Abstract
Understanding the immune responses that explain why infants require multiple doses of pertussis vaccine to achieve protection against infection is a high priority. The objective of this study was to compare the function and phenotypes of antigen-specific CD4+ T cells in adults (n = 12), compared to infants (n = 20), following vaccination with acellular pertussis (DTaP) vaccine. Peripheral blood mononuclear cells (PBMCs) were stimulated with pertussis toxoid (PT), pertactin (PRN) and filamentous haemagglutinin (FHA). Multi-parameter flow cytometry was used to delineate CD4+ T cell populations and phenotypes producing interferon (IFN)-γ, interleukin (IL)-2, tumour necrosis factor (TNF)-α and IL-4. Based on surface CD69 expression, infants demonstrated activation of vaccine antigen-specific CD4+ T cells similar to adults. However, among infants, Boolean combinations of gates suggested that type 1 (Th-1) CD4+ T cell responses were confined largely to TNF-α+IL-2+IFN-γ- or TNF-α+IL-2-IFN-γ-. A significantly lower percentage of polyfunctional T helper type 1 (Th1) responses (TNF-α+IFN-γ+IL-2+) and type 2 (Th2) responses (IL-4) were present in the infants compared to adults. Moreover, a significantly higher percentage of infants' functional CD4+ T cells were restricted to CD45RA-CCR7+CD27+ phenotype, consistent with early-stage differentiated pertussis-specific memory CD4+ T cells. We show for the first time that DTaP vaccination-induced CD4+ T cells in infants are functionally and phenotypically dissimilar from those of adults.
Keywords: CD4 T cells (T helper; Th0, Th1, Th2, Th3, Th17), flow cytometry/FACS, paediatric, vaccines
Introduction
Pertussis remains poorly controlled, causing a total of 16 858 cases and 12 infant deaths in 2009 in the United States [1,2]. Adults contract pertussis due to waning immunity; however, mortality is rare [3]. Nevertheless, in many cases adults may become carriers for the transmission of pertussis to infants and young children [4,5]. Whole cell pertussis vaccine used for child immunization programmes in the past have been replaced in Europe and western countries with diphtheria, tetanus, acellular pertussis (DTaP) vaccination [6–8] and a number of laboratories, including ours, have shown that a robust humoral response is elicited as a result of DTaP vaccination in children, adolescents and adults [6,9,10]. Whereas circulating antibodies may play a role in pertussis toxin neutralization or blocking bacterial adherence to the epithelial cells of the respiratory mucosa, there is growing evidence that antigen-specific CD4+ T cells are required for evoking a long-lasting protective immunity against Bordetella pertussis[11–13]. Moreover, in some cases antibody titres do not always correlate with protection from pertussis [14–16].
CD4+ T cell-dependent protective responses to B. pertussis have been shown to correlate with elicitation of T helper type 1 (Th1) cytokines such as IFN-γ[12,17,18], whereas CD4+ T cells co-producing IL-2 and IFN-γ are thought to be essential contributors to long-lasting pertussis-specific protective immunity [12]. Studies in the past suggested that aP vaccination can promote cell-mediated immunity; however, those observations were made based on traditional antigen-specific T cell proliferation and enzyme-linked immunosorbent assay (ELISA)-based cytokine assays, and lacked information regarding memory generation, functional and/or phenotypic properties of vaccine-induced CD4+ T cells [18–20]. Other studies demonstrated the ability of pertussis toxin and aP vaccine to elicit a mixed Th1 and Th2 CD4+ T cell response [18,21], and that aP vaccine induces predominantly a Th2 CD4+ T cell response in vaccinated children [19,20,22]. In addition, the pattern of cytokine production on a single cell basis in DTaP-vaccinated infants and adults has not been delineated.
The combination of CCR7 and CD45RA has been used extensively for classifying antigen-experienced T cells (effector memory, TEM and central memory, TCM) [23]. More recently, another classification model based on expression of chemokine receptors CCR7 and CD27 has been described [24]. Based on the differences in their telomere lengths, in-vitro stimulation has demonstrated the order of CD4+ T cell differentiation as: naive to CCR7+CD27+ to CCR7-CD27+ to CCR7-CD27-. CCR7+CD27+ cells are least differentiated, whereas CCR7-CD27- are fully differentiated CD4+ T cells due to their shortest telomere lengths. Because characterization of pertussis-specific responses in infants and adults has not been performed previously, we developed a multi-parametric flow cytometry approach and analysis method that allowed simultaneous detection of multi-functionality and phenotypes of CD4+ T cells induced as a result of DTaP vaccination in infants compared to adults.
Material and methods
Subjects and peripheral blood mononuclear cells (PBMC) samples
Healthy infants (n = 20; aged between 9 and 12 months, median age 10 months) who have received three doses of DTaP vaccine (Sanofi Pasteur, Swiftwater, PA, USA) at 2, 4 and 6 months of age were involved in the study. The infants were from a cohort recruited as a part of a prospective study of immunity to respiratory pathogens (NIDCD R0108671). Healthy adult volunteers (n = 12; median age 30·4 years) vaccinated with the same DTaP within the preceding 5 years were given a booster dose before bleeding them at day 7 and for two subjects 3–4 months later for PBMC isolation. Written consent was obtained from parents of the children and the adults in association with a protocol approved by the Rochester General Hospital institutional review board. Heparinized venous blood was drawn and PBMCs isolated using Ficoll gradient according to the manufacturer's instructions. Cells were washed in phosphate-buffered saline (PBS) resuspended at a concentration of 1 × 107 cells/ml in cell recovery freezing media (Gibco, Grand Island, NY, USA) and frozen in liquid nitrogen until used.
