Abstract
Scleroderma (SSc) is a rare connective tissue disease characterized by fibrosis, microvasculopathy and autoimmune features. The role of genetics is limited in SSc, as suggested by similar concordance rates in monozygotic and dizygotic twin pairs, while environmental factors may act through epigenetic changes, as demonstrated for specific genes. Further, sex chromosome changes have been reported in SSc and may explain the female preponderance. In the present study we compared the methylation profile of all X chromosome genes in peripheral blood mononuclear cells from monozygotic twins discordant (n = 7) and concordant (n = 1) for SSc. Methylated DNA immunoprecipitations from each discordant twin pair were hybridized to a custom-designed array included 998 sites encompassing promoters of all X chromosome genes and randomly chosen autosomal genes. Biostatistical tools identified sites with an elevated probability to be consistently hypermethylated (n = 18) or hypomethylated (n = 25) in affected twins. Identified genes include transcription factors (ARX, HSFX1, ZBED1, ZNF41) and surface antigens (IL1RAPL2, PGRMC1), and pathway analysis suggests their involvement in cell proliferation (PGK1, SMS, UTP14A, SSR4), apoptosis (MTM1), inflammation (ARAF) and oxidative stress (ENOX2). In conclusion, we propose that X chromosome genes with different methylation profiles in monozygotic twin pairs may constitute candidates for SSc susceptibility.
Keywords: epigenetics, genome-wide, MeDIP, systemic autoimmunity
Introduction
Scleroderma or systemic sclerosis (SSc) is a multi-system connective tissue disease characterized by widespread skin and visceral fibrosis, microangiopathy and immunological features such as serum autoantibodies and female predominance [1]. Similar to other autoimmune diseases, genetic and environmental factors appear to contribute to disease susceptibility and the comparison of disease concordance rates in monozygotic (MZ) versus dizygotic (DZ) twins is a powerful strategy to estimate their relative weight [2]. Concordance rates for autoimmune diseases in MZ twins are largely below 50% with few exceptions, but remain higher compared to DZ twins or siblings [2]. In the case of SSc, similar concordance rates have been observed in MZ (4·2%) and DZ twins (5·6%) in a cross-sectional study [3], while a recent genome-wide association study (GWAS) has reported significant associations in subgroups of patients [4,5]. Accordingly, environmental factors remain crucial in SSc development and are thought to impact gene expression through epigenetic changes [6–8], particularly DNA methylation, which manifests a partial instability responsible for phenotypic differences across genetic identical organisms [9,10].
An additional clue to SSc pathogenesis comes from its female predominance with a sex ratio as high as 12:1 [11] and from the proposed theories related to X chromosome changes [12]. Peripheral lymphocytes from women with SSc manifest an enhanced rate of X chromosome loss (i.e. X monosomy) [13] and possibly a more frequently skewed X inactivation pattern [14,15], which may contribute to an haplo-insufficiency of X-linked genes predisposing to autoimmunity. Recent experimental evidence suggests that some genes variably escape X chromosome inactivation in women and thus epigenetic differences in X-linked genes could explain both the female preponderance and low monozygotic twin concordance in autoimmune disorders such as SSc [16]. We herein report the first study of the X chromosome-wide DNA methylation profile in the unique model of MZ twins discordant and concordant for SSc. Using this approach, we identify differentially methylated genes that will be useful in dissecting the epigenetic bases of the disease.
Materials and methods
Subjects
Genomic DNA was extracted from peripheral blood mononuclear cells (PBMC) from eight pairs of MZ twins which in seven cases were discordant and in one case concordant for SSc (in the latter case one subject had diffuse and one had limited SSc). The age of discordant twins ranged between 41 and 59 years, while the concordant set was 62 years old at the time of enrolment. Twin sets only included women and have already been described in a previous work, along with the DNA extraction methods [3]. The protocol was approved by the IRB of the University of California at Davis and all subjects provided written informed consent.
Methylated DNA immunoprecipitation (MeDIP)
DNA samples were sheared randomly by sonication to generate fragments between 300 and 500 base pairs (bp), which were immunoprecipitated with a monoclonal mouse antibody against 5-methylcytidine (Ab108005; Abcam, Cambridge, UK). The MeDIP efficiency was verified by polymerase chain reaction (PCR). After the enrichment of MeDIP DNA was validated, genomic MeDIP and control fragments were converted to PCR-amplifiable OmniPlex™ Library (Rubicon Genomics, Ann Arbor, MI, USA) molecules flanked by universal priming sites. The library was then amplified using the Genomeplex Complete WGA 2 Kit (catalogue no. WGA2-50RXN; Sigma, St Louis, MO, USA) by PCR using universal primers with a limited number of cycles.
DNA methylation microarrays
Two to 4 µg of immunoprecipitated and reference DNA were tagged, respectively, with cyanine-5 (Cy5) and Cy3-labelled random 9-mers and hybridized using the NimbleGen Array Hybridization Kit (Roche, Madison, WI, USA). A custom DNA methylation 4-plex array was obtained and utilized to include 998 X chromosome and 18 086 autosomal chromosome promoter sites for methylation analysis for each sample. Oligomers (50–60 nucleotides) used in the microarray hybridization were designed to embrace wide promoter-including regions. The detailed sample preparation protocol is available upon request from Roche Microarray Technical Support.
Data processing and statistical analysis
Our data analysis was limited to the X chromosome sites, but we also report that none of the autosomic chromosome sites met the established consistency criteria for methylation differences (data not shown). Data obtained from Nimblescan software have been processed and converted into a .gff file for each patient containing a P-value for each probe, individuated by a peak start (i.e. the first base of the peak in the chromosome) and a peak end (i.e. the last base of the peak). Because P-values for each twin were distributed in a Gaussian fashion, after the conversion in P-scores (–log10 P-value), we filtered the data set by selecting only the most probably methylated peaks, i.e. with P-score > 1·31 (corresponding to a P-value < 0·05). Next, we have generated a list of methylated sites shared by the concordant twins couple and subsequently determined methylation peaks consistently different in at least three discordant sets, subdivided according to whether sites were exclusively hypermethylated in the affected twins or in healthy twins.
