Abstract
New technologies and interest in cell mechanics are generating exciting new discoveries about how material properties and forces affect biological structure and function. Mechanical forces are transduced via a variety of mechanisms, recently beginning to be revealed, into signals capable of altering cell function and structure. Responses to physical stimuli occur at multiple levels, from changes in the structures of single proteins to global cascades capable of altering cell proliferation and differentiation. This review describes recent findings in which physical stimuli were shown to modulate transcription factor activity, including that of armadillo/β-catenin, serum response factor (SRF), yes-associated protein (YAP) and nuclear factor κB (NF-κB).
Keywords: Mechanotransduction, Transcriptional regulation, Cell mechanics
1. Introduction
The signal transduction cascades initiated by mechanical signals, similarly to those elicited by chemical signals, affect many aspects of cell behavior including division, motility, morphology and protein expression. In this review, we focus on recent experiments in which a mechanical signal has been shown to cause a change in transcription factor activity, and highlight a few recent discoveries about the protein-protein interactions underlying these cascades.
Proof of the principal of mechanically-mediated transcriptional regulation comes from studies in which stem cell lineage commitments changed as the result of growth on 2D substrates (Engler et al., 2006) or in 3D matrices (Pek et al., 2010) of different elastic moduli (Fig. 1A). Limiting the shape of a cell (Fig. 1B) (Gao et al., 2010), exposing cells to a tension gradient (Ruiz and Chen, 2008) or otherwise changing the external physical environment can also drive the alternate differentiation of pluripotent cells (reviewed by Guilak et al., 2009).
Figure 1. Manipulation of Mechanical Forces.
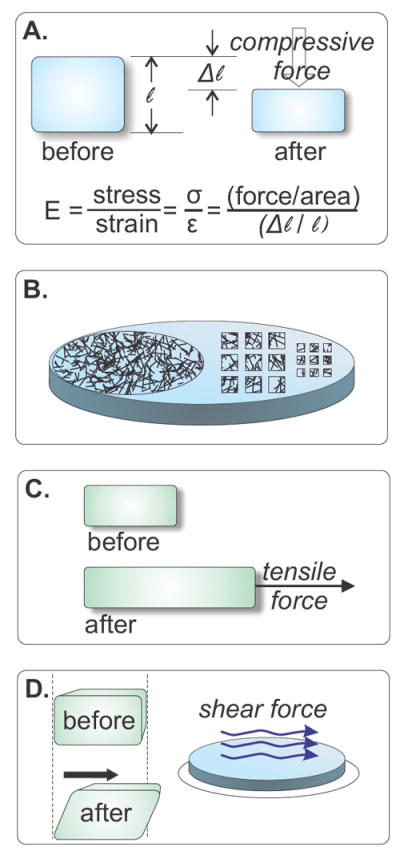
A) Commonly referred to as stiffness, the Young’s or elastic (E) modulus of a material is expressed in Pascals (N/m2) and describes the deformation (strain) that results from a force (stress) that is applied perpendicular the surface of a material. Polyacrylamide (PAA) and other polymeric substrates used in cell culture studies are linearly elastic, meaning that their Young’s modulus is consistent over a wide range of applied forces. B) In order for cells to adhere to PAA, something must be crosslinked to the inert PAA surface with which a cell will interact. Integrin ligands such as collagen or fibronectin are often used for this purpose. Ligands or other molecules may be crosslinked evenly over an entire surface (C, left) or applied in discrete shapes (C, right). Because the non-modified PAA will not allow cells to attach, this technique can be used to limit cells’ ability to spread or crawl. C) Tension is the magnitude of a pulling force divided by the distance over which it is applied, and compression its opposite: the magnitude of a pushing force. (A) illustrates a compressive stress. Pulling forces generate tension. D) Shear forces interact with a surface parallel to that surface, in contrast to extensional and compressive forces, which interact perpendicularly. Fluid flow past a cell is a common source of shear stress in vivo, and is often reported in dynes/cm2; 1 dyne/cm2 = 0.1 Pa.
2. Functions
Cells use a variety of sensors to sample environmental mechanical properties. One way extrinsic physical information is sensed is by structures at the interfaces between the cell and its surroundings. For example, focal adhesions, which mediate cell/substrate attachment, adherens junctions that contribute to cell/cell interactions (Gomez et al., 2011), and actin filament bundles, sometimes called stress fibers (Hayakawa et al., 2008) are altered by changes in tension. Mechanical sensors may also be present on structures that protrude from cell surfaces, such as primary cilia (Hoey et al., 2011) or within the glycocalyx (Shi and Tarbell, 2011).
