Abstract
Experimental studies suggest a role for aspirin in the chemoprevention of prostate cancer and epidemiological evidence supports a modest inverse association between regular aspirin use and prostate cancer risk, especially for advanced disease. In a prospective cohort study of 51,529 health professionals aged 40–75 years at baseline, we evaluated long-term aspirin use and the incidence of total, high-grade (Gleason 8–10, n=488), regionally advanced (T3b-T4 or N1, n=228) and lethal prostate cancer (M1, bony metastases or prostate cancer death, n=580) from 1988–2006. We used Cox proportional hazards regression to evaluate risk associated with frequency (days/week), quantity (tablets/week), recency and duration of aspirin use after multivariable adjustment for confounders and other predictors of prostate cancer risk. A total of 4,858 men were diagnosed with prostate cancer during the 18-year study period. Men taking ≥ 2 adult-strength aspirin tablets a week had a 10% lower risk of prostate cancer (p-for-trend=0.02). For regionally advanced cancer, we observed no significant associations with aspirin use. For high-grade and lethal disease, men taking ≥ 6 adult-strength tablets/week experienced similar reductions in risk (HR=0.72 (95% CI: 0.54, 0.96) and HR=0.71 (95% CI: 0.50, 1.00)). Analytical approaches to address bias from more frequent PSA screening among aspirin users did not yield different conclusions. We observed reductions in the risk of high-grade and lethal prostate cancer associated with higher doses of aspirin, but not with greater frequency or duration, in a large, prospective cohort of health professionals. Our data support earlier observations of modest inverse associations with advanced prostate cancer.
INTRODUCTION
Experimental and animal studies suggest aspirin may act as a chemoprevention agent in prostate cancer 1–6. Results from epidemiological studies are less convincing although meta-analyses show modest reductions (8–10%) in prostate cancer risk 7, 8 with more consistent benefits for daily, adult-strength (≥ 325 mg) aspirin of longer durations 9–11. The epidemiological evidence is stronger for aggressive disease, where daily aspirin use is associated with an approximate one-third lower risk of advanced prostate cancer 8, 12–14.
The potential chemopreventive effects of aspirin and other non-steroidal anti-inflammatory drugs (NSAID’s) may arise from the inhibition of cyclooxygenase (COX) enzymes - COX-1 and COX-2 - and the COX-mediated conversion of arachidonic acid (AA) to inflammatory prostaglandins, which are involved in tumor growth and progression through increased cell proliferation, angiogenesis, invasiveness and motility, and decreased natural killer cell activity and immune surveillance 15. Aspirin and non-aspirin NSAID’s may also exhibit anti-tumorigenic and antioxidant properties that are independent of the COX-pathway 16, 17, including cell cycle inhibition 18, and induction of apoptosis 19 and the NSAID-activated gene 1 20.
We previously examined aspirin use in relation to prostate cancer in the Health Professionals Follow-Up Study (HPFS) with follow-up from 1986–1996, and noted no overall relationship but a suggestive inverse association for men with metastatic prostate cancer 21. We now extend the follow-up to 2006 (increasing the number of total cases from 2479 to 4858), and provide a more detailed assessment of aspirin frequency, dose and duration, and a careful consideration of the influence of PSA screening on the risk of total, high-grade, regionally advanced and lethal prostate cancer in this cohort.
MATERIAL AND METHODS
Study Population
Participants were members of the Health Professionals Follow-up Study (HPFS), a cohort of 51,529 US male dentists, optometrists, osteopaths, podiatrists, pharmacists, and veterinarians, who returned a mailed health questionnaire in 1986. Participants were 40–75 years of age at baseline and the questionnaire included a validated assessment of diet 22 and medical diagnoses, including cancer. Questionnaires are mailed biennially to update anthropometric, physical activity, smoking, medication, vitamin, diet (collected every four years) and other lifestyle factors, and to identify new cases of disease, including prostate cancer. The follow-up rate exceeds 90%. The conduct of this cohort study and these analyses were approved by the Human Subjects Committee of the Harvard School of Public Health.
Ascertainment of Cases
We reviewed the medical records and pathology reports of men who reported prostate cancer on each biennial questionnaire. For all cases, medical charts were abstracted to determine clinical data, including Gleason score, tumor-node-metastasis (TNM) stage 23 and prostate-specific antigen (PSA) levels at the time of diagnosis. Development of bony metastases was ascertained through mailed questionnaires to consenting participants and their treating physicians and was based on the date of a positive bone scan or the date confirmed by the patient’s physician. Deaths were identified through the National Death Index, postal system and next of kin, with virtually complete follow-up 24. A prostate cancer death was based on evidence of extensive metastatic disease and no other plausible cause of death and was determined by a study physician through medical record review.
Assessment of Aspirin Use
Information about aspirin use was collected biennially through mailed questionnaires starting with the questionnaire in 1986 in which men were asked if they currently used aspirin (eg, Anacin, Bufferin, Alka-Seltzer) two or more times per week. Individual medications or brand names were not ascertained. This information was updated every two years. Additional information on frequency and quantity were collected starting in 1992, when men were asked about the number of adult-strength tablets consumed per day or per week (in categories; men were asked to convert 4 baby aspirin into 1 full-strength tablet). In 1993, a supplementary questionnaire was sent to a sample of 211 regular aspirin users to ascertain reasons for aspirin use between 1986 and 1990 (88% response). The major reasons (non-mutually exclusive) for use were cardiovascular disease, 25.4%; to decrease risk for cardiovascular disease, 58.4%; headaches, 25.4%; joint or musculoskeletal pain, 33.0%; and other reasons, 7.0% 25.