The University of California Santa Cruz (UCSC) human genome browser build hg18 (http://genome.ucsc.edu; [17]) was utilized to enrich the data set with chromosomal and genic localization of each identified peak. Promoters and cytosine–phosphate–guanine (CpG) islands were detected using a window of ± 2 kb of the transcription starting site while gene names and symbols approved by the HUGO Gene Nomenclature Committee (HGNC) were used. Information about the function and products of each identified gene was obtained from bibliographical research and the online Gene Expression Atlas consulting the EMBL-EBI (European Molecular Biology Laboratory–European Bioinformatics Institute) database.
Gene ontology clustering
The genes identified as being differentially methylated in SSc were investigated using an unsupervised analysis for gene ontology information by Ingenuity Pathway Analysis (IPA) software (Ingenuity Systems, http://www.ingenuity.com). IPA is a network analysis program for biological data in human, mouse and rat that is based on integrated data to retrieve the putative interactions of genes of interest into known or proposed networks. To start building networks, IPA queries the Ingenuity Pathways Knowledge Database for interactions between identified ‘Focus Genes’, in our case hyper- or hypomethylated genes, and all other gene objects stored in the knowledge base, to generate a set of networks, with maximum network size of 35 genes/proteins. Networks are displayed graphically as gene/genes products (nodes) and the biological relationships between the nodes (edges). All edges are supported by at least one reference from the literature, or from canonical information stored in the Ingenuity Pathways Knowledge Base. In addition, IPA computes a score for each network according to the fit of the user's set of significant genes. The score, representing the – log(P-value), indicates the likelihood of the Focus Genes in a network from Ingenuity Knowledge Database being found together randomly.
Results
We identified X chromosome sites that were consistently hypermethylated (n = 18, Table 1) and hypomethylated (n = 25, Table 2) in affected twins. Within the 5-kb window sampled for each X-linked gene, most of the differentially methylated regions (DMRs) were located in promoter regions or CpG islands while two hypermethylated (48980151–48980208 and 104355356–104355413) and six hypomethylated (103883076–103883125, 47226194–47226247, 134532227–134532289, 134532327–134532376, 134532427–134532476, 134532627–134532676) DMRs were found downstream of the transcription start sites. In all cases, DMRs were associated with known genes and we noticed that IL1RAPL2 was found in both lists (the two hypermethylated sites are downstream of the hypomethylated one). In some cases, multiple DMRs belong to the same gene (as in the case of hypomethylated peaks 134532227–134532289, 134532327–134532376, 134532427–134532476 and 134532627–134532676 onto gene DDX26B) or a specific site is located in a CpG island of a bidirectional promoter for two different genes (i.e. hypomethylated peak 152712287–152712338 for genes SSR4 and IDH3G). Three hypomethylated peaks are associated with intergenic single-nucleotide polymorphisms (SNP), with peak 13087308–13087357, including SNP rs61677044, peak 13087708–13087757 falling in a region 150 bp downstream from SNP rs16978681, peaks 126140539–126140588 and 126140739–126140788 mapping to a SNP-rich region. Genes identified by the hypermethylated and hypomethylated sites encode for proteins that are illustrated in Tables 3 and 4, respectively. The 26 proteins include transcription factors, membrane and soluble enzymes, surface antigens and translocation proteins while in some cases proteins are currently defined only structurally, but not functionally.
Table 1.
DNA sites that were found hypermethylated in twins with systemic sclerosis (SSc) and that had similar profiles in concordant twins. For each site the start–end location, the chromosome position and the target gene are specified. In some cases, multiple sites have the same target gene
| Start | End | Position | Target gene | Gene part | |
|---|---|---|---|---|---|
| 1 | 21868249 | 21868298 | Xp22.11 | SMS | Promoter |
| 2 | 21868349 | 21868398 | Same as 1 | ||
| 3 | 24944225 | 24944274 | Xp21.3 | ARX | CpG island |
| 4 | 24944125 | 24944174 | Same as 3 | ||
| 5 | 47305466 | 47305515 | Xp11.23 | ARAF | CpG island |
| 6 | 47305566 | 47305615 | Same as 5 | ||
| 7 | 48980151 | 48980208 | Xp11.23 | CCDC22 | Body |
| 8 | 52794239 | 52794288 | Xp11.22 | SSX2 | Promoter |
| 9 | 52794039 | 52794100 | Same as 8 | ||
| 10 | 52793839 | 52793895 | Same as 8 | ||
| 11 | 71268623 | 71268672 | Xq13.1 | RGAG4 | CpG island |
| 12 | 77246607 | 77246656 | Xq21.1 | PGK1 | CpG island |
| 13 | 77246607 | 77246656 | Same as 12 | ||
| 14 | 104355356 | 104355413 | Xq22.3 | IL1RAPL2 | Body |
| 15 | 128867440 | 128867489 | Xq25 | UTP14A | CpG island |
| 16 | 148522050 | 148522099 | Xq28 | HSFX1 | Promoter |
| 17 | 149487626 | 149487675 | Xq28 | MTM1 | CpG island |
| 18 | 153360053 | 153360102 | Xq28 | LAGE3 | Promoter |
CpG: cytosine–guanine–dinucleotide.
Table 2.
