Abstract
On April 24–27, 2010, the Motivational Neuronal Networks meeting took place in Wrightsville Beach, North Carolina. The conference was devoted to “Emerging, re-emerging, and forgotten brain areas” of the reward circuit. A central feature of the conference was four scholarly discussions of cutting-edge topics related to the conference's theme. These discussions form the basis of the present review, which summarizes areas of consensus and controversy, and serves as a roadmap for the next several years of research.
Keywords: Subthalamic nucleus, Ventral pallidum, Lateral habenula, Mesopontine rostromedial tegmental nucleus, Subiculum, BNST
1 GENERAL INTRODUCTION
The fifth Motivational Neuronal Networks meeting took place on April 24–27th in Wrightsville Beach, NC. As with the four previous meetings, this event brought together scientists who study the neural circuits that govern motivated behavior, including experts in anatomy, pharmacology, neurophysiology, functional imaging, computational modeling, and animal and human behavior. The theme chosen by this year's organizers was “The Reward Circuit: Emerging, Reemerging, and Forgotten Brain Areas”, with the goal of looking beyond the handful of structures that are most commonly associated with reward and motivation. Four small group workshops were held, each one dedicated to a select set of brain regions; the participants shared findings, raised critical questions and issues, and sought consensus about the current state and future directions of the field. The compiled notes from these group discussions are presented in this review.
The first workshop focused on the subthalamic nucleus and the ventral pallidum; originally considered parts of classical basal ganglia motor circuitry, new evidence from basic and clinical studies points to their roles in reward and motivation. The second workshop addressed the unique contributions of the dorsal and ventral hippocampal formation to contextual conditioning, associative learning, drug-seeking and the pathophysiology of schizophrenia. The focus of the third workshop was the lateral habenula and the rostromedial tegmental nucleus, two structures that may powerfully influence the firing of dopamine neurons. Finally, the fourth workshop was dedicated to the extended amygdala, particularly the bed nucleus of the stria terminalis and its interactions with the lateral hypothalamus. In addition to the group discussions the conference included poster presentations, and plenary lectures by two distinguished guests: Gary Aston-Jones presented recent findings from his group on the role of orexin neurons in drug and natural reward seeking, and Martine Cador presented a historical review of the anatomy and chemoarchitecture of the reward system, given in memory of a major contributor to the field, the late Ann E. Kelley, Ph.D., a colleague and friend of many in attendance.
2 THE SUBTHALAMIC NUCLEUS AND VENTRAL PALLIDUM
2.1 The Subthalamic Nucleus
2.1.1
The subthalamic nucleus (STN) is physically and functionally situated at the interface between the basal ganglia and the limbic system and is reciprocally and topographically connected to both sets of structures [1–3]. Although this nucleus has received less scholarly attention than its neighbors, its function has recently become the subject of great interest. In particular, clinical scientists have become interested in its contributions to the control of motivated behavior and clinical pathologies such as Parkinson's disease (PD) and obsessive compulsive disorder (OCD).
Although it is clear that the STN is heavily recruited in motivated behaviors, its specific role is unknown. STN neurons are reward-sensitive [4, 5], and manipulations of STN activity affect both drug- and natural reward-seeking [6–8]. Reward magnitude modulates neuronal firing in STN, and many STN neurons fire selectively for trials involving only one of multiple rewards present in a given task [9]. High frequency stimulation of the STN (comparable to clinical deep brain stimulation, (DBS) [10, 11]) decreases conditioned place preference (CPP) for cocaine and willingness to work for cocaine in a progressive ratio task, while at the same time increasing the willingness to work for food [6]. The latter may be clinically important because patients receiving DBS therapy often experience weight gain [12].
Several pieces of evidence also link STN with self-control, a decision process with clear implications for addiction [13]. Lesions of the STN result in impairment on three measures of impulsivity: 5-choice serial reaction time tasks, stop signal, and timed reactions [14, 15]. STN lesions also increase compulsive lever-pressing [16], as does functional disconnection of the mPFC-to-STN “hyperdirect” pathway [17]. However, STN lesions also appear to increase willingness to wait for a reward in a delay discounting task [18]. This seemingly paradoxical finding suggests that these measures of impulsivity are served by distinct neuronal mechanisms.
2.1.2 Functional and neuroanatomical subdivisions of the STN
Evidence suggests that the STN consists of two subdivisions: a lateral subdivision (lSTN) associated with the motor circuits of the basal ganglia and a medial subdivision (mSTN) associated with the limbic system and reward seeking; the primate STN may also have an associative segment located between the medial and lateral divisions [19, 20]. These anatomical differences may underlie the functional differences seen in pre-clinical studies and in the effects of deep brain stimulation (DBS) therapy in humans being treated for psychiatric disorders.
Anatomical differences between the medial and lateral STN are substantial. For example, the medial tip of the STN has reciprocal projections with the primate limbic pallidum (ventral pallidum (VP) in rodents), whereas the lSTN preferentially interacts with the external pallidal segment (globus pallidus in rodents) and entopeduncular nucleus [2, 21–23]. In primates (but not rodents), the dendritic field of an individual STN neuron occupies only a small portion of the nucleus, which may help compartmentalize the topographic inputs and outputs [24, 25]. Despite these anatomical distinctions, clear evidence for functional differences between these two subregions has been more elusive. In one nonhuman primate study, Mitchell and colleagues [26] reported an increase in metabolic activity in the dorsolateral STN during experimentally induced chorea, while the ventromedial tip was relatively unaffected. And in rodents, injections of NMDA or the GABA receptor antagonist, bicuculline, into the VP results in Fos activation only in the mSTN [27]. However there is also evidence that functional differentiation of medial and lateral STN might be limited: in nonhuman primates, electrical stimulation of the medial tip of the STN induces both motor and reward-related activity [28] and no differences in reward-related firing have been observed between the medial and lateral STN in rodents [9].
While findings from animal models may be ambiguous, clinical evidence from individuals receiving therapeutic STN DBS supports a medial/lateral distinction, as more medial stimulation evokes strong emotions with little effect on motor behavior [11]. In addition, DBS of the ventromedial STN also relieves the symptoms of OCD [29]. Although compelling, these results must be interpreted with caution. Due to the architecture of the STN and surrounding tissue, it is probable that electrical current spreads away from the body of the STN and directly activates distant neurons, terminals of afferent inputs, or nearby traversing axons [30–32]. Thus, the effects of DBS in the STN could be mediated in whole or in part by effects that occur outside of the STN itself.
Clearly, further research using a wider spectrum of the methodologies available to behavioral, anatomical, molecular and physiological neuroscience is needed to delineate the particular role of the medial and lateral STN. Evaluations across species will be particularly important as in vivo studies of STN subdivisions in rodents are technically difficult because of the small size, location and orientation of the STN.
2.2 The ventral pallidum
The VP is involved several different reward modalities, including drug-seeking, natural rewards, and intracranial self-stimulation [33–35]. Pharmacological disruption of the VP interferes with morphine-induced CPP [36], and with the formation and reinstatement of cocaine CPP [37, 38]. After lesions of the VP, previously palatable foods become aversive [39], and VP inactivation reduces self-stimulation of the medial forebrain bundle [40]. This functional evidence is corroborated by in vivo electrophysiological recordings: VP neurons respond to sucrose delivery and to sucrose-predictive cues [41], and show phasic firing rate changes related to cocaine-seeking lever presses [42]. Finally, a pair of recent studies in nonhuman primates has suggested that the VP may contribute to reward prediction error signaling via its input to the LHb [35, 43]. Although these findings link VP to processing information about rewards, they leave its precise role unclear.
The VP receives strong inputs from both the nucleus accumbens core (NAcc) and the STN [44, 45] and it has been hypothesized that the VP integrates these inputs to generate modality-independent responses. Indeed, there is evidence that single VP neurons encode many different stimuli and events in behavioral tasks [46, 47] – in contrast to the more selective responses found in striatum and NAcc [48, 49]. However, the organization of even these two prominent VP inputs is poorly understood. The NAcc neurons projecting to the VP are GABAergic and also contain opioids and other peptides [50, 51]. In contrast, STN projections to the VP are largely glutamatergic [52]. Physiological and pharmacological evidence suggests that NAcc inputs converge with and modulate other inputs to the VP. For example, exogenous application of opioid agonists modulate cortical- and amygdala-driven excitation in VP neurons [51], whereas inactivation of the NAcc can potentiate responses to cocaine administration [53]. However, the anatomical substrate for these effects is not known.