Men reported use of aspirin and other NSAID’s only if they consumed these medications at least two times a week. As a result, non-users in any given cycle were defined as those using medications less than two times a week, which is consistent with previous analyses of this cohort 21, 25, 26. We also evaluated short-term associations by assessing risk in current and formers users. Men were classified as current users if they reported use in the previous two years, former users if they were non-users in that period but reported previous use, and non-users if they reported no aspirin use or aspirin use less than 2 days a week since the initiation of the study. Although our main results focus on the most recent questionnaire for frequency and quantity, we also assessed the cumulative average of aspirin use for all previous questionnaires up until the time of diagnosis. In any particular cycle, if a man reported aspirin use but did not report a frequency or quantity, his use was captured for analyses of duration but his frequency and quantity for that period were assigned to missing and therefore not incorporated in the respective analyses.
Collection of covariates
The baseline and biennial questionnaires included updated information on demographic, diet, smoking and other lifestyle factors in the cohort. At baseline, demographic information such as weight and height were collected and weight was updated every two years. Information on physical activity was also collected every two years and MET-hours per week was calculated from a list of activities reported in 1986 27. One met-hour is the metabolic expenditure of sitting at rest for one hour. Family history of prostate cancer was based on the 1990 and 1996 questionnaires when participants were asked if they had a father or any biological brothers diagnosed with prostate cancer. Tobacco use, current smoking status, duration and cigarettes per day were collected biennially. Dietary information was collected from a semi-quantitative food-frequency questionnaire, which was updated every four years; the methodology, validation and details are described in detail elsewhere 22, 28. History of prostate-specific antigen (PSA) testing was first asked in 1994 and was updated biennially, with participants being asked if a test had been taken for symptoms or for screening in the prior two years.
Statistical Analysis
At baseline, we excluded men with a prior history of cancer (except non-melanoma skin cancer) and those who died or reported implausible dietary data (outside the range of 800–4200 kcal per day). We evaluated aspirin use two cycles prior to diagnosis (two-year lag) to avoid a potential bias from more frequent use in undiagnosed cases close to the time of diagnosis. A total of 47,271 men accrued follow-up time starting on the month of the 1988 questionnaire return date and ending on the month of date of diagnosis for cases, date of death from other causes for non-cases or January 1, 2006, whichever came first. During follow-up, we excluded cases with a missing date of diagnosis and follow-up time for participants who did not provide information about aspirin use. We calculated duration of aspirin use as the time between return dates of the questionnaires. We focused on simple, updated measurements using the most recent cycle (with the two-year lag) for aspirin frequency, in days per week and quantity, in tablets per week.
Cox proportional hazards regression was used to calculate the hazard ratio (HR) and 95% confidence intervals (CI) while adjusting for age (1-month time intervals), time period (2-year intervals), established risk factors (race and family history) and other covariates shown to be associated with incidence or mortality in HPFS 28: height (<66, 66–67.9, 68–69.9, 70–71.9, 72+ inches), body mass index (<21.0, 21–22.9, 23–24.9, 25–27.4, 27.5–29.9, 30+ kg/m2), smoking (never smoker or quit >10 years, current smoker or quit ≤10 years and <15 cigarettes/day, current smoker or quit ≤10 years and ≥15 cigarettes/day), intake of tomato sauce (<0.25, 0.25–1, 1–2, 2+ servings/week), vitamin D (quintiles), total kilocalories (quintiles, kcal per day), fish (<2/month, 2/month–1/week, >1–<3/week, 3+/week), red meat (quintiles, servings per week), vigorous physical activity (quintiles, hours) and the use of statins (yes/no current user). Covariates were updated every two or four years. Adjusting for a history of myocardial infarction and diabetes, both associated with aspirin use and PSA screening in our cohort, did not change the estimates and were not included in the final models. Tests for linear trend were conducted by assigning the median value in each category of aspirin use (p<0.05).
We assessed the hazard ratio associated with aspirin use and four prostate cancer endpoints, including total, high-grade (Gleason 8–10), regionally advanced (T3b-T4 or N1 and M0) and lethal disease (M1 at diagnosis or development of bony metastases and/or fatal disease during follow-up). The heterogeneity of prostate cancer includes latent forms that remain clinically dormant and more aggressive forms that progress to metastatic and fatal disease. Studies on aspirin 7, 8 and other putative risk factors show varying effects for total and advanced disease and suggest different etiological pathways for different prostate cancer endpoints 28. To evaluate the potential influence of aspirin on these different pathways, we analyzed risks associated with overall prostate cancer and three aggressive sub-types of the disease.
We conducted various sensitivity analyses to address potential confounding due to higher PSA screening rates among aspirin users in our cohort. First, we excluded PSA screen-detected disease (T1c) from overall prostate cancer to address an inflation bias that might arise because aspirin users are more likely to undergo PSA screening than non-users. In another sub-analysis, we restricted the population to men who had ever received a PSA test to reduce detection bias and ensure a similar screening history in cases and non-cases. In a third analysis, we adjusted for PSA screening in the prior 2-year cycle (not the most recent cycle, in which cases would have a higher probability of PSA testing that led to diagnosis). In a fourth sensitivity analysis, we restricted our analysis to men who were screened by PSA in the same 2-year cycle and excluded men who were not screened in that interval to ensure equal opportunities for a prostate cancer diagnosis in any given two-year cycle.
We evaluated whether the association of aspirin on prostate cancer risk varied according to age at diagnosis, body mass index (BMI), fish and red meat intake. Men diagnosed at younger ages tend to have a family history of prostate cancer and exhibit more aggressive forms of the disease, and the influence of aspirin’s anti-inflammatory actions may vary according to age. The low-grade inflammatory state of overweight and/or obesity may modulate the biological efficacy of aspirin and evidence from other sites (eg, endometrial cancer and colorectal adenoma) suggests that the impact of aspirin on risk varies by BMI 29, 30. Fish is inversely 31, 32 and red meat is positively associated with prostate cancer in many but not all studies 33, and both fatty fish and red meat intake are important dietary sources influencing the arachidonic acid pathway for COX-1 and COX-2 enzymatic activity. We used the Wald statistic and likelihood ratio test to determine statistical significance at the p<0.05 level.
