Abstract
Neurofibrillary tangles (NFTs) are one of the pathological hallmarks of Alzheimer’s disease (AD) and are primarily composed of aggregates of hyperphosphorylated forms of the microtubule associated protein tau. It is likely that an imbalance of kinase and phosphatase activities leads to the abnormal phosphorylation of tau and subsequent aggregation. The wide ranging therapeutic approaches that are being developed include to inhibit tau kinases, to enhance phosphatase activity, to promote microtubule stability, and to reduce tau aggregate formation and/or enhance their clearance with small molecule drugs or by immunotherapeutic means. Most of these promising approaches are still in preclinical development whilst some have progressed to Phase II clinical trials. By pursuing these lines of study, a viable therapy for AD and related tauopathies may be obtained.
Keywords: Tau, therapy, kinases, phosphatase, aggregation, immunotherapy
Introduction
Alzheimer’s disease (AD) is a progressive and fatal neurodegenerative disorder characterized by two major neuropathologic hallmarks: extracellular senile plaques composed of amyloid beta (Aβ) and intracellular neurofibrillary tangles (NFT). The cause of this disease is still unknown and is most likely multifactorial. Several genetic and environmental risk factors have been proposed including age, apolipoprotein E4 genotype, diabetes, cardiovascular disease, mutations in the amyloid precursor protein (APP), education levels, elevated cholesterol, SORL1, depression, head injury, mutations in the presenilin genes and others (1). NFT’s were first described by Alois Alzheimer in 1906. In the early sixties, ultrastructural analysis by electron microscopy showed that NFT’s were composed of paired helical filaments (PHF) (2). Tau is a heat stable protein which was found to be essential for microtubule assembly and was identified by its ability to co-purify with microtubules through repeated cycles of polymerization (3). In 1984, Iqbal and colleagues isolated PHF’s from pathologically confirmed AD brain (4). Thereafter, in 1985 it was discovered that tau was a major component and antigenic determinant of PHF (5). This finding was quickly confirmed by several other groups (6–8). At the same time immunoblotting of a supernatant preparation from AD brain with an antibody known as Alz 50, was found to label a band with an apparent molecular weight of 68 kDa. The authors suggested that the Alz 50 antigen was unlikely to be tau (9), but it was later shown that Alz 50 did in fact recognize a phosphorylated tau epitope (10). The novel idea that PHF’s from AD brain were composed of not just tau but phosphorylated tau was first confirmed by Grundke-Iqbal and colleagues and Ihara and colleagues in 1986 (11;12). This finding is of added significance since tau’s phospho-status can affect its microtubule binding ability (13;14), thus many groups have sought to identify the kinases that play a role in PHF tau phosphorylation. More recently, the role of phosphatases leading to abnormal tau phosphorylation has also been investigated (15).
Our current understanding of tau’s function and dysfunction has been aided with the discovery of mutations in the tau gene which are responsible for a range of neurodegenerative disorders collectively termed tauopathies – since they are characterized by the presence of aggregated tau deposits. However, no mutations in the tau gene are associated with AD. With an increasing number of disorders proposed to be caused by tau pathology, there is a very urgent need to develop viable therapeutics. The focus of this minireview will be to assess some of the currently available and potentially novel strategies aimed at targeting tau pathology in neurodegenerative disease.
Phosphorylation of Tau
Tau, a member of the family of microtubule associated proteins (MAPs), is widely expressed in both the central and the peripheral nervous system. Along with various other MAPs, tau is involved in modulating microtubule assembly and maintaining stability within neurons (16). There are over 80 Ser/Thr-Pro motifs in tau which can act as potential phosphorylation sites and it has been shown that increased phosphorylation diminishes tau’s ability to effectively bind microtubules (14). Tau phosphorylation is developmentally regulated, with increased tau phosphorylation in fetal brain compared to adult brain. The phospho-status of tau is controlled by the activities of various kinases and phosphatases. Several kinases including proline directed kinases such as mitogen activated protein kinase (14;17), stress activated kinases (17;18), glycogen synthase kinase 3β(GSK3β) (14);(19), and cyclin dependant kinases cdc2 and cdk5 (20), and non-Ser/Pro kinases, such as calcium-calmodulin dependant protein kinase II (CaMKII), casein kinases I and II and protein kinases A and C (21;22), have been shown to phosphorylate tau in vitro or in situ. The most likely candidates for physiological and pathological conditions are proposed to be glycogen synthase kinase 3 (GSK3), cyclin-dependent kinase 5 (cdk5) and the microtubule-affinity-regulating kinase (MARK) (23). Phosphatases PP1, PP2A, PP2B and PP2C have also been shown to have a role in modulating the phosphorylation state of brain tau. Although tau can undergo other post translational modifications such as glycosylation, ubiquitination and glycation, most of our interest has been directed to the study of tau phosphorylation since tau in an abnormally phosphorylated state is the main component of one of the pathological hallmarks of AD - the intraneuronal neurofibrillary tangle (NFT) that accumulates in the brain of AD patients (24;25). Also, it was found that levels of NFTs rather than amyloid burden, correlated better with the presence of dementia (26;27)
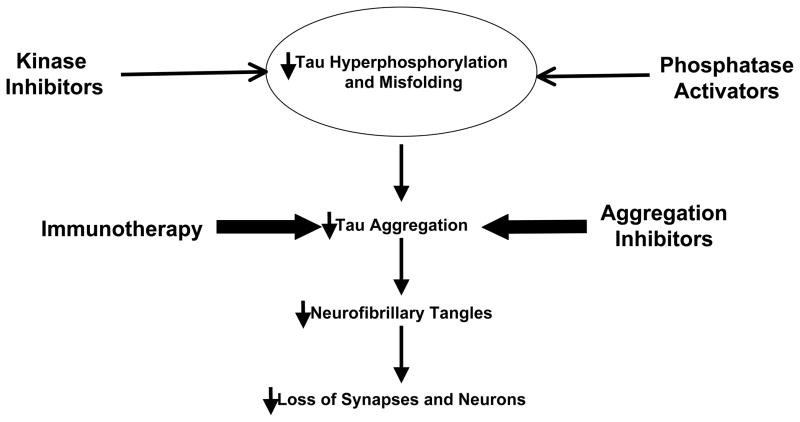
The following has been proposed as the pathogenic series of events that lead to neuronal death. Abnormal tau hyperphosphorylation leads to decreased microtubule binding. This results in destabilized microtubules and a loss of axonal integrity. Detachment of tau results in an increase in the pool of free tau which is available to form misfolded protein aggregates. Based on this model, there are several potential tau based therapeutic targets. For example, the imbalance between kinase and phosphatase activities induces changes in tau phosphorylation, suggesting kinase inibitors and phosphatase activators as a potential therapy targeting tau pathology.
Tau Based Therapies
Since 1990, therapeutic approaches in the AD field have mainly focused on targeting Aβ pathology. The discovery that mutations in the tau gene alone could result in diseases collectively termed tauopathies (28);(29) has reinvigorated efforts on the development of treatment strategies aimed at rectifying tau dysfunction. Tau mutations fall under two categories, a) those that affect the alternative splicing of Exon 10, thereby affecting the ratio of 3 repeat versus 4 repeat tau isoforms, and b) those that affect tau microtubule interactions. Thus, it has been proposed that in the tauopathies there is a loss of tau function i.e. microtubule binding ability, followed by a gain of toxic effect – the enhanced ability to form tau aggregates. To clarify our understanding of the pathophysiology of tau, several transgenic mouse models have been generated. They include the JNPL3 line, which expresses the most common P301L mutation found in frontotemporal dementia and Parkinsonism linked to chromosome 17 (FTDP-17). This model and other similar ones have been shown to develop extensive neurodegeneration (30);(31) and provide a valuable in vivo system to test therapeutic strategies.
