Abstract
Mast cells are involved in the development of psoriatic lesion, but it is not known how mast cells are activated or whether mast cell cytokines are expressed during the lesion development. In this study, the Köbner reaction was induced in uninvolved psoriatic skin of 18 patients using the tape-stripping technique, and a sequence of biopsies was collected at 0 days, 2 h and 3 days or at 0 days, 1 day and 7 days for histochemical analysis. Eight patients developed the Köbner reaction verified at the follow-up visit 2–2·5 weeks later. No significant differences were observed in total tryptase+ mast cells, psoriasis area and severity index and age/sex. Instead, the percentage of tryptase+ mast cells showing interleukin (IL)-6 immunoreactivity was significantly higher in biopsies from Köbner-positive patients than in those from Köbner-negative patients. IL-33 is a known inducer of IL-6 in mast cells, and the number of IL-33+ cells increased significantly in Köbner-positive dermal skin at days 3–7. The number of dermal cells with IL-6 receptor (IL-6R, CD126) also increased in Köbner-positive skin at days 3–7. Unexpectedly, the number of IL-6R+ cells was even higher in Köbner-negative skin at days 3–7. In the chronic plaque of 10 other psoriatic patients, the numbers of IL-6+ mast cells and dermal cells showing IL-6R were higher than those in the non-lesional skin. In conclusion, the positive Köbner reaction is associated with IL-6 in mast cells and appearance of IL-6R+ and IL-33+ dermal cells. This suggests that a previously unrecognized vicious circle may develop in the early psoriatic lesion.
Keywords: IL-33, IL-6 receptor, IL-6, mast cell, psoriasis
Introduction
Mast cells are effector cells in the pathogenesis of cutaneous inflammatory diseases by releasing numerous powerful mediators and by expressing cell surface ligands and receptors [1–3]. In the normal skin, mast cells are predominantly of the MCTC (tryptase+, chymase+) type and their number is increased markedly in psoriasis and basal cell carcinoma [4,5]. During the development of psoriatic lesion the appearance of degranulated mast cells is typical, and is among the earliest morphological features [6,7]. Furthermore, mast cells are in the stage of activation in the psoriatic lesion detected as higher interstitial histamine concentration during intracutaneous microdialysis [8]. In addition to these changes, mast cells in the psoriatic lesion express increased levels of interferon (IFN)-γ[9], CD30 ligand, interleukin (IL)-8 [10], Kit receptor [11] and protease-activated receptor-2 (PAR-2) [12]. Also, increased numbers of tumour necrosis factor (TNF)-α containing mast cells have been found in the lesional psoriatic skin [13]. Besides the interaction between the stem cell factor and Kit receptor [11], the increased morphological contacts between neuropeptide-containing nerves and mast cells, as well as PAR-2 activation by tryptase, may explain the mast cell activation in the psoriatic lesion [11,12,14]. A new IL-1-like cytokine and alarmin, IL-33, expressed constitutively in the nuclei of keratinocytes and endothelial cells in the skin [15], can stimulate the ST2 receptor on mast cells resulting in the release of a range of cytokines [16,17]. Furthermore, recent experimental results in mouse cells show that cultured cells, such as necrotic fibroblasts and keratinocytes, subjected to necrosis by freeze-thawing, release IL-33 and activate mast cells leading to concomitant release of IL-6 and TNF-α[18].
Numerous cell types are involved in the pathogenesis of psoriasis, including T cells, antigen-presenting cells, neutrophils and keratinocytes [19]. Psoriasis has been characterized traditionally by a T helper type 1 (Th1) cell type infiltrate [20]. However, after the discovery of the novel Th17 cell type it was found that this cell is crucial for development of the psoriatic lesion [21–26]. The induction of the development of Th17 lineage from naive T cells requires the presence of transforming growth factor (TGF)-β, IL-6, IL-1β and IL-21 and IL-23 is needed for the stimulation and maintenance of the committed Th17 cell lineage [21–26]. In addition to the essential role for effector Th1 and Th17 cells, regulatory T cells with defective function have also been suggested to play a role in psoriasis [27]. Nevertheless, mast cells can interact with effector and regulatory T cells via soluble mediators and cell surface molecules leading to T cell activation, and under certain circumstances to mast cell activation as well [1,28].
Human mast cells have been shown previously to express IL-1β, IL-6 and TGF-β[29–32], cytokines which can have potent immunomodulatory functions and induce Th17 cell development. The chymotryptic serine proteinase chymase stored in mast cell secretory granules may also have a role in the regulation of these cytokines, as chymase can effectively convert precursor pro-IL-1β to active IL-1β[33], can release latent TGF-β1 complexes from the extracellular matrix [34] and can degrade IL-6 [35]. Conversely, chymase is partially inactivated during the development of psoriatic lesion due probably to the action of protease inhibitors [4,36]. Nevertheless, it is not known to what extent cutaneous mast cells express these cytokines in the inflamed psoriatic skin and whether they can be involved in the psoriatic pathogenesis.