DNA sites that were found hypomethylated in twins with systemic sclerosis (SSc) and that had similar profiles in concordant twins. For each site the start–end location, the chromosome position and the target gene are specified. In some cases, multiple sites have the same target gene
| Start | End | Position | Target gene | Gene part | |
|---|---|---|---|---|---|
| 1 | 103883076 | 103883125 | Xq22.3 | IL1RAPL2 | Body |
| 2 | 103883176 | 103883225 | Same as above | ||
| 3 | 13087308 | 13087357 | Xp22.2 | See results | |
| 4 | 13087708 | 13087757 | Xp22.2 | See results | |
| 5 | 126140539 | 126140588 | Xq25 | See results | |
| 6 | 126140739 | 126140788 | Same as above | ||
| 7 | 2429530 | 2429579 | Xp22.33 | ZBED1 | Promoter |
| 8 | 23982786 | 23982835 | Xp22.11 | EIF2S3 | CpG island |
| 9 | 41077310 | 41077359 | Xp11.4 | DDX3X | CpG island |
| 10 | 47226194 | 47226247 | Xp11.3 | ZNF41 | Body |
| 11 | 118253590 | 118253639 | Xq24 | PGRMC1 | CpG island |
| 12 | 118592857 | 118592906 | Xq24 | UBE2A | CpG island |
| 13 | 129230204 | 129230253 | Xq25 | ZNF280C | CpG island |
| 14 | 129864954 | 129865003 | Xq26.1 | ENOX2 | Promoter |
| 15 | 133759124 | 133759176 | Xq26.3 | FAM122C | Promoter |
| 16 | 133991162 | 133991218 | Xq26.3 | FAM127C | Promoter |
| 17 | 134532227 | 134532289 | Xq26.3 | DDX26B | Body |
| 18 | 134532327 | 134532376 | Same as 17 | ||
| 19 | 134532427 | 134532476 | Same as 17 | ||
| 20 | 134532627 | 134532676 | Same as 17 | ||
| 21 | 148429003 | 148429052 | Xq28 | CXorf40A | Promoter |
| 22 | 148429303 | 148429352 | Same as 21 | ||
| 23 | 148429403 | 148429452 | Same as 21 | ||
| 24 | 152712287 | 152712338 | Xq28 | SSR4 | CpG island |
| IDH3G | CpG island | ||||
| 25 | 152712387 | 152712436 | Same as above |
CpG: cytosine–guanine–dinucleotide.
Table 3.
Proteins encoded by genes identified by hypermethylated sites in affected twins
| Gene symbol | Gene name | Localization | Protein type | Function | Cell or tissue expression | Diseases |
|---|---|---|---|---|---|---|
| SMS | Sspermine synthase | Cytoplasm | Enzyme | Arginine and proline metabolism; methionine metabolism; urea cycle and metabolism of amino groups; β-alanine metabolism | Double-negative T cells, bone marrow cell lines, breast carcinoma, gastrocnemius, myeloma cell lines | Mental retardation, X-linked, Snyder–Robinson type, prostatic carcinoma |
| ARX | Aristaless related homeobox | Nucleus | DNA binding transcription factor | Tissue development, especially of brain | Neural precursor cells, brain, skeletal muscle, heart, liver, testis, Ewing's sarcoma | Lissencephaly X-linked type 2, epileptic encephalopathy early infantile type 1, Partington syndrome, agenesis of the corpus callosum with abnormal genitalia |
| ARAF | V-raf murine sarcoma 3611 viral oncogene homolog | Cytoplasm (mitochondria, nucleus) | Enzyme | Involved in pathway of EGFR1 | Bone marrow, melanoma cell lines, CD34+ cells, acute myeloid leukaemia blast cells, choroidal melanocytes, eye, heart tissue, lymphoblastoid cell lines, squamous cell carcinoma cell lines | – |
| CCDC22 | Coiled-coil domain containing 22 | Unknown | Unknown | Regulation apoptosis (?) | Immature dendritic cells, pancreas, stomach; renal, endometrial and ovarian cancer | Over-expression in malignancies |
| SSX2 | Synovial sarcoma, X breakpoint 2 | Nucleus | Transcription factor | Transcription regulator activity | Testis, thyroid gland | Synovial sarcoma |
| RGAG4 | Retrotransposon gag domain containing 4 | Nucleus | Unknown | Unknown | Ubiquitous | – |
| PGK1 | Phosphoglycerate kinase 1 | Cytoplasm (nucleus, mitochondria) | Enzyme | Glycolysis. May also act as a co-factor for polymerase alpha | Ubiquitous | Aemolytic anaemia, rhabdomyolisis |
| IL1RAPL2 | Interleukin 1 receptor accessory protein-like 2 | Plasma membrane | Co-receptor of IL-1R (?) | Cell communication, signal transduction (?) | Brain, ovary | – |
| UTP14A | UTP14, U3 small nucleolar ribonucleoprotein, homolog A (yeast) | Nucleolus | Possible protein cofactor | 18S rRNA synthesis. Also binds p53 and promotes p53 degradation | – | – |
| HSFX1 | Heat shock transcription factor family, X linked 1 | Nucleus | Transcription factor | Transcription regulator activity | Testis | Male infertility, amyotrophic lateral sclerosis |
| MTM1 | Myotubularin 1 | Cytoplasm | Enzyme | Dual-specificity phosphatase that acts on both phosphotyrosine and phosphoserine | Brain, heart, kidney, liver, placenta, lung, pancreas | X-linked myotubular myopathy |
| LAGE3 | L antigen family, member 3 | Cytoplasm, nucleus | Unknown | Unknown | Ubiquitous | – |
Table 4.