Antigens and antibodies
Purified pertussis toxoid vaccine protein antigen (PT), pertactin (PRN) and filamentous haemagglutinin (FHA) were used for T cell stimulation (gifts from Sanofi Pasteur, Swiftwater, PA, USA). Antibodies used for staining were anti-CD3 Qdot 605 or Pacific blue (clone UCHT1, Invitrogen, Grand Island, NY, USA and Biolegend, San Diego, CA, USA), anti-CD4 allophycocyanin (APC) AlexaFluor 750 (clone RPA T4; eBiosciences, San Diego, CA, USA), APC-conjugated anti-CD69 (clone FN50, BD Biosciences, San Diego, CA, USA), phycoerythrin (PE)-Texas Red anti-CD45RA (clone MEM56; Invitrogen, Grand Island, NY, USA), anti-CCR7 peridinin chlorophyll (PerCP)/cyanin (Cy)5·5 conjugate (clone TG8/CCR7; Biolegend), anti-CD27 Qdot 605 (Invitrogen), PE-Cy7-conjugated anti-IFN-γ (clone B27; BD Biosciences), AlexaFluor 700 anti-IL-2 (clone MQ1-17H12; Biolegend) and PE-conjugated anti-IL-4 (clone 8D4-8; BD Biosciences). Anti-CD28 and anti-CD49d antibodies (clones L293 and L25, respectively) were obtained from BD Biosciences.
PBMC stimulation for detection of intracellular cytokine
Prior to stimulation, frozen PBMCs were thawed quickly in a 37°C water bath followed by slowly adding complete culture medium [RPMI-1640 supplemented with 10% of fetal bovine serum (FBS), 2 mM L-glutamine, 0·1 mM sodium pyruvate, non-essential amino acids, 100 U/ml penicillin, 100 µg/ml streptomycin]. After thawing, frozen cells were washed with RPMI-1640 and rested overnight in complete culture media in 24-well plates. PBMCs were stimulated using a protocol standardized in our laboratory and adapted from elsewhere [25–27]. Briefly, cells were counted and placed in a 96-well flat-bottomed plate and stimulated with either 1 µg/ml final protein concentration of individual antigen or Staphylococcal enterotoxin B (SEB). An optimal dosage for stimulation was determined by absence of detectable cell toxicity, measured by the use of tryptan blue staining and/or flow cytometry analysis after propidium iodide staining (data not shown). Cells were then incubated for 2 h at 37°C in the presence of 5% CO2 for antigen processing. After 2 h, Golgi transport inhibitors (BD Biosciences) were added to preserve cytokines intracellularly and incubated for an additional 4 h. Anti-CD28 and anti-CD49d (1 µg/ml) antibodies were also added to provide co-stimulation and enhance the detection of antigen-specific responses, as described earlier [28,29]. Unstimulated controls received all the reagents except antigens (including anti-CD28 and CD49d antibodies).
Surface and intracellular staining and flow cytometric analysis
Post-stimulation, cells were transferred to 96-well V-bottomed plates and washed once with fluorescence activated cell sorter (FACS) buffer (PBS with 0·5% FBS) before incubating them with various cell surface antibodies for 30 min, followed by washing with FACS buffer. Cells were then permeabilized with fixation and permeabilization solution (both BD Biosciences) for 20 min and a cocktail of antibodies was used to stain intracellularly captured cytokines as a result of stimulation. Because of down-regulation of CD3 on the T cell surface post-stimulation, CD3 staining was carried out intracellularly. After the usual washing steps, cells were acquired on a BD LSR II flow cytometer to collect 1 × 106 events for each sample and data were analysed using FlowJo (TreeStar) software.
Cells were first gated on lymphocytes based on forward- and side-scatter properties followed by sequential gating on CD4+ T cells before gating individual cytokine-positive CD4+ T cells (Fig. 1). For evaluation of cytokine responses, Boolean gating was used to create various combinations of cytokine-producing CD4+ T cells. Sixteen different combinations of gates were formed after combining individual cytokine gates, namely TNF-α, IFN-γ, IL-2 and IL-4. Various Th1 cytokine combinations (IFN-γ+IL2+TNF-α+; IFN-γ+IL-2+TNF-α-; IFN-γ+IL-2-TNF-α+; IFN-γ+IL-2-TNF-α-; IFN-γ-IL-2+TNF-α+; IFN-γ-IL-2+TNF-α-; IFN-γ-IL-2-TNF-α+) were compared among infants and adults. To evaluate Th2 responses, we compared IL4+CD4+ T cells that were negative for IFN-γ, IL-2 and TNF-α (IL-4+IFN-γ-IL-2-TNF-α-) among infants and adults. The percentages of responding cells were calculated by dividing frequencies of cytokine-positive cells with total CD4+ T cell counts for respective stimulation. Responding cells were also analysed with gating on CD69+CD4+ T cell populations and early activation marker of T cells that assists analysis in the multi-parameter protocols by allowing more definitive ‘clustering’ of the true responding cells [26,28]. Background staining, when present, is often only present in the CD69-negative fraction, and thus excluded from consideration. Cytokine producing cells were back-gated on CD69.
Fig. 1.
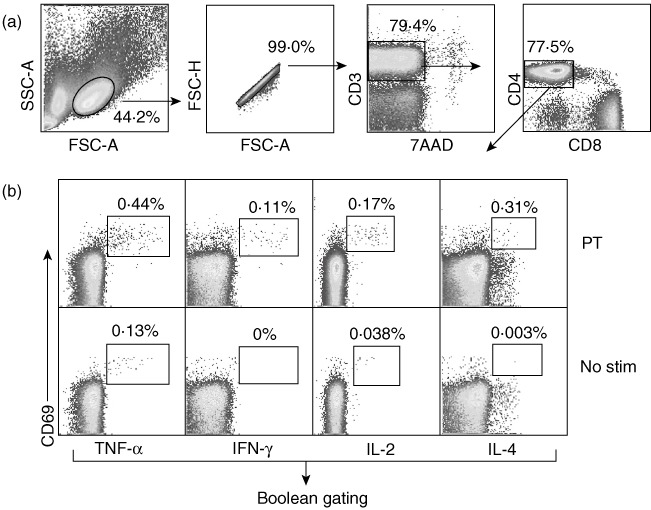
Gating strategy for analysing functional CD4+ T cell responses in infants and adults. Gating tree and analysis for flow cytometry panel used to simultaneously analyse interferon (IFN)-γ, interleukin (IL)-2-, tumour necrosis factor (TNF)- or IL-4 producing CD4+ T cells from peripheral blood mononuclear cells (PBMC) of infants and adults who were stimulated with pertussis toxoid antigen (PT). (a) Initial gating of total events included selection of lymphocytes based on forward- and side-scatter properties followed by gating single cells, live T cells (7AAD-CD3+) and CD4+CD8- T cells. (b) Boolean gating analysis of cytokine-positive populations were used to characterize each cell separately based on its functionality or quality with respect to cytokine production. Individual cytokine-positive CD4+ T cells in each sample were gated as shown and the responding frequencies of gate cut-off were determined based on unstimulated and positive control [Staphylococcal enterotoxin B (SEB)-stimulated] samples. Individual cytokine gates were combined using Boolean combination for all four individual cytokines (TNF-α, IFN-γ, IL-2, IL-4) and frequencies were noted for all 16 combinations of gates. Data for each stimulation were normalized with total CD4+ T cell counts and corrected further for background with respective unstimulated controls.