If the NAcc and STN inputs show a high degree of convergence on VP neurons, it would suggest that VP neurons integrate these sources of information and that single neurons may indeed be capable of encoding multi-modal rewards and cues. In contrast, if the NAcc and STN inputs do not converge in the VP, it would suggest that the VP contains multiple parallel channels that mediate distinct functions. Thus, the function of the VP may rely critically on the organization of its NAcc and STN afferents.
As the NAcc-VP and STN-VP pathways use GABA and glutamate, respectively, they may have distinct – and possibly opposed – functions. As a part of the indirect basal ganglia pathway, the NAcc-VP input may be important for the initiation of goal-directed behaviors. It may also be critical for learning new associations and actions, given the strong influence of the ventral subiculum on both the output neurons and interneurons of the NAcc [54, 55]. In contrast, the input from the STN may act as a “brake,” opposing previously learned actions in order to facilitate reversal learning or response inhibition. Thus, future investigations of the VP and its inputs will benefit by contrasting the effects of novel learning with the effects of reversal learning and extinction.
Given the essential role of dopamine in movement and motivated behaviors, it is possible that many of the functions of VP and STN in reward-seeking behavior are mediated via their actions on the dopamine system. There are two distinct, parallel pathways by which the STN and VP influence DA neuronal firing. First, the VP sends a direct inhibitory projection to the ventral tegmental area (VTA) and the STN sends direct excitatory projections to the substantia nigra pars compacta (SNc). Second, both the STN and the VP project indirectly to dopamine neurons via brainstem cholinergic nuclei. Through these parallel pathways, the VP can influence both the number of active dopamine neurons as well as their tendency for burst firing in the VTA [56]. Adding to the complexity of these interactions is the direct innervation of the STN, VP, and many of their afferent regions by DA [57–59]. Though DA innervation of STN and VP is moderate, local application of DA can modulate the firing of both VP [60] and STN neurons [61]; in the latter case, DA modulates burst firing, a function thought to be particularly important in the pathology of Parkinson's disease [62]. Thus, the VP and STN can directly influence the firing of dopamine neurons but are themselves subject to the modulatory influence of DA.
3 THE SUBICULUM AND RELATED HIPPOCAMPAL AREAS
3.1 The subiculum
The subiculum is a transitional cortical area that lies adjacent to the CA1 subfield of the hippocampus, and along with the entorhinal cortex and hippocampus, is considered to be an integral part of the hippocampal formation. It has been traditionally viewed as an output structure for the hippocampal formation, as its major efferents include numerous cortical and subcortical regions (described below), and its major inputs arise from the CA1 and entorhinal areas [63]. Although the hippocampal formation is typically associated with episodic memory and spatial navigation, the subiculum appears to play a role in stress, contextual conditioning, and drug abuse. Moreover the different subregions of the hippocampal formation appear to have different outputs and different roles: the dorsal hippocampus projects to the NAcc and is implicated in context-based conditioning and reinstatement of drug seeking [64] whereas the ventral subiculum projects to NAcc shell (and other extended amygdala structures) and has a role in stress, and the pathophysiology of schizophrenia [65, 66].
Importantly, the role of the subiculum in reward appears to derive from its interconnections with reward-related structures [67] rather than intrinsic encoding of reward. Neither dorsal nor ventral hippocampal neurons appear to encode value or reward [68, 69], but there is evidence for some post-reward related firing [70]. Neural activity in the subiculum often follows hippocampal CA1 firing with strict temporal correlation but can also persist after CA1 neurons become quiescent. These data suggest that that the subiculum acts as memory buffer for both transfer and retrieval of memory-related information [71], consistent with the idea that the subiculum may act both as an input and output nucleus of the hippocampal formation [72].
3.2 Different roles for dorsal and ventral hippocampus
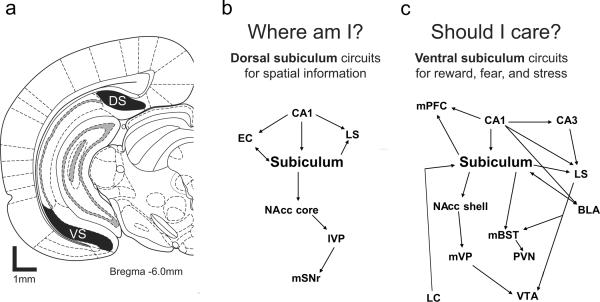
The dorsal subiculum (DSub) is the major output structure for surrounding dorsal hippocampal (DH) areas, connecting the hippocampus to many structures in the cortical reward system (Fig 1a) [73]. DSub in turn receives input from DH CA1, which itself receives input from the entorhinal cortex and lateral septum (LS) (Fig 1b). DSub outputs include the NAcc core, which then projects to the lateral VP and the medial SN (Fig 1b). These outputs allow the DSub to impact and modulate behavioral responses to rewards.
FIGURE 1.
a) Anatomical locations of the dorsal and ventral subiculum. b–c) Schematic diagram to illustrate differences in dorsal (b) and ventral hippocampal (c) connections. The dorsal subiculum (DS) helps determine spatial location, `Where am I?', whereas the ventral subiculum (VS) determines contextual relevance, `Should I care?'. Abbreviations: BLA, basolateral amygdala; EC, entorhinal cortex; LC, locus coeruleus; lVP, lateral ventral pallidum; mBST, medial bed nucleus of the stria terminalis; mPFC, medial prefrontal cortex; mSNr, medial substantia nigra pars reticulate; PVN, hypothalamic paraventricular nucleus.
DH plays a key role in contextual memory processes, including occasion setting [74]. This function of the DH is known to extend to drugs of abuse because DH is involved in ethanol-and cocaine-seeking induced by contextual stimuli. The firing properties of DH neurons are well-suited to providing this detailed contextual information because DH neurons demonstrate finely tuned place sensitive firing and process spatial contexts with very high resolution [75, 76]. Unlike the fine spatial tuning of DH neurons, ventral hippocampal (VH) neurons have broad spatial tuning [77, 78] and are sensitive to head direction [79, 80]. Unlike hippocampal CA1 neurons, subiculum neurons do not “re-map” their place fields as a result of changes in the spatial context in which an animal is placed [77]. This suggests that the place-sensitive firing in the dorsal and ventral hippocampus serve two functionally distinct roles in guiding behaviors [81].
VH and ventral subiculum (VSub) neurons have ideal circuit connections for a role in stress, reward, and fear (Figure 1c). The VSub has substantial outputs to the LS, amygdala nuclei, and bed nucleus of the stria terminalis (BNST), all of which can influence the hypothalamic-pituitary axis by sending projections to the paraventricular nucleus of the hypothalamus [82]. The VSub receives inputs from the ventral tegmental area and basolateral amygdala and sends efferents to the prelimbic and infralimbic medial prefrontal regions and to NAcc shell [83–87], both of which also receive amygdala and VTA inputs.
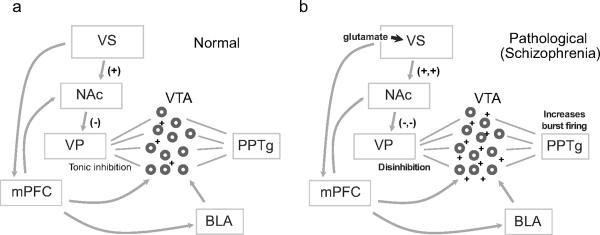
Consistent with these diverse anatomical connections, the VH has been implicated in a host of reward-related functions. VH lesions and inactivation decrease cue- drug- and contextual-induced reinstatement of cocaine-seeking [88–91], effects likely mediated by VH outputs to the medial prefrontal cortex or ventral striatum [92–94]. Additional studies have shown that stimulation of the VSub increases locomotor activity and dopamine release in the NAcc, prefrontal cortex and VTA [93, 94]. Via a multi-synaptic circuit through the NAcc and VP, the VSub also regulates the number of tonically active dopamine neurons in the VTA [95, 96]. Disruption of this pathway in particular may be relevant to the pathophysiology of schizophrenia (Figure 2, [92]), as hallucinations and paranoia have been attributed to elevated levels of tonic dopamine. In addition, high frequency stimulation of the subiculum increases dopamine levels in the NAcc, though this may be mediated by local activation of dopamine terminals [97]. Finally, dopamine afferents to the vSub are important in instrumental learning and motivation [83]. In short, the neural circuitry of the VH is distinct from the DH and has ideal neural connections for a role in stress, affect, and reward.