RESULTS
Over an 18-year follow-up period (727,061 person-years), 4,858 men were diagnosed with incident prostate cancer. At the start of the follow-up (1988), 31.3% of men took aspirin at least twice a week; these men were older, heavier, more likely to smoke, less often engaged in vigorous physical activity and more frequently had a history of myocardial infarction, arthritis or diabetes than non-users (data not shown; p < .01). At the mid-point of the follow-up period (1996), 43.0% of the men took aspirin at least twice a week and these men though older and heavier, were engaged in more vigorous physical activity, less likely to smoke and more often reported a routine physical, digital rectal exam and prostate-specific antigen test in the previous two years (p<.0001) than non-users (Table I).
Table I.
Demographic and other characteristics of aspirin users and non-users in 1996 (mid-point) in the Health Professionals Follow-up Cohort (HPFS), 1988–2006.
| Regular users (≥ 2 days/wk) (n=18,570) |
Non-regular users (< 2 days/wk) (n=24,494) |
||
|---|---|---|---|
| % (N) or age-adj. mean (SE) | % (N) or age-adj. mean (SE) | p-value† | |
|
| |||
| Age, yrs (SD) | 64.8 (9.2) | 62.9 (9.5) | <.0001 |
| BMI, kg/m2 | 26.1 (.03) | 26.0 (.02) | 0.21 |
| missing | 8 (0.04) | 18 (0.07) | |
| Recent history of: | |||
| PSA test | 75.5 (14024) | 49.1 (12032) | <.0001 |
| Digital rectal exam | 76.6 (14214) | 51.9 (12722) | <.0001 |
| General physical exam | 85.5 (15883) | 58.4 (14295) | <.0001 |
| Family history of prostate cancer | |||
| Yes | 5.6 (1040) | 5.4 (1329) | 0.43 |
| No | 94.4 (17529) | 94.9 (23,165) | |
| Smoking status | |||
| Never‡ | 46.1 (8568) | 50.7 (12423) | |
| Past | 48.5 (9002) | 42.1 (10320) | <.0001 |
| Current | 5.4 (1000) | 7.2 (1751) | <.0001 |
| Vigorous physical activity, METS*/wk | 13.72 (0.19) | 13.71 (0.16) | 0.03 |
| Red meat, servings/wk** | 0.50 (.004) | 0.44 (.003) | <.0001 |
| Fish intake, servings/wk** | 0.30 (.002) | 0.25 (.002) | <.0001 |
| Total calories, kcal/day** | 2017 (4.87) | 2013 (4.6) | 0.97 |
| Medical history of: | |||
| Myocardial Infarction | 13.1 (2433) | 5.1 (1241) | <.0001 |
| Rheumatoid & other arthritis | 16.1 (2998) | 12.7 (3116) | <.0001 |
| Diabetes | 7.5 (1394) | 6.0 (1470) | <.0001 |
F-test for analysis of variance adjusted for age at diagnosis
Includes n=3329 non-users with unknown past use
MET=metabolic equivalent task
Based on 1994 questionnaire (diet collected every 4 years)
Total prostate cancer
We observed a 10% lower risk of overall prostate cancer for men who consumed at least two adult-strength aspirin tablets a week (HR=0.90 for 2–5 tablets/week; 95% CI: 0.80, 1.02 and HR=0.90 for ≥ 6 tabs/week; 95% CI: 0.83, 0.99) after multivariate adjustment (Table II). We found no material difference in risk for higher levels of frequency (HR = 0.94 for 6–7 days/week; 95% CI: 0.87, 1.02), duration (HR = 0.99 for ≥ 10 years; 95% CI: 0.87, 1.12) or recency (current and former) of use (HR = 0.98 for current; 95% CI: 0.91, 1.05). To address a potential detection bias due to increased PSA surveillance among aspirin users, we excluded cases detected by PSA screening (T1c) and observed no significant dose-response trends, although the magnitude of associations remained similar (Table II).
Table II.
Patterns of aspirin use * and the risk of total prostate cancer (n=4858) in the Health Professionals Follow-up Study, 1988–2006
| Aspirin use | Cases | P-yrs | All cases (n=4858) | Excluding T1c disease (n=3140) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age-adjusted hazard ratios (HR) | Multivariate** | Multivariate** | |||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||||||
| Frequency of use, †(days/week) | |||||||||||
| < 2 days/wk | 1844 | 237,834 | 1 .00 (referent) | 1 .00 (referent) | 1 .00 (referent) | ||||||
| 2–3 days/wk | 282 | 36,937 | 0.94 | 0.83 | 1.07 | 0.92 | 0.81 | 1.04 | 0.92 | 0.77 | 1.08 |
| 4–5 days/wk | 179 | 22,610 | 0.97 | 0.83 | 1.13 | 0.94 | 0.80 | 1.10 | 0.82 | 0.66 | 1.02 |
| 6–7 days/wk | 1045 | 116,560 | 0.96 | 0.89 | 1.03 | 0.94 | 0.87 | 1.02 | 0.92 | 0.83 | 1.02 |
| p for trend = 0.15 | p for trend = 0.09 | ||||||||||
| Quantity of use, † (tablets/week) | |||||||||||
| Non-user | 1844 | 237,834 | 1 .00 (referent) | 1 .00 (referent) | 1 .00 (referent) | ||||||
| <2 tabs/week | 441 | 46,462 | 1.03 | 0.93 | 1.14 | 1.02 | 0.92 | 1.13 | 0.99 | 0.86 | 1.13 |