Cytometric bead array (CBA) for detection of secreted cytokines
For CBA assay, PBMCs were thawed and rested overnight as described previously before negative isolation of CD4+ T cells using magnetic activated cell sorter (MACS) columns (Miltenyi Biotech, Auburn, CA, USA) from each sample. The non-CD4+ T cell fraction was then pulsed with 1 µg/ml of antigen (PT, PRN or FHA individually) for 2 h and washed extensively with PBS before adding back to respective autologous CD4+ T cells. Finally the cells were suspended in complete RPMI-1640 media, as described above, and cultures were incubated for 18 h before collecting the supernatants for IL-4 quantitation using a CBA kit (BD Biosciences). An acquisition template provided by BD Biosciences was used to acquire cytometric beads on a LSRII flow cytometer and data were analysed using BD FACS DIVA and FlowJo software. Standard graphs were plotted and unknown sample values were extrapolated on linear regression plots with GraphPad Prism version 5 software.
Statistical analysis
P-values were calculated with two-tailed Mann–Whitney U-test using Graph Pad Prism version 5 software. Overall expression of CD69 following DTaP antigen stimulations (PT, PRN and FHA) was compared with one-way analysis of variance (anova).
Results
CD4+ T cells among vaccinated infants demonstrate pertussis antigen-specific activation as in adults
Following antigen encounter, CD69 expression on the surface of CD4+ T cells provides an early sign of T cell activation [30]. Therefore, we investigated if CD4+ T cells among infants demonstrate an increase in CD69 surface expression after ex-vivo stimulation with DTaP antigens. As depicted in Fig. 2a, stimulation with all pertussis antigens (PT, PRN and FHA) resulted in the up-regulation of CD69 expression among individual vaccinated infants compared to their respective unstimulated controls. Percentages of total CD69 expression on CD4+ T cells were variable among individual infants; however, as a result of antigenic stimulation all infants showed CD69 up-regulation over unstimulated controls (P = 0·007) (Fig. 2a).
Fig. 2.
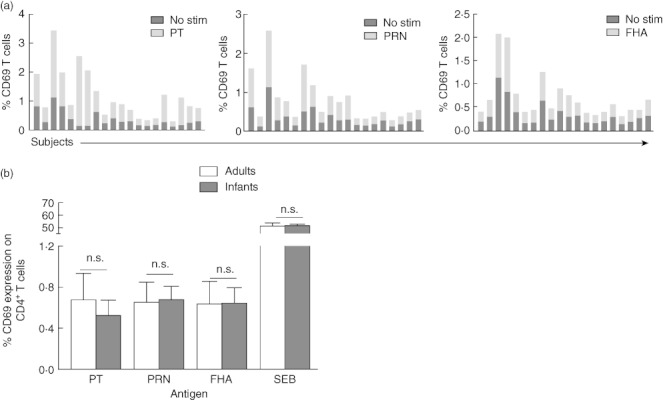
CD69 expression levels on CD4+ T cells after antigen stimulations. After stimulation with each antigen [pertussis toxoid (PT), pertactin (PRN) and filamentous haemagglutinin (FHA)], percentages of CD69 expressing CD4+ T cells among 20 infants were enumerated and plotted against respective unstimulated controls (a). The overall CD69 expression was also compared between infants and adults groups. Shown are percentages of CD4+ T cells expressing CD69 (mean ± standard error of the mean) in response to various diphtheria, tetanus, acellular pertussis (DTaP) antigens and Staphylococcal enterotoxin B (SEB) as a positive control in infants and adults (b).
Among adults, percentages of CD4+ T cells expressing CD69 were also increased post-antigenic and SEB stimulations. Of note, no significant differences were found in the percentages of CD4+ T cells expressing CD69 between infants and adults groups for each antigen stimulation (Fig. 2b).
Infants develop type 1 CD4+ T cell responses but with limited multi-function to pertussis antigens
Because intracellular expression of type 1 cytokines is thought to be critical for protective immunity against pertussis [12,17], we evaluated Th1 cytokine responses among infants and adults post-antigenic stimulations. As type 1 CD4+ T cell responses are determined primarily by the production of mainly IFN-γ, TNF-α and IL-2 [31], we examined if infants' CD4+ T cells were capable of expressing those cytokines in response to pertussis antigens. Dot-plots show that individual Th1 cytokine responses among adults and infants were restricted to CD69-up-regulated cells (Fig. 3a). However, in physiological conditions different combinations of Th1 responses can be produced by individual CD4+ T cells; hence we analysed various combinations of gates for Th1 cytokines rather than comparing individual cytokines (IFN-γ, TNF-α or IL-2). Such gating revealed that infants' CD4+ T cells produce high levels of TNF-α (IFN-γ-IL-2-TNF-α+) in response to vaccinated antigens followed by TNF-α+IL-2+ (IFN-γ-) (Fig. 3b). A similar trend was found among adults where antigen-specific CD4+ T cells produced TNF-α followed by TNF-α+IL-2+ as observed in infants (Fig. 3a,b). Conversely, among adults a significantly higher percentage of antigen-specific CD4+ T cells expressing multi-functional (TNF-α+IFN-γ+IL-2+) were present for all the stimulations (Fig. 3b,c).
Fig. 3.