FIGURE 2.
a) Projections from the ventral subiculum (VS) to the NAcc and VP normally provide tonic inhibition of firing of ventral tegmental area dopamine neurons. b) However, when the VS is activated by glutamate injection, or is overactive as in schizophrenia, this leads to disinhibition of the VP-to-VTA pathway, allowing more VTA dopamine neurons to fire in response to pedunculopontine tegmental nucleus (PPTg) input. This ultimately increases burst firing in the VTA. This over-responsivity in VTA neurons may lend inappropriate salience to stimuli that would otherwise be irrelevant, a process that may underlie the development of psychotic delusions and ideas of reference.
3.3 Summary
The DSub and VSub have distinct contributions to motivated behavior, arising from their distinct firing properties and anatomical connections. The DH encodes specific spatial and episodic information that may then facilitate conditioning based on fine spatial discrimination or specific configurations of discrete cues. The VH, with less-specific spatial encoding and outputs to the extended amygdala and (indirectly) to dopamine neurons, may modulate arousal or mood according to memories of context. In other words, the DH answers the question, “Where am I?”, whereas the VH addresses, “Should I care?” (Figure 1). To reconcile these accounts of hippocampal formation function with the more well-known function of the hippocampus in episodic memory, it was suggested during our discussions that the hippocampal formation may be particularly tuned for encoding memories of rewarding events. Specifically, it has been hypothesized that rewarding events may increase dopamine release within the hippocampus, which may promote late phase LTP and embed experiences into long term memory [98].
4 THE LATERAL HABENULA AND MESOPONTINE ROSTROMEDIAL TEGMENTAL NUCLEUS
4.1 The lateral habenula
The lateral habenula (LHb) is a bilateral telencephalic nucleus located dorsal to thalamus, adjacent to the third ventricle. Its primary inputs are from the VP and internal segment of the globus pallidus [99, 100], as well as from the lateral hypothalamus and preoptic hypothalamic areas [101]. The outputs of the LHb are to mesencephalic structures, including the VTA and SNc, median and dorsal raphe nuclei, and the laterodorsal and pedunculopontine tegmental nuclei [102–104]. The majority of LHb neurons, including those that project to the ventral midbrain, are glutamatergic [105, 106]. Thus, the LHb links the hypothalamus and striatopallidal output nuclei with the dopamine, serotonin, and acetylcholine neurotransmitter systems – critical systems implicated in arousal, voluntary movement, attention, learning, mood, and drug abuse.
Recent findings suggest that the LHb is a unique contributor to reward prediction signals generated by dopamine neurons. In electrophysiological recordings, presumed dopamine neurons initially exhibit increased firing rates in response to primary rewards, but shift their firing rate increases to occur at the earliest cue that predicts reward as the predictor-reward association is learned [107, 108]. If the reward is omitted following the presentation of a predictor, presumed dopamine neurons decrease firing, signaling a reward prediction error [107]. In contrast, LHb neurons phasically increase firing following reward omission and inhibit firing in response to reward-predicting cues [109]. Given that LHb stimulation decreases presumed dopamine neuron firing, Matsumoto and Hikosaka [109] proposed the LHb as the source of negative reward prediction error signals to the dopaminergic midbrain.
While these results suggest a role for LHb in aversive conditioning and negative affect, under some circumstances, the LHb supports positive reinforcement. Rats will engage in intracranial self-stimulation of the LHb [110], and LHb lesions attenuate the efficacy of self-stimulation of lateral hypothalamus, VTA, and other sites [111]. Furthermore, in a particularly compelling case study, a patient suffering from drug-resistant depression was successfully treated via high-frequency DBS of the LHb [112]. Stimulation decreased depression symptoms (assessed by the Hamilton Rating Scale for Depression), but only after 12 weeks of continuous treatment. Interestingly, during an occasion when the stimulator battery failed, the patient's symptoms returned. The symptoms subsided after a new battery was installed, again after a delay of several weeks. LHb DBS may act via a stimulation-induced inactivation of the LHb (as appears to be the case for DBS of the STN [113] and consequent alteration of the VTA and mesocorticolimbic pathways. However, stimulation-induced depression is thought to occur only with high stimulation frequencies (100Hz or greater, [113]), and so this mechanism is unlikely to be the basis for low-frequency LHb self-stimulation in rodents. Thus, the LHb may have a yet-undefined role in positive reinforcement, as well as in negative reward prediction error.
To explain the suppression of VTA neuronal firing by LHb activation, it was originally hypothesized that LHb neurons project directly to VTA and SNc neurons. However, the projection from the LHb to the VTA is relatively sparse [114], primarily glutamatergic, and forms synapses equally onto GABAergic and dopaminergic neurons of the VTA [106]. Therefore, it is unclear how activation of the LHb can inhibit VTA neuronal firing. One possibility, discussed below, is that the LHb may inhibit VTA neurons via an intermediary structure, e.g., the mesopontine rostromedial tegmental nucleus (RMTg).
Additional anatomical projections of the LHb may also mediate negative reward signals. The LHb provides excitatory input to the dorsal raphe [115], a brain region that has long been implicated in signaling aversive events. Further, the dorsal raphe projects directly to the VTA and this may provide inhibitory input that contributes to negative reward signals. However, it is not known whether the dorsal raphe encodes reward-omissions or if activation of the dorsal raphe-to-VTA projection can inhibit VTA dopamine neurons.
4.2 The mesopontine rostromedial tegmental nucleus (RMTg)
The RMTg is a midbrain structure that lies adjacent to the interpeduncular nucleus and expands upward behind the retrorubal area into the paramedian region, to further ascend toward the most medial aspect of the pedunculopontine tegmental nucleus [104]. Although it is located caudal to the VTA and has been referred to as the “tail” of the VTA (tVTA) [116], the RMTg is a reticular structure. Its neurons are not distinguishable from those of the surrounding tissue and its boundaries can only be identified by immunohistochemical localization of markers like the mu-opioid receptor, somatostatin, or Fos (when evoked by external stimuli or pharmacological challenge, see below [104, 117]).
RMTg afferents arrive from a vast array of cortical and subcortical sources, including prelimbic cortex, cingulate cortex, NAcc, VP, ventral BNST, periaqueductal gray, lateral hypothalamus and preoptic areas, and habenula [104]. RMTg projects most densely to the VTA and SNc but also to dorsal raphe nucleus, pedunculopontine tegmental nucleus, lateral dorsal tegmental nucleus, locus coeruleus, and reticular formation [104, 118]. RMTg projections to VTA form symmetrical GABAergic synapses onto dopaminergic neurons. In this way, the connections of the RMTg are similar to those of the LHb, receiving descending inputs from many structures including the hypothalamus and ventral striatal-pallidal system, and subsequently providing descending projections to critical components of the ascending arousal system. However, unlike the LHb, the RMTg appears to use GABA as its primary neurotransmitter.
Several studies link the RMTg to aversion and negative affect. For example, Jhou and colleagues [118] showed that Fos protein was highly expressed in RMTg neurons in rats following foot shock, or following cues that had been previously associated with foot shock. In the same study, lesions of the RMTg disrupted freezing in response to cued foot shock or predatory odor and increased the proportion of time spent in open arms of an elevated plus maze. However, the same lesions increased defensive treading, suggesting that the RMTg primarily governs passive behaviors such as freezing and avoidance rather than active behaviors such as escape or defensive treading [118]. In RMTg neurons that project to the VTA, Fos immunoreactivity increases after administration of psychostimulants [117, 119], but not morphine [120]; this elevated Fos expression may be related to stress or arousal, rather than the positive hedonic properties of psychostimulants.
