conversely, CD8+ T cells appeared to be slightly increased (see Fig. 1). As a result the CD4/CD8 ratio was decreased significantly (1·4% versus 2·1%).
Fig. 1.
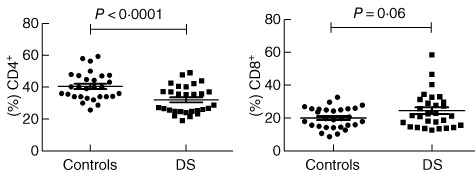
Circulating T cell-subset representation. In Down syndrome (DS) subjects CD4+ cells were decreased compared to HD (32·05 ± 1·591% versus 40·40 ± 1·606%, respectively, P = 0.0005, n = 29). No outliers were found in these summaries. Conversely, CD8+ T cells appear slightly increased, but with no statistical relevance (23·84 ± 2·029% versus 19·35 ± 1·178%, respectively, P = 0.0610, n = 29); one outlier was found but not removed due to statistical irrelevance. As a result the CD4 : CD8 ratio was decreased significantly (1·4% versus 2·1%).
CD4+CD25highFoxP3+ T cells are over-represented in DS patients
Flow cytometric analysis in DS patients highlighted a more represented CD4+CD25high population compared to HD (DS mean 2·692 ± 0·3808%, n = 29, HD mean 1·246 ± 0·119%, n = 29), P = 0.0007. FoxP3 was over-expressed in the CD4+CD25high population of DS subjects (mean 79·21 ± 3·376%, n = 29) compared to HD (mean 59·75 ± 4·496%, n = 29), P = 0.0015. Moreover, CD4+CD25+FoxP3+ cells were increased in peripheral blood from DS subjects (DS mean 5·231 ± 0·6065 n = 29, HD mean 3·076 ± 0·3140, n = 29), P = 0.0026 (see Fig. 2). We found a slightly increased CD4+CD25+FoxP3+ population in Down patients with autoimmune disorders (mean population with autoimmunity: 5·7 ± 1·5% n = 9), but with no statistical relevance. We then analysed and compared the CD4+CD25+ population for expression of the CD127 marker (α-chain of the IL-7 receptor). Low expression of this marker is associated strongly with the Treg phenotype, with functional suppressive features [28]. We found that the vast majority of CD4+CD25high were also CD127low (see Fig. 3), with no statistical difference in the proportion between HD and DS patients. Moreover, a high percentage of FoxP3+ was seen in the CD4+CD25highCD127low cell subset (98·3% in DS and 96·4% in HD).
Fig. 2.
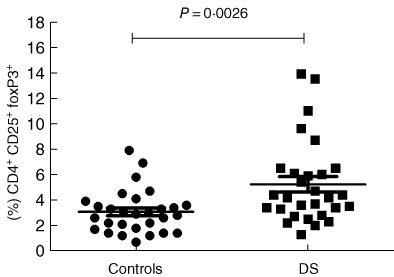
CD4+CD25+forkhead box protein 3 (FoxP3+ ) cells in peripheral blood. We can see an increased natural regulatory T cell (nTreg) population in Down syndrome (DS) subjects compared to healthy donors (HD) (DS mean 5·231 ± 0·6065, n = 29, HD mean 3·076 ± 0·3140, n = 29, P = 0.0026); no outliers were found.
Fig. 3.
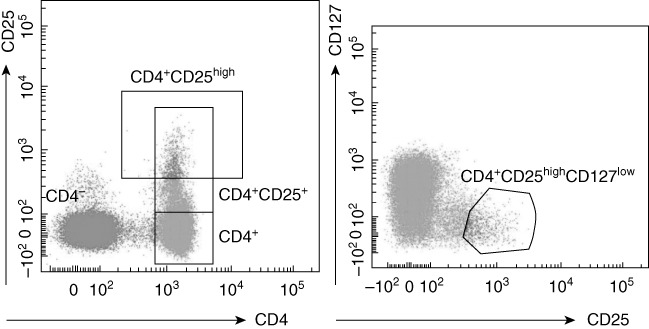
Correlation between CD4+CD25high T cells and CD127 expression in a representative subject. The majority of CD4+CD25high T cells display a CD127low expression phenotype. The CD4+CD25highCD127low T cell subset in one Down syndrome (DS) expressed 98·3% of forkhead box protein 3 (FoxP3); the CD4+CD25highCD127low T cell subset in one control expressed 96·4% of FoxP3 (data not shown).