| 2–5 tabs/wk | 328 | 42,251 | 0.93 | 0.83 | 1.05 | 0.90 | 0.80 | 1.02 | 0.81 | 0.68 | 0.95 |
| 6+ tabs/wk | 700 | 83,171 | 0.93 | 0.85 | 1.01 | 0.90 | 0.83 | 0.99 | 0.91 | 0.81 | 1.03 |
| p for trend = 0.02 | p for trend = 0.10 | ||||||||||
| Duration of use, (years) | |||||||||||
| Non-user‡ | 1600 | 286,418 | 1 .00 (referent) | 1 .00 (referent) | 1 .00 (referent) | ||||||
| < 5 years | 1772 | 244,346 | 1.00 | 0.93 | 1.07 | 1.01 | 0.94 | 1.08 | 1.01 | 0.93 | 1.10 |
| 5–9.9 years | 915 | 103,354 | 0.99 | 0.91 | 1.08 | 0.98 | 0.89 | 1.07 | 0.89 | 0.79 | 1.00 |
| 10+ years | 416 | 43,106 | 1.02 | 0.90 | 1.15 | 0.99 | 0.87 | 1.12 | 0.95 | 0.81 | 1.11 |
| p for trend = 0.67 | p for trend = 0.09 | ||||||||||
| Current & Former use | |||||||||||
| Non-user‡ | 1599 | 286,034 | 1 .00 (referent) | 1 .00 (referent) | 1 .00 (referent) | ||||||
| Former | 1099 | 135,375 | 1.02 | 0.94 | 1.11 | 1.04 | 0.96 | 1.13 | 1.04 | 0.94 | 1.15 |
| Current | 2004 | 255,430 | 0.99 | 0.92 | 1.06 | 0.98 | 0.91 | 1.05 | 0.95 | 0.87 | 1.03 |
updated in each cycle
adjusted for age (months), period (2-year intervals), family history (yes/no), race (Asian, Caucasian or African-American), height (<66, 66–67.9, 68–69.9, 70–71.9, 72+ inches), BMI (<21, 21–22.9, 23–24.9, 25–27.4, 27.5–29.9, 30+ kg/m2), tomato sauce (<0.25, 0.25 – <1, 1 –<2, 2+/wk), vigorous physical activity (quintiles, hours), smoking (never or quit>10 yrs, current or quit < 10 yrs & < 15 cigs/day, current or quit < 10 yrs & > 15 cigs/day), vitamin D (quintiles, ), fish (<2/mo, 2/mo-1/wk, > 1– <3/wk, 3+/wk), red meat (quintiles), cholesterol-lowering drugs (non-user, current user) & total kcal (quintiles)
Based on a sub-cohort of men starting in 1992 when more detailed data on days/week and tablets/week were available (total N= 3488, excluding T1c N= 2076)
Non-users in every 2-year cycle
Quantity of use is number of adult-strength aspirin tablets per week (baby aspirin tablets were converted to adult-strength equivalents).
High-grade prostate cancer
For high-grade prostate cancer (Gleason 8–10), modest decreases in risk were observed for most measurements of aspirin use and a significant dose-relationship emerged for tablets per week with the heaviest users (≥ 6/week) experiencing a 28% decreased risk of high-grade disease compared to non-users (95% CI: 0.54, 0.96; Table III). High-grade prostate cancer risk was 31% lower among aspirin users of up to 5 years (95% CI: 0.55, 0.86) yet there was no further risk reduction for additional years of use (p-for-trend = 0.25). Similarly, no dose-response patterns emerged for frequency of use in days per week (p-for-trend=0.11) or for combinations of quantity and duration (p-for-trend=0.81, data not shown).
Table III.
Patterns of aspirin use * and the risk of high-grade, regionally advanced and lethal prostate cancer in the Health Professionals Follow-up Study, 1988–2006
| High-grade prostate cancer (Gleason 8–10, n=488) | Regionally advanced prostate cancer (T3b-T4 or N1 and M0, n=228) | Lethal prostate cancer (M1 or PCadeath, n=580) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Multivariate HR** | 95% CI | Multivariate HR** | 95% CI | Multivariate HR** | 95% CI | |||||||
| Frequency of use, † (days/week) | ||||||||||||
| < 2 days/wk | 187 | 1 .00 (reference) | 62 | 1 .00 (reference) | 151 | 1 .00 (reference) | ||||||
| 2–3 days/wk | 28 | 0.84 | 0.56 | 1.26 | 9 | 0.81 | 0.40 | 1.65 | 19 | 0.73 | 0.45 | 1.19 |
| 4–5 days/wk | 18 | 0.87 | 0.53 | 1.43 | 8 | 1.32 | 0.62 | 2.80 | 10 | 0.63 | 0.33 | 1.20 |
| 6–7 days/wk | 102 | 0.81 | 0.63 | 1.05 | 43 | 1.31 | 0.87 | 1.97 | 77 | 0.86 | 0.64 | 1.14 |
| p for trend = 0.11 | p for trend = 0.18 | p for trend = 0.21 | ||||||||||
| Quantity of use, † (tablets/week) | ||||||||||||
| Non-user | 187 | 1 .00 (reference) | 62 | 1 .00 (reference) | 151 | 1 .00 (reference) | ||||||
| <2 tabs/week | 48 | 1.02 | 0.74 | 1.42 | 16 | 1.27 | 0.72 | 2.23 | 33 | 0.97 | 0.65 | 1.42 |
| 2–5 tabs/wk | 31 | 0.77 | 0.52 | 1.14 | 12 | 1.01 | 0.54 | 1.89 | 26 | 0.80 | 0.52 | 1.23 |
| 6+ tabs/wk | 64 | 0.72 | 0.54 | 0.96 | 31 | 1.27 | 0.81 | 1.99 | 45 | 0.71 | 0.50 | 1.00 |
| p for trend = 0.02 | p for trend = 0.38 | p for trend = 0.04 | ||||||||||
| Duration of use, (years) | ||||||||||||
| Non-user‡ | 193 | 1 .00 (reference) | 101 | 1 .00 (reference) | 254 | 1 .00 (reference) | ||||||