The increase in IL-6 expression in the psoriatic skin lesion and plasma has long been known [37]. Recently, Goodman et al. [38] reported that IL-6 released by dendritic cells and endothelial cells in the psoriatic lesion may cause the escape of effector T cells from the suppressive effects of regulatory T cells. In addition, IL-6 and IL-21 have been reported to inhibit the expression of TGF-β-induced forkhead box protein 3 (FoxP3), a transcription factor that is characteristic to regulatory T cells [26]. To clarify the possible involvement of mast cell IL-6 in psoriasis, we have induced the development of psoriatic lesion in the healthy-looking skin by using the tape-stripping technique, and have consequently taken skin biopsies at different time-points for histochemical analyses. The Köbner-negative group, i.e. patients without lesion development, acted as a control group for the Köbner-positive group showing lesion development. In addition, skin biopsies from chronic psoriatic lesions were collected and analysed histochemically. Furthermore, the IL-6 receptor (IL-6R, CD126) was analysed immunohistochemically and IL-6R+ cells were counted in these biopsies. IL-33+ cells were also counted, because IL-33 has been shown previously to be expressed in the dermal and epidermal skin and to be increased in the psoriatic lesion, and that it can stimulate mast cells for IL-6 production in vitro[15–18].
Materials and methods
Chemicals
Mouse monoclonal anti-human IL-6 antibody (clone 1936), rat monoclonal anti-human IL-33 antibody (clone 390412) and recombinant human IL-6 (rh-IL-6) were purchased from R&D Systems Europe Ltd (Abingdon, UK), and a mouse monoclonal anti-IL-6R (CD126) antibody (catalogue number LS-C45194) from LifeSpan BioSciences (Seattle, WA, USA). The enzyme-histochemical substrate of tryptase, Z-Gly-Pro-Arg-4-methoxy-2-naphthylamide (Z-Gly-Pro-Arg-MNA) was obtained from Bachem (Bubendorf, Switzerland) and the chromogens, Fast Garnet GBC and Fast black K, from Sigma (St Louis, MO, USA). Reagents for immunohistochemistry were purchased from Vector Laboratories (Burlingame, CA, USA).
Induction of the development of psoriatic lesion and collection of skin biopsies
The study included two groups of psoriatic patients. The first group consisted of 18 subjects (four females and 14 males, age 24–77 years) with chronic psoriasis. The psoriasis area and severity index (PASI) score varied between 1·8 and 18·6. After obtaining consent the development of psoriatic lesion, i.e. the Köbner reaction, was induced using the tape-stripping technique described previously [4,14]. Briefly, a target area, about 2·5 cm × 5–7 cm in size, on the lateral aspect of the arm was tape-stripped with an adhesive tape about 30–40 times until the skin showed slight redness. On the first (day 0) a 4-mm punch biopsy was taken, after local anaesthesia with 1% lidocaine and adrenaline from a skin site that was just outside the tape-stripped area. Thereafter, the study subjects were divided randomly into two biopsing groups: in the first group the 4-mm skin biopsies were taken at time-points of 2 h and 3 days and in the second group at 1 day and 7 days. All subjects were evaluated for the development of psoriatic lesion in the follow-up visit about 2–2·5 weeks later from the tape-stripping. The subject was judged to belong to the Köbner-positive group (eight of 18 subjects) if an identifiable reddish change could be seen on the tape-stripped area, but outside the skin biopsy sites.
The second group consisted of 10 subjects (four females and six males, age 41–85 years) with chronic plaque psoriasis. A 4-mm punch biopsy was taken from the untreated lesional skin and from the healthy-looking skin at least 2 cm apart from the lesions.
The patients had not received any effective systemic or local treatment in the preceding month. After removal, the skin biopsies were embedded immediately in Optimal Cutting Temperature (OCT) compound (Miles Scientific, Naperville, IL, USA) and frozen for preparing 5-µm-thick cryosections, as described previously [4,14]. The methods used were approved by the Ethics Committee of Kuopio University Hospital, Kuopio, Finland.
Staining and counting of mast cells
The mast cells in skin specimens were identified by staining the mast cell-specific serine proteinase tryptase. In this enzyme-histochemical method, 1 mM Z-Gly-Pro-Arg-MNA was used as the substrate for tryptase and Fast black K as the chromogen, as described [4,5]. The number of mast cells was counted on two cryosections in an area of 1·0 mm (width) × 0·6 mm (depth) just adjacent to the epidermis, and the results are expressed as cells/mm2.
Sequential double-staining method for the localization of IL-6 in tryptase-positive mast cells
The method has been described previously [9–13]. Briefly, tryptase-positive mast cells were first identified enzyme-histochemically, but using Fast Garnet GBC as the chromogen, followed by photographing at least at 10 random sites per skin sample in the upper dermis lining the epidermis using a ×20 objective. Then, the red azo dye was dissolved away with 15% Tween 20 overnight, and the same sections were fixed in cold acetone and stained immunohistochemically using 25 µg/ml anti-IL-6 monoclonal antibody (mAb) and the Vectastain Elite ABC kit. The stainings were controlled by unrelated immunoglobulin. After re-photographing at exactly the same sites as the previous pictures, the positively stained cells were counted by comparing the photographs side by side. The results are expressed as the mean percentage of tryptase+ mast cells showing IL-6 immunoreactivity.