Kinases as a Therapeutic Target
More than 20 protein kinases can phosphorylate tau in vitro and in cells. The imbalance of the activities of kinases and phosphatases most likely leads to the aberrant hyperphosphorylation of tau seen in tauopathy. Hence an obvious and desirable therapeutic target would be to inhibit tau kinases, but it is essential to identify which ones to target (32–35).
GSK3β, cdk5 and MARK, have been proposed as the three main tau kinases responsible for phosphorylating the majority of epitopes that are present in PHF-tau (36);(37). Of these three kinases GSK3β and cdk5 have received particular attention, and are the primary targets for drug discovery efforts.
GSK3 Inhibitors
GSK3 is a serine-threonine kinase first identified as an enzyme that phosphorylates glycogen synthase in the glycogen synthesis pathway. There are two isoforms of GSK3 encoded by two independent genes, GSK3α and GSK3β. GSK3β is especially abundant in brain (38). GSK is highly conserved, ubiquitously expressed and found in all eukaryotes. It can phosphorylate tau, neurofilament protein and several transcription factors such as CREB, c-Jun, c-myc, c-Myb and HSF-1 (Heat shock transcription factor) in vitro and in vivo (39;40). Furthermore, it has been identified as a participant in diverse cellular processes including promoting cell death (41) or survival (42;43), signaling pathways, metabolic control, and oncogenesis. In addition, dysregulation of GSK3β has been implicated in a wide range of neurological and degenerative diseases such as bipolar mood disorder and AD (44;45). It has also been shown that inhibition of GSK3 facilitates long term potentiation (46). In AD brain GSK3β has been shown to co-localize with dystrophic neurites and NFT’s (47–49). The active form of GSK3 was found in neurons with pre-tangles (50). Based on these findings it is evident that GSK3β is a promising candidate to be targeted for developing new drugs for AD and other tauopathies (51). Several classes of GSK inhibitors have been identified (52), in addition to peptides (53) and metal ions (54). Some of these molecules have shown efficacy in different animal models (55).
Paullones, indirubines and bisindol-maleimides represent some of the first class of GSK3 inhibitors discovered by high throughput screening. However since these inhibitors compete with ATP for its binding site, these compounds could readily inhibit other kinases such as CDK1 and CDK2. Importantly, knowledge gained from the crystal structure of GSK3β (56;57), has allowed various potential structure based pharmacophores to be designed (58;59).
Lithium as a GSK3 Inhibitor
The alkali metal lithium was discovered in 1817, and utilized as a remedy for multiple human diseases (60), including as effective therapy for manic–depressive illness (61;62). Lithium is selective for GSK3, but its mechanism of action is not well understood (63). Cell culture and in vivo studies have clearly shown that lithium treatment can effectively inhibit the enzyme and reduce tau phosphorylation levels (63–65), thus suggesting lithium as a potential therapeutic agent for AD and other tauopathies (66). However, a recent study found no effect of lithium on Aβ load or memory deficits, despite the reduction of phosphorylated-tau in a triple transgenic mouse model of AD (67).
Lithium has been used as a treatment for various psychiatric disorders for over 50 years, but its use as a potential therapy for AD has only been explored more recently. A pilot study examined the feasibility and tolerability of lithium carbonate in mild to moderate AD. Patients were treated for upto a year and their cognition and adverse effects were assessed (68). The pilot study showed no difference in deaths, drop outs or change in mini mental state exam (MMSE) between those receiving lithium and the comparison group, however discontinuation rates were high and many study participants reported contraindications; thus the authors concluded that lithium therapy for AD may have limited potential (68).
The interest in finding inhibitors of GSK has resulted in the development of several classes of small molecule inhibitors. Bhat and colleagues from AstraZeneca have written a very comprehensive review on GSK3 inhibitors for the treatment of AD and readers are encouraged to refer to this review for details about the various inhibitor classes. In this review we will briefly outline these classes of inhibitors (69).
Other GSK3 Inhibitors
The amino-thiazole small molecule known as AR-A0114418 (Astra Zeneca) has been described as a selective GSK3 inhibitor which acts in an ATP competitive manner (70). It has been shown to inhibit tau phosphorylation on Ser 396 in cells overexpressing the long isoform of human tau (4 repeat tau) (70). It also reduces tau phosphorylation, accumulation and axonal degeneration in a tauopathy mouse model (71). Inhibition of cell death by this molecule correlates with reduced GSK3 activity. These results indicate that GSK-inhibitors can be neuroprotective.
Aminopyrazinyl-2-carboxamide (AZ11125357; Astra Zeneca) is a novel small-molecule GSK3 inhibitor identified through a high-throughput biochemical screen using purified recombinant human GSK3. It inhibits GSK3 mediated tau phosphorylation in cells by competing with ATP binding and it inhibits CDK2 as well (69).
Oxindolequinazoline (AZ10316813; Astra Zeneca) inhibits GSK3β by competing with ATP binding and demonstrates selectivity against more than ten other kinases tested, including IGF1R, ZAP70, PTK2, SRC, JAK3, Abl, p38, JNK1, PKA, MAP2K1 and CHK1 but not CDK2.
Thiadiazolidindiones (TDZD’s) represents the first selective GSK3 inhibitor entering phase I clinical trial (Neuropharma) as a new therapeutic agent which inhibits GSK3 and tau phosphorylation in AD (72;73). Oral treatment of animals with TDZD’s decrease tau phosphorylation in a dose and time dependent way (74).
SRN-003-556 (Sirenade) inhibits ERK2/cdc2, GSK3β, PKA and PKC, and has been shown to reduce soluble hyperphosphorylated and aggregated tau in a P301L tau mouse model (75).
SB-216763 and SB-415286 developed by SmithKline Beecham are maleimide ATP-competitive inhibitors that inhibit GSK3 (76). In primary cerebellar granule neurones, an inhibition of Thr181 and Ser202 tau phosphorylation was shown with both these compounds. In HEK293 cells overexpressing recombinant tau and GSK3β, SB-216763 and SB-415286 treatment led to a reduction in tau phosphorylation at Ser202 (77). SB-216763 and SB-415286 have been shown to cause a decrease in inhibitory Ser9 phosphorylation of GSK3β. This effect is possibly via activation of an upstream kinase p90rsk, that has been shown to inhibit tau phosphorylation at the GSK3-specific site Ser396 (78;79).
Cdk 5 Inhibitors
In 1993, Kobayashi et al., discovered that Tau protein kinase II is cdk5 (20). It is expressed ubiquitously (80;81) and along with GSK, it has been suggested to play a significant role in tau phosphorylation and contribute to the formation of NFT’s in AD brain (82;83). Cdk5 phosphorylates several substrates, and is involved in such processes as insulin exocytosis (84), neurotransmitter release (85), dopamine signaling pathways (86), and neurite outgrowth (87). Cdk5 is a 33 kDa proline-directed protein kinase that phosphorylates serine and threonine residues immediately preceding a proline residue (88–92). Previous studies have suggested that cdk5 activity plays an important role in axonal growth, neuronal migration, synaptic function, neurite outgrowth, synapse formation at the neuromuscular junction and myogenesis (93;94). Many of the substrates of cdk5 are cytoskeleton proteins, such as microtubule associated protein tau. Purified cdk5 was shown in vitro to phosphorylate several tau epitopes using multiple methods explored by different laboratories, such as mass spectrometry, two dimensional gel electrophoresis using radioisotope labeling and phosphotau dependent antibodies (95–97). It can phosphorylate tau at several pathologically relevant residues such as: Ser202, Thr205, Thr212, Thr217, Ser235, Ser396 and Ser404 (98–100).