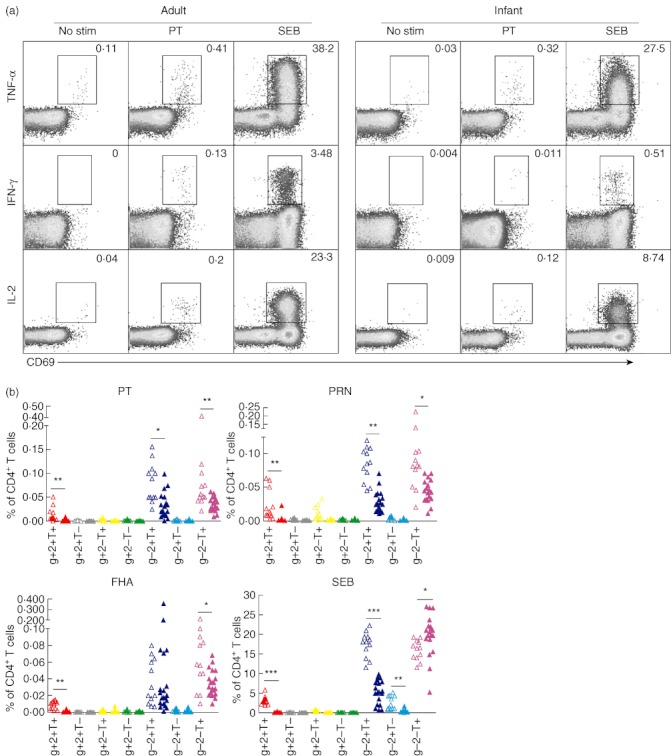
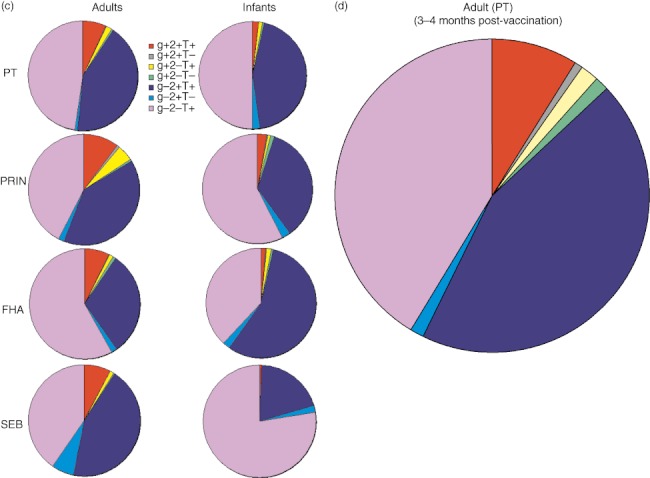
Type 1 CD4+ T cell response in infants and adults. (a) Flow cytometry dot-plots show co-expression of early T cell activation marker CD69, with various individual cytokines [tumour necrosis factor (TNF)-α, interferon (IFN)-γ and interleukin (IL-2)] among infants and adults that were gated previously on CD4+ T cells, as described in Fig. 1a. Frequencies of various T helper type 1 (Th1) cytokine combinations generated with Boolean gating were normalized with total CD4+ T cells in the respective samples, background-corrected with unstimulated controls and then presented as a bar graph (described in Methods) Open triangles represent infants while closed triangles represent adults. (b) or pie charts (c) & (d). The bar graphs show mean percentages of CD4+ T cells (all IL-4-) expressing IFN-γ+IL-2+TNF-α+ (depicted as g+2+T+); IFN-γ+IL-2+TNF-α- (g+2+T–); IFN-γ+IL-2-TNF-α+ (g+2–T+); IFN-γ+IL-2-TNF-α- (g+2–T–); IFN-γ-IL-2+TNF-α+ (g-2+T+); IFN-γ-IL-2+TNF-α- (g–2+T–) and IFN-γ-IL-2-TNF-α+ (g–2–T+) among infants and adults. P-values were calculated using the Mann–Whitney U-test; *<0·05; **<0·005; ***<0·0005.
CD4+ T cells Th2 responses to pertussis toxoid in infants were lower than adults
We evaluated the percentages of DTaP-specific IL-4 expressing CD4+ T cells among infants and adults. To enumerate Th2 cells specifically, using Boolean gating we considered IL-4+ cells that did not express any Th1 cytokines (IL-4+IFN-γ-IL-2-TNF-α-). We observed that, overall, infants had lower frequencies of IL-4 expressing DTaP antigen-specific CD4+ T cells than adults (Fig. 4a). To confirm this further, we used the CBA method to examine differences in IL-4 secretion after short-term (18-h) stimulation with pertussis antigens. Levels of IL-4 secreted in the cell culture supernatants were significantly lower in the CD4+ T cell cultures from infants compared to adults for all stimulations (Fig. 4b).
Fig. 4.
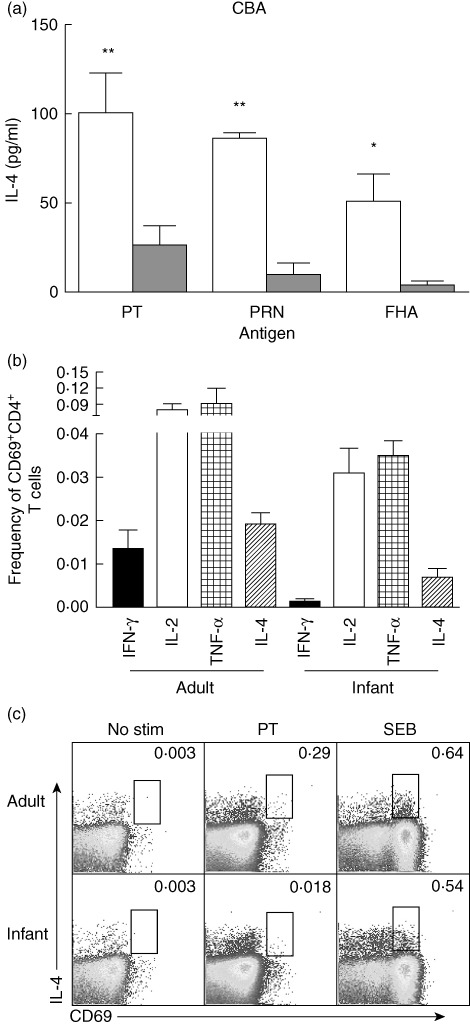
Diphtheria, tetanus and pertussis (DTP) antigen-specific interleukin (IL)-4 production among infants is lower than adults. (a) Comparison of the mean percentages of IL-4 expressing CD4+ T cells shows that adults had significantly higher pertussis toxoid (PT) and pertactin (PRN) antigen-specific CD4+ T cells compared to infants. IL-4 secretion was also measured using the cytometric bead array method for infants and adults. The bar graphs show mean concentrations (pg/ml) of IL-4 secreted in the culture supernatant of the infants and adults peripheral blood mononuclear cells (PBMCs) after stimulation with various diphtheria, tetanus, acellular pertussis (DTaP) antigens (b). Flow cytometry graphs show expression of IL-4 in the pertussis antigen-specific CD4+ T cells in the PBMCs of infant and adult samples (c). P-values were calculated using the Mann–Whitney U-test; *<0·05; **<0·005.
