Abstract
In our previous study, we showed that treatment with an anti-interleukin (IL)-12/23p40 antibody inhibits acute cardiac allograft rejection via inhibiting production of interferon (IFN)-γ and IL-17a. However, the impact of this antagonistic anti-p40 antibody on chronic cardiac rejection was unclear. Hearts of B6.C-H2bm12/KhEg mice were transplanted into major histocompatibility complex (MHC) class II-mismatched C57Bl/6J mice (wild-type, γδTCR –/– and IL-17–/–), which is an established murine model of chronic allograft rejection without immunosuppression. The mice were treated with control immunoglobulin (Ig)G or 200 µg anti-p40 monoclonal antibody on post-operative days, respectively. Abdominal palpation and echocardiography were used to monitor graft survival. The mice administered with anti-p40 antibody showed a significant promotion in graft survival (median survival time >100 days), and histological analyses revealed that cardiac allograft rejection was attenuated. Quantitative real-time polymerase chain reaction (qRT–PCR) and immunofluorescence analyses demonstrated that anti-p40 antibody down-regulated the level of ingraft cytokine and chemokine expression (IL-6, IFN-γ, IL-17a, CCL2 and CCL20). Flow cytometry analyses showed that γδ T cells are an important ingraft source of IFN-γ and IL-17a and inhibit the production of inflammation cytokine by anti-p40 antibody. Compared with the wild-type group, the graft survival time in the γδ T cell receptor–/– and IL-17–/– mice was prolonged significantly. Therefore we propose that, in the chronic allograft rejection model, treatment with anti-p40 antibody prolongs graft survival possibly by reducing the amount of reactive inflammatory cells, especially γδ T cells.
Keywords: chronic allograft rejection, cytokines, T cells
Introduction
Heart transplantation is the most effective treatment for patients with end-stage heart disease, and since 1982 this procedure has been performed on more than 85 000 patients [1]. With the development of immunosuppressive therapy regimens that target adaptive immunity over several decades, the long-term survival of heart transplant recipients has been reported worldwide. However, the long-term survival rates have not changed significantly. According to the International Society of Heart and Lung Transplantation (ISHLT) registry, angiographic studies have reported chronic allograft rejection in 8% of recipients within the first year, in 32% of recipients within the first 5 years and in almost 43% of recipients within the first 8 years after the transplant [2]. Although the pathological features of chronic allograft rejection is characterized clearly by interstitial fibrosis and the concentric proliferation of neointimal cells in the allograft arteries, the mechanism of rejection is complicated and remains poorly understood, which hinders the development of effective therapies against chronic allograft rejection.
Previous studies have confirmed that the immune responses against grafts are involved in the process of allograft rejection. During this process, T cells including CD4+ T helper cells (Th1, Th2 and Th17) and regulatory T cells (Treg) play a crucial role in mediating the graft rejection. These T cell responses are characterized by the expression of specific transcription factors and the production of proinflammatory mediators, e.g. Th1 cells [T-bet and interferon (IFN)-γ], Th2 cells [guanine, adenine, thymine, adenine (GATA-3) and IL-4, -5, -10 and -13], Th17 cells [retinoic acid-related orphan receptor (ROR)-γt and IL-17] and Treg cells [forkhead box protein 3 (FoxP3), IL-10 and transforming growth factor (TGF)-β][3]. IFN-γ and IL-17, which are produced by Th1 and Th17 cells, are known to be responsible for allograft rejection, whereas IL-10, IL-4 and TGF-β, which are secreted by Th2 and Treg cells, are known to inhibit graft rejection [4,5]. In a previous study, we have investigated these cytokines and immune cells in a clinical setting [5]. Recently, reports have demonstrated that depending on the local cytokine milieu, CD4+ T cell lineages exhibit plasticity [3]. It appears that the down-regulation of proinflammatory cytokines attenuates T cell responses and the induction of antigen-specific Treg response inhibits graft rejection.
IL-12 and IL-23 are important proinflammatory cytokines, and their levels are elevated in autoimmune and inflammatory diseases [6,7]. It has been reported that IL-12 and IL-23 play an essential role in Th1 cell development, which mediates allograft rejection [8,9]. These two cytokines, which are essential for Th17 cell differentiation and for IL-17 production by γδ T cells, are composed of a p35/p40 subunit (IL-12) and a p19/p40 subunit (IL-23), respectively, and the cytokines are secreted by activated antigen-presenting cells [10–12]. The administration of anti-p40 antibody effectively inhibits the immune response mediated by Th1 and Th17 cells in human and non-human autoimmune diseases, including psoriasis, Crohn's disease, multiple sclerosis (MS) and experimental autoimmune encephalomyelitis [13]. Given that IL-12 and IL-23 share a p40 subunit and play a critical role in the development of graft rejection-related Th1- and IL-17-producing T cell responses, targeting the p40 subunit should attenuate the allograft rejection mediated by Th1 and Th17 cells. We have confirmed that targeting the p40 subunit inhibits acute allograft rejection in a murine model [14]; however, few studies have focused on the effectiveness of the treatment with anti-p40 antibodies in chronic allograft rejection. In this study, we used an established single major histocompatibility complex (MHC) class II-mismatched model of cardiac allograft rejection without immunosuppression (Bm12→Bm6) to evaluate the effects of the treatment with anti-p40 monoclonal antibody on attenuating chronic allograft rejection, and we provide insight into the possible mechanism.