Cdk5 requires phosphorylation of the activation loop for full activation (101), and depends on the presence of p35 (102;103) or p39 (104). p25 is generated from p35 by proteolytic cleavage (102;105;106), that can lead to neuronal cell death by unknown mechanisms and has been implicated in the progression of AD (107–111). p25 is more effective than p35 in activating cdk5 to phosphorylate tau (112). Overexpression of cdk5 and its activators in neurons and cell lines can induce tau phosphorylation on multiple epitopes (113–115). These findings have been validated in mouse models, in which overexpression of activated cdk5 or p25 leads to the formation of NFTs and impairment of cognitive functions (116–118). P301L mice crossed with p25 mice have a fivefold increase in NFT (119). Interestingly, triple Tg mice that overexpress cdk5, human p35 and tau do not show an increase in tau phosphorylation (120). While non-selective cdk inhibitors have been profiled in preclinical and clinical studies, clinical trials have yet to be initiated on selective cdk5 inhibitors.
The crystal structure of active cdk5/p25 kinase complexed with the three inhibitors, R-roscovitine (121–123), aloisine (124), and indirubin-3′-oxime (125) has been reported. These chemicals are ATP antagonists, and R-roscovitine is entering phase I and II clinical trials against glomerulonephritis and cancer, respectively (123).
MARK/PAR1 as a Target for Inhibition
The microtubule affinity regulating kinase (MARK/PAR1) phosphorylates microtubule associated proteins (MAPs) such as MAP2, MAP4 and tau, resulting in their dissociation from microtubules (126;127). Human PAR-1 is encoded by four genes, giving rise to the isoforms MARK1 (PAR-1c), MARK2 (PAR-1b/EMK), MARK3 (PAR-1a/p78/C-TAK1) and MARK4 (PAR-1d/MARKL1), encoded respectively on chromosomes 1, 11, 14, and 19 (128). Members of the MARK/PAR-1 kinase family play crucial roles in cellular functions such as polarity and cell-cycle control (129;130). MARK 1 is involved in axonal transport and neurite growth (131;132). MARKs phosphorylate tau, MAP2 and MAP4 at KXGS motifs in the repeat regions, and reduce their affinity for microtubules which then become more unstable (131). Phosphorylation of KXGS motifs in the repeat region of tau was found to occur at an early pre-tangle state in AD brains (133). Also, fluorescence resonance energy transfer experiments showed that MARK was responsible for phosphorylation of the Ser262 epitope in PHF’s from AD brain (134). The Mandelkow group has created an inducible mutant tau transgenic mouse (ΔK280), which replicates MARK phosphorylation of the Ser262 site at an early age (135).
Due to its role in phosphorylating tau within the microtubule binding region, MARK maybe another target for therapeutic intervention in AD and other tauopathies. Staurosporine and hymenialdisine have been used in situ as non-selective pharmacological inhibitors of MARK and its activating kinase (MARKK) (131;136).
Interestingly, GSK3β was shown to phosphorylate MARK and inhibit its activity. Since GSK3β can also phosphorylate tau, the net effect of these two kinases would be to alter the status of tau phosphorylation in AD (137).
Phosphatases
The restoration of tau phosphatase activity by targeting its subunits is another approach to reduce hyperphosphorylated tau. Tau is dephosphorylated by protein phsophatase 2A (PP2A), and to lesser effect by PP1, PP2B and PP5 (138;139). PP2A is a major phosphatase implicated in tau phosphorylation in vitro. In addition to dephosphorylating the kinase substrate, PP2A regulates the activity of protein kinase. Kinases and phosphatases act synergistically on tau phosphorylation because the phosphorylation of tau by GSK3 or Cdk5 is overridden by inhibition of PP2A (140).
Phosphatase activity (PP2A and PP2B) is lower in AD brain compared to controls (141;142). Okadaic acid inhibits PP2A and PP1, and in rat brain slice cultures increases tau phosphorylation but does not lead to the formation of tangles (143). Transgenic mice with reduced PP2A activity show a somatodendritic accumulation of hyperphosphorylated and aggregated tau in cortical pyramidal cells and cerebellar purkinje cells (144). Memantine, a noncompetitive N-methyl-D-Aspartate (NMDA) receptor antagonist already approved for the treatment of AD, many also influence PP2A. Using a hippocampal slice culture model, Li et al., showed that Memantine could reverse tau hyperphosphorylation induced by pre-treatment with okadaic acid, most likely through its effect on PP-2A signaling pathway (145). This novel finding from a slice culture model should be evaluated further clinically.
Other Proteins Affecting Tau Phosphorylation
Another potential target for tau therapy is Pin1 (protein interacting with NIMA1) which regulates tau phosphorylation. Tau and Pin1 bind when tau is phosphorylated at Thr231 (146). In AD, more tangles were observed in pyramidal neurons with a lower amount of Pin1, and no tangles were detected in neurons expressing high levels of Pin1 (147). Pre-incubation of phosphorylated tau with Pin1 was shown to restore the ability of abnormally phosphorylated tau to promote microtubule assembly (146). Furthermore, Pin1 knockout mice have been shown to develop hyperphosphorylated tau, tau aggregates and neuronal degeneration (147) suggesting that Pin1 activators could be a potential therapy for tauopathies.
Aggregation of Tau
Under normal conditions, tau is a very soluble protein that does not readily aggregate into filaments and phosphorylated tau does not spontaneously form PHF’s in vitro. However, its fibrillogenicity is influenced by post translational modifications such as enzymatic cleavage (148), and phosphorylation can promote or inhibit aggregation depending on which epitopes are involved (149).
Methylene Blue
In 2008 Wischik and colleagues piqued the interest of the AD field when they presented data from a Phase II trial using the drug methylene blue (MB) (RemberTM). This was the first clinical study to examine inhibitors of tau aggregation as a potential therapy for treating AD. MB is a heterocyclic aromatic which belongs to a class of compounds known as phenothiazines. Its uses are varied and numerous. They include as a dye for staining, a redox indicator, a cheap and effective treatment for malaria, as an antiseptic and even as an antipsychotic drug (150;151). In 1996 it had been determined that MB (phenotiazines) could be used to inhibit tau-tau binding through the repeat domain (152). This finding suggested that the self assembly of tau into pathological PHF’s could be prevented.
The data presented at the ICAD in 2008 examined 321 people with mild or moderate AD and compared the effect of three different doses of MB versus placebo on cognitive abilities as measured by ADAS-Cog (153). Amazingly, patients with moderate AD and treated with MB had a significant improvement in tests of cognitive function at six months. Three different MB doses were compared and by 50 weeks of treatment 60 mg MB resulted in an 81% reduction in rate of decline compared to placebo treated.
More recently, it has been reported that Wischik’s team has developed and patented a new form of MB called leuco-methylthioninium or LMT, which is no longer blue and renders the drug more bioavailable and less toxic at higher dose (Alzforum 2009 and web http://www.alzforum.org/new/detail.asp?id=2203).
N744
The lab of Jeff Kuret has also made headway in identifying compounds to prevent tau oligomerizarion or fibrillization. They have screened small molecule libraries to find fibrillization antagonists. One of these is a cyanine dye compound called N744. It was found to inhibit tau fibrillization and promote disaggregation of mature synthetic filaments (154).
Another way to identify potential tau aggregation inhibitors has been to screen large compound libraries which have sufficient structural diversity. This then allows any potential “hits” to be optimized by medicinal chemists and further developed to have appropriate inhibitory properties and pharmacokinetics (155). The Mandelkow group have screened a random library of 200,000 compounds, from which they were able to identify 77 compounds that were found to inhibit PHF formation and inhibit tau aggregation as validated by using a thioflavine S fluorescence assay. Further screening identified rhodanines, antraquinones and N-phenylamines as classes of aggregate inhibitors. The efficacy of these compounds was tested using a neuronal cell model and the compounds were found to successfully prevent tau aggregation (156–160).