In a different set of analyses, we compared the balance of PT-specific Th1/Th2 cytokines among infants and adults. Percentage frequencies of CD69+CD4+ T cells were enumerated and compared for PT-specific individual cytokines (for IFN-γ, IL-2, TNF-α and IL-4). Although overall frequencies of PT-specific IFN-γ was lower than IL-4, a preponderance of other PT-specific Th1 cytokine-producing CD4+ T cells (IL-2 and TNF-α) was found among both cohorts (Fig. 4c). A similar difference in the Th1/Th2 cytokines was observed for other DTaP antigens (data not shown).
Phenotype discrepancies of pertussis toxoid-specific type 1 CD4+ T cells among infants and adults
To delineate the pertussis-specific CD4+ T cell phenotypes, cell surface characteristics of pertussis-specific functional type 1 CD4+ T cells were assessed in infants and adults. Based on the expression of CCR7 and CD27, the stage-specific differentiation of CD4+ T cells can be learned [24]. We evaluated stage-specific phenotypes of pertussis-specific CD4+ T cells within CD45RAlow cells of infants and adults. A significantly higher percentage of IL-2- and IFN-γ-producing CD4+ T cells in infants were found to be co-expressing CD27 and CCR7 (CD27+CCR7+), representing the earliest differentiated cell pattern. Infants had significantly lower percentages of fully differentiated cells, represented as CD45RA-CCR7-CD27-[24]. Conversely, nearly 50% of the PT-specific CD4+ T cells in adults were fully differentiated (CCR7-CD27-) (Fig. 5). A similar difference in IL-4 cytokine-producing PT-specific CD4+ T cells was observed among infants and adults (data not shown). For IL-2-expressing CD4+ T cells, no significant difference was found between the surface phenotypes CCR7-CD27+ and CCR7+CD27- cells, whereas IFN-γ-producing cells demonstrated a significantly higher percentage of the surface expression pattern of CCR7+CD27- cells compared to infants (Fig. 5). To assess whether the differences in PT-specific CD4+ T cell multi-functionality was due to the earlier timing of PBMC sampling from adults we performed the same analysis on CD4+ T cell responses at 3–4 months post-vaccination in adults and found the same results as day 7 post-vaccination, showing clear functional and phenotypic differences compared to infants (data not shown).
Fig. 5.
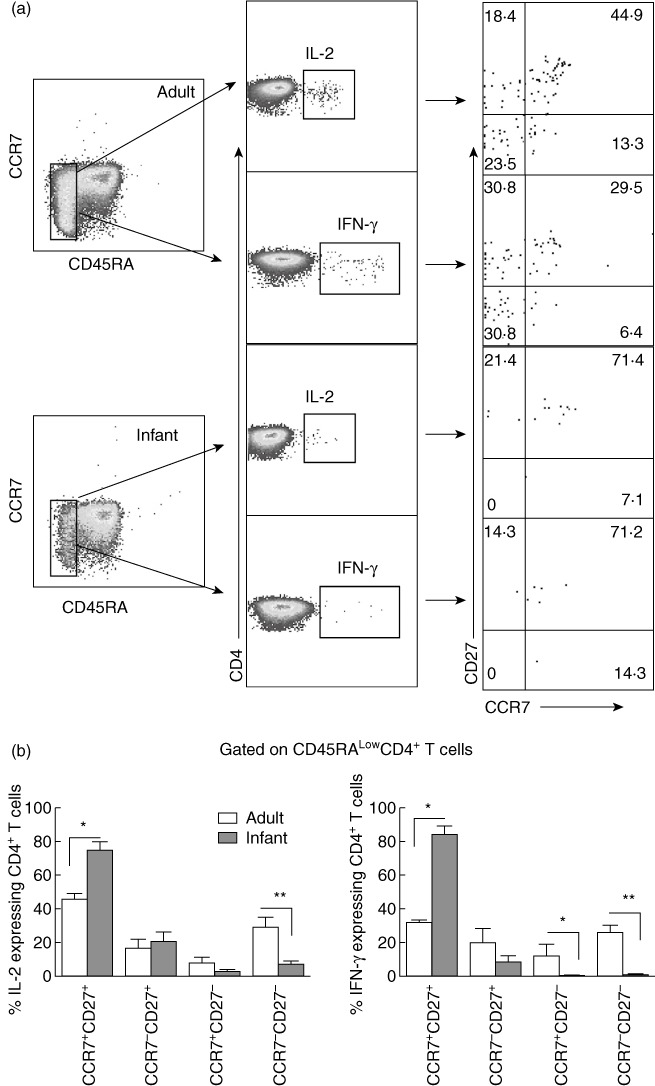
Phenotype characterization of diphtheria, tetanus and pertussis (DTP)-specific CD4+ T cells in infants and adults. CD45RAlow cells were gated and a CCR7/CD27 combination was used to compare the phenotypes of pertussis toxoid (PT)-specific T helper type 1 (Th1) [interleukin (IL)-2 and interferon (IFN)-γ+] CD4+ T cells among infants and adults. Comparative dot-plots show frequencies of PT-specific IL-2+ and IFN-γ+ expressing CD4+ T cells among infants and adults (a). The bar graphs represent comparative percentages of PT-specific CD4+ T cells expressing IL-2 and IFN-γ based on their CCR7 and CD27 expression patterns (b). P-values were calculated using the Mann–Whitney U-test; *<0·05; **<0·005; ***<0·0005.