Sensors can sample force directly or by way of intermediate biochemical reactions. Direct transducers are structurally modified by force, for example a gated ion channel stretch receptor that physically opens when stretched (Grillet et al., 2009) or the titin-associated systems in Z-discs in which tugging forces unfold the protein (Miller et al., 2004). Alternatively, cells can respond to biochemical changes such as F-actin depolymerization, which will be discussed below and can be triggered by multiple inputs, some of which are mechanical.
3. Cascades and Key Molecules
3.1 Armadillo and twist
During early embryogenesis differentiation is driven by two overarching sets of morphogenetic signals, position-based gene expression and rearrangement by cell motility. To test whether cell rearrangement involves transmission of force from motile to non-motile cells, Farge applied unilateral compression (Fig. 1A, C) to whole drosophila embryos. These studies showed that armadillo, a transcriptional coactivator and component of the cadherin complex that is homologous to human β-catenin, translocates to the nucleus in response to compressive stress, where it subsequently activates twist (Fig. 1A; Farge, 2003). During normal embryogenesis, twist expression is greatest in cells that are visibly deformed. In order to determine whether compressive forces are sufficient to cause armadillo’s nuclear translocation and the subsequent upregulation of twist expression, experiments were conducted in mutant drosophila incapable of generating compressive forces. The cells in the mutant flies that would have been deformed by movement in a normal fly exhibited abnormally low twist expression, and the local application of a pushing force rescued that expression (Farge, 2003). These results show that force, in this case generated by the movement of a motile cell, is transduced into a cascade that results in the activation of a transcription factor.
3.2 SRF
Serum response factor (SRF) is a transcription factor that regulates many adhesion-related genes, and is itself regulated by a number of independent pathways. Studies of an SRF-modulating pathway that is affected by actin dynamics lead to identification of a G-actin-associated cofactor, MAL (Megakaryoblastic leukemia 1; (Sotiropoulos et al., 1999, Miralles et al., 2003). Other results suggested that SRF-MAL activity could also be regulated by cell-migration-associated forces (Somogyi and Rorth, 2004). Eventually it was discovered that in the nucleus, the interaction of MAL with G-actin inhibits MAL binding to SRF (Vartiainen et al., 2007). Factors that drive F-actin polymerization decrease the pool of G-actin, and thereby increase MAL availability and the likelihood that it will activate SRF (Fig. 2B).
Figure 2. Mechanotransduction pathways lead to transcriptional regulation.
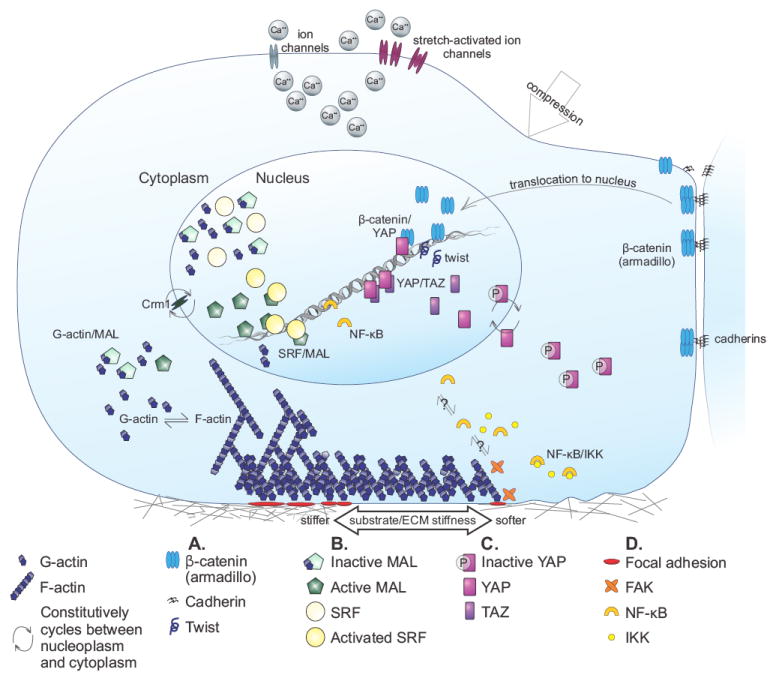
A) Twist activation follows activation of the drosophila homolog of β-catenin, armadillo, in response to compressive force. B) Interaction with unpolymerized actin inactivates MAL. F-actin polymerization depletes the G-actin pool and removes G-actin inhibition of MAL, leaving MAL available to interact with SRF. SRF thus activated binds to DNA and induces transcription. C) Phosphorylated YAP is constitutively expelled from the nucleus. Nuclear non-phosphorylated YAP interacts with TAZ, a closely related DNA-binding coactivator, and induces transcription in a manner that is modulated by substrate stiffness. YAP also interacts with nuclear β-catenin. D) NF-κB is released from IKK-mediated inactivation in a FAK dependent manner.