Taniguchi and colleagues also identified phenothiazines, porphyrins and polyphenols as classes of compounds that were successful at inhibiting heparin induced tau filament formation (161).
One caveat that must be borne in mind when contemplating the potential benefits of tau aggregation and fibrillization inhibitors is that more toxic oligomers may be generated, in the course of preventing the formation of PHF like tau aggregates. If this is the case then the usefulness of these aggregate inhibitors would be limited.
Microtubule Stabilising Drugs
Tau in a pathological state is abnormally phosphorylated and this is proposed to result in a loss of one of its classical functions – the binding of microtubules leading to decreased microtubule stability (162). In fact it was shown immunohistochemically that levels of acetylated α-tubulin, an indicator of stable microtubules, were decreased in tangle bearing neurons in AD brain tissue (163). This impairment in tau–cytoskeleton interactions may have consequences on axonal integrity and cellular transport of organelles and vesicles (164). Thus, another valid treatment strategy that has gained some attention is the use of drugs to complement the microtubule stabilising function of tau such as paclitaxel. Early studies by Michaelis et al., showed the promise of this approach in culture models using low doses of microtubule stabilizing drugs including paclitaxel (Taxol®) (165), which is already widely used as an anti cancer drug. Further work carried out in the group of Virginia Lee and John Trojanowski showed that injections of paclitaxel could restore fast axonal transport deficits and improve motor impairments in a murine tauopathy model (166). However paclitaxel does not readily cross the blood brain barrier (BBB), and its beneficial effect was proposed to be a result of uptake at the neuromuscular junction and retrograde transport to spinal motor neurons (167). Its ability to access the brain is limited because of its high affinity to the efflux transporter ABCB1 (p-glycoprotein 170, p-gp) (168–170). By blocking ABCB1 mediated efflux, it would be possible to increase the concentration of ABCB1 substrates such as paclitaxel in the brain (171–173). New third generation p-gp modulators have been developed, which aim to achieve the delivery of efficacious brain concentrations of paclitaxel and overcome potential systemic toxic side effects of these powerful antimitotic agents (174). Recently, Brunden and colleagues tested the efficacy of Epothilone D (EpoD), a brain penetrant microtubule stabilizing compound. PS19 tau transgenic mice were treated for EpoD for three months. Low doses of EpoD were well tolerated and resulted in significantly fewer dystrophic axons and increased microtubule density in the optic nerve. Additionally, EpoD treated PS19 mice appeared to perform better than vehicle treated mice in the Barnes maze - a test of cognitive function (175).
The octapeptide NAPVSIPQ (NAP) has also been proposed to be a microtubule stabilizing agent, it can cross the BBB and has been shown to promote microtubule stability (176), as discussed in more detail below.
NAP (NAPVSIPQ) is an eight amino acid peptide derived from activity-dependent neuroprotective protein (ADNP) (177–179). The group of Illana Gozes has carried out most of its in vitro and in vivo studies. NAP treatment in tissue culture and in situ models has been shown to protect neurons against a wide variety of toxins and stresses such as Aβ peptide, oxidative stress and zinc intoxication (180;181). NAP was also found to associate with tubulin and enhance proper microtubule assembly (181). Based on these in situ studies it was suggested that NAP may be a promising drug candidate for the treatment of tauopathy in vivo, as tau dysfunction may lead to loss of microtubule integrity. Using the triple transgenic AD model [31], Matsuoka et al., demonstrated that presymptomatic treatment with NAP protected against increased tau hyperphosphorylation and lowered levels of Aβ 1-40 and 1-42 (182). Additionally, triple transgenic mice that received a six month course of NAP treatment showed improved cognitive performance in the Morris water maze (MWM) and an increase in heat-stable soluble tau (presumably containing microtubule-associated tau) (183). Studies with a “pure” tauopathy mouse model expressing two tau mutations (P301S and K257T), reinforced that mice treated with NAP performed better in the Morris water maze, had reduced levels of phosphorylated tau and NFT pathology and had increased levels of soluble tau (184). Allon pharmaceuticals have developed Davunetide, a compound based on NAP, delivered as a nasal spray, and have evaluated its effectiveness in patients with mild cognitive impairment. Their study reported at ICAD 2008 indicated that patients treated with AL108 (NAP) had a significant improvement over placebo treatment in two measures of memory, but not on the primary or secondary endpoints in other cognitive tests. Headache and nasopharyngeal events were reported more frequently by NAP-treated subjects. However, the overall incidence of adverse events was similar between placebo- and NAP-treated groups ((185); www.allontherapeutics.com).
Targeting Glycosylation and Chaperones
Thus far we have discussed the major post-translational modification that tau undergoes –namely phosphorylation. Another modification that tau undergoes and that has been shown to reciprocally affect tau’s phosphorylation state is glycosylation, for example abnormally hyperphosphorylated tau and PHF tau have been found to be glycosylated (186). A specific form of glycosylation, O-linked β-N-acetylglucosaminylation (O-GlcNAc), which was only discovered in 1983 (187), may have a significant role in AD pathophysiology. In the healthy adult brain, tau is extensively O-GlcNAcylated at more than 12 sites but the O-GlcNAcylation level in AD brain extracts has been shown to be decreased compared to controls (188). Interestingly, O-GlcNAcylation of human tau was found to negatively regulate its phosphorylation in a site-specific manner both in vitro and in vivo (188). Thus it has been suggested that by increasing tau O-GlcNAc levels, the hyperphosphorylation of tau could be blocked and the accumulation of toxic tau species prevented (189). To test this, Yuzwa and colleagues have synthesized a potent inhibitor of human O-GlcNAcase, thiamet-G. Thiamet-G decreased phosphorylation of tau in PC-12 cells at pathologically relevant sites including Thr231 and Ser396. Thiamet-G also efficiently reduced tau phosphorylation at Thr231, Ser396 and Ser422 in both rat cortex and hippocampus, and thereby provides an alternative route to block pathological hyperphosphorylation of tau in AD (189).
Since AD is characterized by the accumulation of abnormally aggregated proteins, several labs have examined the role of chaperones in contributing to misfolded protein disorders and also as a potential site for therapeutic intervention. Molecular chaperones are proteins that regulate the correct handling, conformation and transport of other proteins, as well as facilitating the removal of “misbehaving” proteins by transporting them to the proteosome to be degraded (190;191). One class of chaperones are the heat shock proteins (HSP). Immunohistochemical and biochemical studies showed that HSP27 expression was elevated in AD brains, particularly in astrocytes (192). HSP27 has also been shown to directly bind to hyperphosphorylated tau, decreasing its concentration and thus protect against hyperphosphorylated tau induced cell death in a neuronal cell line (193). Levels of HSP70 and HSP90, the two main chaperone scaffolds, which protect cytosolic proteins against stress-induced unfolding and aggregation (194), were also elevated in affected regions from AD brain tissue (195;196). The upregulation of these proteins may suggest that the brain is attempting to actively clear the buildup of aggregated or pathological proteins. Thus the interest in modulating HSP’s as a therapeutic target for AD and other neurodegenerative diseases. The Petrucelli and Chiosis groups have been actively pursuing the development of HSP inhibitors. Dickey et al., used a mutant P301L tau expressing CHO cell line to assess the effect of various small HSP90 inhibitors. These compounds reduced levels of tau phosphorylated at proline-directed Ser/Thr sites (pSer202/Thr205, pSer396/Ser404) and conformationally altered (MC-1) tau species, but tau phosphorylated at Ser262/Ser356 within the tau microtubule binding domain was more resistant to degradation (197). Luo et al., used a novel HSP90 inhibitor, PU24FCl, to treat cortical neurons and a derivative, PU-DZ8, which can more readily enter the brain, to treat JNPL3 mice, and showed that levels of phosphorylated and aggregated tau could be reduced (198).