Methods and materials
Animals
C57BL/6 (H-2b) mice were considered as wild-type (WT) and were purchased from the Institute of Laboratory Animal Sciences of Chinese Academy of Medical Sciences (Beijing, China). C57BL/6 (H-2b) IL-17-deficient mice were provided by Yoichiro Iwakura (University of Tokyo, Tokyo, Japan) and C57BL/6 (H-2b) γδ-deficient mice were provided by Yin Zhinan (Yale School of Medicine). B6.C-H-2bm12KhEg (H-2bm12) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Both C57BL/6 and B6.C-H-2bm12KhEg mice are genetically identical, but differ in their expression of the MHC class II molecule, I-Ab. Eight-week-old male mice, weighing 25–30 g, were housed in a specific pathogen-free facility and regular food and water were provided ad libitum. The Institutional Animal Care and Use Committee at Tongji Medical College (Wuhan, China) approved the experiments.
Cardiac transplantation model and antibody treatment
Vascularized heterotopic cardiac transplantation was performed using a procedure described by Corry et al. [15], with some modifications. Anaesthesia was induced by intraperitoneal injection (i.p.) injection of a combination of atropine (0·04 mg/kg) and 3% chloral hydrate (300 mg/kg). The end-to-side anastomoses were performed using an 11-0 nylon suture. Adult B6.C-H-2bm12KhEg (H-2bm12) mice were used as the donors, and C57BL/6(H-2b), IL-17–/–, γδ T cell receptor (TCR)–/– mice were used as the recipients. All the recipient mice were treated intraperitoneally with 300 µg rat anti-p40 monoclobal antibody (mAb) or control immunoglobulin (Ig)G (both from eBioscience, San Diego, CA, USA) on post-operative days 1, 3, 7 and 10, and the mice were administered with antibody at 3-day intervals. In addition to monitoring the viability of the donor grafts by direct abdominal palpation, a high-frequency ultrasound scanner system was used to assess the overall function of donor grafts.
Histological evaluation
The allografts were harvested at days 45 and 100, when a dysfunctional allograft was identified using left ventricular ejection fraction (LVEF), and the allografts were embedded in paraffin and then stained using haematoxylin and eosin (H&E) or Elastica van Gieson (EVG) stains. The severity of each chronic allograft rejection [parenchymal rejection and graft arterial disease (GAD)] was scored using procedures outlined in previous studies [16,17], which included the assessment of luminal occlusion and the percentage of diseased vessels. A portion of allograft was embedded in octreotide (OCT) compound (Sakura Finetek, Torrance, CA, USA), sectioned for immunofluorescence staining using goat anti-mouse IL-17 and IFN-γ (R&D Systems, Minneapolis, MN, USA) as primary antibodies. Secondary detection was performed using phycoerythrin (PE)-conjugated rat anti-goat IgG (Invitrogen, San Diego, CA, USA). Images were captured using a confocal laser scanning microscope (Nikon, Tokyo, Japan). Immunohistochemical staining for IL-23 receptor (Novus Biologicals, Littleton, CO, USA) was performed as described previously [14].
Cardiac graft echocardiographic monitoring
The function of the cardiac grafts was assessed using two-dimensional echocardiography (Vevo 2100; VisualSonics Inc., Toronto, Ontario, Canada), which was performed using a 30-MHz ultrasound probe. The mice were anaesthetized by the administration of oxygen containing isoflurane, and their abdominal hair was removed cleanly. To obtain high-quality images, the position and orientation of the graft was estimated by palpation, and the short- and long-axis views of the cardiac graft were performed and recorded. To assess the anatomy, the ventricular wall function and the LVEF of the grafts, B-mode, M-mode and Doppler spectra were performed. The detailed procedures and the technical specifications that were followed can be found in the reports by Scherrer-Crosbie et al. and Zhou et al. [18,19].
Isolation of infiltrated cells in the allograft and flow cytometry
The cells that had infiltrated into the allograft were isolated in accordance with the procedures described by Gorbacheva et al., with some modifications. The allografts were minced in phosphate-buffered saline (PBS) containing 10% fetal calf serum (FCS) (Gibco Laboratories, Grand Island, NY, USA) and digested with 2 mg/ml collagenase IV (Sigma-Aldrich, St Louis, MO, USA) at 37°C for 30 min with gentle, continuous vortexing. After processing, cell strainers (40 µm) were used to filter the suspensions. The resultant cells were washed and counted.