The specificity of anti-IL-6 mAb was confirmed further by incubating 20 or 50 µg/ml rh-IL-6, dissolved in 0·1% bovine serum albumin and phosphate-buffered saline (PBS) or diluent control with 25 µg/ml anti-IL-6 mAb at room temperature for 30 min followed by immunohistochemical staining of lesional psoriatic cryosections. As a result, the blocking of anti-IL-6 mAb by rh-IL-6 almost completely abolished the staining reaction on cryosections.
Immunohistochemical staining of IL-6 receptor and IL-33
For the staining of IL-6R (CD126) or IL-33, the skin cryosections were fixed in cold acetone followed by immunohistochemistry using 20 µg/ml anti-IL-6R mAb or 20 µg/ml anti-IL-33 mAb and the Vectastain Elite ABC kit [9–13]. The number of IL-6R+ or IL-33+ cells was counted on two cryosections with a microscope in a dermal area of 1·0 mm (width) × 0·6 mm (depth) just adjacent to the epidermis. The results are expressed as cells/mm2.
Statistics
The results were analysed using paired or unpaired t-test, and P < 0·05 was considered statistically significant.
Results
No differences in age, mean age of disease onset, PASI score and total mast cell number between Köbner-positive and -negative groups
As shown in Table 1, the age, mean age of disease onset and PASI score of the subjects in the Köbner-positive group did not differ significantly from those in the Köbner-negative group. In addition, both groups included two female subjects, and therefore there was no difference in gender. Two subjects in the Köbner-positive group and three in the Köbner-negative group reported joint symptoms, and all subjects had scaly skin psoriasis on the trunk and/or extremities without pustular changes or erythroderma. However, in the Köbner-positive group seven of eight subjects had the age of disease onset of <30 years, but in the Köbner-negative group only three of 10 subjects revealed this age of disease onset.
Table 1.
The age, mean age of disease onset, psoriasis area and severity index (PASI) score and number of tryptase-positive mast cells in the upper dermis in Köbner-positive and -negative groups of psoriatic patients
| Tryptase-positive mast cells at different time-points (cells/mm2) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age | Age of onset | PASI score | 0 days | 2 h | 1 day | 3 days | 7 days | |
| Köbner-positive (n = 8) | 46 ± 15 | 25 ± 14 | 8·3 ± 4·4 | 149 ± 85 (n = 8) | 157 ± 75 (n = 5) | 161 ± 141 (n = 3) | 95 ± 47 (n = 5) | 127 ± 13 (n = 3) |
| Köbner-negative (n = 10) | 54 ± 15 | 40 ± 17 | 6·7 ± 5·0 | 145 ± 122 (n = 10) | 173 ± 59 (n = 4) | 107 ± 49 (n = 6) | 97 ± 13 (n = 3) | 107 ± 36 (n = 6) |
The results are expressed as the mean ± standard deviation. Statistically no significant changes were observed within or between Köbner groups (paired and unpaired t-test).
When comparing the number of tryptase+ mast cells between Köbner groups, no significant differences could be seen in any time-point biopsies. However, it appears that the number of mast cells tends to decrease in 3- and 7-day biopsies in both groups, although the change was not statistically significant. Presumably, this change may reflect mast cell activation in a response to slight cutaneous trauma during the tape-stripping, resulting in the appearance of degranulated phantom mast cells without detectable tryptase.
Mast cells are more immunopositive for IL-6 in the Köbner-positive than in the Köbner-negative skin
To clarify whether there are changes in IL-6 expression in mast cells during the Köbner response, the sequential double-staining method was used (Fig. 1). Even though the percentage of IL-6+ mast cells apparently did not change after the tape-stripping of healthy-looking skin in either Köbner groups (Table 2), an interesting finding is that mast cells are more immunopositive for IL-6 in all time-point biopsies in the Köbner-positive group than in those in the Köbner-negative group, and a statistically significant difference was observed in 2-h–1-day biopsies (Table 2).
Fig. 1.
