Abstract
The transferrin (Tf) family of iron binding proteins includes important endogenous modulators of the immune function that may modulate autoimmune diseases. To define more clearly the role of apotransferrin (apoTf) in type 1 diabetes we determined the impact of this protein on type 1 diabetes as investigated in islet cells, animal models and patient sera. First, we demonstrated that recombinant apoTf counteracts the cytokine-induced death of murine pancreatic islet cells. Secondly, human apoTf administration favourably influences the course of type 1 diabetes in animal models, resulting in protection against disease development that was associated with reduction of insulitis and reduced levels of proinflammatory cytokines. Finally, we confirmed that patients with newly diagnosed type 1 diabetes manifest significantly lower apoTf serum levels compared to healthy controls and patients with long-lasting disease. In conclusion, our data suggest the apoTf pivotal role in the perpetuation of type 1 diabetes pathology.
Keywords: biomarker, insulitis, iron, NOD
Introduction
Type 1 diabetes mellitus (T1DM) is a chronic immunoinflammatory disease resulting from the destruction of insulin-producing pancreatic beta cells mediated by autoreactive T lymphocytes, natural killer (NK) cells and macrophages [1]. A complex interplay of genetic susceptibility, environmental factors and immunological dysfunctions controls the development of type 1 diabetes both in humans and rodent models [1]. Among the latter, type 1 diabetes is characterized by an impaired balance between the predominant proinflammatory type 1, T helper type 17 (Th17) cytokines and anti-inflammatory type 2 [interleukin (IL)-4, IL-10] and type 3 [transforming growth factor (TGF-β] cytokines in patients and rodent models [2,3]. Proinflammatory cytokines exert their function on cell viability and recruitment while favouring the NF-κB-dependent development of reactive oxygen species [4], which can be counteracted by endogenous anti-inflammatory mediators such as vitamin D [5,6] and iron-binding proteins [7,8]. Among these proteins, apotransferrin (apoTf) represents an endogenous immune modulator [9]. Numerous studies have provided evidence for clinical relevance of Tf in diseases that are associated with lower plasma transferring concentrations, as well as with Tf polymorphisms. These include pathologies with an inflammatory component such as renal ischaemia reperfusion injury, diabetes and diabetes-related complications, stroke, Alzheimer's disease, cancer and atransferrinaemia (reviewed in [10]).
In the case of type 1 diabetes, experimental reports support the presence of apoTf dysfunctions based on reduced plasma levels in patients with long-lasting disease [11], but the significance of apoTf in the disease pathogenesis remains largely unknown. We report herein experimental results from pancreatic islet cells, animal models and sera from patients with different disease duration to define this issue more clearly. In particular, the data demonstrate that apoTf counteracts the cytokine-induced cell death of murine pancreatic islets and also prevents disease development in well-established type 1 diabetes models while modulating the cytokine profile at different diabetogenic stages. Further, we confirmed that patients with a new diagnosis of type 1 diabetes manifest significantly lower serum levels of apoTf compared to patients with long-lasting disease and that levels correlate with glycaemic homeostasis.
Materials and methods
Apotransferrin (apoTf)
Recombinant human (rh) apoTf used for in-vitro studies was purchased from Calbiochem (Merck KGaA, Dramstadt, Germany), while human plasma-derived apoTf used for in-vivo experiments was derived by Kedrion (Barga, Italy) from fraction IV-1,4 of the Cohn plasma fractionation process. This fraction was dissolved in water and, after centrifugation, the supernatant was treated with a mixture of solvent/detergent as virus-inactivation step. The resulting solution was filtered, concentrated and diafiltered before chromatographic step. The obtained solution was loaded onto Q Sepharose XL column and Tf was eluted with a step gradient using 25 mM Tris/HCl buffer (pH 7·5) with 100 mM NaCl. The eluted solution was treated with an ion chelator to obtain pure apoTf which was then prefiltered using a 0·1 µm filter before using a 20-nm nanofilter as virus-removal step resulting in endotoxin contents consistently below 50 EU (endotoxin units)/ml.
In-vitro effects of apoTf on cytokine-induced β cell death
Rat insulinoma RINm5F cells were kindly donated by Dr Karsten Buschard (Bartholin Instituttet, Copenhagen, Denmark). Cells were grown in HEPES-buffered RPMI-1640 medium supplemented with 10% fetal calf serum (FCS). After a conventional trypsinization procedure, cells were seeded onto 96-well plates (2 × 104 cells/well) and cultured overnight to be exposed to a proinflammatory cytokine cocktail made of IL-1β, tumour necrosis factor (TNF)-α and interferon (IFN)-γ (10 ng/ml each, all from eBioscience, San Diego, CA, USA) for 24 h in the presence or absence of recombinant human apoTf (25 µg/ml), followed by cell viability assay.
Islets were isolated from C57BL/6 mouse pancreas using the collagenase digestion method. Briefly, the organs were minced into smaller pieces and subsequently incubated with collagenase type V solution (1 mg/ml; Sigma, St Louis, MO, USA) in Hanks's balanced salt solution (HBSS) at 37°C for 10–15 min with vigorous shaking. Following cold HBSS addition to stop the digestion, the islets were handpicked and seeded into 96-well flat-bottomed plates (30/well) in culture medium (RPMI-1640 + 0·5% FCS). After overnight rest, pancreatic islets were treated with apoTf (25 µg/ml) and added to the proinflammatory cytokine cocktail for the next 24 h to be then analysed for cell viability.