Connelly and colleagues further investigated SRF and the role of mechanotransduction using a model of epidermal stem cell differentiation (Connelly et al., 2010). They ‘painted’ micropatterned islands of extracellular matrix (ECM) onto glass coverslips (Fig. 1B). Cells could adhere to the ECM islands, but not to the ‘unpainted’ background. In this manner, the area available to each cell could be altered, or a constant area maintained while the shape was changed. Cells on the smallest islands were more likely to differentiate than cells allowed greater freedom to spread, but varying the ligand to which the cells could adhere or the density of the ligand did not alter cell fate (Connelly et al., 2010). This result demonstrates that mechanical signaling can dominate integrin-based biochemical signaling. Another study, in which mesenchymal stem cell differentiation was monitored in cells cultured on various combinations of substrate stiffness and ligand composition, showed that substrate stiffness does not always dominate over ligand-generated chemical cues, but that interplay exists between the two systems (Rowlands et al., 2008).
One explanation for these two findings may be that cells growing on very small islands, even on a very hard substrate, cannot generate the cytoskeletal tension commensurate with their substrate, and in this way are similar to cells grown on soft substrates. Whereas maximally spread cells exhibited many stress fibers and relatively little G-actin, cells grown on restrictively-sized islands had few stress fibers and more G-actin (Connelly et al., 2010). The difference of G-actin levels suggested that MAL might be involved, and therefore the expression of two SRF target genes, one requiring MAL activation (JUNB) and the other not (FOS), were assayed. Both JUNB and FOS were expressed, showing that SRF activation was modulated by at least two pathways in these cells (Connelly et al., 2010). Together, these results suggest that synergies between chemical and mechanical signals might become apparent as we learn more about crosstalk between the various signalling systems.
3.3 YAP
Yes-associated protein (YAP) is a DNA-binding transcriptional coactivator subject to regulation by many kinases including those of the hippo pathway. Phosphorylation inactivates YAP by promoting its exclusion from the nucleus. When YAP is nuclear, it interacts with a number of coactivators, including its paralog TAZ (transcriptional coactivator with PDZ-binding motif; Lei et al., 2008). YAP’s involvement in mechanotransduction was recognized following a screen of transcripts that were differentially expressed by a variety of human cell types plated on soft versus stiff substrates (Dupont et al., 2011). In cells grown on stiff substrates, YAP transcription and the nuclear localization of YAP protein increase (Fig. 2C). F-actin is required for these effects, as its depolymerization by Latrunculin A prevents these changes, but in contrast to the regulation of SRF/MAL, the ratio of F- to G-actin is not the regulatory factor. Furthermore, under these conditions YAP inactivation on soft substrates does not result primarily from phosphorylation by the LATS kinases of the hippo pathway, which has heretofore been the best undestood YAP modulation cascade (Dupont et al., 2011). Dupont et al. also showed that when the hippo pathway is activated by formation of cell/cell interactions, hippo signalling overrides the hippo-independent mechanotransduced signals.
The latter result might be related to another recent study of epithelial cells, which in culture propagate in characteristic clusters that allow formation of cell/cell junctions. These cells normally express cadherins that extracellularly mediate cell-cell junctions and intercellularly link via a number of intermediates to the cytoskeleton. Two of those intermediates, α- and β-catenin, in addition to contributing to cell adhesion, are also transcription factors capable of nuclear localization in response to signalling. On glass substrates, E-cadherin ligation, as well as homophilic binding, drives hippo-mediated YAP transcriptional inhibition by a mechanism that requires α- and β-catenin (Kim et al., 2011). Whereas the requirement for β-catenin in E-cadherin-mediated YAP signalling involves only the cadherin-linked, but not the nuclear, β-catenin, β-catenin also interacts with active YAP in the nucleus, and YAP phosphorylation and inhibition by hippo pathway kinases negatively regulate gene activation by β-catenin (Heallen et al., 2011).