Proteins encoded by genes identified by hypomethylated sites in affected twins
| Gene symbol | Gene name | Localization | Protein type | Function | Sites of expression | Diseases |
|---|---|---|---|---|---|---|
| IL1RAPL2 | Interleukin 1 receptor accessory protein-like 2 | See Table 3 | ||||
| ZBED1 | Zinc finger, BED-type containing 1 | Nucleus | Transcription factor | Binds to DNA elements found in the promoter regions of several genes related to cell proliferation, such as histone H1 | Ubiquitous | – |
| EIF2S3 | Eukaryotic translation initiation factor 2, subunit 3 gamma, 52 kDa | Cytoplasm | Translational factor | Subunit gamma of GTP-binding protein involved in the recruitment of methionyl-tRNA to the 40S ribosomal subunit | Ubiquitous | Neurodegenerative disorders, prostate cancer |
| DDX3X | DEAD (Asp-Glu-Ala-Asp) box polypeptide 3, X-linked | Nucleus | Enzyme | RNA helicases involved in expression of virus-stimulated Type I interferons | Ubiquitous | Amyotrophic lateral sclerosis |
| Cell growth and apoptosis | ||||||
| ZNF41 | Zinc finger protein 41 | Nucleus | DNA binding protein | Transcription regulator activity | Ubiquitous | Mental retardation |
| PGRMC1 | Progesterone receptor membrane component 1 | Plasma membrane | Receptor | Cell viability and death | Endometrium, kidney, liver, monocytes | Tumorigenesis of reproductive tissues, Huntington's disease |
| UBE2A | Ubiquitin-conjugating enzyme E2A | Nucleus, cytoplasm | Enzyme | Protein degradation | Tumour cell lines, embryonic cell lines, heart tissues, pancreatic tissues, placental tissue | Mental, retardation; UBE2A deficiency syndrome |
| ZNF280C | Zinc finger protein 280C | Nucleus | Transcription factor (?) | |||
| ENOX2 | Ecto-NOX disulfphide-thiol exchanger 2 | Plasma membrane, excreted | Enzyme | Catalysis of hydroquinone or NADH oxidation, and protein disulphide interchange | Ubiquitous | Serum and cellular marker of tumour progression and body ageing |
| FAM122C | Family with sequence similarity 122C | Unknown | Unknown | Unknown | – | – |
| FAM127C | Family with sequence similarity 127, member C | Unknown | Unknown | Unknown | Ubiquitous | – |
| DDX26B | DEAD/H (Asp-Glu-Ala-Asp/His) box polypeptide 26B | Unknown | Unknown | Unknown (RNA helicase activity?) | – | – |
| CXorf40A | Chromosome X open reading frame 40A | Cytoplasm, nucleus | Unknown | Involved in cell proliferation and inflammation (?) | Colon, heart, kidney, liver, placenta, small intestine, spleen | Parkinson's disease |
| SSR4 | Signal sequence receptor, delta (translocon-associated protein delta) | Endoplasmic reticulum | Subunit of TRAP complex | Protein translocation through endoplasmic reticulum membrane | Brain, heart, kidney, liver, placenta, lung, pancreas, monocytes, B cells | – |
| IDH3G | Isocitrate dehydrogenase 3 gamma subinit | Mitochondrion | Enzyme | Reversible decarboxylation of isocitrate to 2-oxoglutarate | Ubiquitous | – |
NADH: nicotinamide adenine dinucleotide.
We explored possible functional relationships between the 26 genes using the IPA Knowledge Database. Unsupervised IPA network analysis identified a single cluster of 25 genes that included seven of our 26 genes and 18 additional genes, which was unlikely to occur by chance (P = 10−13). The plausible biological network generated is shown in Fig. 1 and we note that seven of our 26 genes were gathered in a highly significant network revolving around interleukin 6 (IL-6).
Fig. 1.
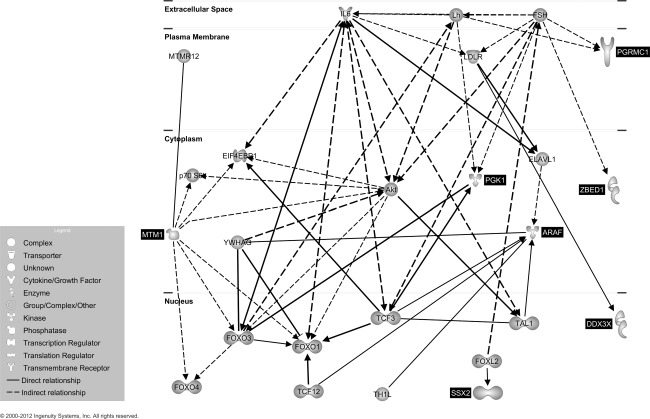
Putative gene network derived from Ingenuity Pathway Analysis (IPA) software analysis. Edges are displayed with labels that describe the nature of the relationship between nodes while lines connecting genes represent known interactions (with solid lines representing direct interactions and dashed lines representing indirect interactions). Nodes are displayed using various shapes that represent the functional class of the gene product (see insert). Black highlighting indicates the seven genes differentially methylated in our analysis and non-highlighted genes are those identified by IPA.
Discussion
This is the first report of a genome-wide fine mapping of DNA methylation in MZ twins discordant and concordant for SSc. Interestingly, we found that consistent differences between the studied twins affect only genes located on the X chromosome, thus possibly contributing to the aetiology of SSc female predominance. The study of individual susceptibility to autoimmune diseases is hampered by numerous issues which apply well to SSc, including the rare prevalence, the long latency between the exposure to specific environmental factors and disease onset and the limited applicability of GWAS data gathered in recent studies [18–25]. These limitations are well represented by the variable concordance rates in MZ twins for specific autoimmune diseases and suggest that epigenetic changes may constitute the missing link between individual susceptibility and environmental factors. Data on the epigenetics of SSc are limited to the observation that DNA from CD4+ T cell of patients with SSc is hypomethylated significantly compared to healthy controls, along with a reduced expression of enzymes crucial to DNA methylation such as DNMT1, MBD3 and MBD4 [26]. More specifically, the FL1 promoter is down-regulated by CpG methylation in SSc fibroblasts, thus influencing the expression of collagen alpha 1 and other matrix proteins [27]. Conversely, a growing amount of data is being produced by epigenome-wide studies of peripheral blood cells from MZ twins discordant for autoimmune diseases such as type 1 diabetes [28], multiple sclerosis [29], systemic lupus erythematosus [30] and psoriasis [31]. These studies are performed mainly on effector cell subpopulations (monocytes, T cells) to identify differentially expressed genes possibly preceding disease onset [28].