CD4+CD25highCD127low T cells from patients with DS exhibit impaired suppressive function in vitro
In order to investigate the regulatory potential of Treg (CD4+CD25highCD127low) in DS patients, T cell proliferation assays were set up in which DS and HD Treg were incubated with autologous Teff (CD4+CD25–) stimulated in vitro by a pan-T stimulus (anti-CD2-, anti-CD3- and anti-CD28-coated beads). No statistical difference between the proliferation rate of DS CD4+CD25- and HD T cells was found after the pan-stimulus in the absence of Treg (72·8% versus 79·5%, respectively, P = 0.41). Conversely, when Treg cells from the same subject were added to the culture, a significantly reduced inhibition of T cell proliferation was observed for DS-derived Treg compared to HD-derived Treg. Indeed, the percentage of inhibition (see Fig. 4) by 10 healthy control Treg was 58·9 ± 4·157% (co-culture Teff : Treg 1:1) and 45·10 ± 5·858% (co-culture Teff : Treg 1:0·5), whereas the percentage of inhibition by 10 DS Treg was 39·8 ± 4·788% (co-culture Teff : Treg 1:1), P = 0.0075 and 24·10 ± 5·517% (co-culture Teff : Treg 1:0·5), P = 0.0177.
Fig. 4.
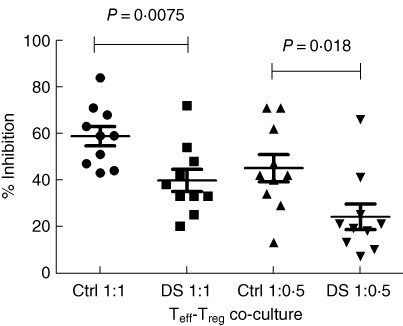
Co-culture effector T cell–regulatory T cell (Teff–Treg) median inhibitory rate. In co-culture Teff : Treg 1:1 the median percentage of inhibition by Treg in control subjects was 58·9 ± 4·157%, whereas in Down syndrome (DS) subjects was 39·8 ± 4·788% (n = 10, P = 0.0075); in co-culture Teff : Treg 1:0·5 the median percentage of inhibition by Treg in control subjects was 45·10 ± 5·858%, whereas in DS subjects was 24·10 ± 5·517% (n = 10, P = 0.0177), calculated as described in Materials and methods. One outlier was identified from the DS 1:0·5 Teff–Treg co-culture reference interval data. This data point had a Z-value of 2·40 (P < 0·05), while the critical value of Z for this data set was 2·29. The data were checked and confirmed by a second control; they were not removed because no technical reason was found.
Discussion
DS patients have a characteristically altered immunological asset [9,10]. In our DS population we found a decreased number of circulating CD4+ and an increased number of circulating CD8+ cells [29]. In this study we show that the proliferative capacity of T cells was not altered significantly compared to T cells from normal individuals, provided that the activation stimulus was exerted through several co-stimulatory molecules such as insoluble anti-CD2, anti-CD3 and anti-CD28 monoclonal antibodies (72·8% versus 79·5%, respectively, P = 0.41). As outlined previously, autoimmune diseases are represented much more in Down syndrome than in healthy controls [1–3]. Although several hypotheses have been put forward in the past to explain the high incidence of autoimmune diseases in DS patients [30,31], the immunological basis of this event is still unclear. An impaired function of nTreg cells has been shown in many human and murine autoimmune subjects [32]. Mice that lack Treg (scurfy mice) and individuals with altered expression of FoxP3 gene develop a severe autoimmune-like disease which progresses rapidly to death [immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome][33]. Natural Treg cells are generated in the thymus [18], but the DS thymus presents profound anatomical and architectural abnormalities which may alter the maturation process of these cells. This phenomenon has been documented in patients with Omenn syndrome, a severe congenital immunodeficiency, who carry a severe Treg defect and a profound anatomical alteration of the thymus gland [34]. DS children also have decreased T cell receptor excision circles (TREC), which are DNA by-products of a TCR recombination that reflect production of new T cells in the thymus [35]. Similarly, decreased TRECs as a measure of decreased thymopoiesis are seen in infants with congenital T cell defects [36]. Treg may be particularly sensitive to this altered maturation process and thus exit the thymus with functional defects. The high incidence of autoimmune diseases in DS may be related at least partially to the functional impairment of Treg. The mechanisms of suppression by nTreg include modulation of the cytokine microenvironment, metabolic disruption of the target cell and alteration of dendritic cell activation capacity and cytolysis [37]. In our study we have assessed only their suppressive function, using the dilution of CFSE by proliferating T target cells. Natural Treg cells which arise in the thymus co-operate with induced Treg cells which are generated in the periphery following CD4+ T cell activation [22].
In our DS group the circulating Treg cells are increased in number compared to HD, whereas their function is impaired. In IPEX syndrome, nTreg cells are present but dysfunctional [38].