The viability of RINm5F cells, as well as of pancreatic islets, was assessed using the mitochondrial-dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) to formazan. Cells were washed with phosphate-buffered saline (PBS) to remove non-adherent dead cells, and MTT (0·5 mg/ml) was added to the remaining adherent cells. Pancreatic islets were then collected, centrifuged and the pellets dissolved in MTT solution for 60 min at 37°C. After incubation, dimethyl sulphoxide (DMSO) was added to the adherent insulinoma cells, or pellets of pancreatic islets to dissolve the formazan crystals. Absorbance was finally measured at 570 nm wavelength, with a correction at 690 nm, using an automated microplate reader (LKB 5060-006; LKB, Vienna, Austria). The results of the MTT assay are presented as the proportion of control values obtained in untreated cell cultures. Data are expressed as the mean ± standard deviation (s.d.) of values obtained in at least five, three and seven individual experiments for mouse pancreatic islets or RINm5F cells, respectively.
Type 1 diabetes animal models and glucose monitoring
All animal experiments were conducted in accordance with national and local regulations regarding animal welfare, and with the approval of the institutional animal care and use committee (IACUC).
Female non-obese diabetic (NOD) mice (8–9 weeks old) and male diabetes-prone (DP) BB rats (5–6 weeks old) were purchased from Charles River, Milano (Italy). Rodents were housed under standard conditions with ad libitum food and water at the University of Catania (Italy). All animals were housed for 1 week prior to study initiation and then randomized into five per cage corresponding to one specific experimental condition. NOD mice and DP-BB rats were screened for glycosuria twice per week starting at the ages of 8–9 and 5–6 weeks, respectively.
The effects of apoTf on the development of animal type 1 diabetes
Two animal models of type 1 diabetes were used for these experiments. First, male DP-BB rats (36–45 days of age) were divided into four groups (n = 14 per group) to be treated with human apoTf at different concentrations (1·25, 2·5 or 5 mg/kg) or PBS for 7 consecutive weeks. During the study period the rats were checked twice a week for the onset of diabetes by measuring urine and plasma glucose levels [12]. Secondly, 8–9-week-old euglycaemic female NOD mice were divided into four 16-mice experimental groups treated with human apoTf at doses of 0·1, 1 and 2·5 mg/kg or PBS six times a week for 12 consecutive weeks [13]. These treatment regimens were chosen on the basis of the different natural course of disease development in the DP-BB rats and the NOD mouse. Most female NOD mice, which exhibit a higher incidence of the disease than males, develop hyperglycaemia by the age of 35 weeks after a prolonged prediabetic period characterized from progressive insulitis that initiates from the age of 4–5 weeks [14]. In contrast, T1DM, that has a similar incidence in male and female DP-BB rats, is characterized from a more rapid course than that observed in the NOD mouse, with most of the animals developing diabetes by the age of 120 days after a short period of insulitis that develops in a non-synchronous manner between the ages of 30 and 60 days [15]. Accordingly, both in the NOD mice and the DP-BB rats, we initiate treatment under a ‘late prophylactic’ at a time when most of the animals have developed signs of insulitis.
As established previously, type 1 diabetes was diagnosed in the presence of 2 consecutive days of detectable glycosuria and plasma glucose levels ≥200 mg/dl [12] using a FreeStyle Glucometer (Abbot, Abbot Park, IL, USA) and all experiments were performed in duplicate. Animals were killed when the diagnosis was made.
Effects of apoTf on insulitis, cytokines and cells
To evaluate the impact of apoTf on the development of insulitis and the production of cytokines, euglycaemic 5-week-old female NOD mice were treated for 12 consecutive weeks with either apoTf (2·5 mg/kg, n = 24) or its vehicle (n = 20) and then killed to collect pancreas, blood samples, spleens and pancreatic lymph nodes for histological and immunological analyses [16].
For the histological examination of pancreatic islets, samples were fixed in Bouin's solution embedded in paraffin for light microscopy [17]. Serial sections (5 µm thick) were stained with haematoxylin and eosin and only sections containing 10 or more islets were selected to be graded blindly by two observers (0, no infiltrate; 1, periductular infiltrate; 2 peri-islet infiltrate; 3 intra-islet infiltrate; and 4, intra-islet infiltrate associated with beta cell destruction) [18].
Pancreatic lymph nodes and spleens were isolated aseptically and minced to yield single-cell suspensions in culture medium with RPMI-1640 added with 10% fetal bovine serum (FBS; Sigma), 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 100 units/ml penicillin and 5 µg/ml streptomycin (Gibco, Grand Island, NY, USA). After centrifuging spleen cell suspensions at 300 g for 10 min, red blood cells were lysed with 3 ml of chilled red blood cell lysis buffer (Sigma) on ice for 5 min and then washed three times with chilled culture medium. Lymphocytes from pooled lymph nodes (5 × 105/well) or one spleen (106/well) were cultured in duplicate with or without concanavalin A (Con A; 2·5 µg/ml) in 1 ml of culture medium for 48 h, and conditioned medium was collected and stored at −80°C until analysed. Using the cardiac puncture method following CO2 euthanasia serum was collected and TNF-α, IL-2, IL-1β, IFN-γ (BD Biosciences, San Diego, CA, USA) and IL-17 (BioLegend, San Diego, CA, USA) levels measured using commercially available enzyme-linked immunosorbent assays (ELISAs) in duplicate for each mouse.