Investigating DMRs in peripheral blood mononuclear cells (PBMC) from MZ twins discordant and concordant for SSc to search for aetiological factors and biomarkers for SSc is expected to be a powerful tool in spite of the limited number of samples examined. First, the limited number of samples should not be considered as a limit of this study based on the low prevalence of the disease in the general population, ranging from 71 to 433 cases per million [32], the low rate of MZ twinning (approximately three to four per 1000 pregnancies) [33] and the low concordance for SSc in such twins [3]; these factors suggest that our series of twins is representative of a general population of 10 million individuals. Secondly, several studies investigated the expression signature in PBMC from patients affected by complex diseases [34–36], including SSc, with reported correlations with defined subsets of SSc and different organ involvements [37]. Among such identified markers, interferon (IFN)-induced protein 44 seems to be one of the most highly differentially expressed gene in SSc monocytes and CD4+ T cells as well as IL-1α and IL-16 [38]. While we could not identify genes from this previous report as differentially methylated in MZ twins, our approaches are similar in providing support that peripheral blood is the ideal target tissue for the development of SSc biomarkers.
While our custom-designed array included 998 sites from the X chromosome and several thousand sites from autosomal chromosomes, only X chromosome sites were found to be consistently hypermethylated (n = 18) or hypomethylated (n = 25) in twins with SSc while sharing identical methylation profiles in the one set concordant for the disease. These sites corresponded to 26 genes that were investigated further for their function and their putative pathways involved; such genes could be expected to be expressed differentially based on their methylation profile although an inverse correlation between hypermethylation and reduced expression can only be hypothesized and should be investigated by reverse transcription–polymerase chain reaction (RT–PCR) in the future if additional blood samples can be obtained for this purpose.
Among differentially methylated sites, we found some of particular interest. First, the spermine synthase (SMS) gene encodes for an enzyme involved in polyamine synthesis and recycling [39]. The synthesis is directed by two aminopropyltransferases, i.e. spermidine synthases converting putrescine into spermidine and SMS which converts spermidine into spermine, with decarboxylated S-adenosylmethionine (SAM) as the aminopropyl donor in both cases [40]. A ‘polyamine hypothesis’ has been proposed recently by Wesley Brooks, who suggests that alterations in polyamine synthesis may lead to disease phenotype secondary to abnormalities in DNA methylation status when SAM is in excess [41]. Secondly, among hypomethylated sites, four refer to the gene encoding for DDX26B, a member of the DEAD/H-box helicases involved in various steps of RNA metabolism and chromatin dynamics [42,43], particularly in the Drosophila mode [44]. Of note, DDX proteins are involved in virus-induced innate immunity acting as a scaffold for protein–protein interactions in the signalling cascade that controls IFN type 1 [45] as a critical component of the TANK-binding kinase-1 that activates the transcription factor IFN regulatory factor (IRF)-1 and IRF-7 to promote the expression of IFN-α and -β[46]. Accordingly, changes in DDX3X expression mediated by altered methylation may support the correlation between viral infections and SSc [47]. Thirdly, the association between SSc and ENOX2 hypomethylation is of particular interest, as tissue hypoxia is a major feature of SSc tissues. ENOX2 is a membrane copper-containing enzyme that catalyzes electron transfer from hydroquinone or nicotinamide adenine dinucleotide (NADH) to molecular oxygen, thus playing a role in the induction of reactive oxygen species [48] which have a direct profibrogenic effects on fibroblasts, thus favouring fibrosis [49]. Fourthly, four differentially methylated sites mapped within gene bodies (intragenic methylation) and the process is now recognized to variably affect gene expression, as observed in plants [50,51] and mammals [52–54]. Among these genes, IL1RALP2 belongs to a novel class of IL-1/Toll-like receptor family characterized by the presence of a 150 amino acids carboxy terminus that has no significant homology with any protein of known function [55]. Signalling experiments indicate that neither ILRAPL2 nor its homologue ILRAPL1 can mediate transcriptional activation of nuclear factor (NF)-κB in response to IL-1α, IL-1β or IL-18 [56] and the protein biological features remain to be defined, but a functional study suggests that the ILRAPL1 intracellular domain is crucial to neutrophil function during the inflammatory response [57]. Finally, we were intrigued by the observation that several genes associated with hypomethylated sites have been linked with neurological disorders and mental retardation. While we can only speculate on the putative mechanisms justifying this association, we are traced back to earlier reports of the co-existence of phenylketonuria-associated mental retardation and scleroderma [58]. Further, there are data suggesting that patients with SSc may have disturbances of the nervous system as represented by an impaired response to stress [59], the detection of central nervous system ischaemic vasculopathy at magnetic resonance imaging [60,61] and the changes in cerebrovascular reactivity [62].
To determine the mechanistic implications of our findings we utilized IPA in an unsupervised manner to allow the identification of gene–gene relationship without a priori assumptions. This analysis linked seven of our 26 genes in a highly significant network around IL-6. This is not surprising, as IL-6 seems to be involved in SSc endothelial dysfunction and fibrogenesis [63]. Increased IL-6 levels have been found in serum [64] and lesional skin specimens from patients with SSc [65]. Further, an increased B cell-mediated IL-6 production has been reported from SSc lung fibroblasts in vitro with a parallel increased collagen production, and the pharmacological B cell depletion in SSc leads to a decrease in skin score paralleled by decreased IL-6 serum levels [66]. Finally, SSc serum is able to induce IL-6-mediated endothelial activation and apoptosis [67]. Nevertheless, we should also note that the genes identified in the present work do not include interleukins or their receptors, possibly based on a more indirect effect of other genes [68].