Several lines of evidence suggest that the RMTg may play an intermediary role in a circuit mediating aversion. First, the RMTg receives a massive projection from the LHb. Similar to the LHb, a subpopulation of RMTg neurons exhibits increased firing rate following reward omission or cues that predict the absence of reward [118]. In experiments that measured firing in the LHb, RMTg and VTA, reward omission increased firing in the LHb and the RMTg and suppressed firing in the VTA (T.C. Jhou, personal communication). Based on these anatomical and physiological findings, it was proposed that activation of GABAergic RMTg neurons by glutamatergic LHb neurons results in inhibition of VTA dopaminergic neurons. However, this model cannot account for the finding that (when compared across several studies) the onset latency of RMTg neurons to a conditioned stimulus appears to be approximately 20 ms faster than the onset latency in LHb neurons [T.C. Jhou, personal communication, 118, 121] Thus, while the anatomical evidence suggests that the RMTg is an interlocutor between LHb and the VTA, physiological evidence suggests a more complex role for the RMTg.
4.3 Different roles for LHb and RMTg?
Aversive events can include both punishment (the arrival of an unpleasant stimulus) and disappointment (the omission of reward). While both can induce negative affect, they are different phenomena and could be represented by separate brain circuits or by distinct nodes of a network that also include common points of signal integration. In the latter case, the RMTg may be able to integrate signals involved in both punishment and disappointment, as it receives input from the extended amygdala and periaqueductal grey, structures involved in processing aversive stimuli [116], as well as the LHb, which responds to reward omissions [122]. If this model is correct, then the circuits that couple the LHb and amygdala/ periaqueductal grey to dopamine neurons, though they respond to different classes of negative stimuli, would both use the RMTg as a common node. Otherwise, the RMTg may be selectively involved in encoding a specific subclass of negative events.
Another possibility proposed is that the RMTg may selectively regulate the motor component of responses to disappointment, punishment or both. According to this hypothesis, the strong inhibitory projection from the RMTg to the VTA and SNc acts as a “brake” or no-go signal for motor actions. Acting in parallel, the weak excitatory input from the LHb to the VTA/SNc provides input that encodes the positive or negative valence or external events. Supporting such a distinction between motivational and motor aspects of RMTg function is the observation that RMTg lesions selectively affect passive—but not active—defense behaviors [118].
4.4 Dopamine release and aversive events
As reviewed above, there is ample evidence that the RMTg may suppress firing in dopamine neurons in response to aversive events. However, primary or conditioned aversive stimuli can increase extrasynaptic (or tonic) dopamine release in terminal regions such as NAcc and prefrontal cortex, as measured by microdialysis [123, 124]. This apparent contradiction may be explained by the differential effects of tonic and phasic dopamine neuron firing on dopamine release. Changes in dopamine release measured with microdialysis appear to be driven primarily by increases in the number of tonically active dopamine neurons, and by local activation of dopamine terminals by excitatory inputs within terminal regions [56, 96, 125, 126]. In contrast, phasic burst firing of dopamine neurons (traditionally linked to reward-related stimuli) does not appear to make a substantial contribution to tonic dopamine levels [56]. Thus, tonic and phasic transmission may be two distinct and quasi-independent signals through which the dopamine system processes rewarding or aversive events. Whereas rewarding stimuli may enhance both tonic and phasic dopamine activity, aversive stimuli may preferentially suppress phasic dopamine activity via activation of the RMTg, while concurrently increasing extrasynaptic dopamine efflux through other mechanisms.
5 THE EXTENDED AMYGDALA, THE BED NUCLEUS OF THE STRIA TERMINALIS, AND THE HYPOTHALAMUS
The extended amygdala has been defined as a continuum of inter-related basal forebrain structures that includes the central amygdaloid nucleus (CEA), the sublenticular extended amygdala nuclei, the bed nucleus of the stria terminalis (BNST), and the shell of the NAcc [127–129]. These nuclei share much in common and are involved in many motivated behaviors including reward-based operant conditioning and stress- or cue-induced reinstatement of drug seeking in animals [130–134].
Among the extended amygdala structures, the BNST appears to be particularly important in motivated behaviors, including those driven by pharmacological agents [135–137]. The BNST has been characterized as a cluster of approximately 12 interconnected nuclei [127, 138, 139]. Whether BNST nuclei should be referred to a single unitary structure is a matter of debate. However, each of the neuronal clusters currently considered as BNST subnuclei are highly interconnected and have similar neuronal electrophysiological signatures, suggesting it may be appropriate to consider the BNST as a unit [140–147]. These BNST nuclei are intricately interconnected with major components of the brain reward circuitry such as the VTA, the ventral striatum, and the hypothalamus [c.f. 144 and others from these authors].
The nuclei of the BNST have clear lateral/medial and ventral/dorsal gradients based on their function and anatomical connectivity. Lateral BNST nuclei preferentially connect with the lateral hypothalamus and lateral regions of the CEA, and medial BNST nuclei connect with medial CEA and medial hypothalamus [127, 128]. While the medial and lateral BNST networks are thought to influence endocrine function and motivated behaviors, respectively, anatomically specific lesions or pharmacological interventions will be required to clarify this issue.
There are also clear neurochemical distinctions between the ventral and dorsal BNST. Dopamine-containing terminals are restricted to BNST regions dorsal to the anterior commissure, whereas noradrenaline-containing terminals are preferentially, although not exclusively, located in the ventral BNST [148–151]. As with the medial and lateral parts of the BNST, these anatomical distinctions may correspond to different functions: pharmacological manipulations of the dorsal BNST interfere with appetitive-motivated behaviors such as drug self-administration whereas noradrenergic antagonists in the ventral BNST reduces withdrawal-induced place aversion [135–137, 152].
The BNST contributes to coding the appetitive outcome of a given situation, responding to stimuli associated with both positive and negative affect. However, there is a relatively clear distinction between the types of stimuli that activate the BNST and those that activate other regions of the extended amygdala such as the CEA. Whereas the CEA is critical for discrete cue-induced conditioning, the BNST seems to play a special role in contextual conditioning [153–156]. As such, the BNST is thought to contribute to a general `awareness' loosely linked to a particular context, rather than the prediction of specific outcomes by discrete cues [157]. Consistent with this idea, the BNST, but not the CEA, receives a strong input from the VSub, which plays a key role in contextual conditioning [158, 159] (see Section 3).
One key feature of the BNST and other extended amygdala structures are their bidirectional anatomical connections with hypothalamic nuclei. Indeed, the extended amygdala is the main input to hypothalamic nuclei and, in return, several hypothalamic nuclei project back to regions of the extended amygdala [127, 128, 141–146]. The extended amygdala seems to act as an important relay and integration center between cortical regions and hypothalamic nuclei [160, 161]. For example, the BNST projection to the paraventricular nucleus may be a pathway that allows contextual information (from the ventral subiculum) to influence motivated behavior. Of particular relevance to motivated behaviors is the relationship between the lateral hypothalamus and the lateral extended amygdala. Both the ventral BNST and the NAcc shell send projections to the lateral hypothalamus, which contains orexinergic neurons recently identified as important for drug-seeking behaviors [162]. The lateral hypothalamus in turn projects back to the ventral BNST, though the functional significance of this reciprocal projection is not known (but see [163]).
6 CONCLUSION
More than just recognizing understudied brain regions, the purpose of this meeting was to expand what we think of as the “reward circuit”. Indeed, the importance of reward in all aspects of our decisions –what food to eat, what job to take – belies the notion that reward processing is only a small part of our brain. The many, diverse brain structures discussed above are at the center of a current blizzard of studies, comprising work in anatomy, behavioral pharmacology, in vivo and in vitro electrophysiology, neuroimaging, and theoretical modeling. From these studies is emerging a new understanding of the essential players in motivation and reward.