Finally, single-cell suspensions of splenocytes were used for flow cytometry and stained with allophycocyanin (APC) anti-CD4 (clone RM4-5), peridinin chlorophyll-cyanin 5·5 (PerCP-Cy5·5) anti-CD25 (PC61) and phycoerythrin (PE) anti-forkhead box protein 3 (FoxP3) (clone MF23) monoclonal antibodies to be analysed on a fluorescence activated cell sorter (FACSCalibur) flow cytometer using CellQuest software (all from BD Biosciences).
ApoTf in sera from patients with type 1 diabetes
Sera were obtained from different groups of patients with type 1 diabetes at different stages, i.e. newly diagnosed (ND, n = 20), clinical remission (CR, n = 18) or long-standing (LS, n = 10), and 12 healthy unrelated control subjects. All patients were followed at the Clinic for Endocrinology, Diabetes and Metabolic Diseases, CCS in Belgrade, Serbia, between January 2008 and June 2009.
All patients with ND-T1D fulfilled the diagnostic criteria reported by the Expert Committee of American Diabetes Association [19], including the presence of autoantibodies to glutamic acid decarboxylase (GADA) and/or to the tyrosine phosphatase insulinoma antigen-2 (IA-2A). At the time of the study enrolment, all patients were in satisfactory metabolic control (15 with ketosis). The insulin-requiring state (IRS) in patients with type 1 diabetes was defined as the necessity for insulin therapy in order to maintain euglycaemia and all patients were treated with intensified insulin therapy, multiple daily (subcutaneous) injection, four daily doses, human rapid-acting insulin (Actrapid HM 100 Novolet; Novo Nordisk, Bagsvaerd, Denmark) before the meals and neutral protamine Hagedorn (NPH) insulin (Insulatard HM 100 Novolet; Novo Nordisk) at bedtime. Clinical remission (CR) was defined as optimal metabolic control without the need for insulin lasting more than 30 days; these patients belong to newly diagnosed cases and pertain to the ‘honeymoon phase’. LS type 1 diabetes patients had a disease duration exceeding 5 years with unsatisfactory metabolic control (HbA1c > 7·5%). Control subjects (n = 12) had fasting blood glucose less than 110 mg/dl (normal levels), no family history of type 1 diabetes, undetectable serum type 1 diabetes-specific autoantibodies and a negative oral glucose tolerance test (OGTT) [20]. None of the participating subjects had clinical or laboratory signs of ongoing infections, allergic or autoimmune disease during the 6 months prior to blood draw nor had they used immunomodulatory drugs for at least 3 months prior to enrolment. The subject characteristics are illustrated in Table 1. Of note, subject groups did not differ in terms of age, sex and body mass index [analysis of variance (anova) Bonferroni P = 0·705, P = 0·403, P = 0·147; respectively]. The study design and procedures were approved by the local Human Ethical Committee, following the ethical guidelines of the most recent Declaration of Helsinki (Edinburgh, 2000), and all participants gave their written consent.
Table 1.
Characteristics of patients with newly diagnosed type 1 diabetes (ND-T1D) in insulin–requiring state (IRS) at the onset, in the state of clinical remission (CR T1D), long-standing T1D (LS T1D) and healthy controls included in apotransferrin analysis in peripheral blood
| T1D ND | T1D CR | T1D LS | Healthy controls | |
|---|---|---|---|---|
| n (male/female) | 20 (11/9) | 18 (9/9) | 10 (4/6) | 12 (3/9) |
| Age (years) | 28·15 ± 5·53 | 26·78 ± 4·91 | 26·3 ± 2·06 | 28·42 ± 7·68 |
| BMI (kg/m2) | 20·36 ± 2·53 | 21·3 ± 2·60 | 22·83 ± 1·59 | 22·90 ± 0·38 |
| Lost of BW (kg) | 4·77 ± 5·88 | – | – | – |
| Duration of symptoms prior Dg (months) | 1·78 ± 1·44 | – | – | – |
| Duration of diabetes (years) | – | – | 9·0 ± 2·54 | – |
| Duration of CR (months) | 4·86 ± 3·14 | |||
| C-peptide 0 min (nmol/l) | 0·28 ± 0·29 | 0·39 ± 0·20 | 0·05 ± 0·5 | – |
| C-peptide 6 min (nmol/l) | 0·57 ± 0·62 | 0·72 ± 0·39 | 0·07 ± 0·5 | – |
| HbA1c (%) | 10·83 ± 2·51 | 7·17 ± 0·63 | 9·63 ± 1·47 | – |
| GADA (U/ml) | 49·67 ± 70·58 | 36·15 ± 53·5 | – | – |
| IA-2 (U/ml) | 5·59 ± 13·96 | 0·47 ± 0·58 | – |
Data are n, means ± standard deviation. BW, body weight; GADA, glutamic acid decarboxylase.