It remains to be discovered whether this particular E-cadherin-mediated pathway transduces a force or whether this is purely a chemical signalling mechanism. Recent experiments suggest that it may be both. When cardiac myocytes are plated on N-cadherin-coated substrates – i.e. substrates that allow attachment only by N-cadherin-mediated processes – they reorganize their cytoskeletons, generate traction forces and modulate their cortical stiffness in response to substrate stiffness (Chopra et al., 2011).
3.4 NF-κB
It has been recognized that mechanical loading causes a biochemical response in bone for more than 100 years, although the exact mechanisms are only more recently becoming clear (Duncan and Turner, 1995). Many changes in transcription factor localization or activity result from altered exposure to force (Liedert et al., 2006). One of the most studied transcription regulators, NF-κB, is a protein complex that comprises a family of transcription factors that are controlled by an array of activators, inhibitors and degradation signals (Hoffmann et al., 2006).
Work in bone highlights the difficulty in distinguishing experimentally between a compressive stress and one generated by fluid flow (Fig. 1A, D), particularly in vivo. Shear stress is generated by fluid flow, and cells in bone experience shear stress as fluid is pushed out of interstitial spaces during compression. In both endothelial cells (Khachigian et al., 1995) and bone (Chen et al., 2003), shear stress causes NF-κB to translocate to the nucleus and participate in gene induction. Two more recent examples of protein/protein interactions leading to NF-κB activation are the responses of chondrocytes to dynamic compressive strain (Nam et al., 2009) and of cultured osteoblasts to oscillatory, but not continuous, fluid flow (Young et al., 2010). In both cell types, NF-κB was released from inactivation by IKK (IκB kinase) and translocated to the nucleus in response to a range of stresses, but not in response to stresses of lower magnitude (Fig. 2D). These biphasic responses indicate that at least one other mechanically-mediated protein is involved in the response. Focal adhesion kinase (FAK), a known mediator of mechanotransduction (Zebda et al., 2011), is one such protein. In FAK-null osteoblasts, only an upstream portion of the NF-κB cascade is activated by mechanical stress, whereas the cells respond to cytokine application no differently than FAK-normal cells. This result suggests that a mechanically-mediated pathway is perturbed when FAK is not available.
4. Associated pathologies and therapeutic implications
Defining the effect of matrix or tissue stiffness on cell function has utility both in defining how mechanical changes impact the progression of diseases like fibrosis, atherosclerosis or cancer, and in developing biomaterials with physical and chemical properties tuned for specific applications. For example, measurements of liver viscoelasticity during experimentally-triggered fibrosis showed that liver stiffness increased before there were any histological signs of either increased matrix deposition or altered cell morphology (Georges et al., 2007). Such results suggest that changes in tissue mechanics precede, and therefore might contribute to, development of the pathologic state. Similarly, consideration of stiffness effects on cells can direct the design of improved softer, biocompatible materials for tissue engineering.
Signaling Network Facts.
Mechanotransduction is the conversion of force into a biochemical or genetic response.
Forces are generated by molecular motor activity; fluid flow; changes in membrane tension caused by osmotic gradients; and tissue stretch or compression. Cells transduce information about the magnitude, direction, and rate of external stress, e.g. whether flow is constant, pulsatile or oscillating, and respond to differences in the resistance of their environment to forces they apply to it.
Like other environmental conditions such as temperature or nutrient availability, mechanical inputs are constitutively sampled.
Cell biological studies on glass or plastic substrates place the cell in a mechanically non-physiologic setting because these materials are much stiffer (GPa) than soft tissues in vivo, which generally range from 1 to 100 kPa.