One consistent point that became apparent from our discussions was that brain regions cannot be simply labeled as either contributing, or not contributing, to motivated behavior; rather, it's necessary to consider the specific circumstances under which the region is being engaged. For example, while the large inhibitory input from the RMTg to the VTA is clearly of functional significance, this pathway may only drive passive defensive responses such as freezing or avoidance (See Section 4 and Jhou et al 2009a). Thus, a major thread in our discussions was whether these regions were tied to specific classes of behaviors or specific kinds of reward. A second major thread was that the anatomy of these forgotten regions – afferent and efferent connections, cytoarchitecture, local neurotransmitters and peptides – can provide important clues to their function, and can shape our hypotheses and interpretations. However, when crucial anatomical details are lacking, it is that much more difficult to understand their function. In the case of the medial STN (Section 2), its direct excitatory projection to the VP may powerfully influence cortical-striatal-pallidal transmission; but without knowing how the inputs from the STN are arranged relative to other major VP afferents, the functions of this pathway can only be guessed. In this way, our discussions showed that to expand the reward circuit, two questions must be answered: Under what specific circumstances are these brain regions required for reward and motivation? And how does their anatomy make this possible?
The consensus, common ground, and unresolved issues from our discussions are summarized in the reviews above. If nothing else, they show that the reward system is large and multi-faceted – that it may not be a unitary system with clear borders, but rather may be interwoven throughout the structure of the brain, influencing all aspects of cognition and behavior. The borders – or lack thereof – of the reward circuit will continue to be important points of discussion at the next MNN conference scheduled for 2012, and in our continued gatherings in the future.
Research Highlights
The 2010 Motivational Neuronal Networks conference took place on April 24–27.
Discussions focused on understudied brain regions involved in reward and motivation.
Topics included subthalamic nucleus, subiculum, lateral habenula, and several others.
These discussions are summarized in this review.
ACKNOWLEDGEMENTS
The authors wish to thank Lisa Swetz for assistance organizing the conference. D.S. Zahm and A.A. Grace provided helpful comments on the manuscript. Funding for the conference was made possible (in part) by NIDA 1 R13 DA030034-01
ABBREVIATIONS
- BNST
bed nucleus of the stria terminalis
- CEA
central amygdaloid nucleus
- CPP
conditioned place preference
- DA
dopamine
- DBS
deep brain stimulation
- DH
dorsal hippocampal formation
- DSub
dorsal subiculum
- LHb
lateral habenula
- LS
lateral septum
- lSTN
lateral subthalamic nucleus
- mSTN
medial subthalamic nucleus
- NAcc
nucleus accumbens
- OCD
obsessive compulsive disorder
- PD
Parkinson's Disease
- RMTg
mesopontine rostromedial tegmental nucleus
- SNc
substantia nigra pars compacta
- STN
subthalamic nucleus
- tVTA
tail of the VTA
- VH
ventral hippocampal formation
- VP
ventral pallidum
- VSub
ventral subiculum
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Carpenter MB, Carleton SC, Keller JT, Conte P. Connections of the subthalamic nucleus in the monkey. Brain Res. 1981;224:1–29. doi: 10.1016/0006-8993(81)91113-6. [DOI] [PubMed] [Google Scholar]
- [2].Groenewegen HJ, Berendse HW. Connections of the subthalamic nucleus with ventral striatopallidal parts of the basal ganglia in the rat. J Comp Neurol. 1990;294:607–22. doi: 10.1002/cne.902940408. [DOI] [PubMed] [Google Scholar]
- [3].Shink E, Bevan MD, Bolam JP, Smith Y. The subthalamic nucleus and the external pallidum: Two tightly interconnected structures that control the output of the basal ganglia in the monkey. Neuroscience. 1996;73:335–57. doi: 10.1016/0306-4522(96)00022-x. [DOI] [PubMed] [Google Scholar]
- [4].Darbaky Y, Baunez C, Arecchi P, Legallet E, Apicella P. Reward-related neuronal activity in the subthalamic nucleus of the monkey. Neuroreport. 2005;16:1241–4. doi: 10.1097/00001756-200508010-00022. [DOI] [PubMed] [Google Scholar]
- [5].Matsumura M, Kojima J, Gardiner TW, Hikosaka O. Visual and Oculomotor Functions of Monkey Subthalamic Nucleus. Journal of Neurophysiology. 1992;67:1615–32. doi: 10.1152/jn.1992.67.6.1615. [DOI] [PubMed] [Google Scholar]
- [6].Baunez C, Dias C, Cador M, Amalric M. The subthalamic nucleus exerts opposite control on cocaine and `natural' rewards. Nat Neurosci. 2005;8:484–9. doi: 10.1038/nn1429. [DOI] [PubMed] [Google Scholar]
- [7].Rouaud T, Lardeux S, Panayotis N, Paleressompoulle D, Cador M, Baunez C. Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proc Natl Acad Sci U S A. 2010;107:1196–200. doi: 10.1073/pnas.0908189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baunez C, Gubellini P. Effects of GPi and STN inactivation on physiological, motor, cognitive and motivational processes in animal models of Parkinson's disease. Prog Brain Res. 2010;183:235–58. doi: 10.1016/S0079-6123(10)83012-2. [DOI] [PubMed] [Google Scholar]
- [9].Lardeux S, Pernaud R, Paleressompoulle D, Baunez C. Beyond the reward pathway: coding reward magnitude and error in the rat subthalamic nucleus. J Neurophysiol. 2009;102:2526–37. doi: 10.1152/jn.91009.2008. [DOI] [PubMed] [Google Scholar]
- [10].Benabid AL, Krack PP, Benazzouz A, Limousin P, Koudsie A, Pollak P. Deep brain stimulation of the subthalamic nucleus for Parkinson's disease: methodologic aspects and clinical criteria. Neurology. 2000;55:S40–4. [PubMed] [Google Scholar]
- [11].Mallet L, Schupbach M, N'Diaye K, Remy P, Bardinet E, Czernecki V, et al. Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proc Natl Acad Sci U S A. 2007;104:10661–6. doi: 10.1073/pnas.0610849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Barichella M, Marczewska AM, Mariani C, Landi A, Vairo A, Pezzoli G. Body weight gain rate in patients with Parkinson's disease and deep brain stimulation. Mov Disord. 2003;18:1337–40. doi: 10.1002/mds.10543. [DOI] [PubMed] [Google Scholar]
- [13].Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- [14].Baunez C, Humby T, Eagle DM, Ryan LJ, Dunnett SB, Robbins TW. Effects of STN lesions on simple vs choice reaction time tasks in the rat: preserved motor readiness, but impaired response selection. Eur J Neurosci. 2001;13:1609–16. doi: 10.1046/j.0953-816x.2001.01521.x. [DOI] [PubMed] [Google Scholar]
- [15].Baunez C, Robbins TW. Bilateral lesions of the subthalamic nucleus induce multiple deficits in an attentional task in rats. Eur J Neurosci. 1997;9:2086–99. doi: 10.1111/j.1460-9568.1997.tb01376.x. [DOI] [PubMed] [Google Scholar]
- [16].Winter C, Flash S, Klavir O, Klein J, Sohr R, Joel D. The role of the subthalamic nucleus in `compulsive' behavior in rats. Eur J Neurosci. 2008;27:1902–11. doi: 10.1111/j.1460-9568.2008.06148.x. [DOI] [PubMed] [Google Scholar]
- [17].Chudasama Y, Baunez C, Robbins TW. Functional disconnection of the medial prefrontal cortex and subthalamic nucleus in attentional performance: evidence for corticosubthalamic interaction. J Neurosci. 2003;23:5477–85. doi: 10.1523/JNEUROSCI.23-13-05477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Uslaner JM, Robinson TE. Subthalamic nucleus lesions increase impulsive action and decrease impulsive choice - mediation by enhanced incentive motivation? Eur J Neurosci. 2006;24:2345–54. doi: 10.1111/j.1460-9568.2006.05117.x. [DOI] [PubMed] [Google Scholar]
- [19].Karachi C, Grabli D, Baup N, Mounayar S, Tande D, Francois C, et al. Dysfunction of the subthalamic nucleus induces behavioral and movement disorders in monkeys. Mov Disord. 2009;24:1183–92. doi: 10.1002/mds.22547. [DOI] [PubMed] [Google Scholar]
- [20].Karachi C, Yelnik J, Tande D, Tremblay L, Hirsch EC, Francois C. The pallidosubthalamic projection: an anatomical substrate for nonmotor functions of the subthalamic nucleus in primates. Mov Disord. 2005;20:172–80. doi: 10.1002/mds.20302. [DOI] [PubMed] [Google Scholar]
- [21].Carpenter MB, Baton RR, 3rd, Carleton SC, Keller JT. Interconnections and organization of pallidal and subthalamic nucleus neurons in the monkey. J Comp Neurol. 1981;197:579–603. doi: 10.1002/cne.901970404. [DOI] [PubMed] [Google Scholar]