Discussion
We demonstrate that acellular pertussis (DTaP)-specific CD4+ T cell responses among infants differ quantitatively from those of adults. Our data suggest that CD4+ T cells of infants can up-regulate CD69 expression in response to DTaP antigens similar to adults. Because CD69 is an early activation marker of all T cells [30,32] the data suggest that, similar to adults, CD4+ T cells of infants are capable of antigen-specific activation in terms of CD69 expression. Up-regulation of CD69+ T cells has been shown recently on aP-specific CD8+ T cells among DTaP booster vaccinated among adolescents; however, the study could not find a difference in CD69-up-regulation on CD4+ T cells that was evaluated post-48 h [33]. After antigen stimulation, CD69 expression on T cells is a very early event and, hence, its up-regulation should be analysed within a few hours of T cell stimulation [30].
After stimulation with DTaP antigens (PT, PRN and FHA), CD4+ T cells of infants as well as adults primarily expressed TNF-α alone or IFN-γ-IL-2+TNF-α+. Lower percentages of antigen-specific IFN-γ-expressing CD4+ T cells could be detected (IFN-γ+IL-2+TNF-α+) among adults, which was significantly lower among infants. This could be attributed to the inherent inability of infant CD4+ T cells to express IFN-γ[34,35]. When quantified on a per-cell basis, type 1 CD4+ T cells producing multiple cytokine combinations (IFN-γ+IL-2+TNF-α+) are by far the most significant contributor to the protective immunity against many diseases [36,37]. We speculate that significantly higher levels of type 1 multi-functional CD4+ T cell responses to pertussis antigens may elicit a greater degree of protection among adults against B. pertussis infections. Earlier experiments in neonatal mouse models have demonstrated minimal Th 1 function and a preponderance of Th 2 activity to a variety of antigens, as assessed by the inability of antigen to induce IFN-γ responses [34,38–41]. Moreover, CD4+ T cells among human neonates are reported to be Th 2 biased to the antigens, while adults are capable of eliciting potent Th 1 responses to a variety of stimulations [20,22,41,42]. Exceptions to this paradigm have been noted during infection and/or following vaccination with certain antigens [43–45]. In a limited number of adults, we stimulated PBMCs with DTaP antigens obtained 3–4 months after DTaP vaccination and found the same profile of responses as adult PBMCs obtained 7 days post-vaccination. In other studies by our laboratory, similar differences were found in adults when their PBMCs were collected at random time-points and stimulated with Streptococcus pneumoniae surface protein, PspA (data not shown). Hence, our studies, in association with others, suggest that functional quality of vaccine-specific CD4+ T cell responses in infants are largely affected by maturational status of their immune system.
To evaluate DTaP vaccine-induced Th2 responses, we analysed percentages of IL-4-expressing CD4+ T cells. We found that both groups (infants and adults) had low frequencies of antigen-specific IL-4 responses. One could argue that intracellular cytokine staining may not be a sensitive approach for evaluating IL-4 production; therefore, we measured secreted IL-4 using a bead array method that yielded a similar result. We considered observing other Th2 cytokine responses such as IL-10; however, the presence of IL-10 is precarious, and does not always inform about type 2 CD4+ T cell responses [46,47]. Earlier studies that evaluated acellular pertussis-specific T cell responses suggested that infants and young children had a predisposition to Th2 responses, with an overall response skew towards Th1 occurring gradually with age [20,22]. However, those studies relied upon traditional ELISA methods for cytokine estimations, and it is not feasible to look selectively at single cell functionality by such methods. Hence, in contrast to the earlier studies, our results clearly define that TNF-α+ and IL-2+ Th1 responses predominate following DTaP vaccination in infants. While adults develop significantly higher IFN-γ+ polyfunctional responses than infants, both groups had further higher percentages of DTaP-specific IL-2-expressing CD4+ T cells. We hypothesize that because pertussis neither persists in the body, nor is there frequent exposure to pertussis in the environment, most of the DTaP vaccine-induced CD4+ T cells are largely IFN-γ-IL-2+TNF-α+ or IFN-γ-IL-2-TNF-α+ in adults and infants. This is in contrast to bacilli Calmette–Guérin (BCG) vaccination, where it has been shown that IFN-γ levels are high due to persistent antigen or environmental exposure [48].
As loss of surface expression of CD45RA is considered to be a characteristic of vaccine-induced memory CD4+ T cells [23], we divided CD45RA- cytokine-producing CD4+ T cells further based on their ability to co-express CCR7 and CD27. We observed that most of the functional CD4+ T cells that were DTaP-specific expressed CCR7 and CD27 in infants that were significantly higher than adults. Conversely, adults had significantly higher percentages of CCR7-CD27- cells that were considered as fully differentiated CD4+ T cells [24]. We conclude that, in infants, the phenotype of DTaP-specific CD4+ T cells is predominantly an early differentiated phenotype, while in adults CD4+ T cells due to multiple vaccinations transform into fully differentiated CD4+ T cells (CCR7-CD27-). Earlier reports have demonstrated phenotypic changes in influenza vaccine-specific CD8+ T cells among young children and adults that were imparted due to maturational status, as well as the number of doses of vaccinations [49].
In summary, the capacity of CD4+ T cells in infants to express the CD69 activation marker in response to vaccine DTaP antigens or SEB is not impaired. Infants lack pertussis-specific polyfunctional CD4+ T cells (TNF-α+IL-2+IFN-γ+) and mainly express IFN-γ- Th1 cells. Several mechanisms might account for this relative deficiency at the early stages of life, including an immature state of the APCs, as suggested previously [50,51]. However, we speculate that infants' T cells have the ability to perceive the signals provided by APCs that are required for early T cell activation (CD69 up-regulation) upon antigen encounter. Further studies are under way to determine if this interaction is transient and the APC–T cell synapse formation is somehow not prolonged, which is required for functional CD4+ T cell generation [52]. More studies are needed to understand the comprehensive intrinsic defects of T cells in the early stages of life.