Tau Immunotherapy
A novel therapeutic approach for Alzheimer’s disease (AD) that is currently being intensively pursued is immune modulation to clear amyloid-β (Aβ). Studies in transgenic mice showed that immunization with aggregated Aβ1-42 was able to reduce Aβ levels and associated pathology (199). Additional studies indicated that the mechanism for this effect was likely to be antibody-mediated (200;201). The promise shown by these vaccination studies led to the initiation of a Phase I clinical study in AD patients using Aβ1-42 (AN 1792). The subsequent Phase IIa study had to be halted because 6% of the patients developed meningoencephalitis (202). Despite the suspension of the clinical trial, there were several positive outcomes. Neuropathological examination of three cases stunningly showed that Aβ had been removed and there was a resolution of dystrophic neurites (203). Also, cognitive testing of the Zurich cohort indicated that of the patients who were immunized and generated antibodies to Aβ there was a significantly slower rate of decline of cognitive functions and activities of daily living (204). But this promising finding was not detected in the overall analysis of all the study sites, although a trend for cognitive stabilization was observed (205). And disappointingly, more recent autopsy data from a few subjects from the trials indicates that complete clearance of Aβ plaques does not appear to halt the progression of AD dementia (206). This approach is being refined and further trials are underway. Interestingly, passive immunization targeting Aβ was recently shown to reduce amyloid detected with in vivo PET imaging (207). However, Aβ clearance in the AN 1792 trial did not appear to affect tangle pathology (208;209), although plaque-associated tau pathology was decreased (203;210;211). Autopsy data is not yet available from the second generation Aβ immunotherapy trials. Using the 3XTg mouse model, immunotherapy targeting Aβ cleared early tau pathology but hyperphosphorylated or aggregated tau was not affected (212). Together, these findings further emphasize the need for therapy targeting neurofibrillary tangles.
As detailed above, therapeutic approaches targeting tau pathology have concentrated on reducing its level of phosphorylation primarily by targeting tau kinases. An alternative novel approach, from our lab has used active immunization with peptides containing pathologically hyperphosphorylated tau epitopes or corresponding antibody, resulting in a decrease in levels of aggregated tau which importantly includes prevention of cognitive impairments and reduction in other functional impairments in two tauopathy mouse models (213–218). Hence, clearance of NFTs or their precursors could reduce the amount of synaptic and neuronal loss associated with AD. Importantly, the functional outcome correlated well with reduction of tau aggregates (219). Studies from related fields have helped to add credence to our tau immunotherapy approach as a viable treatment strategy. While our studies were underway, vaccination with recombinant α-synuclein was shown to lead to clearance of intraneuronal aggregates of the same sequence in transgenic Parkinson’s disease model mice (220), and subsequently anti-Aβ antibodies were shown to be taken up into neurons in culture and promote the clearance of intracellular Aβ aggregates via the endosomal-lysosomal pathway (221). The idea that an immunological approach could be used to target an intracellular protein has met with a fair deal of skepticism. However, we have showed that following intracarotid injections of FITC-labeled anti-tau antibodies in tangle model mice, these antibodies are detected within brain neurons bound to tau aggregates (213). Furthermore, clearance of extracellular tau may also be beneficial, as it may be toxic and/or have a role in the spread of tau pathology throughout the brain. The addition of various recombinant tau constructs to the culture media of SH-SY5Y cells has been shown to increase cell death (222). More recently it was reported that extracellular tau aggregates, but not monomer, can be taken up by cultured cells and displace tubulin, co-localize with dextran, a marker of fluid-phase endocytosis, and induce fibrillization of intracellular full-length tau (223). This interesting finding was extended by an in vivo study that indicated that extracellular tau mediates the spread of tau pathology through anatomically connected regions (224). These findings clearly enhance the value of an immunotherapeutic approach targeting tau as these antibodies should have better access to the extracellular pool of tau compared to its intracellular one. The potential mechanism of tau immunotherapy is discussed extensively in (219;225).
Conclusion
Currently there is no cure for a multifactorial disease like AD. In this mini review we have described some of the extensive data that supports a causative role for tau protein in the pathogenesis of tauopathies. For targeting the dysregulation of tau protein in an appropriate temporal and spatial manner, the various potential treatment options discussed are likely to have a therapeutic value. Further work on improving the specificity, efficacy and minimizing potential side effects of these treatments remains to be completed, but these are exciting times for the tau research community.
Figure 1. Tau based therapeutic strategies under development.
Kinase inhibitors targeting GSK3β, MARK and cdk5 or phosphatase activators may reduce pathological tau phosphorylation. Small molecule aggregation inhibitors or immunotherapy may prevent the build-up and spread of toxic tau aggregates. These therapies should reduce the levels of tau oligomers, paired helical filaments and/or neurofibrillary tangles. Additionally, microtubule stabilizing drugs may compensate for loss of tau function by maintaining the integrity of the cytoskeleton. The overall aim of these direct or indirect approaches is to maintain synaptic connections and prevent neuronal loss.
Acknowledgments
This manuscript was supported by NIH grants AG032611 (ES), AG020197 (ES), the Alzheimer’s Association (AB, ES, PK), The Alzheimer’s Drug Discovery Foundation / The Association for Frontotemporal Degeneration (ES), and the Irma T. Hirschl/Monique Weill-Caulier Trust (ES).