The isolated cells, splenic cells and co-cultured cells were stained with fluorochrome-conjugated anti-mouse CD4, CD8, γδ TCR, IFN-γ, FoxP3 and IL-17 mAbs (eBioscience). The methods used for the intracellular staining procedures were in accordance with those described in a previous study [14]. Flow cytometry was performed using a fluorescence activated cell sorter (FACS) Calibur (BD FACSAria, San Jose, CA, USA) instrument and the data were analysed using FlowJo version 7.6 software (Tree Star Inc., Ashland, OR, USA).
Immunoblots and enzyme-linked immunosorbent assay (ELISA)
Allograft tissues were lysed using a volume of 1 × sodium dodecyl sulphate (SDS) loading buffer (Bio-Rad, Hercules, CA, USA), supplemented with phenylmethanesulphonyl fluoride (PMSF) (1 mM), and boiled for 5 min. The protein concentration was determined and detailed immunobloting procedures were performed following the protocol recommended by eBioscience. Equal amounts of sample were analysed by SDS-polyacrylamide gel electophoresis (PAGE), transferred electrophoretically to polyvinylidene difluoride membrane (PVDF) and probed with the respective IFN-γ and IL-17a antibodies (1:1000; both purchased from eBioscience). As a loading control, the immunoblot was stripped and reprobed with anti-glyceraldehyde 3-phosphate dehydrogenase (GADPH) antibody (1:1000; Cell Signaling, Beverly, MA, USA). After washing, subsequent incubation (1 h) was carried out with the appropriate horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology). Immunoblots were detected using an enhanced chemiluminescence (ECL) kit (Pierce Chemical Co., Rockford, IL, USA).
Murine serum IL-12/23 p40 was quantified using Ready-SET-Go! ELISA kits (eBioscience), following the manufacturer's protocol.
RNA isolation and quantitative mRNA analysis
Total RNA was extracted from individual samples that were dissected from the allografts or from isolated cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's protocol. The reverse transcription procedure was performed using a high-RNA-to-cDNA kit (Applied Biosystems, Foster City, CA, USA). The specific primers, probes for murine T-bet, RORγt, GATA3, FoxP3, CCL2, CCL20, IFN-γ, IL-2, TGF-β, IL-6, IL-4, IL-10 and IL-12p40 and the housekeeping gene 18S mRNA that were used for this analysis have been described previously [14]. The samples were analysed using the ABI Prism 7900 Sequence Detection System (Applied Biosystems). The relative expression levels were normalized to the expression of GAPDH. The detailed methods and procedures have been described previously [14].
Statistical analysis
The data are expressed as mean ± standard error of the mean. Kaplan–Meier methods were used to calculate the graft survival. The differences between groups were assessed using a one-way analysis of variance followed by Bonferroni's correction. To determine the statistical significance between two groups of mice, an unpaired Student's t-test was performed. A P-value < 0·05 was considered statistically significant. The GraphPad version 5.0 statistical program (GraphPad Software Inc., San Diego, CA, USA) was used for the statistical analyses and for preparation of the statistical graphics.
Results
Treatment with anti-IL-12/23p40 antibody prolongs survival of a cardiac allograft and retains its function following transplantation
A heterotrophic cardiac transplantation in a single MHC class II-mismatched model was performed to determine the effect of targeting the IL-12/23-shared p40 subunit in alleviating chronic rejection of a cardiac allograft. In the wild-type control IgG-injected group and no-administration group, the median allograft survival of 66 and 67 days was observed after transplantation. The group treated with the anti-IL-12/23p40 antibody showed a statistically significant prolonged allograft survival of >100 days (P < 0·05, Fig. 1a). The dynamic accurate function of the allograft was evaluated using serial echocardiography. In the first 100 days, the parameters of the LVEF in the allograft remained stable in the p40 antibody-treated group. In contrast, starting on day 45, the LVEF declined significantly in the control group (Fig. 1b,c).
Fig. 1.

Administration of anti-p40 monoclonal antibody (mAb) prolonged graft survival and retained functions of the allograft in a single major histocompatibility complex (MHC) class II-mismatched murine model. (a) Survival curves of cardiac allografts transplanted in recipients treated with the anti-p40 mAb, with the control immunoglobulin (Ig)G and no administration. (b,c) Serial echocardiography was used to monitor left ventricular ejection fraction (LVEF) in cardiac allograft recipients treated with the anti-p40 mAb, with the control IgG and no administration. (d) Serum level of protein interleukin (IL)-12/23p40 was detected by enzyme-linked immunosorbent assay (ELISA). (e) Pathological features of allografts from anti-p40 mAb- and control IgG-treated groups at 45 and 100 days post-transplantation. The paraffin sections were stained using Elastica van Gieson (EVG) or haematoxylin and eosin (H&E) (×400). (f,g) In accordance with the scales described in the Materials and methods, the parenchymal rejection (PR) (f) and the graft coronary artery disease (GAD) scores for the allograft tissue sections from the different groups of mice were evaluated on days 45 and 100 post-transplantation (*versus the anti-p40 group, P < 0·05).