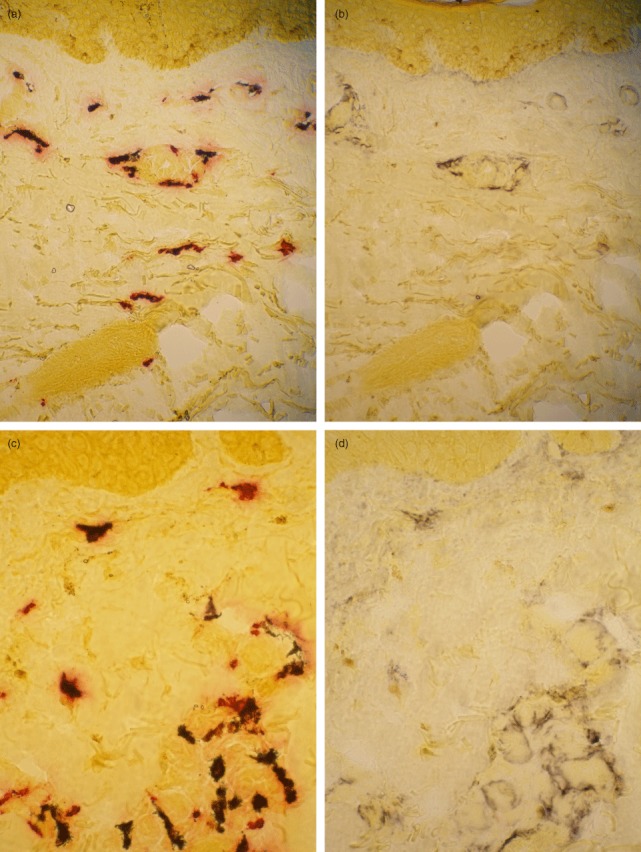
Localization of interleukin (IL)-6 in tryptase-positive mast cells in psoriatic skin using the sequential double-staining technique. The skin cryosection was stained first (a,c) enzyme-histochemically for tryptase. After photographing, the red staining product was dissolved away with 15% Tween20 overnight followed by (b,d) staining immunohistochemically for IL-6 and re-photographing. (a,b) Non-lesional psoriatic skin at day 0; (c,d) Köbner-positive psoriatic skin at day 3.
Table 2.
The percentage of mast cells showing interleukin (IL)-6 immunoreactivity in the upper dermis in Köbner-positive and -negative groups of psoriatic patients
| IL-6-positive mast cells at different time-points (%) | |||||
|---|---|---|---|---|---|
| 0 days | 2 h | 1 day | 3 days | 7 days | |
| Köbner-positive (n = 8) | 42 ± 9 (n = 8) | 41 ± 6 (n = 5) | 51 ± 12 (n = 3) | 37 ± 13 (n = 5) | 49 ± 19 (n = 3) |
| Köbner-negative (n = 10) | 33 ± 15 (n = 10) | 20 ± 8 (n = 4) | 32 ± 13 (n = 6) | 30 ± 14 (n = 3) | 31 ± 13 (n = 6) |
| P-value (unpaired t-test) between Köbner groups | 0·152 | 0·00526 | 0·125 | ||
The results are expressed as the mean ± standard deviation. Statistically, no significant changes were observed within Köbner groups (paired t-test).
It is noteworthy to point out that there are numerous other cells than mast cells which show IL-6 immunoreactivity, e.g. endothelial cells and other unidentified cells just beneath the epidermis (Fig. 1).
Dermal cells immunopositive for IL-6 receptor are increased in the Köbner-positive skin within 3–7 days but the Köbner-negative skin shows even higher IL-6R+ cell numbers
Because mast cell IL-6 associated with the induction of the Köbner reaction, the receptor for IL-6 was stained (Fig. 2). As shown in Table 3, the number of IL-6R+ cells increased in the upper dermis in the Köbner-positive skin, and statistical significance was reached in 3–7-day biopsies compared to corresponding 0-day biopsies. Unexpectedly, IL-6R+ dermal cells were considerably numerous in the Köbner-negative skin, and a statistically significant difference between Köbner groups was reached in 3–7-day biopsies.
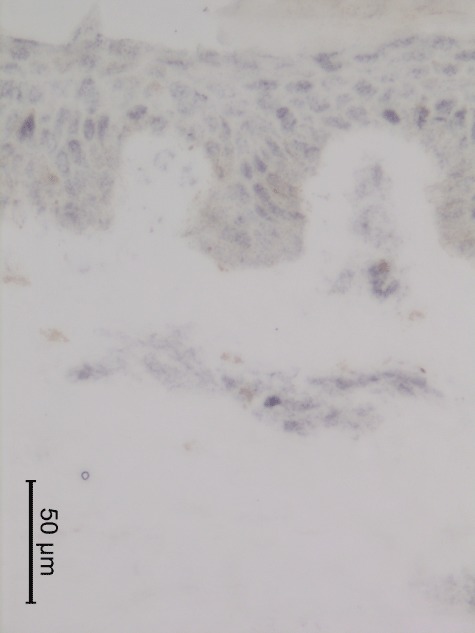
Fig. 2.

Immunohistochemical staining of interleukin (IL)-6 receptor in a Köbner-negative skin biopsy taken at day 7 after the tape-stripping.
Table 3.
The number of cells showing interleukin (IL)-6 receptor immunoreactivity in the upper dermis in Köbner-positive and -negative groups of psoriatic patients
| IL-6R+ cells at different time-points (cells/mm2) | |||||
|---|---|---|---|---|---|
| 0 days | 2 h | 1 day | 3 days | 7 days | |
| Köbner-positive (n = 8) | 11 ± 14 (n = 8) | 10 ± 12 (n = 5) | 27 ± 21 (n = 3) | 45 ± 37* (n = 5) | 52 ± 17* (n = 3) |
| Köbner-negative (n = 10) | 93 ± 169 (n = 10) | 41 ± 65 (n = 4) | 140 ± 125 (n = 6) | 100 ± 60 (n = 3) | 260 ± 222 (n = 6) |
| P-value (unpaired t-test) between Köbner groups | 0·191 | 0·0594 | 0·0374 | ||
The results are expressed as the mean ± standard deviation.