All serum samples were stored at −20°C until analysed and apoTf levels were measured by the nephelometric method (Siemens Mod BN™: BN 100) and the radial immunodiffusion method performed on plates ‘NOR Partigen Transferrin’ (Siemens, Erlangen, Germany). Calibration curves were obtained with the calibrator N Protein Standard SL and the sensitivity limit of the test was 0·513 g/l.
Statistical methods
Continuous variables were expressed as mean ± s.d. and Student's t-tests were used to compare continuous variables between groups. All analyses were two-tailed and performed using spss version 18·0 for Macintosh (IBM Company, Chicago, IL, USA). P-values <0·05 were considered statistically significant.
Results
Effects of apoTf on pancreatic islet cells and type 1 diabetes animal models
Overnight exposure of pancreatic islet and RINm5F cells to a cytokine cocktail, including IFN-γ, IL-1β and TNF-α, decreased significantly cell viability measured using the MTT assay. When recombinant apoTf was added to the experimental setting, it protected pancreatic islets significantly, as well as insulinoma cells, from the deleterious effect of the cytokine cocktail (Fig. 1).
Fig. 1.
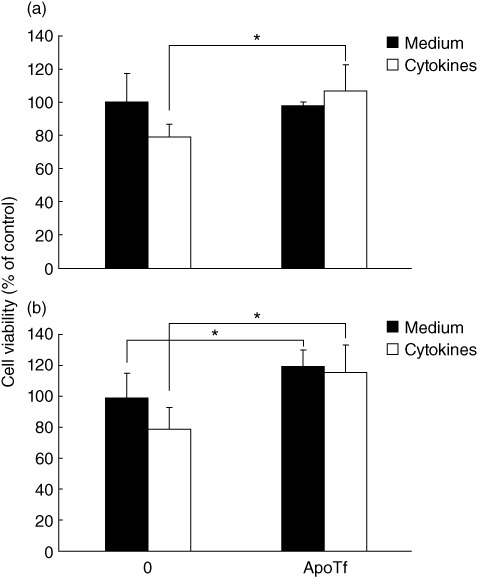
In-vitro effects of apotransferrin (apoTf) on survival of primary pancreatic islets and insulinoma cell lines. Mouse pancreatic islets (a) or RINm5F cells (b) were grown in culture medium without (medium) or with cytokine cocktail (cytokines) made of interleukin (IL)-1β + tumour necrosis factor (TNF)-α + interferon (IFN)-γ (10 µg/ml each) in the absence or presence of 25 µg/ml apo. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay was performed after 24 h of cultivation and the results are presented as the percentage of control absorbance values obtained for cultures grown in medium alone. Mean values ± standard deviation of values obtained in five (a) and seven (b) individual experiments are presented. *P < 0·05.
As mentioned previously, two models of type 1 diabetes were used to dissect the role of apoTf on disease onset. In the first model, untreated DP-BB rats developed type 1 diabetes based on glycosuria and blood glucose levels higher than 200 mg/dl in 11 of 14 cases (79%) within 12 weeks (Table 2 and Fig. 2a). In contrast, the prophylactic treatment with 5 mg/kg human apoTf reduced type 1 diabetes prevalence significantly by 12 weeks (64·3% versus 79%; P < 0·05) (Fig. 2a) and delayed the age of diabetes onset (88·9 ± 6·8 days versus 78·6 ± 6·6 days in control rats; P < 0·01) (Table 2). Recombinant apoTf at doses of 2·5 and 1·25 mg/kg was not associated with statistically significant differences in diabetes phenotype in this rat model.
Table 2.
Effect of apotransferrin on diabetes incidence and onset in a spontaneous model of type 1 diabetes in BB rats
| Treatment | Dose (mg/kg) | Diabetes incidence | Age at onset (mean ± s.d.) |
|---|---|---|---|
| Apotransferrin | 5 | 9/14 (64·3%) | 88·9 ± 6·8* |
| Apotransferrin | 2·5 | 8/14 (57·1%) | 81·2 ± 11·1 |
| Apotransferrin | 1·25 | 8/14 (57·1%) | 78·3 ± 9·2 |
| Vehicle (PBS) | – | 11/14 (78·6%) | 78·6 ± 6·6 |
P < 0·01 versus vehicle by t-test. PBS, phosphate-buffered saline; s.d., standard deviation.
Fig. 2.
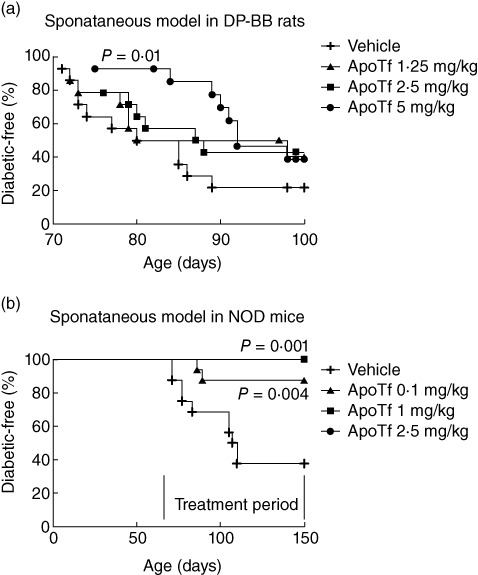
Effects of apotransferrin (apoTf) on diabetes incidence in a spontaneous model of type 1 diabetes, i.e. DP-BB rats (a) and spontaneous (b) non-obese diabetic (NOD) mouse models of diabetes. Thirty-six to 45-day-old male DP-BB rats were treated with either apoTf (1·25, 2·5 or 5 mg/kg) or phosphate-buffered saline (PBS) for 7 consecutive weeks (a). Eight to 9-week-old female NOD mice were treated with apoTf at doses of 0·1, 1 and 2·5 mg/kg or its vehicle (PBS) once daily (six times a week) for 12 consecutive weeks (b). During the study period the animals were checked twice a week or the onset of diabetes by measuring glucosuria. Diabetic mice were killed on the day that diagnosis was confirmed. The log-rank test was performed to evaluate statistical significance.