Treatment with anti-IL-12/23p40 antibody alleviates chronic allograft rejection
To investigate the histological changes in the cardiac allograft, H&E and EVG staining were performed on the allografts 45 days after transplantation in the controls, and 45 and 100 days after transplantation in the anti-p40 antibody-treated mice (Fig. 1e). To confirm the neutralized bioactivity, serum expression of protein IL-12/23 p40 was detected by ELISA, revealing low levels of protein IL-12/23 p40 in the administration group (P < 0·05) (Fig. 1d). The average scores for the PR and GAD in the anti-p40 group observed on day 45 were significantly lower than in the control group (P < 0·05) (Fig. 1f,g).
Treatment with anti-IL-12/23p40 antibody reduces inflammatory cell infiltration and cytokine and chemokine expression
Infiltration of the host leucocytes into the allografts is a hallmark of chronic allograft rejection. Therefore, flow cytometry was performed to detect the numbers of the different leucocyte subsets (CD4+, CD8+, γδ TCR+ and CD11b+) on days 7, 45, 80 and 100 after transplantation (Fig. 2a). The results demonstrate that significantly reduced numbers of leucocytes (CD4+, CD8+, γδ TCR+ and CD11b+) infiltrated into the allografts in the anti-p40 antibody-treated recipients (P < 0·05) compared with the control IgG-treated recipients from day 7. The population of CD4+, CD8+, γδ TCR+ and CD11b+ infiltrated cells was similar (Fig. 2b). Furthermore, a decrease in the expression levels of the specific transcription factor T-bet (Th1 cells) and RORγt (IL-17-producing T cells) was observed in the anti-p40-treated mice. No significant difference in the expression levels of FoxP3 and GATA3 mRNA was observed between the two groups (Fig. 3c).
Fig. 2.
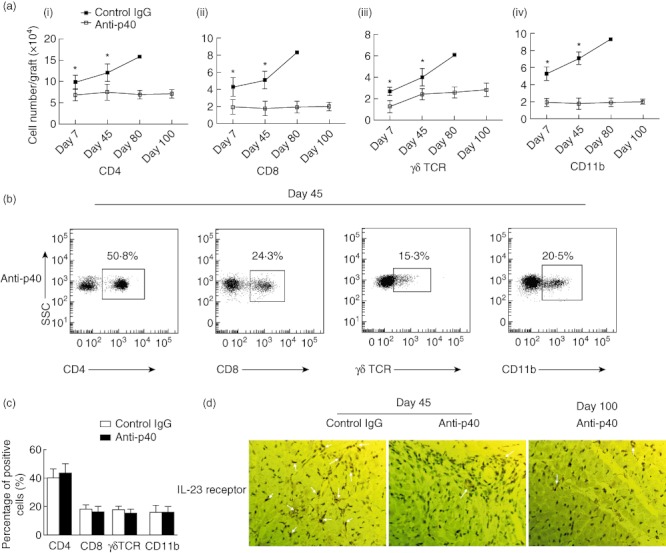
Dynamic analyses of the infiltrated immune subset cells. (ai, ii, iii, iv). Flow cytometry dynamic analyses of the number of infiltrated immune subsets cells [CD4+, CD8+, γδ T cell receptor (TCR)+ and CD11b+] in anti-p40 monoclonal antibody (mAb)- and control immunoglobulin (Ig)G-treated groups at days 45 and 100 post-transplantation. (b,c) Flow cytometry analyses of the percentage of infiltrated immune subsets cells (CD4+, CD8+, γδTCR+ and CD11b+). (c) The paraffin sections were stained using immunohistochemical analysis (IHC) of interleukin (IL)-23 receptor (×400) in cardiac allograft (×400) (*versus the anti-p40 group, P < 0·05).
Fig. 3.

Treatment with the anti-p40 monoclonal antibody (mAb) reduced the expression of ingraft cytokine, chemokine and transcription factors. (a) Immunofluorescence analysis of interleukin (IL)-17a and interferon (IFN)-γ populations in cardiac allografts on days 45 and 100 post-transplantation (×400); the relative gene expression of chemokine (b), transcription factors specific for the T cell subsets (c) and cytokine profiling (f) in the allografts were detected using quantitative real-time polymerase chain reaction (qRT–PCR) (*versus the anti-p40 group, P < 0·05).