P = 0·0115 (paired t-test) when comparing the cell numbers in 3–7-day biopsies to those in corresponding 0-day biopsies.
Dermal cells immunopositive for IL-6 receptor are increased in the chronic psoriatic lesion, whereas the percentage of IL-6+ mast cells is not
To compare the results obtained in the developing psoriatic lesion (Köbner-positive group) with those in the chronic lesion, a new set of non-lesional and lesional skin biopsies from 10 other psoriatic patients was analysed. The number of IL-6R+ cells was significantly higher in the upper dermis of chronic psoriatic lesion than in that of healthy-looking skin (165 ± 207 cells/mm2versus 16 ± 6 cells/mm2, P = 0·041, paired t-test, respectively). However, even though in seven cases an increased percentage of IL-6+ mast cells was found in the lesional skin, the difference between lesional and non-lesional skin was not statistically significant in the whole group of 10 cases (44 ± 12 % versus 36 ± 14%, P = 0·205, paired t-test, respectively). Hence, the results obtained in the chronic lesion resemble those found in the early developing lesion.
Dermal cells immunopositive for IL-33 are increased in the Köbner-positive skin within 3–7 days
IL-33 has been shown to stimulate mast cells for IL-6 production [17,18]. Hence, IL-33 was stained immunohistochemically (Fig. 3) and IL-33+ cells were counted in dermis (Table 4). Interestingly, the number of IL-33+ cells seemed to decrease in 1-day biopsies in the Köbner-positive group but increased significantly thereafter in 3–7-day biopsies. In contrast, the changes in IL-33+ cells in the Köbner-negative skin were clearly less marked. Unexpectedly, the number of IL-33+ cells was slightly, although significantly, higher in 0-day biopsies in the Köbner-negative group than in that in the Köbner-positive group.
Fig. 3.
Immunohistochemical staining of interleukin (IL)-33 in a 7-day-old psoriatic lesion (Köbner-positive skin). Note that the IL-33 staining is predominantly nuclear in the epidermis, but both nuclear and cytoplasmic stainings are seen in the dermis.
Table 4.
The number of cells showing interleukin (IL)-33 immunoreactivity in the upper dermis in Köbner-positive and -negative groups of psoriatic patients
| IL-33+ cells at different time-points (cells/mm2) | |||||
|---|---|---|---|---|---|
| 0 days | 2 h | 1 day | 3 days | 7 days | |
| Köbner-positive (n = 8) | 76 ± 28 (n = 8) | 69 ± 32 (n = 5) | 42 ± 11 (n = 3) | 92 ± 28* (n = 5) | 152 ± 5* (n = 3) |
| Köbner-negative (n = 10) | 101 ± 27 (n = 10) | 88 ± 41 (n = 4) | 88 ± 30 (n = 6) | 70 ± 28 (n = 3) | 116 ± 35 (n = 6) |
| P-value (unpaired t-test) between Köbner groups | 0·0381 | 0·127 | 0·651 | ||
The results are expressed as the mean ± standard deviation.
P = 0·0236 (paired t-test) when comparing the cell numbers in 3–7-day biopsies to those in corresponding 0-day biopsies.
Because IL-33 can stimulate mast cells for IL-6 synthesis, the association of IL-33+ cell numbers with the percentage of IL-6+ mast cells was analysed in 0-day and 3–7-day biopsies in both Köbner groups. However, no significant positive or negative correlation was observed.
Discussion
The Köbner reaction in uninvolved psoriatic skin is a well-known phenomenon, and usually develops in 38–76% of patients in 7–14 days after slight skin injury [39]. However, it should be kept in mind that the Köbner reactivity may be represented by a subgroup of psoriatic patients. In this study, eight of 18 patients (44%) developed the Köbner reaction, and this percentage falls well within the percentage limits reported previously. Even though no significant differences in age, mean age of disease onset, sex, PASI score, joint symptoms and total number of mast cells in 0-day biopsies were found between Köbner-positive and -negative groups, it is still possible that the positive Köbner reactivity has an association with the early onset psoriasis in this study. However, the number of subjects is too low to draw a definite conclusion.
In a previous study, where mast cells were stained with a metachromatic stain in formalin-fixed biopsies, the number of mast cells increased temporarily within 6 h after scarification and then increased again on day 4 [40]. In contrast, no increase in the number of mast cells stained enzyme-histochemically for tryptase on cryosections was observed in this study but, similarly, a somewhat slight decrease in the cell count in both Köbner groups. In our previous study, where mast cell tryptase was stained identically and biopsies were taken at 1, 2 and 3 weeks after tape-stripping, both Köbner-positive and -negative groups showed similar mast cell counts prior to tape-stripping, and some increase in cell number was observed at 2–3 weeks in the Köbner-positive group. However, no increase was seen in 1-week biopsies [4]. Thus, the present results on tryptase+ mast cells parallel those of our previous study.