In the second type 1 diabetes rodent model, control NOD mice developed diabetes by 11 weeks of age with glycosuria and blood glucose levels higher than 200 mg/dl observed in 63% of mice by the end of the study (Fig. 2b). In contrast, the treatment of the mice with apoTf at 0·1, 1 and 2·5 mg/kg for 12 consecutive weeks led to a significant reduction (12·5% with 0·1 mg/kg dose) or complete prevention (with higher doses) of type 1 diabetes onset (Fig. 2b). In another study aimed at evaluating the impact of apoTf on the development of insulitis and the production of cytokines, euglycaemic 5-week-old female NOD mice were treated for 12 consecutive weeks with the highest dose (2·5 mg/kg) of apoTf, and exhibited a significant reduction in the degree of insulitis compared to the PBS-treated mice (1·13 ± 0·85 versus 1·95 ± 0·83; P < 0·01 by Student's t-test) at the end of the treatment (Fig. 3). Furthermore, the same treatment regimen reduced significantly the levels of IFN-γ, IL-1β, IL-2, IL-17 and TNF-α both in the spleen and pancreatic lymph nodes compared to control mice (Fig. 4a,b). The same differences, with the exception of TNF-α being undetectable, were also observed in murine sera in the same experimental conditions (Fig. 4c). Finally, prolonged treatment with apoTf did not change significantly the proportion of splenic CD4+ regulatory T cells (Treg) (CD4+/CD25+/FoxP3+) cells compared to control mice (Fig. 5).
Fig. 3.
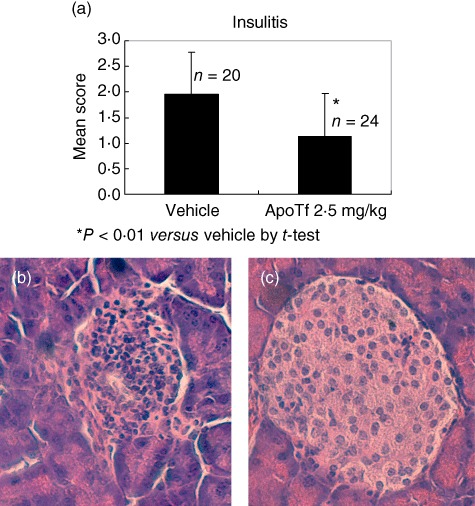
Effects of apotransferrin (apoTf) on insulitis in a spontaneous model of type 1 diabetes. Five-week-old female non-obese diabetic (NOD) mice were treated for 12 consecutive weeks with either apoTf (2·5 mg/kg, n = 24) or its vehicle (n = 20) (a). Histological examinations were performed on pancreatic islets: (b) representative image from a vehicle treated mouse (haematoxylin and eosin staining, ×400). Note massive infiltration of the islet. (c) Representative image from an apo-treated mouse (haematoxylin and eosin staining, ×400). Note well-preserved islet without insulitis.
Fig. 4.

Ex-vivo and in-vivo effects of apotransferrin (apoTf) on cytokine production. Cytokine production by lymphocytes isolated from spleen (a) and pancreatic lymph nodes (b) upon stimulation with concanavalin A for 48 h and in serum (c) was determined via enzyme-linked immunosorbent assay (ELISA). The mean values of duplicate cultures from each mouse were used to calculate the mean ± standard deviation values from several mice per cohort. Student's t-test was used for comparisons. *P < 0·05; **P < 0·01; ***P < 0·0001.
Fig. 5.
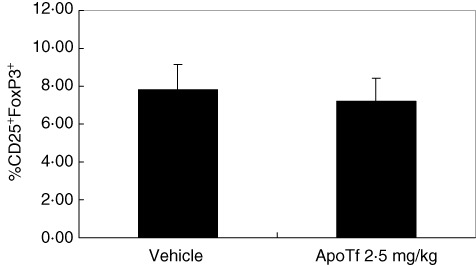
Effect of apotransferrin (apo) on regulatory T cells in a spontaneous model of type 1 diabetes. Five-week-old female non-obese diabetic (NOD) mice were treated for 12 consecutive weeks with either apoTf (2·5 mg/kg, n = 24) or its vehicle (n = 20). Mice were killed and single-cell suspensions of splenocytes were stained to characterize the regulatory T cell population. Data are shown as percentage of CD25+forkhead box protein 3 (FoxP3+) cells among CD4+ cells ± standard deviation of each cohort. Significance evaluated by Student's t-test.