| < 5 years | 148 | 0.69 | 0.55 | 0.86 | 77 | 0.78 | 0.57 | 1.06 | 212 | 0.84 | 0.70 | 1.02 |
| 5–9.9 years | 90 | 0.75 | 0.57 | 0.99 | 31 | 0.78 | 0.50 | 1.22 | 63 | 0.75 | 0.55 | 1.03 |
| 10+ years | 49 | 0.85 | 0.58 | 1.23 | 15 | 0.88 | 0.47 | 1.65 | 31 | 1.40 | 0.89 | 2.19 |
| p for trend = 0.25 | p for trend = 0.32 | p for trend = 0.70 | ||||||||||
| Current & Former use | ||||||||||||
| Non-user‡ | 193 | 1 .00 (reference) | 101 | 1 .00 (reference) | 253 | 1 .00 (reference) | ||||||
| Former | 90 | 0.69 | 0.53 | 0.90 | 29 | 0.59 | 0.38 | 0.91 | 94 | 0.86 | 0.67 | 1.11 |
| Current | 197 | 0.74 | 0.59 | 0.91 | 94 | 0.87 | 0.64 | 1.18 | 212 | 0.84 | 0.69 | 1.02 |
updated in each cycle
adjusted for age (months), period (2-year intervals), family history (yes/no), race (Asian, Caucasian or African-American), height (<66, 66–67.9, 68–69.9, 70–71.9, 72+ inches), BMI (<21, 21–22.9, 23–24.9, 25–27.4, 27.5–29.9, 30+ kg/m2), tomato sauce (<0.25, 0.25 – <1, 1 –<2, 2+/wk), vigorous physical activity (quintiles, hours), smoking (never or quit>10 yrs, current or quit < 10 yrs & < 15 cigs/day, current or quit < 10 yrs & > 15 cigs/day), vitamin D (quintiles, ), fish (<2/mo, 2/mo-1/wk, > 1– <3/wk, 3+/wk), red meat (quintiles), cholesterol-lowering drugs (non-user, current user) & total kcal (quintiles)
Based on a sub-cohort of men starting in 1992 when more detailed data on days/week and tablets/week were available (high-grade N= 343, regionally adv N= 125 and lethal N= 272)
Non-users in every 2-year cycle
Quantity of use is number of adult-strength aspirin tablets per week (baby aspirin tablets were converted to adult-strength equivalents)
Regionally advanced prostate cancer
For regionally advanced disease (T3b-T4 or N1 and M0), there was no overall dose-response association for frequency, quantity or duration of aspirin use (Table III). Former use of aspirin was associated with a 41% reduced risk of regionally advanced disease (95% CI: 0.38, 0.91) whereas current use was not (HR=0.87, 95% CI: 0.64, 1.18). For tablets per week, we observed non-significant elevated risks of regionally advanced prostate cancer and no dose-response trend (Table III).
Lethal prostate cancer
For lethal prostate cancer (M1 at diagnosis or the development of bony metastases or fatal prostate cancer during follow-up), we observed a significant inverse trend (p = 0.04) for increasing number of tablets per week whereby men who consumed less than two adult-strength tablets per week (equivalent to one baby aspirin daily) had a non-significant 3% lower risk of lethal prostate cancer (95% CI: 0.65, 1.42) that further decreased to 20% for 2–5 tablets a week (95% CI: 0.52, 1.23) and 29% (95% CI: 0.50, 1.00) for 6 or more tablets per week, when compared to non-users. For frequency in days per week, risks decreased by 14–37% (2–3 days/week, HR = 0.73, 95% CI: 0.45, 1.19; 4–5 days/week, HR = 0.63, 95% CI: 0.33, 1.20; 6–7 days/week, HR=0.86, 95% CI: 0.64, 1.14) but the estimates did not reach statistical significance and there was no suggestion of a dose-response trend (p = 0.21). Similarly, longer durations or combinations of quantity and duration (p-for-trend=0.26, data not shown) did not significantly reduce a man’s risk of developing lethal disease.
Interaction by age, BMI, diet
We assessed if the association between aspirin and prostate cancer differed by age at diagnosis, body mass index, fish and red meat intake. We found that the association of aspirin use on total disease differed by age (p-for-interaction = 0.005) such that older men (> 65 years) experienced reduced risks (< 2 tabs/week: HR = 0.97 (95% CI: 0.86, 1.10), 2–5 tabs/week: HR = 0.85 (95% CI: 0.73, 0.98), and ≥ 6 tabs/week: HR = 0.85 (95% CI: 0.76, 0.94), p-for-trend = 0.001) whereas men 65 years and younger did not (< 2 tabs/week: HR = 1.16 (95% CI: 0.94, 1.43), 2–5 tabs/week: HR = 1.08 (95% CI: 0.87, 1.34) and ≥ 6 tabs/week: HR = 1.09 (0.91, 1.29), p-for-trend = 0.41). A similar pattern emerged for total disease after excluding T1c cases. We also observed stronger inverse dose-response trends for aspirin tablets per week in men over the age of 65 years for high-grade (p-for-trend = 0.02) and lethal disease (p-for-trend = 0.05) than in younger men although the test for interaction was not statistically significant for these outcomes. We did not find effect modification by body mass index, red meat or fish intake.