To explore the contribution of cytokines and chemokines to the plastic and induced immune cell infiltration following anti-p40 antibody treatment, immunofluorescence staining and quantitative polymerase chain reaction (qPCR) were used to investigate the expression, respectively. As expected, immunofluorescence staining analyses revealed that on day 45 allograft from the anti-p40 antibody group showed low levels of both of IL-17a and IFN-γ and no difference between days 45 and 100 (Fig. 3a). On day 45, levels of the IFN-γ, IL-6, IL-17 and IL-12/23p40 cytokine mRNAs in Th1 cells and IL-17-producing T cells were down-regulated in the allografts that were treated with anti-p40 antibody (P < 0·05). In contrast, there was no significant change in the expression levels of TGF-β, IL-4, IL-10 and IL-2 cytokine mRNAs (Fig. 3d). Finally, a low level of expression chemokine (CCL2 and CCL20) in the allografts treated with anti-p40 antibody was detected (Fig. 3c) (P < 0·05).
Treatment with anti-IL-12/23p40 antibody inhibits infiltrated IFN-γ- and IL-17-producing T cell responses in vivo, particularly γδ T cells
The data suggest that IFN-γ- and IL-17-producing T cells may play a role in prolonging allograft survival after anti-p40 antibody therapy. To test this hypothesis, we determined the number of infiltrated distinct subsets of IFN-γ- and IL-17-producing T cells (CD4+, CD8+ and γδ TCR+) in the different groups of mice on day 45 post-transplantation. The infiltrated lymphocytes were isolated from the allografts and analyses by flow cytometry (Fig. 4a). Compared with the anti-p40 antibody-treated mice, the number of infiltrated IFN-γ-producing T cells (CD4+ and γδ TCR+) in the isotype IgG-treated group increased (P < 0·05) dramatically (Fig. 4d). Furthermore, the treatment with anti-p40 antibody reduced significantly the number of infiltrated IL-17-producing T cells (γδ TCR+) (P < 0·05, Fig. 4e). Conversely, the number of infiltrated Treg cells (CD4+ FoxP3+) in the anti-p40 group was similar (Fig. 4b,e).
Fig. 4.
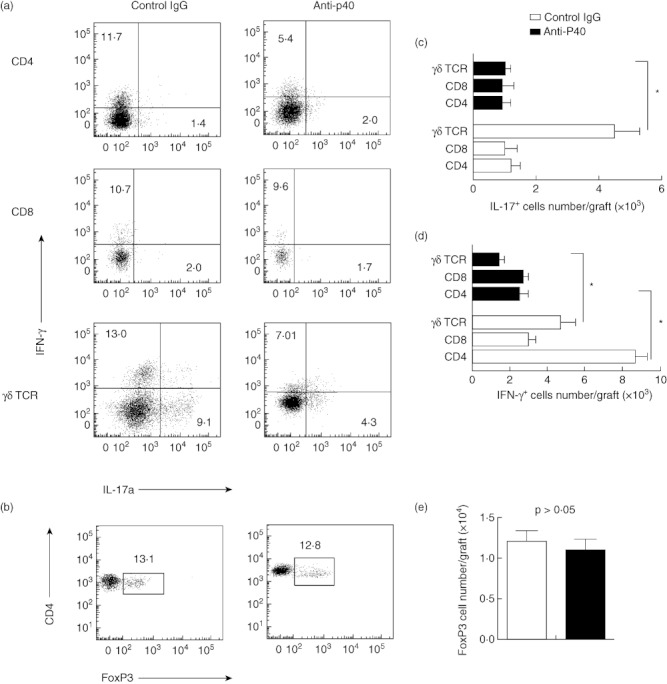
(a,c) Flow cytometry analyses showing the number of infiltrated interferon (IFN)-γ- and interleukin (IL)-17-producting T cells in the CD4–, CD8– and γδ T cell receptor (TCR)-gated population, respectively; (b,d) the number of infiltrated forkhead box protein 3+ T cells in the CD4-gated population (*versus in the anti-p40 group, P < 0·05).
Given the important role of the production of IL-17-producing T cells, immunohistochemical staining was used to detect expression of the IL-23 receptor, and the anti-p40 antibody down-regulated levels of the IL-23 receptor in the graft at days 45 and 100 (Fig. 2c).
According to the above data, we propose that treatment with anti-p40 antibody could decrease IFN-γ and IL-17 production by inhibiting γδ T cells. To investigate this hypothesis, IL-17-deficient and γδ-deficient C57BL/6 (H-2b) mice were used as recipients, respectively. The mean survival time of allografts in the IL-17-deficient and γδ-deficient groups was prolonged to 75 and 91 days, respectively. There was a significant difference in the allograft survival time between the IL-17-deficient or γδ-deficient groups and the wild-type group (P < 0·05, Fig. 5a). However, it was not significantly extended compared with the anti-p40 group. Immunoblot analyses revealed that the level of protein IFN-γ and IL-17 was decreased significantly in the γδ-deficient and anti-p40 group on day 45 (Fig. 5b).
Fig. 5.

