Abstract
The uptake of apoptotic cells by phagocytes is defined as efferocytosis. In this issue of Cell Host & Microbe, Martin et al. and Yang et al. report that macrophage- and neutrophil-mediated efferocytosis of apoptotic cells containing mycobacteria is an innate antibacterial effector mechanism.
The interaction of Mycobacterium tuberculosis (Mtb) with its host cell is complex and the mechanisms underlying these interactions are only beginning to be elaborated. The topic of Mtb-induced cell death, for example, has been very confusing, since depending on the study Mtb could either induce or inhibit host cell death (Briken and Miller, 2008). During the past five years tremendous progress has been made leading to a conceptual framework by which Mtb inhibits host cell apoptosis early during its intracellular lifecycle but induces host cell necrosis later(Behar et al., 2010). Consistently, Mtb mutants deficient in inhibiting host cell apoptosis are less virulent (Ernst, 2012). Furthermore, the increased resistance to Mtb infections of different mouse strains is directly correlated with the capacity of their macrophages to induce apoptosis upon Mtb infection(Briken and Miller, 2008).
The documented importance for Mtb to inhibit host cell apoptosis notwithstanding, it is still unclear how increased host cell apoptosis leads to an increase in host resistance to mycobacterial infections. The study by Martin et al. addresses this question and establishes an elegant experiment system which allows the ex vivo and in vivo analysis of efferocytosis involving Mtb-infected macrophages. CD45.2-expressing macrophages are fluorescently labeled and then infected ex vivo with Mtb expressing a fluorescent (mCherry) protein. Next, the infected macrophages are transferred intratracheally into congenic (CD45.1+) mice. After various times total lung macrophage are harvested and the infected macrophages (mCherry+) can then be sorted into three groups: 1) primary infected macrophages (CD45.2+), 2) secondary infection of recipient macrophage (CD45.1+) and 3) efferocytosis of Mtb-infected, apoptotic primary macrophage (CD45.1+ and CD45.2+) by uninfected recipient macrophage. Martin et al. used this system to demonstrate that in vivo efferocytosis is a mechanism for uptake of Mtb by uninfected macrophages in the mouse lung. Furthermore, in vivo inhibition of efferocytosis via injecting neutralizing antibodies to a receptor involved in efferocytosis leads to increased growth of Mtb. This clearly demonstrates the importance of efferocytosis for the innate immune response during Mtb infection. It will be very interesting to see if this experimental system could be used to investigate the role of efferocytosis on the generation of adaptive immunity to Mtb which would have important implication for vaccine design. Another compelling question that could be addressed is whether other phagocytes besides macrophages are involved in efferocytosis in the lung of Mtb infected mice (the report by Yang et al. points towards neutrophils) and if the outcome (killing of Mtb) is the same.
Furthermore, Martin et al. investigated the molecular mechanism of efferocytosis-mediated killing of Mtb. They compared the nature of the Mtb phagosome in primary infected macrophages to the efferocytic phagosome and show that after efferocytosis the Mtb-containing phagosomes mature. These efferocytic phagosomes now accumulate the lysosomal marker Lamp1, recruit the vATPase and acidify. This is in strong contrast to the primary phagosome in which Mtb resides, and where phagosome maturation is inhibited (Figure 1). Using a novel LIVE/DEAD Mtb reporter strain, Martin et al. also demonstrate that most of the bacteria contained in efferocytic phagosomes are dead. The authors propose that the loss of Mtb's capacity to inhibit phagosome maturation in efferocytic phagosomes may be due to the extra lipid bilayer that separates Mtb from its host cell protein targets needed for this manipulation. Another aspect that has not yet been addressed is whether there is a difference in host cell signaling after efferocytosis of a dead uninfected macrophage versus a dead Mtb-infected macrophage, similar to the differences reported in dendritic cells after uptake of apoptotic bodies generated from uninfected or mycobacteria-infected cells (Winau et al., 2006).