Reference List
- 1.Alzheimer’s association. 2009 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2009;5:234–70. doi: 10.1016/j.jalz.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Kidd M. Paired helical filaments in electron microscopy of Alzheimer’s disease. Nature. 1963 Jan 12;197:192–3. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- 3.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–62. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iqbal K, Zaidi T, Thompson CH, Merz PA, Wisniewski HM. Alzheimer paired helical filaments: bulk isolation, solubility, and protein composition. Acta Neuropathol. 1984;62(3):167–77. doi: 10.1007/BF00691849. [DOI] [PubMed] [Google Scholar]
- 5.Brion JP. Immunological demonstration of tau protein in neurofibrillary tangles of Alzheimer’s disease. J Alzheimers Dis. 2006;9(3 Suppl):177–85. doi: 10.3233/jad-2006-9s321. [DOI] [PubMed] [Google Scholar]
- 6.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986 May 5;261(13):6084–9. [PubMed] [Google Scholar]
- 7.Delacourte A, Defossez A. Alzheimer’s disease: Tau proteins, the promoting factors of microtubule assembly, are major components of paired helical filaments. J Neurol Sci. 1986 Dec;76(2–3):173–86. doi: 10.1016/0022-510x(86)90167-x. [DOI] [PubMed] [Google Scholar]
- 8.Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4044–8. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolozin BL, Pruchnicki A, Dickson DW, Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986 May 2;232(4750):648–50. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- 10.Nukina N, Ihara Y. One of the antigenic determinants of paired helical filaments is related to tau protein. J Biochem. 1986 May;99(5):1541–4. doi: 10.1093/oxfordjournals.jbchem.a135625. [DOI] [PubMed] [Google Scholar]
- 11.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986 May 5;261(13):6084–9. [PubMed] [Google Scholar]
- 12.Ihara Y, Nukina N, Miura R, Ogawara M. Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer’s disease. J Biochem. 1986 Jun;99(6):1807–10. doi: 10.1093/oxfordjournals.jbchem.a135662. [DOI] [PubMed] [Google Scholar]
- 13.Gustke N, Trinczek B, Biernat J, Mandelkow EM, Mandelkow E. Domains of tau protein and interactions with microtubules. Biochemistry. 1994 Aug 16;33(32):9511–22. doi: 10.1021/bi00198a017. [DOI] [PubMed] [Google Scholar]
- 14.Mandelkow EM, Biernat J, Drewes G, Gustke N, Trinczek B, Mandelkow E. Tau domains, phosphorylation, and interactions with microtubules. Neurobiol Aging. 1995 May;16(3):355–62. doi: 10.1016/0197-4580(95)00025-a. [DOI] [PubMed] [Google Scholar]
- 15.Gong CX, Grundke-Iqbal I, Iqbal K. Dephosphorylation of Alzheimer’s disease abnormally phosphorylated tau by protein phosphatase-2A. Neuroscience. 1994 Aug;61(4):765–72. doi: 10.1016/0306-4522(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 16.Matus A. Microtubule-associated proteins: their potential role in determining neuronal morphology. Annu Rev Neurosci. 1988;11:29–44. doi: 10.1146/annurev.ne.11.030188.000333. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds CH, Utton MA, Gibb GM, Yates A, Anderton BH. Stress-activated protein kinase/c-jun N-terminal kinase phosphorylates tau protein. J Neurochem. 1997 Apr;68(4):1736–44. doi: 10.1046/j.1471-4159.1997.68041736.x. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins SM, Zinnerman M, Garner C, Johnson GV. Modulation of tau phosphorylation and intracellular localization by cellular stress. Biochem J. 2000 Jan 15;345(Pt 2):263–70. [PMC free article] [PubMed] [Google Scholar]
- 19.Utton MA, Vandecandelaere A, Wagner U, Reynolds CH, Gibb GM, Miller CC, et al. Phosphorylation of tau by glycogen synthase kinase 3beta affects the ability of tau to promote microtubule self-assembly. Biochem J. 1997 May 1;323(Pt 3):741–7. doi: 10.1042/bj3230741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi S, Ishiguro K, Omori A, Takamatsu M, Arioka M, Imahori K, et al. A cdc2-related kinase PSSALRE/cdk5 is homologous with the 30 kDa subunit of tau protein kinase II, a proline-directed protein kinase associated with microtubule. FEBS Lett. 1993 Dec 6;335(2):171–5. doi: 10.1016/0014-5793(93)80723-8. [DOI] [PubMed] [Google Scholar]
- 21.Kuret J, Johnson GS, Cha D, Christenson ER, DeMaggio AJ, Hoekstra MF. Casein kinase 1 is tightly associated with paired-helical filaments isolated from Alzheimer’s disease brain. J Neurochem. 1997 Dec;69(6):2506–15. doi: 10.1046/j.1471-4159.1997.69062506.x. [DOI] [PubMed] [Google Scholar]
- 22.Olsen MK, Reszka AA, Abraham I. KT5720 and U-98017 inhibit MAPK and alter the cytoskeleton and cell morphology. J Cell Physiol. 1998 Sep;176(3):525–36. doi: 10.1002/(SICI)1097-4652(199809)176:3<525::AID-JCP9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 23.Mazanetz MP, Fischer PM. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat Rev Drug Discov. 2007 Jun;6(6):464–79. doi: 10.1038/nrd2111. [DOI] [PubMed] [Google Scholar]
- 24.Brion JP, Passareiro H, Nunez J, Flament-Durand J. Mise en evidence immunologique de la proteine tau au niveau des lesions de degenerescence neurofibrillaire de la maladie Alzheimer. Arch Biol. 1985;95:229–35. [Google Scholar]
- 25.Wood JG, Mirra SS, Pollock NJ, Binder LI. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–3. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992 Mar;42(3 Pt 1):631–9. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 27.Wilcock GK, Esiri MM. Plaques, tangles and dementia. A quantitative study. J Neurol Sci. 1982 Nov;56(2–3):343–56. doi: 10.1016/0022-510x(82)90155-1. [DOI] [PubMed] [Google Scholar]
- 28.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998 Jun 18;393(6686):702–5. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 29.Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, et al. Tau is a candidate gene for chromosome 17 frontotemporal dementia [published erratum appears in Ann Neurol 1998 Sep;44(3):428] Ann Neurol. 1998 Jun;43(6):815–25. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- 30.Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000 Aug;25(4):402–5. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 31.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular A beta and synaptic dysfunction. Neuron. 2003 Jul 31;39(3):409–21. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 32.Hanger DP, Byers HL, Wray S, Leung KY, Saxton MJ, Seereeram A, et al. Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J Biol Chem. 2007 Aug 10;282(32):23645–54. doi: 10.1074/jbc.M703269200. [DOI] [PubMed] [Google Scholar]
- 33.Mandelkow EM, Thies E, Trinczek B, Biernat J, Mandelkow E. MARK/PAR1 kinase is a regulator of microtubule-dependent transport in axons. J Cell Biol. 2004 Oct 11;167(1):99–110. doi: 10.1083/jcb.200401085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cruz JC, Tsai LH. Cdk5 deregulation in the pathogenesis of Alzheimer’s disease. Trends Mol Med. 2004 Sep;10(9):452–8. doi: 10.1016/j.molmed.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Anderton BH, Betts J, Blackstock WP, Brion JP, Chapman S, Connell J, et al. Sites of phosphorylation in tau and factors affecting their regulation. Biochem Soc Symp. 2001;(67):73–80. doi: 10.1042/bss0670073. [DOI] [PubMed] [Google Scholar]
- 36.Geschwind DH. Tau phosphorylation, tangles, and neurodegeneration: the chicken or the egg? Neuron. 2003 Oct 30;40(3):457–60. doi: 10.1016/s0896-6273(03)00681-0. [DOI] [PubMed] [Google Scholar]
- 37.Chung SH. Aberrant phosphorylation in the pathogenesis of Alzheimer’s disease. BMB Rep. 2009 Aug 31;42(8):467–74. doi: 10.5483/bmbrep.2009.42.8.467. [DOI] [PubMed] [Google Scholar]