(a) Survival curves of cardiac allografts transplanted in wild-type, interleukin (IL)-17-deficient, γδ-deficient and treated with the anti-p40 monoclonal antibody (mAb) recipients, respectively (*versus in the anti-p40 group, P < 0·05). (b) Immunoblot analyses for IL-17 and interferon (IFN)-γ in cardiac allograft from each group on day 45 post-transplantation. (c) Pathological features of allografts from wild-type, IL-17-deficient, γδ-deficient and treated with the anti-p40 mAb recipients at day 45 post-transplantation.
Discussion
Previous reports show that cytokines govern the differentiation of CD4+ T cells in the microenvironment [3]. A number of studies confirm that IL-12 and IL-23 play pivotal roles in the generation of Th1 and Th17 cells, respectively. It has been reported that IL-23 induces and maintains the generation of IL-17-producing γδ T cells [20]. The p40 subunit, which is shared by both IL-12 and IL-23, binds to the IL-12Rβ1 chain [21]. Previous studies have confirmed that targeting the p40 subunit neutralizes the biological activity of IL-12 and IL-23 in both animal models and clinical trials [7,22]. Furthermore, IL-12 and IL-23 play vital roles in allograft rejection. Collectively, these analyses establish the molecular basis for the targeting of the p40 subunit in the investigation of chronic allograft rejection, which is mediated by IFN-γ- and IL-17-producing T cells [6,23,24].
In this study, we focused on the effects of the anti-p40 antibody on the attenuation of chronic allograft rejection. In a single MHC class II-mismatched model, treatment with the anti-p40 antibody inhibited significantly chronic allograft rejection and prolonged allograft survival through the reduction of the immune responses to the allograft that could be mediated by IFN-γ- and IL-17-producing T cells (CD4+ and γδ TCR+). The mice administered with the anti-p40 antibody exhibited a significantly reduced number of infiltrated immune cells (CD4+, CD8+, γδ TCR+ and CD11b+), with a concomitant reduction in the levels of relevant proinflammatory mediators (IFN-γ, IL-6, IL-17 and IL-12/23p40). Furthermore, analysis of the number of infiltrated T cell subsets revealed that treatment with anti-p40 antibody reduced significantly the number of IFN-γ- and IL-17-producing T cells [CD4 and γδ T cells (γδ TCR+)]. Further investigation of the source of IFN-γ and IL-17 showed that IL-17-deficient and γδ-deficient groups could prolong significantly the allograft survival time, and the results were similar. The levels of both the IL-17-deficient and γδ-deficient groups could be reduced. Therefore, we propose that infiltrated γδ T cells are an important source of IFN-γ and IL-17 in chronic allograft rejection, the production of which could be inhibited by anti-p40. The humoral response to the allograft in the pathogenesis of chronic rejection is mediated by Th2 cells; however, our data did not show that treatment with anti-p40 could change significantly the expression of mRNA GATA3 and IL-4-related Th2 cells [25].
Our observations reveal that targeting the p40 subunit influences some aspects of molecular and cellular expression in the chronic allograft rejection model and provides new insight into the pathological processes of chronic allograft rejection. Given that the anti-p40 antibody neutralizes the activity of both IL-12 and IL-23, we will discuss their influence on the immune responses of chronic rejection separately. There is no doubt that the anti-p40 mAb inhibits IL-12-mediated immune responses and that it drives cell-mediated immunity by activating natural killer cells, inducing CD8+ T cells, triggering IFN-γ production and T cell proliferation by Th1 cells [10,26,27]. These IL-12-regulated immune cells, especially the natural killer cells, are implicated in the development of chronic allograft rejection [28]. Uehara et al. proposed that the anti-p40 antibody down-regulates the expression of IFN-γ secreted by natural killer (NK) cells. Therefore, it seemed likely that an anti-p40 antibody would regulate the IL-12-signalling pathway, which is activated by the phosphorylation of a signal transducer and which activates the signal transducer and activation of transcription (STAT)4 and STAT6 genes to attenuate the process of chronic allograft rejection [13]. We did not investigate this signalling pathway and have little evidence to support this theory; however, our data demonstrate that the IL-12/23-p40 subunit has little effect on the development of Th2 cells in the process of allograft rejection, which is consistent with other published studies [29].