In conclusion, we report herein X chromosome genes that may contribute to SSc susceptibility through differential gene methylation, and thus expression, independent of genomic SNPs or variants. This is particularly relevant provided that data were gathered from MZ twins which recognize an identical genomic sequence. We are aware that expression data and cell subpopulation analysis need to be sought to confirm the proposed associations which could be masked by cumulative cell population effects or be modulated by additional mechanisms which include but are not limited to histone post-translational modifications and non-coding RNAs (such as microRNAs). This limitation is well represented by the lack of changes observed in DNA methylation, possibly leading to different interleukin expression, as reported in SSc peripheral blood [68]. Nevertheless, we are convinced that genome-wide epigenomic studies have the unique potential to provide new evidences on the aetiopathogenesis of complex diseases while possibly proposing novel clinical biomarkers and therapeutic targets.
Acknowledgments
This study was supported by the generous contribution of the Scleroderma Foundation Starting Investigator Grant.
Disclosure
The authors have nothing to disclose.
References
- 1.Chighizola C, Shoenfeld Y, Meroni PL. Systemic sclerosis. Introduction. Autoimmun Rev. 2011;10:239–40. doi: 10.1016/j.autrev.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Bogdanos DP, Smyk DS, Rigopoulou EI, et al. Twin studies in autoimmune disease: genetics, gender and environment. J Autoimmun. 2012;38:J156–69. doi: 10.1016/j.jaut.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Feghali-Bostwick C, Medsger TA, Jr, Wright TM. Analysis of systemic sclerosis in twins reveals low concordance for disease and high concordance for the presence of antinuclear antibodies. Arthritis Rheum. 2003;48:1956–63. doi: 10.1002/art.11173. [DOI] [PubMed] [Google Scholar]
- 4.Bossini-Castillo L, Martin JE, Broen J, et al. A GWAS follow-up study reveals the association of the IL12RB2 gene with systemic sclerosis in Caucasian populations. Hum Mol Genet. 2012;21:926–33. doi: 10.1093/hmg/ddr522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allanore Y, Saad M, Dieude P, et al. Genome-wide scan identifies TNIP1, PSORS1C1, and RHOB as novel risk loci for systemic sclerosis. PLoS Genet. 2011;7:e1002091. doi: 10.1371/journal.pgen.1002091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Santis M, Selmi C. The therapeutic potential of epigenetics in autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:92–101. doi: 10.1007/s12016-011-8293-8. [DOI] [PubMed] [Google Scholar]
- 7.Meda F, Folci M, Baccarelli A, Selmi C. The epigenetics of autoimmunity. Cell Mol Immunol. 2011;8:226–36. doi: 10.1038/cmi.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu Q, Renaudineau Y, Cha S, et al. Epigenetics in autoimmune disorders: highlights of the 10th Sjogren's syndrome symposium. Autoimmun Rev. 2010;9:627–30. doi: 10.1016/j.autrev.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Wong AH, Gottesman, Petronis A. Phenotypic differences in genetically identical organisms: the epigenetic perspective. Hum Mol Genet. 2005;14:R11–8. doi: 10.1093/hmg/ddi116. (Special issue no. 1) [DOI] [PubMed] [Google Scholar]
- 10.Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA. 2005;102:10604–9. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–80. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 12.Invernizzi P, Pasini S, Selmi C, Gershwin ME, Podda M. Female predominance and X chromosome defects in autoimmune diseases. J Autoimmun. 2009;33:12–6. doi: 10.1016/j.jaut.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Invernizzi P, Miozzo M, Selmi C, et al. X chromosome monosomy: a common mechanism for autoimmune diseases. J Immunol. 2005;175:575–8. doi: 10.4049/jimmunol.175.1.575. [DOI] [PubMed] [Google Scholar]
- 14.Ozbalkan Z, Bagislar S, Kiraz S, et al. Skewed X chromosome inactivation in blood cells of women with scleroderma. Arthritis Rheum. 2005;52:1564–70. doi: 10.1002/art.21026. [DOI] [PubMed] [Google Scholar]
- 15.Uz E, Loubiere LS, Gadi VK, et al. Skewed X-chromosome inactivation in scleroderma. Clin Rev Allergy Immunol. 2008;34:352–5. doi: 10.1007/s12016-007-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selmi C, Brunetta E, Raimondo MG, Meroni PL. The X chromosome and the sex ratio of autoimmunity. Autoimmun Rev. 2012;11:A531–7. doi: 10.1016/j.autrev.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Kent WJ, Sugnet CW, Furey TS, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorlova O, Martin JE, Rueda B, et al. Identification of novel genetic markers associated with clinical phenotypes of systemic sclerosis through a genome-wide association strategy. PLoS Genet. 2011;7:e1002178. doi: 10.1371/journal.pgen.1002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gourh P, Agarwal SK, Martin E, et al. Association of the C8orf13-BLK region with systemic sclerosis in North-American and European populations. J Autoimmun. 2010;34:155–62. doi: 10.1016/j.jaut.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito I, Kawaguchi Y, Kawasaki A, et al. Association of a functional polymorphism in the IRF5 region with systemic sclerosis in a Japanese population. Arthritis Rheum. 2009;60:1845–50. doi: 10.1002/art.24600. [DOI] [PubMed] [Google Scholar]
- 21.Ito I, Kawaguchi Y, Kawasaki A, et al. Association of the FAM167A-BLK region with systemic sclerosis. Arthritis Rheum. 2010;62:890–5. doi: 10.1002/art.27303. [DOI] [PubMed] [Google Scholar]