- 38.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990 Aug;9(8):2431–8. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xavier IJ, Mercier PA, McLoughlin CM, Ali A, Woodgett JR, Ovsenek N. Glycogen synthase kinase 3beta negatively regulates both DNA-binding and transcriptional activities of heat shock factor 1. J Biol Chem. 2000 Sep 15;275(37):29147–52. doi: 10.1074/jbc.M002169200. [DOI] [PubMed] [Google Scholar]
- 40.He B, Meng YH, Mivechi NF. Glycogen synthase kinase 3beta and extracellular signal-regulated kinase inactivate heat shock transcription factor 1 by facilitating the disappearance of transcriptionally active granules after heat shock. Mol Cell Biol. 1998 Nov;18(11):6624–33. doi: 10.1128/mcb.18.11.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turenne GA, Price BD. Glycogen synthase kinase3 beta phosphorylates serine 33 of p53 and activates p53’s transcriptional activity. BMC Cell Biol. 2001;2:12. doi: 10.1186/1471-2121-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang MH, Chuang DM. Regulation and function of glycogen synthase kinase-3 isoforms in neuronal survival. J Biol Chem. 2007 Feb 9;282(6):3904–17. doi: 10.1074/jbc.M605178200. [DOI] [PubMed] [Google Scholar]
- 43.Carmichael J, Sugars KL, Bao YP, Rubinsztein DC. Glycogen synthase kinase-3beta inhibitors prevent cellular polyglutamine toxicity caused by the Huntington’s disease mutation. J Biol Chem. 2002 Sep 13;277(37):33791–8. doi: 10.1074/jbc.M204861200. [DOI] [PubMed] [Google Scholar]
- 44.Kockeritz L, Doble B, Patel S, Woodgett JR. Glycogen synthase kinase-3--an overview of an over-achieving protein kinase. Curr Drug Targets. 2006 Nov;7(11):1377–88. doi: 10.2174/1389450110607011377. [DOI] [PubMed] [Google Scholar]
- 45.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001 Nov;65(4):391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 46.Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T, et al. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur J Neurosci. 2007 Jan;25(1):81–6. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- 47.Imahori K, Uchida T. Physiology and pathology of tau protein kinases in relation to Alzheimer’s disease. J Biochem. 1997 Feb;121(2):179–88. [PubMed] [Google Scholar]
- 48.Pei JJ, Tanaka T, Tung YC, Braak E, Iqbal K, Grundke-Iqbal I. Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J Neuropathol Exp Neurol. 1997 Jan;56(1):70–8. doi: 10.1097/00005072-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguchi H, Ishiguro K, Uchida T, Takashima A, Lemere CA, Imahori K. Preferential labeling of Alzheimer neurofibrillary tangles with antisera for tau protein kinase (TPK) I/glycogen synthase kinase-3 beta and cyclin-dependent kinase 5, a component of TPK II. Acta Neuropathol. 1996 Sep;92(3):232–41. doi: 10.1007/s004010050513. [DOI] [PubMed] [Google Scholar]
- 50.Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, et al. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol. 1999 Sep;58(9):1010–9. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 51.Huang HC, Klein PS. Multiple roles for glycogen synthase kinase-3 as a drug target in Alzheimer’s disease. Curr Drug Targets. 2006 Nov;7(11):1389–97. doi: 10.2174/1389450110607011389. [DOI] [PubMed] [Google Scholar]
- 52.Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol. 2003 Dec;10(12):1255–66. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 53.Plotkin B, Kaidanovich O, Talior I, Eldar-Finkelman H. Insulin mimetic action of synthetic phosphorylated peptide inhibitors of glycogen synthase kinase-3. J Pharmacol Exp Ther. 2003 Jun;305(3):974–80. doi: 10.1124/jpet.102.047381. [DOI] [PubMed] [Google Scholar]
- 54.Gomez-Ramos A, Diaz-Nido J, Smith MA, Perry G, Avila J. Effect of the lipid peroxidation product acrolein on tau phosphorylation in neural cells. J Neurosci Res. 2003 Mar 15;71(6):863–70. doi: 10.1002/jnr.10525. [DOI] [PubMed] [Google Scholar]
- 55.Martinez A. Preclinical efficacy on GSK-3 inhibitors: towards a future generation of powerful drugs. Med Res Rev. 2008 Sep;28(5):773–96. doi: 10.1002/med.20119. [DOI] [PubMed] [Google Scholar]
- 56.Dajani R, Fraser E, Roe SM, Young N, Good V, Dale TC, et al. Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001 Jun 15;105(6):721–32. doi: 10.1016/s0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 57.ter HE, Coll JT, Austen DA, Hsiao HM, Swenson L, Jain J. Structure of GSK3beta reveals a primed phosphorylation mechanism. Nat Struct Biol. 2001 Jul;8(7):593–6. doi: 10.1038/89624. [DOI] [PubMed] [Google Scholar]
- 58.Dessalew N, Bharatam PV. Structure based de novo design of novel glycogen synthase kinase 3 inhibitors. Bioorg Med Chem. 2007 Jun 1;15(11):3728–36. doi: 10.1016/j.bmc.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 59.Kim HJ, Choo H, Cho YS, No KT, Pae AN. Novel GSK-3beta inhibitors from sequential virtual screening. Bioorg Med Chem. 2008 Jan 15;16(2):636–43. doi: 10.1016/j.bmc.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 60.Johnson G. Lithium. Med J Aust. 1984 Oct 27;141(9):595–601. [PubMed] [Google Scholar]
- 61.Cade JF. Lithium salts in the treatment of psychotic excitement. 1949. Bull World Health Organ. 2000;78(4):518–20. [PMC free article] [PubMed] [Google Scholar]
- 62.Schou M. Lithium as a prophylactic agent in unipolar affective illness: comparison with cyclic antidepressants. Arch Gen Psychiatry. 1979 Jul 20;36(8 Spec No):849–51. doi: 10.1001/archpsyc.1979.01780080023006. [DOI] [PubMed] [Google Scholar]
- 63.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996 Aug 6;93(16):8455–9. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Munoz-Montano JR, Moreno FJ, Avila J, Diaz-Nido J. Lithium inhibits Alzheimer’s disease-like tau protein phosphorylation in neurons. FEBS Lett. 1997 Jul 14;411(2–3):183–8. doi: 10.1016/s0014-5793(97)00688-1. [DOI] [PubMed] [Google Scholar]
- 65.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996 Dec 1;6(12):1664–8. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 66.Alvarez G, Munoz-Montano JR, Satrustegui J, Avila J, Bogonez E, Diaz-Nido J. Regulation of tau phosphorylation and protection against beta-amyloid-induced neurodegeneration by lithium. Possible implications for Alzheimer’s disease. Bipolar Disord. 2002 Jun;4(3):153–65. doi: 10.1034/j.1399-5618.2002.01150.x. [DOI] [PubMed] [Google Scholar]
- 67.Caccamo A, Oddo S, Tran LX, LaFerla FM. Lithium reduces tau phosphorylation but not A beta or working memory deficits in a transgenic model with both plaques and tangles. Am J Pathol. 2007 May;170(5):1669–75. doi: 10.2353/ajpath.2007.061178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Macdonald A, Briggs K, Poppe M, Higgins A, Velayudhan L, Lovestone S. A feasibility and tolerability study of lithium in Alzheimer’s disease. Int J Geriatr Psychiatry. 2008 Jul;23(7):704–11. doi: 10.1002/gps.1964. [DOI] [PubMed] [Google Scholar]
- 69.Bhat RV, Berg S, Burrows J, Lindquit J. GSK-3 Inhibitors for treatment of Alzheimer’s disease. Topics in Medicinal Chemistry. 2008;2:137–74. [Google Scholar]
- 70.Bhat R, Xue Y, Berg S, Hellberg S, Ormo M, Nilsson Y, et al. Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J Biol Chem. 2003 Nov 14;278(46):45937–45. doi: 10.1074/jbc.M306268200. [DOI] [PubMed] [Google Scholar]
- 71.Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci U S A. 2005 May 10;102(19):6990–5. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martinez A, Alonso M, Castro A, Perez C, Moreno FJ. First non-ATP competitive glycogen synthase kinase 3 beta (GSK-3beta) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J Med Chem. 2002 Mar 14;45(6):1292–9. doi: 10.1021/jm011020u. [DOI] [PubMed] [Google Scholar]