Acknowledgments
We thank our collaborator, Dr Tim Mosmann, Director, Vaccine Research Center at University of Rochester for his help in the flow cytometry data analysis. The work is supported by the grant NIDCD R01 08671 and the Thrasher Fund to M.E.P.
Disclosure
None declared.
References
- 1.Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. Morb Mortal Wkly Rep. 2011;60:13–5. [PubMed] [Google Scholar]
- 2.Kuehn BM. Panel backs wider pertussis vaccination to curb outbreaks, prevent deaths. JAMA. 2010;304:2684–6. doi: 10.1001/jama.2010.1812. [DOI] [PubMed] [Google Scholar]
- 3.Gidengil CA, Sandora TJ, Lee GM. Tetanus–diphtheria–acellular pertussis vaccination of adults in the USA. Exp Rev Vaccines. 2008;7:621–34. doi: 10.1586/14760584.7.5.621. [DOI] [PubMed] [Google Scholar]
- 4.von Konig CH, Halperin S, Riffelmann M, Guiso N. Pertussis of adults and infants. Lancet Infect Dis. 2002;2:744–50. doi: 10.1016/s1473-3099(02)00452-8. [DOI] [PubMed] [Google Scholar]
- 5.Wendelboe AM, Njamkepo E, Bourillon A, et al. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J. 2007;26:293–9. doi: 10.1097/01.inf.0000258699.64164.6d. [DOI] [PubMed] [Google Scholar]
- 6.Pichichero ME, Bernstein H, Blatter MM, Schuerman L, Cheuvart B, Holmes SJ. Immunogenicity and safety of a combination diphtheria, tetanus toxoid, acellular pertussis, hepatitis B, and inactivated poliovirus vaccine coadministered with a 7-valent pneumococcal conjugate vaccine and a Haemophilus influenzae type b conjugate vaccine. J Pediatr. 2007;151:43–9. doi: 10.1016/j.jpeds.2007.02.013. 49. [DOI] [PubMed] [Google Scholar]
- 7.Pichichero ME, Blatter MM, Kennedy WA, Hedrick J, Descamps D, Friedland LR. Acellular pertussis vaccine booster combined with diphtheria and tetanus toxoids for adolescents. Pediatrics. 2006;117:1084–93. doi: 10.1542/peds.2005-1759. [DOI] [PubMed] [Google Scholar]
- 8.Plotkin SA, Liese J, Madhi SA, Ortiz E. A DTaP-IPV//PRP approximately T vaccine (Pentaxim): a review of 16 years' clinical experience. Exp Rev Vaccines. 2011;10:981–1005. doi: 10.1586/erv.11.72. [DOI] [PubMed] [Google Scholar]
- 9.Pichichero ME, Rennels MB, Edwards KM, et al. Combined tetanus, diphtheria, and 5-component pertussis vaccine for use in adolescents and adults. JAMA. 2005;293:3003–11. doi: 10.1001/jama.293.24.3003. [DOI] [PubMed] [Google Scholar]
- 10.Wood N, McIntyre P, Marshall H, Roberton D. Acellular pertussis vaccine at birth and one month induces antibody responses by two months of age. Pediatr Infect Dis J. 2010;29:209–15. doi: 10.1097/INF.0b013e3181bc98d5. [DOI] [PubMed] [Google Scholar]
- 11.Feunou PF, Bertout J, Locht C. T- and B-cell-mediated protection induced by novel, live attenuated pertussis vaccine in mice. Cross protection against parapertussis. PLoS ONE. 2010;5:e10178. doi: 10.1371/journal.pone.0010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leef M, Elkins KL, Barbic J, Shahin RD. Protective immunity to Bordetella pertussis requires both B cells and CD4(+) T cells for key functions other than specific antibody production. J Exp Med. 2000;191:1841–52. doi: 10.1084/jem.191.11.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolfe DN, Kirimanjeswara GS, Harvill ET. Clearance of Bordetella parapertussis from the lower respiratory tract requires humoral and cellular immunity. Infect Immun. 2005;73:6508–13. doi: 10.1128/IAI.73.10.6508-6513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kowalzik F, Barbosa AP, Fernandes VR, et al. Prospective multinational study of pertussis infection in hospitalized infants and their household contacts. Pediatr Infect Dis J. 2007;26:238–42. doi: 10.1097/01.inf.0000256750.07118.ee. [DOI] [PubMed] [Google Scholar]
- 15.Kretzschmar M, Teunis PF, Pebody RG. Incidence and reproduction numbers of pertussis: estimates from serological and social contact data in five European countries. PLoS Med. 2010;7:e1000291. doi: 10.1371/journal.pmed.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storsaeter J, Hallander HO, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine. 1998;16:1907–16. doi: 10.1016/s0264-410x(98)00227-8. [DOI] [PubMed] [Google Scholar]
- 17.Ryan M, Murphy G, Gothefors L, Nilsson L, Storsaeter J, Mills KH. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J Infect Dis. 1997;175:1246–50. doi: 10.1086/593682. [DOI] [PubMed] [Google Scholar]
- 18.Ryan M, Murphy G, Ryan E, et al. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology. 1998;93:1–10. doi: 10.1046/j.1365-2567.1998.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holt PG, Rudin A, Macaubas C, et al. Development of immunologic memory against tetanus toxoid and pertactin antigens from the diphtheria–tetanus–pertussis vaccine in atopic versus nonatopic children. J Allergy Clin Immunol. 2000;105:1117–22. doi: 10.1067/mai.2000.105804. [DOI] [PubMed] [Google Scholar]
- 20.White OJ, Rowe J, Richmond P, et al. Th2-polarisation of cellular immune memory to neonatal pertussis vaccination. Vaccine. 2010;28:2648–52. doi: 10.1016/j.vaccine.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Ryan M, McCarthy L, Rappuoli R, Mahon BP, Mills KH. Pertussis toxin potentiates Th1 and Th2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the co-stimulatory molecules B7-1, B7-2 and CD28. Int Immunol. 1998;10:651–62. doi: 10.1093/intimm/10.5.651. [DOI] [PubMed] [Google Scholar]
- 22.Rowe J, Macaubas C, Monger T, et al. Heterogeneity in diphtheria–tetanus–acellular pertussis vaccine-specific cellular immunity during infancy: relationship to variations in the kinetics of postnatal maturation of systemic th1 function. J Infect Dis. 2001;184:80–8. doi: 10.1086/320996. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 24.Fritsch RD, Shen X, Sims GP, Hathcock KS, Hodes RJ, Lipsky PE. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J Immunol. 2005;175:6489–97. doi: 10.4049/jimmunol.175.10.6489. [DOI] [PubMed] [Google Scholar]