ApoTf in patients with type 1 diabetes
ApoTf plasma levels were significantly lower in patients with ND-type 1 diabetes compared to matched controls, while this difference was not observed comparing patients with CR or LS disease (Fig. 6).When biochemical and clinical features of ND-type 1 diabetes were correlated with apoTf levels we found a significant association with HbA1c determined at disease onset using both laboratory methods (r = −0·452, P = 0·045 with RID; r = −0·564, P = 0·01 with nephelometry) but not with basal or stimulated C peptide levels, GADA and IA2 antibodies, weight loss prior to diagnosis or symptom duration (data not shown). No correlation with any of the analysed clinical and biochemical features was encountered in patients with LS or CR type 1 diabetes (data not shown).
Fig. 6.
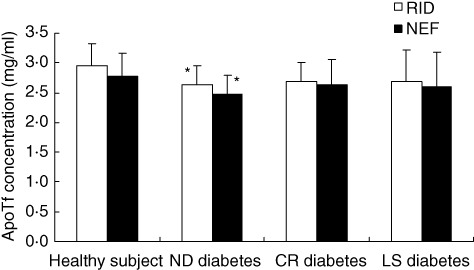
Apotransferrin (apoTf) plasma levels in patients with type 1 diabetes. Blood samples were collected from patients with newly diagnosed (ND), clinical remission (CR) and long-standing (LS) type 1 diabetes. For comparison, blood samples were also collected from sex- and age-matched healthy subjects. Plasma levels of apoTf were measured by both the nephelometric method (NEF) and the radial immunodiffusion method (RID). Student's t-test was used for comparisons. *P < 0·05 versus healthy subjects.
Discussion
The data presented herein were obtained from different murine and cellular models as well as human samples to demonstrate for the first time that recombinant human apoTf or human-derived apoTf acts to inhibit significantly the inflammatory pathways leading to diabetes. The affected pathways included cytokine-induced beta cell death in vitro and disease onset in well-established models. In particular, apoTf was associated with milder signs of insulitis and profound modulation of cytokine secretory profile in NOD mice.
Several findings may prove significant in our understanding of type 1 diabetes pathogenesis and the role of apoTf. First, the prolonged ex-vivo treatment with apoTf leads to down-modulation of the destructive Th1 and Th17 autoimmune responses [17,21,22] that produce IL-1β, IL-2, TNF-α, IFN-γ, IL-17 and IL-18 [23], which are crucial to diabetes development in the NOD mouse. Th1, Th17 and Treg are thought to be regulated reciprocally and, therefore, changes in Treg could be expected in the immune modulating activity we observed during apoTf treatment in NOD mice [24]. Nevertheless, we could not observe significant changes in the prevalence of Treg (CD4+/CD25+/FoxP3+) cells in the spleen of animals treated for 12 weeks. Further studies are being carried out to demonstrate whether ApoTf exerts its anti-diabetogenic effect by up-regulating Treg function without modifying their numbers or whether it acts via Treg-independent pathways.
We further observed that the apoTf administration prevents the spontaneous forms of type 1 diabetes both in the NOD mice and the DP-BB rats. Despite the numerous limitations of the translation of animal observations into clinical implications for patients with type 1 diabetes [25], these data are in support of the possible use of ApoTf in subjects at high risk for developing type 1 diabetes [26]. Nevertheless, we cannot rule out the possibility that the prolonged use of recombinant human ApoTf might prove immunogenic in both the DP-BB rats and the NOD mouse with potential reduction of its immunomodulatory effects and this would probably strengthen the clinical anti-diabetogenic potential of ApoTf.
In general terms, apoTf may be beneficial in the early stages of human type 1 diabetes, as suggested by its low plasma levels in newly diagnosed patients included in the present study. The reduced apoTf levels and defective iron-binding capacity have been described previously in patients with long-standing type 1 diabetes [11]. While we can only speculate on the reasons for this discrepancy with this previous report [11], we note that the apoTf levels of newly diagnosed type 1 diabetes patients included in our study manifested a correlation with HbA1C as a type 1 diabetes clinical marker [27] to suggest that the apoTf iron binding capacity may influence the glycaemic status of patients. Indeed, iron depletion improves insulin resistance in patients with non-alcoholic fatty liver disease and diabetes resulting in increased glucose uptake in vitro[28,29]. The use of the iron chelator desferroxiamine on HepG2 cells induced the constitutive glucose transporter Glut1, while iron depletion increased insulin receptor activity, with an effect counteracted by iron supplementation [29].
A third observation is derived from the experimental data and is represented by the modulation of glucose homeostasis by endogenous apoTf deficiency that may indirectly amplify and accelerate type 1 diabetes onset. Indeed, it is well established that elevated glucose levels contribute to beta cell destruction by inducing expression of autoantigens and fatty acid synthase (FAS), thus favouring the cell-mediated immune responses and apoptosis via FAS–FAS ligand interaction [30]. Based on the data from human sera we may further hypothesize that these mechanisms are limited to the early and possibly preclinical stages of type 1 diabetes, and we encourage a study aiming at measuring ApoTf blood levels in individuals who are at high risk for developing type 1 diabetes. Thus, if endogenous apoTf plays a protective role in type 1 diabetes, we suggest that the treatment with recombinant apoTf may also prove beneficial in prediabetic individuals or newly diagnosed type 1 diabetes patients. An additional mechanism for the apoTf qualitative involvement in type 1 diabetes is based on the defective apoTf secondary to the protein glycation that follows the prolonged hyperglycaemic conditions, and impairs the protein iron binding capacity [30]. This impairment may thus lead to increased circulating free iron that, in turn, enhances the production of free radicals, crucial for the development of the long-term complications of type 1 diabetes and beta cell destruction [30]. Free iron is found at higher levels in patients with type 1 diabetes [30] and exogenous apoTf may prevent iron to engage in reactions that lead to production of hydroxyl radicals and consequent oxidative stress, as represented by the effects of desferrioxamine (DFO) treatment on hypoxia-inducible factor (HIF)-1α and vascular endothelial growth factor (VEGF) expression in encapsulated human islets [27], oxidative stress in mouse pancreatic beta cells [30] and chronic allograft damage [31].