Sensitivity analyses to address potential PSA screening bias
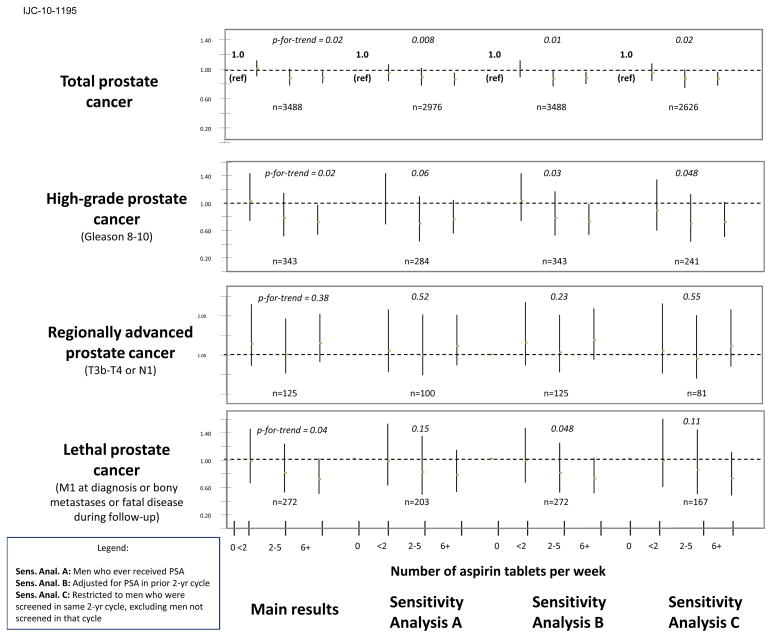
We used several approaches to address a potential bias due to different PSA screening practices of aspirin users and non-users during the follow-up period; Figure I summarizes the results obtained from each of these approaches (A–C) for total, high-grade, regionally advanced and lethal disease. In one sub-analysis (A), we restricted the population to men who had ever received a PSA test (n = 43,932 since 1994 when this question was first asked) to ensure a similar opportunity for screening, and found essentially the same results for total (significant p-for-trend = 0.008), high-grade (borderline significant inverse, p = 0.06) and regionally advanced disease (null, p = 0.52). For lethal prostate cancer, the pattern remained the same but due to smaller numbers, the estimates were non-significant (p = 0.15; Figure I). In a second analysis (B), we adjusted for PSA screening in the prior 2-year cycle (excluding the most recent two years when cases are much more likely to have had a PSA than non-cases) and the overall set of results did not change (total, p = 0.01; high-grade, p = 0.03; regionally advanced, p = 0.23 and lethal, p = 0.048). Finally, when we restricted our analysis to men who were screened by PSA in the same 2-year cycle (C - excluding men who were not screened in that interval), the overall associations and patterns remained the same, with changes in significance level due to smaller numbers (total, p = 0.02; high-grade, p = 0.048; regionally advanced, p = 0.55; lethal, p = 0.11).
Figure 1. Sensitivity analyses (A–C) to address potential PSA screening bias.
Sens. Anal. A: Men who ever received PSA
Sens. Anal. B: Adjusted for PSA in prior 2-yr cycle
Sens. Anal. C: Restricted to men who were screened in same 2-yr cycle, excluding men not screened in that cycle
DISCUSSION
We observed a 10% reduced risk of prostate cancer associated with the consumption of at least two adult-strength aspirin tablets per week that was further reduced in men over the age of 65 years (15%). We also observed significant decreases in high-grade (28%) and lethal (29%) prostate cancer risk with dose-response associations for number of tablets per week. A recent international consensus statement stated that aspirin has the potential for chemoprevention given its cardiovascular benefit and known safety profile, and that more studies are needed to define the optimal dose, duration, frequency and sub-populations with maximum benefit34. We used detailed data on aspirin over an 18-year follow-up period to address some of these considerations.
Previous findings
Our results on aspirin use are in line with several studies and two meta-analyses 7, 8 estimating an 8–10% decrease in total prostate cancer risk, a 27–36% decrease in advanced prostate cancer risk 12–14, 21 and stronger inverse associations (for NSAID use) in older men 7, 8, 35, 36. Recent data suggest that aspirin use may lower PSA levels and delay detection of disease 38, which might result in poor long-term outcomes for aspirin use. However we found a significantly reduced risk of developing metastatic or fatal prostate cancer among aspirin users.
Sensitivity analyses to address potential PSA screening bias
We utilized multiple approaches to address potential confounding due to PSA screening (Figure I) but found little evidence of a strong bias. If PSA screening was inducing a detection bias whereby aspirin users who undergo more frequent exams were less likely to have worse disease, one would expect stronger inverse associations in men diagnosed with regionally advanced (T3b-T4 or N1) or positive associations for low-grade or organ-confined disease since these cancers would be over-sampled by PSA screening. However, this was not the case in our data (adult-strength tablets per week: low-grade disease (n=2497)- HR’s = 1.03 (0.91, 1.17), 0.97 (0.84, 1.11) and 0.93 (0.84, 1.03); organ-confined disease (n=2451)- HR’s = 1.03 (0.91, 1.17), 0.90 (0.78, 1.04) and 0.92 (0.83, 1.02); data not shown). Finally, recent results from large, randomized trials 39, 40 suggest that PSA screening may only moderately reduce prostate cancer mortality, and therefore, may have limited potential to confound the risk of lethal disease.
Sub-analyses on grade vs. stage, dose vs. duration, short-term vs. long-term
High-grade and advanced stage prostate cancer may represent heterogeneous etiologies 28 and the mechanism by which aspirin acts on the initiation of poorly-differentiated disease may be distinct from its influence on the development of advanced disease. To tease out the associations on grade and stage, we excluded men with extra-prostatic disease from the group of high-grade cases and men with high-grade tumors from the group who developed lethal disease; in both instances, inverse associations remained but were non-significant (6+ tabs/week: organ-confined, high-grade disease: HR=0.82 (0.57,1.16) and low-grade, lethal disease: HR=0.80 (0.53,1.18) suggesting that aspirin’s apparent influence on high-grade and lethal prostate cancer is only partially captured through stage or histologic grade. However, grade is measured imperfectly and may impede our ability to separate grade and stage 41. For combinations of quantity and duration, no clear dose-response trends emerged and when we evaluated men who consumed on average, almost one adult-strength aspirin/day (6+ tablets/week) for more than 4 years, we found no significant association (HR = 0.96; 95% CI: 0.87,1.06). Although we observed the most consistent and robust dose-response associations for aspirin use in relation to lethal disease, residual confounding due to unmeasured factors associated with aspirin use and fatal prostate cancer may account for part of our observations, although we adjusted for the strongest predictors of prostate cancer mortality in this cohort. A potential bias arising from the association between aspirin use and cardiovascular disease (CVD) mortality was addressed first by excluding men with a history of CVD at baseline, second by evaluating effect modification of CVD (p>0.05), third by stratifying on CVD history (no change in results) and fourth, by adjusting for a history of myocardial infarction, diabetes and stroke (no change in results). An alternative explanation is that aspirin use before diagnosis is a surrogate for aspirin patterns after diagnosis and perhaps the post-diagnostic time period is also influential on prostate cancer progression and survival.