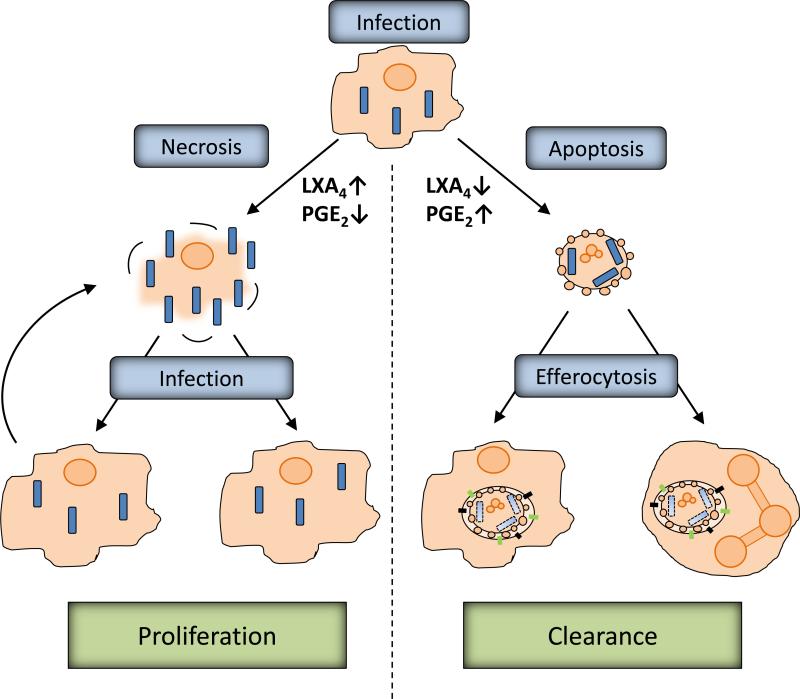
Figure 1. Efferocytosis is an innate host defense mechanism.
The genotype of host cell and mycobacterium both determine if infection induces either necrotic or apoptotic cells death. For example, virulent Mtb strains are able to increase lipoxin A4 (LXA4) and decrease prostaglandin E2 (PGE2) levels, whereas the inverse is true for attenuated strains of Mtb. Apoptotic macrophages can be ingested by uninfected macrophages or neutrophils in a process defined as efferocytosis. The Mtbphagosome generated during efferocytosis is enriched in lysosomal markers, recruits the vacular ATPase and becomes acidic. This process is defined as phagosome maturation and leads to killing of bacteria combined with the increase in reactive oxygens species generated by the host cell NOX2 complex. In contrast, necrosis of host macrophages releases bacteria which can then infect other phagocytes, inhibit phagosome maturation, replicate and induce host cell necrosis to repeat the cycle.
Zebrafish infected with the natural the fish pathogen M. marinum (Mm) is a powerful model system to study innate immune responses early during mycobacterial infection (Tobin and Ramakrishnan, 2008). Initially, Yang et al. discovered that the gfp gene driven by the lysozyme C promoter in transgenic zebrafish is only expressed in neutrophils starting at 48h after fertilization whereas earlier GFP positive cells also include macrophages. This discovery opened the door to use the the zebrafish model to specifically analyze the temporal and spatial response of neutrophils to mycobacterial infections, which is currently not well understood. Using this system the first surprising finding by Yang et al. was that neutrophils, despite their presence, did not appear to phagocytose extracellular mycobacteria at the site of infection, which was in stark contrast to neutrophils at the site of infection with Pseudomonas aeruginosa. Nevertheless, 2-4 days post infection neutrophils with intracellular mycobacteria could be detected, and they were always found at the anatomical sites of granuloma formation in the fish. In a demonstration of the advantages of the zebrafish model Yang et al. used live in vivo imaging to visualize the uptake of infected macrophages by uninfected neutrophils. The morphology suggested that these macrophages were undergoing apoptosis. The use of apoptosis inhibitors demonstrated that the recruitment of neutrophils to granulomas itself is dependent upon apoptosis of cells within the granuloma.