- [22].Feger J, Robledo P. The Effects of Activation or Inhibition of the Subthalamic Nucleus on the Metabolic and Electrophysiological Activities Within the Pallidal Complex and Substantia Nigra in the Rat. Eur J Neurosci. 1991;3:947–52. doi: 10.1111/j.1460-9568.1991.tb00030.x. [DOI] [PubMed] [Google Scholar]
- [23].Haber SN, Lynd-Balta E, Mitchell SJ. The organization of the descending ventral pallidal projections in the monkey. J Comp Neurol. 1993;329:111–28. doi: 10.1002/cne.903290108. [DOI] [PubMed] [Google Scholar]
- [24].Hammond C, Yelnik J. Intracellular labelling of rat subthalamic neurones with horseradish peroxidase: computer analysis of dendrites and characterization of axon arborization. Neuroscience. 1983;8:781–90. doi: 10.1016/0306-4522(83)90009-x. [DOI] [PubMed] [Google Scholar]
- [25].Yelnik J, Percheron G. Subthalamic neurons in primates: a quantitative and comparative analysis. Neuroscience. 1979;4:1717–43. doi: 10.1016/0306-4522(79)90030-7. [DOI] [PubMed] [Google Scholar]
- [26].Mitchell IJ, Jackson A, Sambrook MA, Crossman AR. The role of the subthalamic nucleus in experimental chorea. Evidence from 2-deoxyglucose metabolic mapping and horseradish peroxidase tracing studies. Brain. 1989;112(Pt 6):1533–48. doi: 10.1093/brain/112.6.1533. [DOI] [PubMed] [Google Scholar]
- [27].Turner MS, Gray TS, Mickiewicz AL, Napier TC. Fos expression following activation of the ventral pallidum in normal rats and in a model of Parkinson's Disease: implications for limbic system and basal ganglia interactions. Brain Struct Funct. 2008;213:197–213. doi: 10.1007/s00429-008-0190-4. [DOI] [PubMed] [Google Scholar]
- [28].Baunez C, Gubellini P. Effects of GPi and STN inactivation on physiological, motor, cognitive and motivational processes in animal models of Parkinson's disease. Prog Brain Res. 183:235–58. doi: 10.1016/S0079-6123(10)83012-2. [DOI] [PubMed] [Google Scholar]
- [29].Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121–34. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- [30].Tehovnik EJ, Tolias AS, Sultan F, Slocum WM, Logothetis NK. Direct and indirect activation of cortical neurons by electrical microstimulation. J Neurophysiol. 2006;96:512–21. doi: 10.1152/jn.00126.2006. [DOI] [PubMed] [Google Scholar]
- [31].Ranck JB., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975;98:417–40. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- [32].Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–9. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Panagis G, Miliaressis E, Anagnostakis Y, Spyraki C. Ventral pallidum self-stimulation: a moveable electrode mapping study. Behav Brain Res. 1995;68:165–72. doi: 10.1016/0166-4328(94)00169-g. [DOI] [PubMed] [Google Scholar]
- [34].Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25:8637–49. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bromberg-Martin ES, Matsumoto M, Hong S, Hikosaka O. A pallidus-habenula-dopamine pathway signals inferred stimulus values. Journal of Neurophysiology. 2010;104:1068–76. doi: 10.1152/jn.00158.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dallimore JE, Mickiewicz AL, Napier TC. Intra-ventral pallidal glutamate antagonists block expression of morphine-induced place preference. Behav Neurosci. 2006;120:1103–14. doi: 10.1037/0735-7044.120.5.1103. [DOI] [PubMed] [Google Scholar]
- [37].Gong W, Neill D, Justice JB., Jr 6-Hydroxydopamine lesion of ventral pallidum blocks acquisition of place preference conditioning to cocaine. Brain Res. 1997;754:103–12. doi: 10.1016/s0006-8993(97)00059-0. [DOI] [PubMed] [Google Scholar]
- [38].McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cromwell HC, Berridge KC. Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res. 1993;624:1–10. doi: 10.1016/0006-8993(93)90053-p. [DOI] [PubMed] [Google Scholar]
- [40].Waraczynski M, Demco C. Lidocaine inactivation of the ventral pallidum affects responding for brain stimulation reward more than it affects the stimulation's reward value. Behav Brain Res. 2006;173:288–98. doi: 10.1016/j.bbr.2006.06.040. [DOI] [PubMed] [Google Scholar]
- [41].Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009;196:155–67. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Root DH, Fabbricatore AT, Ma S, Barker DJ, West MO. Rapid phasic activity of ventral pallidal neurons during cocaine self-administration. Synapse. 2010;64:704–13. doi: 10.1002/syn.20792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron. 2008;60:720–9. doi: 10.1016/j.neuron.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Haber SN, Lynd E, Klein C, Groenewegen HJ. Topographic organization of the ventral striatal efferent projections in the rhesus monkey: an anterograde tracing study. J Comp Neurol. 1990;293:282–98. doi: 10.1002/cne.902930210. [DOI] [PubMed] [Google Scholar]
- [45].Zahm DS, Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. J Comp Neurol. 1990;302:437–46. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- [46].Jaeger D, Gilman S, Aldridge JW. Neuronal activity in the striatum and pallidum of primates related to the execution of externally cued reaching movements. Brain Research. 1995;694:111–27. doi: 10.1016/0006-8993(95)00780-t. [DOI] [PubMed] [Google Scholar]
- [47].Tindell AJ, Berridge KC, Aldridge JW. Ventral pallidal representation of pavlovian cues and reward: population and rate codes. J Neurosci. 2004;24:1058–69. doi: 10.1523/JNEUROSCI.1437-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Cue-evoked firing of nucleus accumbens neurons encodes motivational significance during a discriminative stimulus task. J Neurophysiol. 2004;91:1840–65. doi: 10.1152/jn.00657.2003. [DOI] [PubMed] [Google Scholar]
- [49].Opris I, Hampson RE, Deadwyler SA. The encoding of cocaine vs. natural rewards in the striatum of nonhuman primates: categories with different activations. Neuroscience. 2009;163:40–54. doi: 10.1016/j.neuroscience.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zahm DS, Zaborszky L, Alones VE, Heimer L. Evidence for the coexistence of glutamate decarboxylase and Met-enkephalin immunoreactivities in axon terminals of rat ventral pallidum. Brain Res. 1985;325:317–21. doi: 10.1016/0006-8993(85)90331-2. [DOI] [PubMed] [Google Scholar]
- [51].Napier TC, Mitrovic I. Opioid modulation of ventral pallidal inputs. Ann N Y Acad Sci. 1999;877:176–201. doi: 10.1111/j.1749-6632.1999.tb09268.x. [DOI] [PubMed] [Google Scholar]
- [52].Turner MS, Lavin A, Grace AA, Napier TC. Regulation of limbic information outflow by the subthalamic nucleus: excitatory amino acid projections to the ventral pallidum. J Neurosci. 2001;21:2820–32. doi: 10.1523/JNEUROSCI.21-08-02820.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Johnson PI, Napier TC. Contribution of the nucleus accumbens to cocaine-induced responses of ventral pallidal neurons. Synapse. 1996;22:253–60. doi: 10.1002/(SICI)1098-2396(199603)22:3<253::AID-SYN8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- [54].French SJ, Totterdell S. Quantification of morphological differences in boutons from different afferent populations to the nucleus accumbens. Brain Res. 2004;1007:167–77. doi: 10.1016/j.brainres.2004.02.018. [DOI] [PubMed] [Google Scholar]
- [55].O'Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–39. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–73. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- [57].Francois C, Savy C, Jan C, Tande D, Hirsch EC, Yelnik J. Dopaminergic innervation of the subthalamic nucleus in the normal state, in MPTP-treated monkeys, and in Parkinson's disease patients. J Comp Neurol. 2000;425:121–9. doi: 10.1002/1096-9861(20000911)425:1<121::aid-cne10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- [58].Hassani OK, Francois C, Yelnik J, Feger J. Evidence for a dopaminergic innervation of the subthalamic nucleus in the rat. Brain Research. 1997;749:88–94. doi: 10.1016/s0006-8993(96)01167-5. [DOI] [PubMed] [Google Scholar]
- [59].Parent A, Smith Y. Differential dopaminergic innervation of the two pallidal segments in the squirrel monkey (Saimiri sciureus) Brain Research. 1987;426:397–400. doi: 10.1016/0006-8993(87)90896-1. [DOI] [PubMed] [Google Scholar]
- [60].Napier TC, Potter PE. Dopamine in the rat ventral pallidum/substantia innominata: biochemical and electrophysiological studies. Neuropharmacology. 1989;28:757–60. doi: 10.1016/0028-3908(89)90163-9. [DOI] [PubMed] [Google Scholar]