These characteristics are present in all our DS individuals, irrespective of their actual autoimmune status. We cannot be strongly affirmative on the pathogenic role of the functional defect of these Treg because different subpopulations of Tregs exist, many of them differentiating in the periphery [32]. We should also take into account that different types of Treg regulate different types of T helper type 1 (Th1)-, Th2- and Th17-mediated immunity [39–42]: in our in-vitro experiments we used a pan-stimulus for T cells. A functional impairment of these cells is often associated with organ-specific autoimmunity and these disorders can be blocked by the infusion of new Treg[43]. In DS patients the organ-specific autoimmunity (T1D; Hashimoto thyroiditis and coeliac disease) is remarkably increased in incidence while generalized autoimmunity, such as systemic lupus erythematosus, is present with similar frequency, as in kariotypically normal individuals [44].
Acknowledgments
The Authors would like to thank the participating children and their parents. They also thank all the physicians who collaborated with the Down syndrome follow-up programme, in particular, Monica Ugolini, Elisa Di Natale, Lucia Morando and all the nursing staff of the Pediatric Day Hospital Center of Ospedale Filippo Del Ponte, Varese. A special thanks to the General Pathology and Immunology Laboratory of Insubria, in particular to Alessandra Tedeschi, for the valuable collaboration. This study was supported financially by grants from the University of Insubria-Varese, Italy.
Disclosure
None of the Authors has conflicts of interest to declare.
Ethics approval
The study protocol was approved by the Ethics Committee of the Insubria University (Protocol number 0048558). All parents provided a written informed consent prior to participation in the study.
References
- 1.Carnicer J, Farré C, Varea V, Vilar P, Moreno J, Artigas J. Prevalence of coeliac disease in Down's syndrome. Eur J Gastroenterol Hepatol. 2001;13:263–67. doi: 10.1097/00042737-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Anwar AJ, Walker JD, Frier BM. Type 1 Diabetes mellitus and Down's syndrome: prevalence, management and diabetic complications. Diabet Med. 1998;15:160–63. doi: 10.1002/(SICI)1096-9136(199802)15:2<160::AID-DIA537>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics. 2001;107:442–49. [Google Scholar]
- 4.Epstein CJ, Korenberg JR, Annerén G, et al. Protocols to establish genotype–phenotype correlations in Down syndrome. Am J Hum Genet. 1991;49:207–35. [PMC free article] [PubMed] [Google Scholar]
- 5.Reymond A, Marigo V, Yaylaoglu MB, et al. Human chromosome 21 gene expression atlas in the mouse. Nature. 2002;420:582–6. doi: 10.1038/nature01178. [DOI] [PubMed] [Google Scholar]
- 6.Lyle R, Béna F, Gagos S, et al. Genotype–phenotype correlations in Down syndrome identified by array CGH in 30 cases of partial trisomy and partial monosomy chromosome 21. Eur J Hum Genet. 2009;17:454–66. doi: 10.1038/ejhg.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down's syndrome in the USA from 1983 to 1997: a population-based study. Lancet. 2002;359:1019–25. doi: 10.1016/s0140-6736(02)08092-3. [DOI] [PubMed] [Google Scholar]
- 8.Garrison MM, Jeffries H, Christakis DA. Risk of death for children with Down syndrome and sepsis. J Pediatr. 2005;147:748–52. doi: 10.1016/j.jpeds.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 9.Burgio GR, Ugazio AG, Nespoli L, Marcioni AF, Bottelli AM, Pasquali F. Derangements of immunoglobulin levels, phytohemagglutinin responsiveness and T and B cell markers in Down's syndrome at different ages. Eur J Immunol. 1975;5:600–3. doi: 10.1002/eji.1830050904. [DOI] [PubMed] [Google Scholar]
- 10.Ram G, Chinen J. Infections and immunodeficiency in Down syndrome. Clin Exp Immunol. 2011;164:9–16. doi: 10.1111/j.1365-2249.2011.04335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin S, Schlesinger M, Handzel Z, et al. Thymic deficiency in Down's syndrome. Pediatrics. 1979;63:80–3. [PubMed] [Google Scholar]
- 12.Murphy M, Friend DS, Pike-Nobile L, Epstein LB. Tumor necrosis factor alpha and IFN-gamma expression in human thymus. J Immunol. 1992;149:2506–12. [PubMed] [Google Scholar]
- 13.Murphy M, Insoft RM, Pike-Nobile L, Epstein LB. A hypothesis to explain the immune defects in Down syndrome. Prog Clin Biol Res. 1995;393:147–67. [PubMed] [Google Scholar]