- 22.Radstake TRDJ, Gorlova O, Rueda B, et al. Genome-wide association study of systemic sclerosis identifies CD247 as a new susceptibility locus (vol 42, pg 426, 2010) Nat Genet. 2010;42:426–9. doi: 10.1038/ng.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rueda B, Gourh P, Broen J, et al. BANK1 functional variants are associated with susceptibility to diffuse systemic sclerosis in Caucasians. Ann Rheum Dis. 2010;69:700–5. doi: 10.1136/ard.2009.118174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuchiya N, Kawasaki A, Hasegawa M, et al. Association of STAT4 polymorphism with systemic sclerosis in a Japanese population. Ann Rheum Dis. 2009;68:1375–6. doi: 10.1136/ard.2009.111310. [DOI] [PubMed] [Google Scholar]
- 25.Zhou XD, Lee JE, Arnett FC, et al. HLA-DPB1 and DPB2 are genetic loci for systemic sclerosis. A genome-wide association study in Koreans with replication in North Americans. Arthritis Rheum. 2009;60:3807–14. doi: 10.1002/art.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei W, Luo Y, Yan K, et al. Abnormal DNA methylation in CD4+ T cells from patients with systemic lupus erythematosus, systemic sclerosis, and dermatomyositis. Scand J Rheumatol. 2009;38:369–74. doi: 10.1080/03009740902758875. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Fan P-S, Kahaleh B. Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum. 2006;54:2271–9. doi: 10.1002/art.21948. [DOI] [PubMed] [Google Scholar]
- 28.Rakyan VK, Beyan H, Down TA, et al. Identification of type 1 diabetes-associated DNA methylation variable positions that precede disease diagnosis. PLoS Genet. 2011;7:e1002300. doi: 10.1371/journal.pgen.1002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baranzini SE, Mudge J, van Velkinburgh JC, et al. Genome, epigenome and RNA sequences of monozygotic twins discordant for multiple sclerosis. Nature. 2010;464:1351–6. doi: 10.1038/nature08990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Javierre BM, Fernandez AF, Richter J, et al. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res. 2010;20:170–9. doi: 10.1101/gr.100289.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gervin K, Vigeland MD, Mattingsdal M, et al. DNA methylation and gene expression changes in monozygotic twins discordant for psoriasis: identification of epigenetically dysregulated genes. PLoS Genet. 2012;8:e1002454. doi: 10.1371/journal.pgen.1002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikpour M, Stevens WM, Herrick AL, Proudman SM. Epidemiology of systemic sclerosis. Best practice and research. Clin Rheumatol. 2010;24:857–69. doi: 10.1016/j.berh.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Judith GH. Twinning. Lancet. 2003;362:735–43. doi: 10.1016/S0140-6736(03)14237-7. [DOI] [PubMed] [Google Scholar]
- 34.Olsen NJ, Moore JH, Aune TM. Gene expression signatures for autoimmune disease in peripheral blood mononuclear cells. Arthritis Res Ther. 2004;6:120–8. doi: 10.1186/ar1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolen CR, Uduman M, Kleinstein SH. Cell subset prediction for blood genomic studies. BMC Bioinformatics. 2011;12:258. doi: 10.1186/1471-2105-12-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Idaghdour Y, Storey JD, Jadallah SJ, Gibson G. A genome-wide gene expression signature of environmental geography in leukocytes of Moroccan amazighs. PLoS Genet. 2008;4:e1000052. doi: 10.1371/journal.pgen.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pendergrass SA, Hayes E, Farina G, et al. Limited systemic sclerosis patients with pulmonary arterial hypertension show biomarkers of inflammation and vascular injury. PLoS ONE. 2010;5:e12106. doi: 10.1371/journal.pone.0012106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duan H, Fleming J, Pritchard DK, et al. Combined analysis of monocyte and lymphocyte messenger RNA expression with serum protein profiles in patients with scleroderma. Arthritis Rheum. 2008;58:1465–74. doi: 10.1002/art.23451. [DOI] [PubMed] [Google Scholar]
- 39.Brown CJ, Carrel L, Willard HF. Expression of genes from the human active and inactive X chromosomes. Am J Hum Genet. 1997;60:1333–43. doi: 10.1086/515488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coffino P. Regulation of cellular polyamines by antizyme. Nat Rev Mol Cell Biol. 2001;2:188–94. doi: 10.1038/35056508. [DOI] [PubMed] [Google Scholar]
- 41.Brooks WH, Le Dantec C, Pers JO, Youinou P, Renaudineau Y. Epigenetics and autoimmunity. J Autoimmun. 2010;34:J207–19. doi: 10.1016/j.jaut.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 42.de la Cruz J, Kressler D, Linder P. Unwinding RNA in Saccharomyces cerevisiae: DEAD-box proteins and related families. Trends Biochem Sci. 1999;24:192–8. doi: 10.1016/s0968-0004(99)01376-6. [DOI] [PubMed] [Google Scholar]
- 43.Gillespie DE, Berg CA. Homeless is required for RNA localization in Drosophila oogenesis and encodes a new member of the DE-H family of RNA-dependent ATPases. Genes Dev. 1995;9:2495–508. doi: 10.1101/gad.9.20.2495. [DOI] [PubMed] [Google Scholar]
- 44.Pal-Bhadra M, Leibovitch BA, Gandhi SG, et al. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science. 2004;303:669–72. doi: 10.1126/science.1092653. [DOI] [PubMed] [Google Scholar]
- 45.Ranji A, Boris-Lawrie K. RNA helicases: emerging roles in viral replication and the host innate response. RNA Biol. 2010;7:775–87. doi: 10.4161/rna.7.6.14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soulat D, Burckstummer T, Westermayer S, et al. The DEAD-box helicase DDX3X is a critical component of the TANK-binding kinase 1-dependent innate immune response. EMBO J. 2008;27:2135–46. doi: 10.1038/emboj.2008.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baum H, Davies H, Peakman M. Molecular mimicry in the MHC: hidden clues to autoimmunity? Immunol Today. 1996;17:64–70. doi: 10.1016/0167-5699(96)80581-0. [DOI] [PubMed] [Google Scholar]