- [61].Mintz I, Hammond C, Feger J. Excitatory effect of iontophoretically applied dopamine on identified neurons of the rat subthalamic nucleus. Brain Research. 1986;375:172–5. doi: 10.1016/0006-8993(86)90971-6. [DOI] [PubMed] [Google Scholar]
- [62].Kreiss DS, Mastropietro CW, Rawji SS, Walters JR. The response of subthalamic nucleus neurons to dopamine receptor stimulation in a rodent model of Parkinson's disease. J Neurosci. 1997;17:6807–19. doi: 10.1523/JNEUROSCI.17-17-06807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10:272–82. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- [64].Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- [65].Belujon P, Grace AA. Hippocampus, amygdala, and stress: interacting systems that affect susceptibility to addiction. Ann N Y Acad Sci. 2011;1216:114–21. doi: 10.1111/j.1749-6632.2010.05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Grace AA. Dopamine system dysregulation by the ventral subiculum as the common pathophysiological basis for schizophrenia psychosis, psychostimulant abuse, and stress. Neurotox Res. 2010;18:367–76. doi: 10.1007/s12640-010-9154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Holscher C, Jacob W, Mallot HA. Reward modulates neuronal activity in the hippocampus of the rat. Behav Brain Res. 2003;142:181–91. doi: 10.1016/s0166-4328(02)00422-9. [DOI] [PubMed] [Google Scholar]
- [69].Berke JD, Breck JT, Eichenbaum H. Striatal versus hippocampal representations during win-stay maze performance. J Neurophysiol. 2009;101:1575–87. doi: 10.1152/jn.91106.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Young B, McNaughton N. Common firing patterns of hippocampal cells in a differential reinforcement of low rates of response schedule. J Neurosci. 2000;20:7043–51. doi: 10.1523/JNEUROSCI.20-18-07043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Deadwyler SA, Hampson RE. Differential but complementary mnemonic functions of the hippocampus and subiculum. Neuron. 2004;42:465–76. doi: 10.1016/s0896-6273(04)00195-3. [DOI] [PubMed] [Google Scholar]
- [72].Naber PA, Witter MP, Lopes Silva FH. Networks of the hippocampal memory system of the rat. The pivotal role of the subiculum. Ann N Y Acad Sci. 2000;911:392–403. doi: 10.1111/j.1749-6632.2000.tb06739.x. [DOI] [PubMed] [Google Scholar]
- [73].Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed Academic Press/Elsevier; Amsterdam ; Boston: 2007. [Google Scholar]
- [74].Holland PC. Occasion Setting in Pavlovian Conditioning. In: Medin DL, editor. Psychology of Learning and Motivation: Advances in Research and Theory. Academic Press; New York, New York: 1992. pp. 69–124. [Google Scholar]
- [75].O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–5. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- [76].Henze DA, Borhegyi Z, Csicsvari J, Mamiya A, Harris KD, Buzsaki G. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J Neurophysiol. 2000;84:390–400. doi: 10.1152/jn.2000.84.1.390. [DOI] [PubMed] [Google Scholar]
- [77].Barnes CA, McNaughton BL, Mizumori SJ, Leonard BW, Lin LH. Comparison of spatial and temporal characteristics of neuronal activity in sequential stages of hippocampal processing. Prog Brain Res. 1990;83:287–300. doi: 10.1016/s0079-6123(08)61257-1. [DOI] [PubMed] [Google Scholar]
- [78].O'Mara S. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. J Anat. 2005;207:271–82. doi: 10.1111/j.1469-7580.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci. 1990;10:436–47. doi: 10.1523/JNEUROSCI.10-02-00436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990;10:420–35. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sharp PE. Subicular place cells generate the same “map” for different environments: comparison with hippocampal cells. Behav Brain Res. 2006;174:206–14. doi: 10.1016/j.bbr.2006.05.034. [DOI] [PubMed] [Google Scholar]
- [82].Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–24. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- [83].Andrzejewski ME, Spencer RC, Kelley AE. Dissociating ventral and dorsal subicular dopamine D1 receptor involvement in instrumental learning, spontaneous motor behavior, and motivation. Behav Neurosci. 2006;120:542–53. doi: 10.1037/0735-7044.120.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].French SJ, Hailstone JC, Totterdell S. Basolateral amygdala efferents to the ventral subiculum preferentially innervate pyramidal cell dendritic spines. Brain Res. 2003;981:160–7. doi: 10.1016/s0006-8993(03)03017-8. [DOI] [PubMed] [Google Scholar]
- [85].French SJ, Totterdell S. Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience. 2003;119:19–31. doi: 10.1016/s0306-4522(03)00150-7. [DOI] [PubMed] [Google Scholar]
- [86].Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–79. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- [87].Naber PA, Lopes da Silva FH, Witter MP. Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus. 2001;11:99–104. doi: 10.1002/hipo.1028. [DOI] [PubMed] [Google Scholar]
- [88].Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 2003;23:10258–64. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Atkins AL, Mashhoon Y, Kantak KM. Hippocampal regulation of contextual cue-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 2008;90:481–91. doi: 10.1016/j.pbb.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur J Neurosci. 2009;30:1370–81. doi: 10.1111/j.1460-9568.2009.06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Rogers JL, See RE. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem. 2007;87:688–92. doi: 10.1016/j.nlm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–30. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Peleg-Raibstein D, Feldon J. Effects of dorsal and ventral hippocampal NMDA stimulation on nucleus accumbens core and shell dopamine release. Neuropharmacology. 2006;51:947–57. doi: 10.1016/j.neuropharm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- [94].Taepavarapruk P, Floresco SB, Phillips AG. Hyperlocomotion and increased dopamine efflux in the rat nucleus accumbens evoked by electrical stimulation of the ventral subiculum: role of ionotropic glutamate and dopamine D1 receptors. Psychopharmacology (Berl) 2000;151:242–51. doi: 10.1007/s002130000376. [DOI] [PubMed] [Google Scholar]
- [95].Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–22. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–7. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- [97].Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci. 2001;21:2851–60. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–68. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- [99].Parent A, De Bellefeuille L. Organization of efferent projections from the internal segment of globus pallidus in primate as revealed by fluorescence retrograde labeling method. Brain Research. 1982;245:201–13. doi: 10.1016/0006-8993(82)90802-2. [DOI] [PubMed] [Google Scholar]
- [100].van der Kooy D, Carter DA. The organization of the efferent projections and striatal afferents of the entopeduncular nucleus and adjacent areas in the rat. Brain Research. 1981;211:15–36. doi: 10.1016/0006-8993(81)90064-0. [DOI] [PubMed] [Google Scholar]
- [101].Kowski AB, Geisler S, Krauss M, Veh RW. Differential projections from subfields in the lateral preoptic area to the lateral habenular complex of the rat. J Comp Neurol. 2008;507:1465–78. doi: 10.1002/cne.21610. [DOI] [PubMed] [Google Scholar]
- [102].Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977;173:123–46. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- [103].Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- [104].Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–96. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–43. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009;30:1239–50. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–9. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- [108].Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- [109].Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–5. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- [110].Vachon MP, Miliaressis E. Dorsal diencephalic self-stimulation: a movable electrode mapping study. Behav Neurosci. 1992;106:981–91. doi: 10.1037//0735-7044.106.6.981. [DOI] [PubMed] [Google Scholar]