Subsequently, the authors used a different transgenic zebrafish line (WHIM), which is deficient in neutrophil trafficking, to investigate the importance of neutrophils in mycobacterial infections. In these transgenic fish neutrophils are absent from the sites of infection and granulomas which led to an increased number of mycobacteria and granuloma relative to wild-type fish. Consistently, Yang et al. could demonstrate that neutrophils are able to kill mycobacteria contained within the ingested macrophages. This killing was dependent on the generation of reactive oxygen species by the phagocyte oxidase NOX2 complex (Figure 1). In summary, these data clearly demonstrate a protective role for neutrophils during the innate phase of the immune response to mycobacteria. The authors argue that this role might have previously been missed because many earlier studies used only ex vivo infection models which were not designed to detect neutrophil killing of mycobacteria via efferocytosis. Other studies demonstrated that lung neutrophils facilitate activation of CD4 T-cells during Mtb infections and Mtb-mediated apoptosis inhibition of infected lung neutrophils is important to avoid accelerated CD4 T-cell responses (Blomgran et al., 2012; Ernst, 2012). Altogether these recent reports and the current study by Yang et al. underline the importance of increasing our understanding of the role of neutrophils during Mtb pathogenesis. Furthermore, Yang et al. provide a second line of evidence in addition to the paper by Martin et al. for the importance of efferocytosis during the innate immune response against mycobacteria in vivo.
Despite setting out to address two apparently very different questions in mycobacterial pathogenesis, both research group have come to one common central finding, which is that efferocytosis helps in the clearance of intracellular bacteria. This expands the well documented role of efferocytosis in the prevention of inflammatory responses to a function in the innate immune defense. In addition, it is likely that efferocytosis also plays a role in the immune defense against other intracellular pathogens (Chlamydia, Legionella) that are known to inhibit host cell apoptosis during part of their lifecycle (Behar et al., 2010; Briken and Miller, 2008). Consequently, one could now hypothesize that enhancing host efferocytosis may not only help to prevent excessive tissue injury during the immune response but also help in clearance of the pathogen, since Martin et al. showed that inhibition of efferocytosis in vivo leads to an increase in bacterial growth. For example, the High Mobility Group Box 1 (HMGB1) protein negatively regulates efferocytosis (Banerjee et al., 2011). Hence, stimulating efferocytosis may be one of the mechanisms by which the treatment of mice with neutralizing HMGB1 antibodies enhanced the clearance of P. aeruginosa in a mouse cystic fibrosis model (Entezari et al., 2012). One could thus envision a novel treatment approach for tuberculosis which would ideally take advantage of the putative synergy of new anti-mycobacterial drugs inhibiting the capacity of Mtb to suppress host cell apoptosis with host immunomodulatory therapeutics stimulating efferocytosis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banerjee S, de Freitas A, Friggeri A, Zmijewski JW, Liu G, Abraham E. Intracellular HMGB1 negatively regulates efferocytosis. J Immunol. 2011;187:4686–4694. doi: 10.4049/jimmunol.1101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Microbiol. 2010;8:668–674. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgran R, Desvignes L, Briken V, Ernst JD. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell Host Microbe. 2012;11:81–90. doi: 10.1016/j.chom.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briken V, Miller JL. Living on the edge: inhibition of host cell apoptosis by Mycobacterium tuberculosis. Future Microbiol. 2008;3:415–422. doi: 10.2217/17460913.3.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entezari M, Weiss DJ, Sitapara R, Whittaker L, Wargo MJ, Li J, Wang H, Yang H, Sharma L, Phan BD, et al. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas Aeruginosa pneumonia in cystic fibrosis. Mol Med. 2012;18:477–485. doi: 10.2119/molmed.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012;12:581–591. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- Tobin DM, Ramakrishnan L. Comparative pathogenesis of Mycobacterium marinum and Mycobacterium tuberculosis. Cell Microbiol. 2008;10:1027–1039. doi: 10.1111/j.1462-5822.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- Martin CJ, Booty MG, Rosebrock TR, Nunes-Alves C, Desjardins DM, Keren I, Fortune SM, Remold HG, Behar SM. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe. 2012 doi: 10.1016/j.chom.2012.06.010. THIS ISSUE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, Sandhoff K, Brinkmann V, Kaufmann SH, Schaible UE. Apoptotic Vesicles Crossprime CD8 T Cells and Protect against Tuberculosis. Immunity. 2006;24:105–117. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Yang C-T, Cambier CJ, Davis JM, Hall CJ, Crosier PS, Ramakrishnan L. Neutrophils Exert Protection in the Early Tuberculous Granuloma by Oxidative Killing of Mycobacteria Phagocytosed From Infected Macrophages. Cell Host Microbe. 2012 doi: 10.1016/j.chom.2012.07.009. THIS ISSUE. [DOI] [PMC free article] [PubMed] [Google Scholar]