Strengths and limitations
One potential limitation of our study is the generalizability of findings from a cohort of health professionals that is not racially diverse (89.8% white, 5.0% other) yet aspirin use and screening patterns in our study population do not materially differ from the general population 13. In 2000, 60% of men in HPFS reported a PSA in the previous 24 months whereas in the general population, 57% of U.S. men aged 50 or older reported a PSA in the previous 12 months in 2001. Although our data suggest that adult-strength (≥ 325 mg) tablets offer the greatest benefit for reducing prostate cancer risk, we did not have detailed information on dose (not collected before 2000) to confirm whether or not lower dose tablets confer the same benefit.
Another limitation is that aspirin use was significantly associated with confounders related to diet, detection of disease, other diseases (eg, myocardial infarction), medications (eg, statins) and lifestyle factors, such as physical activity and smoking (Table I), so there may be residual confounding in the associations. However, we have detailed, updated biennial data on potential confounders (eg, BMI, fish, red meat, physical activity, cigarette smoking, etc.) and when we adjusted for these covariates in our models, the multivariate estimates did not materially differ from the age-adjusted estimates (Table II). Finally, men who took aspirin were more likely to take other medications, such as statins and other NSAID’s; we excluded men who took statins (n = 14,648), and still found significantly reduced risks for the heaviest aspirin users (≥ 6 tablets/week, 33% for high-grade and 40% for lethal disease) and dose-response trends (high-grade disease, p-for-trend = 0.03; lethal disease, p-for-trend = 0.01; data not shown). We did not have adequate data on frequency, quantity or dose of non-aspirin (NA) NSAID’s to separately assess their effects on prostate cancer risk. Although aspirin accounted for the majority of NSAID use in this cohort (84% at baseline), we excluded non-aspirin NSAID use to reduce the likelihood of over-estimating aspirin effects and the magnitude of associations remained the same (for ≥ 6+ tabs/week: HRs=0.89, 0.73 and 0.75 for total, high-grade and lethal disease; data not shown).
Conclusions
We observed modest risk reductions and significant dose-response trends for high-grade and lethal disease in an 18-year prospective cohort study with updated, biennial information on aspirin use. Our data add to increasing evidence that suggests a potential modest benefit of aspirin in reducing the risk of aggressive prostate cancer, in particular for high-grade and lethal disease.
Acknowledgments
We thank Ms. Jill Arnold, Ms. Stacey De Caro, Ms. Elizabeth Frost-Hawes, Ms. Mira Kaufman, Ms. Siobhan Saint Surin, Ms. Laura Sampson, Ms. Barbara Vericker, Ms. Christine Iacconne and Ms. Olga Veysman for their continuing help in the Health Professionals Follow-Up Study.
References
- 1.Gupta S, Adhami VM, Subbarayan M, MacLennan GT, Lewin JS, Hafeli UO, Fu P, Mukhtar H. Suppression of prostate carcinogenesis by dietary supplementation of celecoxib in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2004;64:3334–43. doi: 10.1158/0008-5472.can-03-2422. [DOI] [PubMed] [Google Scholar]
- 2.Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397–403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- 3.Hussain T, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett. 2003;191:125–35. doi: 10.1016/s0304-3835(02)00524-4. [DOI] [PubMed] [Google Scholar]
- 4.Lim JT, Piazza GA, Han EK, Delohery TM, Li H, Finn TS, Buttyan R, Yamamoto H, Sperl GJ, Brendel K, Gross PH, Pamukcu R, et al. Sulindac derivatives inhibit growth and induce apoptosis in human prostate cancer cell lines. Biochem Pharmacol. 1999;58:1097–107. doi: 10.1016/s0006-2952(99)00200-2. [DOI] [PubMed] [Google Scholar]
- 5.Rotem R, Tzivony Y, Flescher E. Contrasting effects of aspirin on prostate cancer cells: suppression of proliferation and induction of drug resistance. Prostate. 2000;42:172–80. doi: 10.1002/(sici)1097-0045(20000215)42:3<172::aid-pros2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Zhu W, Smith A, Young CY. A nonsteroidal anti-inflammatory drug, flufenamic acid, inhibits the expression of the androgen receptor in LNCaP cells. Endocrinology. 1999;140:5451–4. doi: 10.1210/endo.140.11.7246. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Perez A, Garcia Rodriguez LA, Lopez-Ridaura R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: a meta-analysis. BMC Cancer. 2003;3:28. doi: 10.1186/1471-2407-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahmud S, Franco E, Aprikian A. Prostate cancer and use of nonsteroidal anti-inflammatory drugs: systematic review and meta-analysis. Br J Cancer. 2004;90:93–9. doi: 10.1038/sj.bjc.6601416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia Rodriguez LA, Gonzalez-Perez A. Inverse association between nonsteroidal anti-inflammatory drugs and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:649–53. [PubMed] [Google Scholar]
- 10.Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J Natl Cancer Inst. 2007;99:608–15. doi: 10.1093/jnci/djk132. [DOI] [PubMed] [Google Scholar]
- 11.Perron L, Bairati I, Moore L, Meyer F. Dosage, duration and timing of nonsteroidal antiinflammatory drug use and risk of prostate cancer. Int J Cancer. 2003;106:409–15. doi: 10.1002/ijc.11250. [DOI] [PubMed] [Google Scholar]
- 12.Habel LA, Zhao W, Stanford JL. Daily aspirin use and prostate cancer risk in a large, multiracial cohort in the US. Cancer Causes Control. 2002;13:427–34. doi: 10.1023/a:1015788502099. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs EJ, Rodriguez C, Mondul AM, Connell CJ, Henley SJ, Calle EE, Thun MJ. A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J Natl Cancer Inst. 2005;97:975–80. doi: 10.1093/jnci/dji173. [DOI] [PubMed] [Google Scholar]