Physical cues have important differences from chemical cues. For instance, they can be more exclusively unidirectional and extend without attenuation over longer length scales.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chen NX, Geist DJ, Genetos DC, Pavalko FM, Duncan RL. Fluid shear-induced NFkappaB translocation in osteoblasts is mediated by intracellular calcium release. Bone. 2003;33:399–410. doi: 10.1016/s8756-3282(03)00159-5. [DOI] [PubMed] [Google Scholar]
- Chopra A, Tabdanov E, Patel H, Janmey PA, Kresh JY. Cardiac myocyte remodeling mediated by N-cadherin-dependent mechanosensing. Am J Physiol Heart Circ Physiol. 2011;300:H1252–1266. doi: 10.1152/ajpheart.00515.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly JT, Gautrot JE, Trappmann B, Tan DW, Donati G, Huck WT, Watt FM. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat Cell Biol. 2010;12:711–718. doi: 10.1038/ncb2074. [DOI] [PubMed] [Google Scholar]
- Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int. 1995;57:344–358. doi: 10.1007/BF00302070. [DOI] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- Gao L, McBeath R, Chen CS. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells. 2010;28:564–572. doi: 10.1002/stem.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, Mick R, Janmey PA, Furth EE, Wells RG. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1147–1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- Gomez GA, McLachlan RW, Yap AS. Productive tension: force-sensing and homeostasis of cell-cell junctions. Trends Cell Biol. 2011;21:499–505. doi: 10.1016/j.tcb.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Grillet N, Kazmierczak P, Xiong W, Schwander M, Reynolds A, Sakaguchi H, Tokita J, Kachar B, Muller U. The mechanotransduction machinery of hair cells. Sci Signal. 2009;2:pt5. doi: 10.1126/scisignal.285pt5. [DOI] [PubMed] [Google Scholar]
- Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Tatsumi H, Sokabe M. Actin stress fibers transmit and focus force to activate mechanosensitive channels. J Cell Sci. 2008;121:496–503. doi: 10.1242/jcs.022053. [DOI] [PubMed] [Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey DA, Downs ME, Jacobs CR. The mechanics of the primary cilium: An intricate structure with complex function. J Biomech. 2011 doi: 10.1016/j.jbiomech.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Natoli G, Ghosh G. Transcriptional regulation via the NF-kappaB signaling module. Oncogene. 2006;25:6706–6716. doi: 10.1038/sj.onc.1209933. [DOI] [PubMed] [Google Scholar]
- Khachigian LM, Resnick N, Gimbrone MA, Jr, Collins T. Nuclear factor-kappa B interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J Clin Invest. 1995;96:1169–1175. doi: 10.1172/JCI118106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci U S A. 2011;108:11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedert A, Kaspar D, Blakytny R, Claes L, Ignatius A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Commun. 2006;349:1–5. doi: 10.1016/j.bbrc.2006.07.214. [DOI] [PubMed] [Google Scholar]
- Miller MK, Granzier H, Ehler E, Gregorio CC. The sensitive giant: the role of titin-based stretch sensing complexes in the heart. Trends Cell Biol. 2004;14:119–126. doi: 10.1016/j.tcb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- Nam J, Aguda BD, Rath B, Agarwal S. Biomechanical thresholds regulate inflammation through the NF-kappaB pathway: experiments and modeling. PLoS One. 2009;4:e5262. doi: 10.1371/journal.pone.0005262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pek YS, Wan AC, Ying JY. The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials. 2010;31:385–391. doi: 10.1016/j.biomaterials.2009.09.057. [DOI] [PubMed] [Google Scholar]
- Rowlands AS, George PA, Cooper-White JJ. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am J Physiol Cell Physiol. 2008;295:C1037–1044. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- Ruiz SA, Chen CS. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells. 2008;26:2921–2927. doi: 10.1634/stemcells.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ZD, Tarbell JM. Fluid flow mechanotransduction in vascular smooth muscle cells and fibroblasts. Ann Biomed Eng. 2011;39:1608–1619. doi: 10.1007/s10439-011-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi K, Rorth P. Evidence for tension-based regulation of Drosophila MAL and SRF during invasive cell migration. Dev Cell. 2004;7:85–93. doi: 10.1016/j.devcel.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749–1752. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- Young SR, Gerard-O’Riley R, Harrington M, Pavalko FM. Activation of NF-kappaB by fluid shear stress, but not TNF-alpha, requires focal adhesion kinase in osteoblasts. Bone. 2010;47:74–82. doi: 10.1016/j.bone.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebda N, Dubrovskyi O, Birukov KG. Focal Adhesion Kinase Regulation of Mechanotransduction and its Impact on Endothelial Cell Functions. Microvasc Res. 2011 doi: 10.1016/j.mvr.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