IL-23 is a heterodimer comprising of covalently linked p19 and p40 protein subunits, which bind to the effector cell receptors to activate IL-23-specific intracellular signalling pathways, such as the intracellular phosphorylation of STATs [30,31]. IL-23 is required for the development and maintenance of IL-17-producing T cells, including Th17 and γδ T cells [20,32]. Furthermore, bioactivity of IL-23 regulates the expression of RORγτ and RORα transcription factors for IL-17-producing T cells. In accordance with these studies, our observations show that treatment with anti-p40 antibody down-regulates significantly the frequencies of infiltrated IL-17-producing T cells (Th17 and γδ T cells) by inhibiting the bioactivity of IL-23. As an innate source of IL-17, γδ T cells are involved in the pathological process of chronic allograft rejection [23,33]. Our previous studies have demonstrated the effects of the anti-p40 antibody on the immune response mediated by Th17 cells, but not on the response mediated by IL-17-producing γδ T cells. In a single MHC class II-mismatched murine model, the anti-p40 antibody down-regulated the number of infiltrated IL-17-producing γδ T cells. In addition, the level of IL-23 receptors in the anti-p40 group was reduced. Therefore, our results propose that γδ T cells might be involved in the process of chronic allograft vasculopathy (CAV). Previous observation has also demonstrated that NK cells play important roles in chronic allograft rejection [34]. This provides new insight into the development of chronic allograft rejection, that innate immunity composed of γδ T cells and NK cells is involved in the process. Our data show that there was no significant change in the percentage of infiltrated CD8+ T cells and the number of infiltrated CD8+ T cells decreased in the anti-p40 group. According to previous reports on the activity of IL-23 on CD8+ T cells, this could explain the decreased number of infiltrated CD8+ T cells in the anti-p40 group [35].
Interestingly, our study revealed that treatment with the anti-p40 antibody did not influence the number of CD4+ FoxP3+ Treg cells. Analysis of the specific transcription factors using qPCR showed that the expression levels of FoxP3 mRNA in Treg cells are unchanged in the allograft. Recently, research has demonstrated that Treg cells did not express the IL-23 receptor [36]. Given the above, our results suggest that the anti-p40 antibody could not influence the expansion and function of the Treg cells directly.
In addition, and in accordance with previous studies, our results show that targeting the p40 subunit inhibited the infiltration of macrophages, which is an important component of chronic allograft rejection [37,38]. We found that anti-p40 antibody could reduce the expression of CCL2 and CCL20, which is attributed to macrophage recruitment [38]. As a chemoattractant factor, the p40 subunit may recruit macrophages and dendritic cells to sites of inflammation and mediate inflammatory damage [39]. According to a report by Diaz et al. [40], echocardiography is an effective tool for the dynamic monitoring of allograft function. The LVEF parameters remained stable in the group that was treated with anti-p40 antibody during the post-operative period.
In summary, this study illustrates that treatment with the anti-p40 antibody attenuates chronic allograft rejection in a single MHC class II-mismatched murine model through the inhibition of IFN-γ and IL-17-producing T cells (particularly γδ T cells). Therefore, these results suggest a possible strategy to alleviate chronic allograft rejection and to improve the long-term survival of allografts in the heart.
Disclosure
The authors have no financial or commercial conflicts of interest. This work was supported by the National Natural Science Fund of China (Grant No. 81070205), State Key Development Program of Basic Research of China (Grant No. 81130056) and the 973 State Key Development Program of Basic Research of China (Grant No. 2010CB535012).
References
- 1.Taylor DO, Stehlik J, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-sixth official adult heart transplant report-2009. J Heart Lung Transplant. 2009;28:1007–22. doi: 10.1016/j.healun.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Schmauss D, Weis M. Cardiac allograft vasculopathy: recent developments. Circulation. 2008;117:2131–41. doi: 10.1161/CIRCULATIONAHA.107.711911. [DOI] [PubMed] [Google Scholar]
- 3.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–55. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Ingulli E. Mechanism of cellular rejection in transplantation. Pediatr Nephrol. 2008;25:61–74. doi: 10.1007/s00467-008-1020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S, Li J, Xie A, et al. Dynamic changes in Th1, Th17, and FoxP3(+) T cells in patients with acute cellular rejection after cardiac transplantation. Clin Transplant. 2011;25:E177–86. doi: 10.1111/j.1399-0012.2010.01362.x. [DOI] [PubMed] [Google Scholar]
- 6.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205:3133–44. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 8.Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–9. [PubMed] [Google Scholar]
- 9.Ha SJ, Kim DJ, Baek KH, Yun YD, Sung YC. IL-23 induces stronger sustained CTL and Th1 immune responses than IL-12 in hepatitis C virus envelope protein 2 DNA immunization. J Immunol. 2004;172:525–31. doi: 10.4049/jimmunol.172.1.525. [DOI] [PubMed] [Google Scholar]