- 14.Norrish AE, Jackson RT, McRae CU. Non-steroidal anti-inflammatory drugs and prostate cancer progression. Int J Cancer. 1998;77:511–5. doi: 10.1002/(sici)1097-0215(19980812)77:4<511::aid-ijc6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Badawi AF. The role of prostaglandin synthesis in prostate cancer. BJU Int. 2000;85:451–62. doi: 10.1046/j.1464-410x.2000.00507.x. [DOI] [PubMed] [Google Scholar]
- 16.Betts WH, Whitehouse MW, Cleland LG, Vernon-Roberts B. In vitro antioxidant properties of potential biotransformation products of salicylate, sulphasalazine and amidopyrine. J Free Radic Biol Med. 1985;1:273–80. doi: 10.1016/0748-5514(85)90131-x. [DOI] [PubMed] [Google Scholar]
- 17.Upchurch GR, Jr, Ramdev N, Walsh MT, Loscalzo J. Prothrombotic Consequences of the Oxidation of Fibrinogen and their Inhibition by Aspirin. J Thromb Thrombolysis. 1998;5:9–14. doi: 10.1023/a:1008859729045. [DOI] [PubMed] [Google Scholar]
- 18.Maier TJ, Schilling K, Schmidt R, Geisslinger G, Grosch S. Cyclooxygenase-2 (COX-2)-dependent and -independent anticarcinogenic effects of celecoxib in human colon carcinoma cells. Biochem Pharmacol. 2004;67:1469–78. doi: 10.1016/j.bcp.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Eibl G, Reber HA, Wente MN, Hines OJ. The selective cyclooxygenase-2 inhibitor nimesulide induces apoptosis in pancreatic cancer cells independent of COX-2. Pancreas. 2003;26:33–41. doi: 10.1097/00006676-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi K, Lee SH, Eling TE, Baek SJ. Identification of nonsteroidal anti-inflammatory drug-activated gene (NAG-1) as a novel downstream target of phosphatidylinositol 3-kinase/AKT/GSK-3beta pathway. J Biol Chem. 2004;279:49617–23. doi: 10.1074/jbc.M408796200. [DOI] [PubMed] [Google Scholar]
- 21.Leitzmann MF, Stampfer MJ, Ma J, Chan JM, Colditz GA, Willett WC, Giovannucci E. Aspirin use in relation to risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:1108–11. [PubMed] [Google Scholar]
- 22.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. discussion 27–36. [DOI] [PubMed] [Google Scholar]
- 23.Schroder FH, Hermanek P, Denis L, Fair WR, Gospodarowicz MK, Pavone-Macaluso M. The TNM classification of prostate cancer. Prostate Suppl. 1992;4:129–38. doi: 10.1002/pros.2990210521. [DOI] [PubMed] [Google Scholar]
- 24.Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, Hennekens CH. Test of the National Death Index. Am J Epidemiol. 1984;119:837–9. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 25.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med. 1994;121:241–6. doi: 10.7326/0003-4819-121-4-199408150-00001. [DOI] [PubMed] [Google Scholar]
- 26.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–42. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 27.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–6. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121:1571–8. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moysich KB, Baker JA, Rodabaugh KJ, Villella JA. Regular analgesic use and risk of endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2923–8. doi: 10.1158/1055-9965.EPI-05-0457. [DOI] [PubMed] [Google Scholar]
- 30.Viswanathan AN, Feskanich D, Schernhammer ES, Hankinson SE. Aspirin, NSAID, and acetaminophen use and the risk of endometrial cancer. Cancer Res. 2008;68:2507–13. doi: 10.1158/0008-5472.CAN-07-6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mina K, Fritschi L, Johnson KC. An inverse association between preserved fish and prostate cancer: results from a population-based case-control study in Canada. Nutr Cancer. 2008;60:222–6. doi: 10.1080/01635580701684864. [DOI] [PubMed] [Google Scholar]
- 32.Pham TM, Fujino Y, Kubo T, Ide R, Tokui N, Mizoue T, Ogimoto I, Matsuda S, Yoshimura T. Fish intake and the risk of fatal prostate cancer: findings from a cohort study in Japan. Public Health Nutr. 2008:1–5. doi: 10.1017/S1368980008003182. [DOI] [PubMed] [Google Scholar]
- 33.Terry PD, Rohan TE, Wolk A. Intakes of fish and marine fatty acids and the risks of cancers of the breast and prostate and of other hormone-related cancers: a review of the epidemiologic evidence. Am J Clin Nutr. 2003;77:532–43. doi: 10.1093/ajcn/77.3.532. [DOI] [PubMed] [Google Scholar]
- 34.Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, Jankowski J, La Vecchia C, Meyskens F, Senn HJ, Thun M. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–7. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 35.Dasgupta K, Di Cesar D, Ghosn J, Rajan R, Mahmud S, Rahme E. Association between nonsteroidal anti-inflammatory drugs and prostate cancer occurrence. Cancer J. 2006;12:130–5. [PubMed] [Google Scholar]
- 36.Roberts RO, Jacobson DJ, Girman CJ, Rhodes T, Lieber MM, Jacobsen SJ. A population-based study of daily nonsteroidal anti-inflammatory drug use and prostate cancer. Mayo Clin Proc. 2002;77:219–25. doi: 10.4065/77.3.219. [DOI] [PubMed] [Google Scholar]
- 37.Mahmud SM, Tanguay S, Begin LR, Franco EL, Aprikian AG. Non-steroidal anti-inflammatory drug use and prostate cancer in a high-risk population. Eur J Cancer Prev. 2006;15:158–64. doi: 10.1097/01.cej.0000197451.02604.25. [DOI] [PubMed] [Google Scholar]
- 38.Fowke JH, Motley SS, Smith JA, Jr, Cookson MS, Concepcion R, Chang SS, Byerly S. Association of Nonsteroidal Anti-Inflammatory Drugs, Prostate Specific Antigen and Prostate Volume. J Urol. 2009 doi: 10.1016/j.juro.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andriole GL, Grubb RL, 3rd, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, Crawford ED, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 41.Stark JR, Perner S, Stampfer MJ, Sinnott JA, Finn S, Eisenstein AS, Ma J, Fiorentino M, Kurth T, Loda M, Giovannucci EL, Rubin MA, et al. Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3? J Clin Oncol. 2009;27:3459–64. doi: 10.1200/JCO.2008.20.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]