- 14.Sakaguchi S, Tanaka S, Tanaka A, et al. Thymus, innate immunity and autoimmune arthritis: interplay of gene and environment. FEBS Lett. 2011;585:3633–9. doi: 10.1016/j.febslet.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2008;181:225–34. doi: 10.4049/jimmunol.181.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol. 2006;7:709–14. doi: 10.1038/ni1360. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Q, Su H, Knudsen G, Helms W, Su L. Delayed functional maturation of natural regulatory T cells in the medulla of postnatal thymus: role of TSLP. BMC Immunol. 2006;7:6. doi: 10.1186/1471-2172-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe N, Wang YH, Lee HK, et al. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–5. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 19.Wong J, Mathis D, Benoist C. TCR-based lineage tracing: no evidence for conversion of conventional into regulatory T cells in response to a natural self-antigen in pancreatic islets. J Exp Med. 2007;204:2039–45. doi: 10.1084/jem.20070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy M, Lempert MJ, Epstein LB. Decreased level of T cell receptor expression by Down syndrome (trisomy 21) thymocytes. Am J Med Genet. 1999;7:234–7. doi: 10.1002/ajmg.1320370747. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 22.Haribhai D, Williams JB, Jia S, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–22. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and non-regulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–10. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 24.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 2006;25:249–59. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Benson A, Murray S, Divakar P, et al. Microbial infection-induced expansion of effector T cells overcomes the suppressive effects of regulatory T cells via an IL-2 deprivation mechanism. J Immunol. 2012;188:800–10. doi: 10.4049/jimmunol.1100769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adeegbe D, Matsutani T, Yang J, Altman NH, Malek TR. CD4(+) CD25(+) Foxp3(+) T regulatory cells with limited TCR diversity in control of autoimmunity. J Immunol. 2010;184:56–66. doi: 10.4049/jimmunol.0902379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malek TR, Pugliese A. Low-dose IL-2 as a therapeutic agent for tolerance induction. Immunotherapy. 2011;3:1281–4. doi: 10.2217/imt.11.120. [DOI] [PubMed] [Google Scholar]
- 28.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roat E, Prada N, Lugli E, et al. Homeostatic cytokines and expansion of regulatory T cells accompany thymic impairment in children with Down syndrome. Rejuvenation Res. 2008;11:573–83. doi: 10.1089/rej.2007.0648. [DOI] [PubMed] [Google Scholar]
- 30.Shield JPH, Wadsworth EJ, Hassold TJ, Judis LA, Jacobs PA. Is disomic homozygosity at the APECED locus the cause of increased autoimmunity in Down's syndrome? Arch Dis Child. 1999;81:147–50. doi: 10.1136/adc.81.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuadrado E, Barrena MJ. Immune dysfunction in Down's syndrome: primary immunodeficiency or early senescence of the immune system? Clin Immunol Immunopathol. 1996;78:209–14. doi: 10.1006/clin.1996.0031. [DOI] [PubMed] [Google Scholar]
- 32.Buckner JH. Mechanism of impaired regulation by CD4+CD25+FOXP3+ regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–59. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 34.Cassani B, Poliani PL, Moratto D, et al. Defect of regulatory T cells in patients with Omenn syndrome. J Allergy Clin Immunol. 2010;125:209–16. doi: 10.1016/j.jaci.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Bloemers BL, Bont L, de Weger RA, Otto SA, Borghans JA, Tesselaar K. Decreased thymic output accounts for decreased naive T cell numbers in children with Down syndrome. J Immunol. 2011;186:4500–7. doi: 10.4049/jimmunol.1001700. [DOI] [PubMed] [Google Scholar]
- 36.Knutsen AP, Baker MW, Markert ML. Interpreting low T-cell receptor excision circles in newborns with DiGeorge anomaly: importance of assessing naive T-cell markers. J Allergy Clin Immunol. 2011;128:1375–6. doi: 10.1016/j.jaci.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 38.Passerini L, Olek S, Di Nunzio S, et al. Forkhead box protein 3 (FOXP3) mutations lead to increased TH17 cell numbers and regulatory T-cell instability. J Allergy Clin Immunol. 2011;128:1376–9. doi: 10.1016/j.jaci.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Y, Chaudhry A, Kas A, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control Th2 responses. Nature. 2009;458:351–6. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon H, Thierry-Mieg D, Thierry-Mieg J, et al. Analysis of interleukin-21-induced Prdm 1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–52. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–30. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaminitz A, Yolcu ES, Stein J, Yaniv I, Shirwan H, Askenasy N. Killer Treg restore immune homeostasis and suppress autoimmune diabetes in prediabetic NOD mice. J Autoimmun. 2011;37:39–47. doi: 10.1016/j.jaut.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Roizen NJ, Patterson D. Down's syndrome. Lancet. 2003;361:1281–9. doi: 10.1016/S0140-6736(03)12987-X. [DOI] [PubMed] [Google Scholar]