- 25.Perfetto SP, Chattopadhyay PK, Lamoreaux L, et al. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J Immunol Methods. 2006;313:199–208. doi: 10.1016/j.jim.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997;99:1739–50. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma SK, Casey JR, Pichichero ME. Reduced memory CD4+ T-cell generation in the circulation of young children may contribute to the otitis-prone condition. J Infect Dis. 2011;204:645–53. doi: 10.1093/infdis/jir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pitcher CJ, Quittner C, Peterson DM, et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–25. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 29.Waldrop SL, Davis KA, Maino VC, Picker LJ. Normal human CD4+ memory T cells display broad heterogeneity in their activation threshold for cytokine synthesis. J Immunol. 1998;161:5284–95. [PubMed] [Google Scholar]
- 30.Ziegler SF, Ramsdell F, Alderson MR. The activation antigen CD69. Stem Cells. 1994;12:456–65. doi: 10.1002/stem.5530120502. [DOI] [PubMed] [Google Scholar]
- 31.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 32.Petersen CC, Nederby L, Roug AS, et al. Increased expression of CD69 on T cells as an early immune marker for human cytomegalovirus reactivation in chronic lymphocytic leukemia patients. Viral Immunol. 2011;24:165–9. doi: 10.1089/vim.2010.0087. [DOI] [PubMed] [Google Scholar]
- 33.Rieber N, Graf A, Hartl D, Urschel S, Belohradsky BH, Liese J. Acellular pertussis booster in adolescents induces Th1 and memory CD8+ T cell immune response. PLoS ONE. 2011;6:e17271. doi: 10.1371/journal.pone.0017271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 35.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–91. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bansal A, Jackson B, West K, et al. Multifunctional T-cell characteristics induced by a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine regimen given to healthy adults are dependent on the route and dose of administration. J Virol. 2008;82:6458–69. doi: 10.1128/JVI.00068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 38.Adkins B, Bu Y, Guevara P. The generation of Th memory in neonates versus adults: prolonged primary Th2 effector function and impaired development of Th1 memory effector function in murine neonates. J Immunol. 2001;166:918–25. doi: 10.4049/jimmunol.166.2.918. [DOI] [PubMed] [Google Scholar]
- 39.Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996;271:1728–30. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 40.Singh RR, Hahn BH, Sercarz EE. Neonatal peptide exposure can prime T cells and, upon subsequent immunization, induce their immune deviation: implications for antibody vs. T cell-mediated autoimmunity. J Exp Med. 1996;183:1613–21. doi: 10.1084/jem.183.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanna-Wakim R, Yasukawa LL, Sung P, et al. Age-related increase in the frequency of CD4(+) T cells that produce interferon-gamma in response to staphylococcal enterotoxin B during childhood. J Infect Dis. 2009;200:1921–7. doi: 10.1086/648375. [DOI] [PubMed] [Google Scholar]
- 42.van den Biggelaar AH, Richmond PC, Pomat WS, et al. Neonatal pneumococcal conjugate vaccine immunization primes T cells for preferential Th2 cytokine expression: a randomized controlled trial in Papua New Guinea. Vaccine. 2009;27:1340–7. doi: 10.1016/j.vaccine.2008.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marchant A, Goetghebuer T, Ota MO, et al. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette–Guerin vaccination. J Immunol. 1999;163:2249–55. [PubMed] [Google Scholar]
- 44.Jupelli M, Guentzel MN, Meier PA, Zhong G, Murthy AK, Arulanandam BP. Endogenous IF. N-gamma production is induced and required for protective immunity against pulmonary chlamydial infection in neonatal mice. J Immunol. 2008;180:4148–55. doi: 10.4049/jimmunol.180.6.4148. [DOI] [PubMed] [Google Scholar]
- 45.Kollmann TR, Reikie B, Blimkie D, et al. Induction of protective immunity to Listeria monocytogenes in neonates. J Immunol. 2007;178:3695–701. doi: 10.4049/jimmunol.178.6.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 47.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 48.Soares AP, Scriba TJ, Joseph S, et al. Bacillus Calmette–Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol. 2008;180:3569–77. doi: 10.4049/jimmunol.180.5.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He XS, Holmes TH, Mahmood K, et al. Phenotypic changes in influenza-specific CD8+ T cells after immunization of children and adults with influenza vaccines. J Infect Dis. 2008;197:803–11. doi: 10.1086/528804. [DOI] [PubMed] [Google Scholar]
- 50.Upham JW, Rate A, Rowe J, Kusel M, Sly PD, Holt PG. Dendritic cell immaturity during infancy restricts the capacity to express vaccine-specific T-cell memory. Infect Immun. 2006;74:1106–12. doi: 10.1128/IAI.74.2.1106-1112.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Velilla PA, Rugeles MT, Chougnet CA. Defective antigen-presenting cell function in human neonates. Clin Immunol. 2006;121:251–9. doi: 10.1016/j.clim.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ueda H, Morphew MK, McIntosh JR, Davis MM. CD4+ T-cell synapses involve multiple distinct stages. Proc Natl Acad Sci USA. 2011;108:17099–104. doi: 10.1073/pnas.1113703108. [DOI] [PMC free article] [PubMed] [Google Scholar]