Human mast cells have been shown to express IL-6 [31], but it is not known to what extent mast cells express IL-6 in skin diseases. Mast cells are increased in number in the psoriatic lesion and they express increased levels of IFN-γ[9], CD30L, IL-8 [10], Kit [11] and PAR-2 [12]. However, the percentage of mast cells expressing another proinflammatory cytokine, TNF-α, was not increased significantly in the psoriatic plaque, although it was increased in the atopic dermatitis lesion [13]. In addition to psoriasis, the percentage of TNF-α+ mast cells was not increased markedly in the lesional skin of basal cell carcinoma [41]. Even though seven psoriatic cases showed an increased percentage of IL-6+ mast cells in the lesion, statistical significance was not reached, a result which resembles the previous result on TNF-α. This can suggest that mast cells in the psoriatic lesion cannot be induced to produce substantially more IL-6 and TNF-α than they already produce in the uninvolved skin. In our two biopsy series from non-lesional psoriatic skin, the mean ± standard deviation percentages of IL-6+ mast cells were very close to each other: 36 ± 14% and 37 ± 13%, suggesting that the analysis result is reproducible. Nevertheless, the high increase in tryptase+ mast cell number in the psoriatic lesion [4,9], possibly through cell proliferation or new recruitment from circulation, means that the numbers of IL-6+ and TNF-α+ mast cells are evidently also increased.
As in the chronic psoriatic lesion, no significant changes were seen in the percentage of IL-6+ mast cells in either Köbner-positive or -negative groups. Of interest is the finding that Köbner-positive patients showed higher a percentage of IL-6+ mast cells in all time-point biopsies compared to Köbner-negative patients. Therefore, the result suggests that mast cells are readily loaded with IL-6 in patients prone to develop a Köbner reaction. The mechanism for this higher IL-6 expression in mast cells is unclear. Different factors can induce IL-6 expression in mast cells, including IL-33 [17,18], IL-1 [42] and FcεRI-mediated stimulation [43]. The Bcl10–Malt1 complex has been found to be essential for mast cell IL-6 and TNF-α production, and this is independent of degranulation and eicosanoid secretion [44]. However, the mast cell is not the only cell type that expresses IL-6, as apparently endothelial cells and different other cells of the immune system also express IL-6. Nevertheless, mast cells can be potential candidates in providing IL-6 during the very early phase of psoriatic pathogenesis, possibly by promoting the maturation of Th17 cells and inhibition of regulatory T cells [24–26,38].
The IL-6 target, i.e. IL-6R, can be expressed by both effector T cells and regulatory T cells in psoriasis [38]. The number of IL-6R+ cells increased in 3–7-day biopsies in the Köbner-positive group, allowing an increased interaction between IL-6 and IL-6R, and possibly also between mast cells and effector T cells. Unexpectedly, the Köbner-negative group revealed an even higher number of IL-6R+ cells, especially in 3–7-day biopsies. It may be possible that FoxP3+ regulatory T cells express IL-6R and infiltrate the tape-stripped skin site in the Köbner-negative group (Suttle et al., preliminary results), but this needs to be clarified in further studies. In the chronic psoriatic lesion, IL-6R+ cells were significantly more numerous than the cells in the uninvolved skin. Therefore, IL-6+ mast cells and IL-6R+ cells can be involved in the induction, potentiation and maintenance of chronic inflammation.
The higher percentage of IL-6+ mast cells in the Köbner-positive group suggests that these patients may have an IL-6-inducing factor in the skin, such as IL-33. A somewhat unexpected finding was that IL-33+ cells were more numerous in 0-day biopsies in the Köbner-negative group than in those in the Köbner-positive group, and therefore dermal IL-33 may not explain the higher IL-6 percentage in the Köbner-positive skin. However, the immunological activities of IL-33 appear to be more diverse than thought previously. Recently, a novel function for IL-33 has been uncovered, i.e. stimulation of myeloid-derived suppressor cells and regulatory T cells [45], and it could therefore participate in inhibition of lesion development in the Köbner-negative skin. Nevertheless, no significant changes in cell numbers took place during the 1-week period in the Köbner-negative skin. In contrast, the number of IL-33+ cells seemed first to decrease in number on day 1, but then the cell numbers increased significantly in 3–7-day biopsies in the Köbner-positive skin. Hence, this fluctuation in IL-33+ cell number during the first week of lesion development may be related to mast cell IL-6.
To summarize the results of this study, the induction of the Köbner reaction associates with higher percentage of IL-6+ mast cells and increase in IL-33+ and IL-6R+ cells in the dermis at days 3–7, but it does not associate with total mast cell number, PASI score, joint symptoms, age/sex and mean age of disease onset. This result can mean that a vicious circle already develops in the early psoriatic lesion. Unexpected findings were the somewhat higher number of IL-33+ cells in 0-day biopsies, as well as the numerous IL-6R+ cells in 3–7-day biopsies in the Köbner-negative group. These cells may be related to inhibition of lesion development, but further studies are needed to characterize these cells.
Acknowledgments
The authors wish to thank the strategic funding of the Cancer Center of Eastern Finland and University of Eastern Finland, the COST Action BM1007 (mast cells and basophils – targets for innovative therapies), the EVO-funding of Kuopio University Hospital and Abbott Oy Finland. Ms Anne Koivisto is acknowledged for expert technical assistance.