The reduced production of proinflammatory cytokines may have contributed to the anti-diabetogenic effects of apoTf, but we cannot rule out that this effect ensues from the apoTf-related inhibition of other different steps of autoimmune diabetogenesis in vivo that may lead secondarily to the reduced prevalence of these cytokines. The prolonged treatment with apoTf could, primarily, have inhibited other diabetogenic pathways including, but not limited to, leucocyte chemotaxis into pancreatic islets, the generation and maintenance of cytotoxic effectors (macrophages, CD8+ and NK cells) or the induction of tolerogenic cells or cells such as DC1 or M1. It is known that naive B and T cells express low levels of TfR1 (transferrin receptor 1) that increase after stimulation with the mitogen phytohaemagglutinin (PHA), thus suggesting its role in the modulation of the inflammatory process mediated by the binding of transferrin molecules. Moreover, earlier evidence demonstrated that iron-saturated transferrin may decrease the production of granulocyte–macrophage colony-stimulating factors (GM-CSF) by human T lymphocytes that had been stimulated by either PHA or ConA, while no inhibitory effect was observed upon treatment with a monoclonal antibody against transferrin receptors [32]. Based on these observations, we speculate that the effects of exogenous ApoTf may be due partially to its chelation of iron and the subsequent binding to TfR1. Additional immunopharmacological in-vitro and ex-vivo studies are awaited to clarify this point.
In conclusion, the translational findings gathered from our study suggest that apoTf manifests powerful anti-diabetogenic effects in established models of type 1 diabetes and that the blood levels of this protein are reduced significantly in a substantial proportion of newly diagnosed type 1 diabetes with elevated HbA1C. These data warrant further studies on the role of endogenous and exogenous apoTf in autoimmune diabetogenesis and its possible use for the prevention and early treatment of human disease.
Acknowledgments
The authors received a grant support for research from a MIUR (Ministry of Education, University, Research) project (Decree no. 795 of 21 June 2004).
Disclosure
The authors have no financial conflict of interest.
References
- 1.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–26. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 2.Bettini M, Vignali DA. Regulatory T cells and inhibitory cytokines in autoimmunity. Curr Opin Immunol. 2009;21:612–8. doi: 10.1016/j.coi.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coppieters KT, Roep BO, von Herrath MG. Beta cells under attack: toward a better understanding of type 1 diabetes immunopathology. Semin Immunopathol. 2011;33:1–7. doi: 10.1007/s00281-010-0236-6. [DOI] [PubMed] [Google Scholar]
- 4.Davi G, Falco A, Patrono C. Lipid peroxidation in diabetes mellitus. Antioxid Redox Signal. 2005;7:256–68. doi: 10.1089/ars.2005.7.256. [DOI] [PubMed] [Google Scholar]
- 5.Pelajo CF, Lopez-Benitez JM, Miller LC. Vitamin D and autoimmune rheumatologic disorders. Autoimmun Rev. 2010;9:507–10. doi: 10.1016/j.autrev.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Pittas AG, Dawson-Hughes B. Vitamin D and diabetes. J Steroid Biochem Mol Biol. 2010;121:425–9. doi: 10.1016/j.jsbmb.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Recalcati S, Invernizzi P, Arosio P, Cairo G. New functions for an iron storage protein: the role of ferritin in immunity and autoimmunity. J Autoimmun. 2008;30:84–9. doi: 10.1016/j.jaut.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Yin H, Cheng L, Agarwal C, Agarwal R, Ju C. Lactoferrin protects against concanavalin A-induced liver injury in mice. Liver Int. 2010;30:623–32. doi: 10.1111/j.1478-3231.2009.02199.x. [DOI] [PubMed] [Google Scholar]
- 9.Gomme PT, McCann KB, Bertolini J. Transferrin: structure, function and potential therapeutic actions. Drug Discov Today. 2005;10:267–73. doi: 10.1016/S1359-6446(04)03333-1. [DOI] [PubMed] [Google Scholar]
- 10.de Vries B, Walter SJ, von Bonsdorff L, et al. Reduction of circulating redox-active iron by apotransferrin protects against renal ischemia–reperfusion injury. Transplantation. 2004;77:669–75. doi: 10.1097/01.tp.0000115002.28575.e7. [DOI] [PubMed] [Google Scholar]
- 11.van Campenhout A, van Campenhout CM, Lagrou AR, Manuel-y-Keenoy B. Transferrin modifications and lipid peroxidation: implications in diabetes mellitus. Free Radic Res. 2003;37:1069–77. doi: 10.1080/10715760310001600390. [DOI] [PubMed] [Google Scholar]