- 10.Manetti R, Parronchi P, Giudizi MG, et al. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelissen F, van Hamburg JP, Lubberts E. The IL-12/IL-23 axis and its role in Th17 cell development, pathology and plasticity in arthritis. Curr Opin Invest Drugs. 2009;10:452–62. [PubMed] [Google Scholar]
- 12.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–30. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Benson JM, Sachs CW, Treacy G, et al. Therapeutic targeting of the IL-12/23 pathways: generation and characterization of ustekinumab. Nat Biotechnol. 2011;29:615–24. doi: 10.1038/nbt.1903. [DOI] [PubMed] [Google Scholar]
- 14.Xie A, Wang S, Zhang K, et al. Treatment with interleukin-12/23p40 antibody attenuates acute cardiac allograft rejection. Transplantation. 2011;91:27–34. doi: 10.1097/tp.0b013e3181fdd948. [DOI] [PubMed] [Google Scholar]
- 15.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–50. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa Y, Libby P, Stinn JL, Becker G, Mitchell RN. Cold ischemia induces isograft arteriopathy, but does not augment allograft arteriopathy in non-immunosuppressed hosts. Am J Pathol. 2002;160:1077–87. doi: 10.1016/S0002-9440(10)64928-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sayegh MH, Wu Z, Hancock WW, et al. Allograft rejection in a new allospecific CD4+ TCR transgenic mouse. Am J Transplant. 2003;3:381–9. doi: 10.1034/j.1600-6143.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 18.Scherrer-Crosbie M, Glysing-Jensen T, Fry SJ, et al. Echocardiography improves detection of rejection after heterotopic mouse cardiac transplantation. J Am Soc Echocardiogr. 2002;15:1315–20. doi: 10.1067/mje.2002.124644. [DOI] [PubMed] [Google Scholar]
- 19.Zhou YQ, Bishay R, Feintuch A, et al. Morphological and functional evaluation of murine heterotopic cardiac grafts using ultrasound biomicroscopy. Ultrasound Med Biol. 2007;33:870–9. doi: 10.1016/j.ultrasmedbio.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–41. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Presky DH, Yang H, Minetti LJ, et al. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–7. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandhi M, Alwawi E, Gordon KB. Anti-p40 antibodies ustekinumab and briakinumab: blockade of interleukin-12 and interleukin-23 in the treatment of psoriasis. Semin Cutan Med Surg. 2010;29:48–52. doi: 10.1016/j.sder.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Itoh S, Nakae S, Axtell RC, et al. IL-17 contributes to the development of chronic rejection in a murine heart transplant model. J Clin Immunol. 2010;30:235–40. doi: 10.1007/s10875-009-9366-9. [DOI] [PubMed] [Google Scholar]
- 24.Nagano H, Libby P, Taylor MK, et al. Coronary arteriosclerosis after T-cell-mediated injury in transplanted mouse hearts: role of interferon-gamma. Am J Pathol. 1998;152:1187–97. [PMC free article] [PubMed] [Google Scholar]
- 25.Russell PS, Chase CM, Winn HJ, Colvin RB. Coronary atherosclerosis in transplanted mouse hearts. II. Importance of humoral immunity. J Immunol. 1994;152:5135–41. [PubMed] [Google Scholar]
- 26.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–45. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gately MK, Desai BB, Wolitzky AG, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor) J Immunol. 1991;147:874–82. [PubMed] [Google Scholar]
- 28.Uehara S, Chase CM, Kitchens WH, et al. NK cells can trigger allograft vasculopathy: the role of hybrid resistance in solid organ allografts. J Immunol. 2005;175:3424–30. doi: 10.4049/jimmunol.175.5.3424. [DOI] [PubMed] [Google Scholar]
- 29.Bi EG, Shi W, Zou J, et al. IL-12p40 is not required for islet allograft rejection. Acta Pharmacol Sin. 2006;27:1065–70. doi: 10.1111/j.1745-7254.2006.00341.x. [DOI] [PubMed] [Google Scholar]
- 30.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 31.Parham C, Chirica M, Timans J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 32.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–9. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 33.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–89. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 34.Millington TM, Madsen JC. Innate immunity and cardiac allograft rejection. Kidney Int. 2010;119(Suppl):S18–21. doi: 10.1038/ki.2010.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curtis MM, Way SS, Wilson CB. IL-23 promotes the production of IL-17 by antigen-specific CD8 T cells in the absence of IL-12 and type-I interferons. J Immunol. 2009;183:381–7. doi: 10.4049/jimmunol.0900939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petermann F, Rothhammer V, Claussen MC, et al. Gammadelta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–63. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitchens WH, Chase CM, Uehara S, et al. Macrophage depletion suppresses cardiac allograft vasculopathy in mice. Am J Transplant. 2007;7:2675–82. doi: 10.1111/j.1600-6143.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- 38.Saeki K, Kanai T, Nakano M, et al. CCL2-induced migration and SOCS3-mediated activation of macrophages are involved in cerulein-induced pancreatitis in mice. Gastroenterology. 2012;142:1010–20. doi: 10.1053/j.gastro.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 39.Ha SJ, Lee CH, Lee SB, et al. A novel function of IL-12p40 as a chemotactic molecule for macrophages. J Immunol. 1999;163:2902–8. [PubMed] [Google Scholar]
- 40.Diaz JA, Booth AJ, Lu G, Wood SC, Pinsky DJ, Bishop DK. Critical role for IL-6 in hypertrophy and fibrosis in chronic cardiac allograft rejection. Am J Transplant. 2009;9:1773–83. doi: 10.1111/j.1600-6143.2009.02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