- 48.Kelker M, Kim C, Chueh PJ, Guimont R, Morre DM, Morre DJ. Cancer isoform of a tumor-associated cell surface NADH oxidase (tNOX) has properties of a prion. Biochemistry. 2001;40:7351–4. doi: 10.1021/bi010596i. [DOI] [PubMed] [Google Scholar]
- 49.Svegliati S, Cancello R, Sambo P, et al. Platelet-derived growth factor and reactive oxygen species (ROS) regulate Ras protein levels in primary human fibroblasts via ERK1/2. Amplification of ROS and Ras in systemic sclerosis fibroblasts. J Biol Chem. 2005;280:36474–82. doi: 10.1074/jbc.M502851200. [DOI] [PubMed] [Google Scholar]
- 50.Zilberman D, Gehring M, Tran RK, Ballinger T, Henikoff S. Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat Genet. 2007;39:61–9. doi: 10.1038/ng1929. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X, Yazaki J, Sundaresan A, et al. Genome-wide high-resolution mapping and functional analysis of DNA methylation in arabidopsis. Cell. 2006;126:1189–201. doi: 10.1016/j.cell.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Ball MP, Li JB, Gao Y, et al. Targeted and genome-scale strategies reveal gene–body methylation signatures in human cells. Nat Biotechnol. 2009;27:361–8. doi: 10.1038/nbt.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hellman A, Chess A. Gene body-specific methylation on the active X chromosome. Science. 2007;315:1141–3. doi: 10.1126/science.1136352. [DOI] [PubMed] [Google Scholar]
- 54.Rauch TA, Wu X, Zhong X, Riggs AD, Pfeifer GP. A human B cell methylome at 100-base pair resolution. Proc Natl Acad Sci USA. 2009;106:671–8. doi: 10.1073/pnas.0812399106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greenfeder SA, Nunes P, Kwee L, Labow M, Chizzonite RA, Ju G. Molecular cloning and characterization of a second subunit of the interleukin 1 receptor complex. J Biol Chem. 1995;270:13757–65. doi: 10.1074/jbc.270.23.13757. [DOI] [PubMed] [Google Scholar]
- 56.Born TL, Smith DE, Garka KE, Renshaw BR, Bertles JS, Sims JE. Identification and characterization of two members of a novel class of the interleukin-1 receptor (IL-1R) family. Delineation of a new class of IL-1R-related proteins based on signaling. J Biol Chem. 2000;275:29946–54. doi: 10.1074/jbc.M004077200. [DOI] [PubMed] [Google Scholar]
- 57.Nef S, Fiumelli H, de Castro E, Raes MB, Nef P. Identification of neuronal calcium sensor (NCS-1) possibly involved in the regulation of receptor phosphorylation. J Recept Signal Transduct Res. 1995;15:365–78. doi: 10.3109/10799899509045227. [DOI] [PubMed] [Google Scholar]
- 58.Lasser AE, Schultz BC, Beaff D, Bielinski S, Kirschenbaum B. Phenylketonuria and scleroderma. Arch Dermatol. 1978;114:1215–7. [PubMed] [Google Scholar]
- 59.Matsuura E, Ohta A, Suematsu R, et al. Functional disturbance of the stress-adaptation system in patients with scleroderma. Mod Rheumatol. 2011;21:397–405. doi: 10.1007/s10165-010-0412-5. [DOI] [PubMed] [Google Scholar]
- 60.Mohamed RH, Nassef AA. Brain magnetic resonance imaging findings in patients with systemic sclerosis. Int J Rheum Dis. 2010;13:61–7. doi: 10.1111/j.1756-185X.2009.01453.x. [DOI] [PubMed] [Google Scholar]
- 61.Mohammed RH, Sabry YY, Nasef AA. Brain MRI screening showing evidences of early central nervous system involvement in patients with systemic sclerosis. Rheumatol Int. 2011;31:667–71. doi: 10.1007/s00296-009-1325-5. [DOI] [PubMed] [Google Scholar]
- 62.Giuliodori G, Fraticelli P, Bartolini M, et al. Cognitive and cerebral hemodynamic impairment in scleroderma patients. Eur J Neurol. 2009;16:1285–90. doi: 10.1111/j.1468-1331.2009.02714.x. [DOI] [PubMed] [Google Scholar]
- 63.Barnes TC, Anderson ME, Moots RJ. The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int J Rheumatol. 2011;2011:721608. doi: 10.1155/2011/721608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato S, Hasegawa M, Takehara K. Serum levels of interleukin-6 and interleukin-10 correlate with total skin thickness score in patients with systemic sclerosis. J Dermatol Sci. 2001;27:140–6. doi: 10.1016/s0923-1811(01)00128-1. [DOI] [PubMed] [Google Scholar]
- 65.Feghali CA, Bost KL, Boulware DW, Levy LS. Mechanisms of pathogenesis in scleroderma. I. Overproduction of interleukin 6 by fibroblasts cultured from affected skin sites of patients with scleroderma. J Rheumatol. 1992;19:1207–11. [PubMed] [Google Scholar]
- 66.Kondo K, Okada T, Matsui T, et al. Establishment and characterization of a human B cell line from the lung tissue of a patient with scleroderma; extraordinary high level of IL-6 secretion by stimulated fibroblasts. Cytokine. 2001;13:220–6. doi: 10.1006/cyto.2000.0822. [DOI] [PubMed] [Google Scholar]
- 67.Barnes TC, Spiller DG, Anderson ME, Edwards SW, Moots RJ. Endothelial activation and apoptosis mediated by neutrophil-dependent interleukin 6 trans-signalling: a novel target for systemic sclerosis? Ann Rheum Dis. 2011;70:366–72. doi: 10.1136/ard.2010.133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fujii H, Hasegawa M, Takehara K, Mukaida N, Sato S. Abnormal expression of intracellular cytokines and chemokine receptors in peripheral blood T lymphocytes from patients with systemic sclerosis. Clin Exp Immunol. 2002;130:548–56. doi: 10.1046/j.1365-2249.2002.02017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