- [111].Morissette MC, Boye SM. Electrolytic lesions of the habenula attenuate brain stimulation reward. Behav Brain Res. 2008;187:17–26. doi: 10.1016/j.bbr.2007.08.021. [DOI] [PubMed] [Google Scholar]
- [112].Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- [113].Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol. 2001;85:1351–6. doi: 10.1152/jn.2001.85.4.1351. [DOI] [PubMed] [Google Scholar]
- [114].Araki M, McGeer PL, Kimura H. The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain Res. 1988;441:319–30. doi: 10.1016/0006-8993(88)91410-2. [DOI] [PubMed] [Google Scholar]
- [115].Kim U. Topographic commissural and descending projections of the habenula in the rat. J Comp Neurol. 2009;513:173–87. doi: 10.1002/cne.21951. [DOI] [PubMed] [Google Scholar]
- [116].Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- [117].Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20:8620–8. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Geisler S, Marinelli M, Degarmo B, Becker ML, Freiman AJ, Beales M, et al. Prominent activation of brainstem and pallidal afferents of the ventral tegmental area by cocaine. Neuropsychopharmacology. 2008;33:2688–700. doi: 10.1038/sj.npp.1301650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Perrotti LI, Bolanos CA, Choi KH, Russo SJ, Edwards S, Ulery PG, et al. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur J Neurosci. 2005;21:2817–24. doi: 10.1111/j.1460-9568.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- [121].Mileykovskiy BY, Morales MF. Responses of typrosine hydroxylase-positive neurons in the ventral tegmental area to aversive conditioned signals. Society of Neuroscience Annual Meeting; Chicago, IL: Society for Neuroscience; 2009. [Google Scholar]
- [122].Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008;28:11825–9. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Young AM, Joseph MH, Gray JA. Latent inhibition of conditioned dopamine release in rat nucleus accumbens. Neuroscience. 1993;54:5–9. doi: 10.1016/0306-4522(93)90378-s. [DOI] [PubMed] [Google Scholar]
- [124].Wilkinson LS, Humby T, Killcross AS, Torres EM, Everitt BJ, Robbins TW. Dissociations in dopamine release in medial prefrontal cortex and ventral striatum during the acquisition and extinction of classical aversive conditioning in the rat. Eur J Neurosci. 1998;10:1019–26. doi: 10.1046/j.1460-9568.1998.00119.x. [DOI] [PubMed] [Google Scholar]
- [125].Howland JG, Taepavarapruk P, Phillips AG. Glutamate receptor-dependent modulation of dopamine efflux in the nucleus accumbens by basolateral, but not central, nucleus of the amygdala in rats. J Neurosci. 2002;22:1137–45. doi: 10.1523/JNEUROSCI.22-03-01137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Valenti O, Lodge DJ, Grace AA. Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci. 2011;31:4280–9. doi: 10.1523/JNEUROSCI.5310-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].De Olmos JS, Beltramin CA, Alheid GF. Amygdala and extended amygdala of the rat: A cytoarchitectonical, fibroarchitectonical, and chemoarchitectonical survey. In: Paxinos G, editor. The rat nervous system. Elsevier Academic Press; San Diego: 2004. pp. 509–604. [Google Scholar]
- [128].Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- [129].Zahm DS. The evolving theory of basal forebrain functional-anatomical 'macrosystems'. Neurosci Biobehav Rev. 2006;30:148–72. doi: 10.1016/j.neubiorev.2005.06.003. [DOI] [PubMed] [Google Scholar]
- [130].McGregor A, Roberts DC. Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain Res. 1993;624:245–52. doi: 10.1016/0006-8993(93)90084-z. [DOI] [PubMed] [Google Scholar]
- [131].Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res. 1995;692:47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- [132].Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- [135].Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–9. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- [136].Epping-Jordan MP, Markou A, Koob GF. The dopamine D-1 receptor antagonist SCH 23390 injected into the dorsolateral bed nucleus of the stria terminalis decreased cocaine reinforcement in the rat. Brain Res. 1998;784:105–15. doi: 10.1016/s0006-8993(97)01190-6. [DOI] [PubMed] [Google Scholar]
- [137].Eiler WJ, 2nd, Seyoum R, Foster KL, Mailey C, June HL. D1 dopamine receptor regulates alcohol-motivated behaviors in the bed nucleus of the stria terminalis in alcohol-preferring (P) rats. Synapse. 2003;48:45–56. doi: 10.1002/syn.10181. [DOI] [PubMed] [Google Scholar]
- [138].Ju G, Swanson LW. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. Cytoarchitecture. J Comp Neurol. 1989;280:587–602. doi: 10.1002/cne.902800409. [DOI] [PubMed] [Google Scholar]
- [139].Ju G, Swanson LW, Simerly RB. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: II. Chemoarchitecture. J Comp Neurol. 1989;280:603–21. doi: 10.1002/cne.902800410. [DOI] [PubMed] [Google Scholar]
- [140].Rainnie DG. Neurons of the bed nucleus of the stria terminalis (BNST). Electrophysiological properties and their response to serotonin. Ann N Y Acad Sci. 1999;877:695–9. doi: 10.1111/j.1749-6632.1999.tb09304.x. [DOI] [PubMed] [Google Scholar]
- [141].Dong H, Petrovich GD, Swanson LW. Organization of projections from the juxtacapsular nucleus of the BST: a PHAL study in the rat. Brain Res. 2000;859:1–14. doi: 10.1016/s0006-8993(99)02246-5. [DOI] [PubMed] [Google Scholar]
- [142].Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- [143].Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436:430–55. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- [144].Dong HW, Swanson LW. Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: implications for cerebral hemisphere regulation of ingestive behaviors. J Comp Neurol. 2003;463:434–72. doi: 10.1002/cne.10758. [DOI] [PubMed] [Google Scholar]
- [145].Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004;471:396–433. doi: 10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- [146].Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004;468:277–98. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- [147].Hammack SE, Mania I, Rainnie DG. Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. J Neurophysiol. 2007;98:638–56. doi: 10.1152/jn.00382.2007. [DOI] [PubMed] [Google Scholar]
- [148].Phelix CF, Liposits Z, Paull WK. Monoamine innervation of bed nucleus of stria terminalis: an electron microscopic investigation. Brain Res Bull. 1992;28:949–65. doi: 10.1016/0361-9230(92)90218-m. [DOI] [PubMed] [Google Scholar]
- [149].Kozicz T. Axon terminals containing tyrosine hydroxylase- and dopamine-beta-hydroxylase immunoreactivity form synapses with galanin immunoreactive neurons in the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Res. 2001;914:23–33. doi: 10.1016/s0006-8993(01)02770-6. [DOI] [PubMed] [Google Scholar]
- [150].Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- [151].Freedman LJ, Cassell MD. Distribution of dopaminergic fibers in the central division of the extended amygdala of the rat. Brain Res. 1994;633:243–52. doi: 10.1016/0006-8993(94)91545-8. [DOI] [PubMed] [Google Scholar]
- [152].Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–4. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- [153].Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–83. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–8. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- [156].Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- [157].Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1291–308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- [159].McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann N Y Acad Sci. 1999;877:309–38. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- [160].Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: possible role of the bed nucleus of the stria terminalis. J Comp Neurol. 2005;481:363–76. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- [161].Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29:7330–40. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–9. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- [163].Zhang W, Zhang N, Sakurai T, Kuwaki T. Orexin neurons in the hypothalamus mediate cardiorespiratory responses induced by disinhibition of the amygdala and bed nucleus of the stria terminalis. Brain Res. 2009;1262:25–37. doi: 10.1016/j.brainres.2009.01.022. [DOI] [PubMed] [Google Scholar]