Disclosure
Abbott Oy, Finland has financially supported this study.
References
- 1.Harvima IT, Nilsson G, Suttle M-M, Naukkarinen A. Is there a role for mast cells in psoriasis? Arch Dermatol Res. 2008;300:461–78. doi: 10.1007/s00403-008-0874-x. [DOI] [PubMed] [Google Scholar]
- 2.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–42. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 3.Henz BM, Maurer M, Lippert U, Worm M, Babina M. Mast cells as initiators of immunity and host defense. Exp Dermatol. 2001;10:1–10. doi: 10.1034/j.1600-0625.2001.100101.x. [DOI] [PubMed] [Google Scholar]
- 4.Harvima IT, Naukkarinen A, Paukkonen K, et al. Mast cell tryptase and chymase in developing and mature psoriatic lesions. Arch Dermatol Res. 1993;285:184–92. doi: 10.1007/BF00372007. [DOI] [PubMed] [Google Scholar]
- 5.Diaconu N-C, Kaminska R, Naukkarinen A, Harvima RJ, Harvima IT. The increase in tryptase- and chymase-positive mast cells is associated with partial inactivation of chymase and increase in protease inhibitors in basal cell carcinoma. J Eur Acad Dermatol Venereol. 2007;21:908–15. doi: 10.1111/j.1468-3083.2006.02100.x. [DOI] [PubMed] [Google Scholar]
- 6.Schubert C, Christophers E. Mast cells and macrophages in early relapsing psoriasis. Arch Dermatol Res. 1985;277:352–8. doi: 10.1007/BF00509232. [DOI] [PubMed] [Google Scholar]
- 7.Brody I. Mast cell degranulation in the evolution of acute eruptive guttate psoriasis vulgaris. J Invest Dermatol. 1985;82:460–4. doi: 10.1111/1523-1747.ep12260955. [DOI] [PubMed] [Google Scholar]
- 8.Petersen LJ, Hansen U, Kristensen JK, Nielsen H, Skov PS, Nielsen HJ. Studies on mast cells and histamine release in psoriasis: the effect of ranitidine. Acta Derm Venereol (Stockh) 1998;78:190–3. doi: 10.1080/000155598441503. [DOI] [PubMed] [Google Scholar]
- 9.Ackermann L, Harvima IT, Pelkonen J, et al. Mast cells in psoriatic skin are strongly positive for interferon-gamma. Br J Dermatol. 1999;140:624–33. doi: 10.1046/j.1365-2133.1999.02760.x. [DOI] [PubMed] [Google Scholar]
- 10.Fischer M, Harvima IT, Carvalho RFS, et al. Mast cell CD30 ligand is up-regulated in cutaneous inflammation and mediates degranulation-independent chemokine secretion. J Clin Invest. 2006;116:2748–56. doi: 10.1172/JCI24274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huttunen M, Naukkarinen A, Horsmanheimo M, Harvima IT. Transient production of stem cell factor in dermal cells but increasing expression of Kit receptor in mast cells during normal wound healing. Arch Dermatol Res. 2002;294:324–30. doi: 10.1007/s00403-002-0331-1. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho RFS, Nilsson G, Harvima IT. Increased mast cell expression of PAR-2 in skin inflammatory diseases and release of IL-8 upon PAR-2 activation. Exp Dermatol. 2010;19:117–22. doi: 10.1111/j.1600-0625.2009.00998.x. [DOI] [PubMed] [Google Scholar]
- 13.Ackermann L, Harvima IT. Mast cells of psoriatic and atopic dermatitis skin are positive for TNF-α and their degranulation is associated with expression of ICAM-1 in epidermis. Arch Dermatol Res. 1998;290:353–9. doi: 10.1007/s004030050317. [DOI] [PubMed] [Google Scholar]
- 14.Naukkarinen A, Järvikallio A, Lakkakorpi J, Harvima IT, Harvima RJ, Horsmanheimo M. Quantitative histochemical analysis of mast cells and sensory nerves in psoriatic skin. J Pathol. 1996;180:200–5. doi: 10.1002/(SICI)1096-9896(199610)180:2<200::AID-PATH632>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 15.Moussion C, Ortega N, Girard J. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS ONE. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theoharides TC, Zhang B, Kempuraj D, et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci USA. 2010;107:4448–53. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iikura M, Suto H, Kajiwara N, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–8. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 18.Enoksson M, Lyberg K, Möller-Westerberg C, Falon PG, Nilsson G, Lunderius-Andersson C. Mast cells as sensors of cell injury through IL-33 recognition. J Immunol. 2011;186:2523–8. doi: 10.4049/jimmunol.1003383. [DOI] [PubMed] [Google Scholar]
- 19.Sabat R, Philipp S, Höflich C, et al. Immunopathogenesis of psoriasis. Exp Dermatol. 2007;16:779–98. doi: 10.1111/j.1600-0625.2007.00629.x. [DOI] [PubMed] [Google Scholar]
- 20.Uyemura K, Yamamura M, Fivenson DF, Modlin RL, Nickoloff BJ. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993;101:701–5. doi: 10.1111/1523-1747.ep12371679. [DOI] [PubMed] [Google Scholar]