- 12.Nicoletti F, Zaccone P, Di Marco R, et al. Paradoxical antidiabetogenic effect of gamma-interferon in DP-BB rats. Diabetes. 1998;47:32–8. doi: 10.2337/diab.47.1.32. [DOI] [PubMed] [Google Scholar]
- 13.Nicoletti F, Zaccone P, Di Marco R, et al. Effects of sodium fusidate in animal models of insulin-dependent diabetes mellitus and septic shock. Immunology. 1995;85:645–50. [PMC free article] [PubMed] [Google Scholar]
- 14.Driver JP, Serreze DV, Chen YG. Mouse models for the study of autoimmune type 1 diabetes: a NOD to similarities and differences to human disease. Semin Immunopathol. 2011;33:67–87. doi: 10.1007/s00281-010-0204-1. [DOI] [PubMed] [Google Scholar]
- 15.Ramanathan S, Poussier P. BB rat lyp mutation and Type 1 diabetes. Immunol Rev. 2001;184:161–71. doi: 10.1034/j.1600-065x.2001.1840115.x. [DOI] [PubMed] [Google Scholar]
- 16.Nicoletti F, Di Marco R, Zaccone P, et al. Endogenous interleukin-12 only plays a key pathogenetic role in non-obese diabetic mouse diabetes during the very early stages of the disease. Immunology. 1999;97:367–70. doi: 10.1046/j.1365-2567.1999.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicoletti F, Zaccone P, Di Marco R, et al. The effects of a nonimmunogenic form of murine soluble interferon-gamma receptor on the development of autoimmune diabetes in the NOD mouse. Endocrinology. 1996;137:5567–75. doi: 10.1210/endo.137.12.8940385. [DOI] [PubMed] [Google Scholar]
- 18.Kosiewicz MM, Auci DL, Fagone P, et al. HE3286, an orally bioavailable synthetic analogue of an active DHEA metabolite suppresses spontaneous autoimmune diabetes in the non-obese diabetic (NOD) mouse. Eur J Pharmacol. 2011;658:257–62. doi: 10.1016/j.ejphar.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl. 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 20.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 21.Debray-Sachs M, Carnaud C, Boitard C, et al. Prevention of diabetes in NOD mice treated with antibody to murine IFN gamma. J Autoimmun. 1991;4:237–48. doi: 10.1016/0896-8411(91)90021-4. [DOI] [PubMed] [Google Scholar]
- 22.Cailleau C, Diu-Hercend A, Ruuth E, Westwood R, Carnaud C. Treatment with neutralizing antibodies specific for IL-1beta prevents cyclophosphamide-induced diabetes in nonobese diabetic mice. Diabetes. 1997;46:937–40. doi: 10.2337/diab.46.6.937. [DOI] [PubMed] [Google Scholar]
- 23.Marleau AM, Sarvetnick NE. IL-18 is required for self-reactive T cell expansion in NOD mice. J Autoimmun. 2011;36:263–77. doi: 10.1016/j.jaut.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandenbark AA, Offner H. Critical evaluation of regulatory T cells in autoimmunity: are the most potent regulatory specificities being ignored? Immunology. 2008;125:1–13. doi: 10.1111/j.1365-2567.2008.02900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roep BO, Peakman M. Surrogate end points in the design of immunotherapy trials: emerging lessons from type 1 diabetes. Nat Rev Immunol. 2010;10:145–52. doi: 10.1038/nri2705. [DOI] [PubMed] [Google Scholar]
- 26.Roep BO, Atkinson M, von Herrath M. Satisfaction (not) guaranteed: re-evaluating the use of animal models of type 1 diabetes. Nat Rev Immunol. 2004;4:989–97. doi: 10.1038/nri1502. [DOI] [PubMed] [Google Scholar]
- 27.Larsen ML. The clinical usefulness of glucated haemoglobin in diabetes care evaluated by use of a medical technology assessment strategy. Dan Med Bull. 1997;44:303–15. [PubMed] [Google Scholar]
- 28.Valenti L, Fracanzani AL, Bugianesi E, et al. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2010;138:905–12. doi: 10.1053/j.gastro.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Dongiovanni P, Valenti L, Fracanzani AL, Gatti S, Cairo G, Fargion S. Iron depletion by deferoxamine up-regulates glucose uptake and insulin signaling in hepatoma cells and in rat liver. Am J Pathol. 2008;172:738–47. doi: 10.2353/ajpath.2008.070097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Campenhout A, Van Campenhout C, Lagrou AR, Moorkens G, De Block C, Manuel-y-Keenoy B. Iron-binding antioxidant capacity is impaired in diabetes mellitus. Free Radic Biol Med. 2006;40:1749–55. doi: 10.1016/j.freeradbiomed.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Bradley B, Prowse SJ, Bauling P, Lafferty KJ. Desferrioxamine treatment prevents chronic islet allograft damage. Diabetes. 1986;35:550–5. doi: 10.2337/diab.35.5.550. [DOI] [PubMed] [Google Scholar]
- 32.Broxmeyer HE, Lu L, Bognacki J. Transferrin, derived from an OKT8-positive subpopulation of T lymphocytes, suppresses the production of granulocyte-macrophage colony-stimulatory factors from mitogen-activated T lymphocytes. Blood. 1983;62:37–50. [PubMed] [Google Scholar]