- 21.Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 cells. J Invest Dermatol. 2008;128:1207–11. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 22.Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 23.Ma HL, Liang S, Li J, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Laurence A, O'Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin Immunol. 2007;19:400–8. doi: 10.1016/j.smim.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–50. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 26.Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature. 2008;454:350–2. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugiyama H, Gyulai R, Toichi E, et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–73. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayed BA, Brown MA. Mast cells as modulators of T-cell responses. Immunol Rev. 2007;217:53–64. doi: 10.1111/j.1600-065X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson G, Svensson V, Nilsson K. Constitutive and inducible cytokine mRNA expression in the human mast cell line HMC-1. Scand J Immunol. 1995;42:76–81. doi: 10.1111/j.1365-3083.1995.tb03628.x. [DOI] [PubMed] [Google Scholar]
- 30.Lorentz A Schwengberg S, Sellge G, Manns MP, Bischoff SC. Human intestinal mast cells are capable of producing different cytokine profiles: role of IgE receptor cross-linking and IL-4. J Immunol. 2000;164:43–8. doi: 10.4049/jimmunol.164.1.43. [DOI] [PubMed] [Google Scholar]
- 31.Krüger-Krasagakes S, Möller A, Kolde G, Lippert U, Weber M, Henz BM. Production of interleukin-6 by human mast cells and basophilic cells. J Invest Dermatol. 1996;106:75–9. doi: 10.1111/1523-1747.ep12327815. [DOI] [PubMed] [Google Scholar]
- 32.Kanbe N, Kurosawa M, Nagata H, Saitoh H, Miyachi Y. Cord blood-derived human cultured mast cells produce transforming growth factor β1. Clin Exp Allergy. 1999;29:105–13. doi: 10.1046/j.1365-2222.1999.00459.x. [DOI] [PubMed] [Google Scholar]
- 33.Mizutani H, Schechter NM, Lazarus GS, Black RA, Kupper TS. Rapid and specific conversion of precursor interleukin 1β (IL-1β) to an active IL-1 species by human mast cell chymase. J Exp Med. 1991;174:821–5. doi: 10.1084/jem.174.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taipale J, Lohi J, Saarinen J, Kovanen PT, Keski-Oja J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-β1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem. 1995;270:4689–96. doi: 10.1074/jbc.270.9.4689. [DOI] [PubMed] [Google Scholar]
- 35.Zhao W, Oskeritzian CA, Pozez AL, Schwartz LB. Cytokine production by skin-derived mast cells: endogenous proteases are responsible for degradation of cytokines. J Immunol. 2005;175:2635–42. doi: 10.4049/jimmunol.175.4.2635. [DOI] [PubMed] [Google Scholar]
- 36.Harvima IT, Haapanen L, Ackermann L, Naukkarinen A, Harvima RJ, Horsmanheimo M. Decreased chymase activity is associated with increased levels of protease inhibitors in mast cells of psoriatic lesions. Acta Derm Venereol (Stockh) 1999;79:98–104. doi: 10.1080/000155599750011282. [DOI] [PubMed] [Google Scholar]
- 37.Grossman RM, Krueger J, Yourish D, et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci USA. 1989;86:6367–71. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183:3170–6. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farber EM, Nall ML. The natural history of psoriasis in 5600 patients. Dermatologica. 1974;148:1–18. doi: 10.1159/000251595. [DOI] [PubMed] [Google Scholar]
- 40.Toruniowa B, Jablonska S. Mast cells in the initial stages of psoriasis. Arch Dermatol Res. 1988;280:189–93. doi: 10.1007/BF00513956. [DOI] [PubMed] [Google Scholar]
- 41.Diaconu N-C, Kaminska R, Naukkarinen A, Harvima RJ, Nilsson G, Harvima IT. Increase in CD30 ligand/CD153 and TNF-α expressing mast cells in basal cell carcinoma. Cancer Immunol Immunother. 2007;56:1407–15. doi: 10.1007/s00262-007-0290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kandere-Grzybowska K, Kempuraj D, Cao J, Cetrulo CL, Theoharides TC. Regulation of IL-1-induced selective IL-6 release from human mast cells and inhibition by quercetin. Br J Pharmacol. 2006;148:208–15. doi: 10.1038/sj.bjp.0706695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castellani ML, Perrella A, Kempuraj DJ, et al. Immunological activation of human umbilical cord blood mast cells induces tryptase secretion and interleukin-6, and histidine decarboxylase mRNA gene expression. Pharmacol Res. 2007;55:57–63. doi: 10.1016/j.phrs.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Rivera J. Adaptors discriminate mast-cell cytokine production from eicosanoid production and degranulation. Trends Immunol. 2006;27:251–3. doi: 10.1016/j.it.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Turnquist HR, Zhao Z, Rosborough BR, et al. IL-33 expands suppressive CD11b+ Gr-1int and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J Immunol. 2011;187:4598–610. doi: 10.4049/jimmunol.1100519. [DOI] [PMC free article] [PubMed] [Google Scholar]