- 73.Martinez A, Alonso M, Castro A, Dorronsoro I, Gelpi JL, Luque FJ, et al. SAR and 3D-QSAR studies on thiadiazolidinone derivatives: exploration of structural requirements for glycogen synthase kinase 3 inhibitors. J Med Chem. 2005 Nov 17;48(23):7103–12. doi: 10.1021/jm040895g. [DOI] [PubMed] [Google Scholar]
- 74.Martin-Aparicio E, Fueters A, Perez-Puerto MJ, Alonso M, Martinez A, Medina M. TDZD’s: GSK3-beta inhibitors as therapeutic agents for Alzheimer’s disease and other tauopathies. IX International Conference of Alzheimer’s Disease and Related Disorders; Pheladelphia, Pennsylvania. 2004. [Google Scholar]
- 75.Le CS, Klafki HW, Plesnila N, Hubinger G, Obermeier A, Sahagun H, et al. An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc Natl Acad Sci U S A. 2006 Jun 20;103(25):9673–8. doi: 10.1073/pnas.0602913103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000 Oct;7(10):793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 77.Cross DA, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J Neurochem. 2001 Apr;77(1):94–102. doi: 10.1046/j.1471-4159.2001.t01-1-00251.x. [DOI] [PubMed] [Google Scholar]
- 78.Zhang F, Phiel CJ, Spece L, Gurvich N, Klein PS. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J Biol Chem. 2003 Aug 29;278(35):33067–77. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
- 79.Lochhead PA, Coghlan M, Rice SQ, Sutherland C. Inhibition of GSK-3 selectively reduces glucose-6-phosphatase and phosphatase and phosphoenolypyruvate carboxykinase gene expression. Diabetes. 2001 May;50(5):937–46. doi: 10.2337/diabetes.50.5.937. [DOI] [PubMed] [Google Scholar]
- 80.Ino H, Ishizuka T, Chiba T, Tatibana M. Expression of CDK5 (PSSALRE kinase), a neural cdc2-related protein kinase, in the mature and developing mouse central and peripheral nervous systems. Brain Res. 1994 Oct 24;661(1–2):196–206. doi: 10.1016/0006-8993(94)91197-5. [DOI] [PubMed] [Google Scholar]
- 81.Tsai LH, Takahashi T, Caviness VS, Jr, Harlow E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development. 1993 Dec;119(4):1029–40. doi: 10.1242/dev.119.4.1029. [DOI] [PubMed] [Google Scholar]
- 82.Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci. 2007 Jan;25(1):59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Flaherty DB, Soria JP, Tomasiewicz HG, Wood JG. Phosphorylation of human tau protein by microtubule-associated kinases: GSK3beta and cdk5 are key participants. J Neurosci Res. 2000 Nov 1;62(3):463–72. doi: 10.1002/1097-4547(20001101)62:3<463::AID-JNR16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 84.Lilja L, Yang SN, Webb DL, Juntti-Berggren L, Berggren PO, Bark C. Cyclin-dependent kinase 5 promotes insulin exocytosis. J Biol Chem. 2001 Sep 7;276(36):34199–205. doi: 10.1074/jbc.M103776200. [DOI] [PubMed] [Google Scholar]
- 85.Tomizawa K, Ohta J, Matsushita M, Moriwaki A, Li ST, Takei K, et al. Cdk5/p35 regulates neurotransmitter release through phosphorylation and downregulation of P/Q-type voltage-dependent calcium channel activity. J Neurosci. 2002 Apr 1;22(7):2590–7. doi: 10.1523/JNEUROSCI.22-07-02590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999 Dec 9;402(6762):669–71. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 87.Li BS, Zhang L, Gu J, Amin ND, Pant HC. Integrin alpha(1) beta(1)-mediated activation of cyclin-dependent kinase 5 activity is involved in neurite outgrowth and human neurofilament protein H Lys-Ser-Pro tail domain phosphorylation. J Neurosci. 2000 Aug 15;20(16):6055–62. doi: 10.1523/JNEUROSCI.20-16-06055.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheng K, Ip NY. Cdk5: a new player at synapses. Neurosignals. 2003 Sep;12(4–5):180–90. doi: 10.1159/000074619. [DOI] [PubMed] [Google Scholar]
- 89.Cruz JC, Tsai LH. Cdk5 deregulation in the pathogenesis of Alzheimer’s disease. Trends Mol Med. 2004 Sep;10(9):452–8. doi: 10.1016/j.molmed.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 90.Paglini G, Caceres A. The role of the Cdk5--p35 kinase in neuronal development. Eur J Biochem. 2001 Mar;268(6):1528–33. [PubMed] [Google Scholar]
- 91.Shelton SB, Johnson GV. Cyclin-dependent kinase-5 in neurodegeneration. J Neurochem. 2004 Mar;88(6):1313–26. doi: 10.1111/j.1471-4159.2003.02328.x. [DOI] [PubMed] [Google Scholar]
- 92.Tang D, Wang JH. Cyclin-dependent kinase 5 (Cdk5) and neuron-specific Cdk5 activators. Prog Cell Cycle Res. 1996;2:205–16. doi: 10.1007/978-1-4615-5873-6_20. [DOI] [PubMed] [Google Scholar]
- 93.Smith DS, Greer PL, Tsai LH. Cdk5 on the brain. Cell Growth Differ. 2001 Jun;12(6):277–83. [PubMed] [Google Scholar]
- 94.Smith DS, Tsai LH. Cdk5 behind the wheel: a role in trafficking and transport? Trends Cell Biol. 2002 Jan;12(1):28–36. doi: 10.1016/s0962-8924(01)02181-x. [DOI] [PubMed] [Google Scholar]
- 95.Paudel HK, Lew J, Ali Z, Wang JH. Brain proline-directed protein kinase phosphorylates tau on sites that are abnormally phosphorylated in tau associated with Alzheimer’s paired helical filaments. J Biol Chem. 1993 Nov 5;268(31):23512–8. [PubMed] [Google Scholar]
- 96.Lund ET, McKenna R, Evans DB, Sharma SK, Mathews WR. Characterization of the in vitro phosphorylation of human tau by tau protein kinase II (cdk5/p20) using mass spectrometry. J Neurochem. 2001 Feb;76(4):1221–32. doi: 10.1046/j.1471-4159.2001.00130.x. [DOI] [PubMed] [Google Scholar]
- 97.Sakaue F, Saito T, Sato Y, Asada A, Ishiguro K, Hasegawa M, et al. Phosphorylation of FTDP-17 mutant tau by cyclin-dependent kinase 5 complexed with p35, p25, or p39. J Biol Chem. 2005 Sep 9;280(36):31522–9. doi: 10.1074/jbc.M504792200. [DOI] [PubMed] [Google Scholar]
- 98.Flaherty DB, Soria JP, Tomasiewicz HG, Wood JG. Phosphorylation of human tau protein by microtubule-associated kinases: GSK3beta and cdk5 are key participants. J Neurosci Res. 2000 Nov 1;62(3):463–72. doi: 10.1002/1097-4547(20001101)62:3<463::AID-JNR16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 99.Ishiguro K, Sato K, Takamatsu M, Park J, Uchida T, Imahori K. Analysis of phosphorylation of tau with antibodies specific for phosphorylation sites. Neurosci Lett. 1995 Dec 29;202(1–2):81–4. doi: 10.1016/0304-3940(95)12206-0. [DOI] [PubMed] [Google Scholar]
- 100.Baumann K, Mandelkow EM, Biernat J, Piwnica-Worms H, Mandelkow E. Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett. 1993 Dec 28;336(3):417–24. doi: 10.1016/0014-5793(93)80849-p. [DOI] [PubMed] [Google Scholar]
- 101.Mapelli M, Massimiliano L, Crovace C, Seeliger MA, Tsai LH, Meijer L, et al. Mechanism of CDK5/p25 binding by CDK inhibitors. J Med Chem. 2005 Feb 10;48(3):671–9. doi: 10.1021/jm049323m. [DOI] [PubMed] [Google Scholar]
- 102.Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, et al. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994 Sep 29;371(6496):423–6. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 103.Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994 Sep 29;371(6496):419–23. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 104.Humbert S, Lanier LM, Tsai LH. Synaptic localization of p39, a neuronal activator of cdk5. Neuroreport. 2000 Jul 14;11(10):2213–6. doi: 10.1097/00001756-200007140-00030. [DOI] [PubMed] [Google Scholar]
- 105.Ishiguro K, Kobayashi S, Omori A, Takamatsu M, Yonekura S, Anzai K, et al. Identification of the 23 kDa subunit of tau protein kinase II as a putative activator of cdk5 in bovine brain. FEBS Lett. 1994 Apr 4;342(2):203–8. doi: 10.1016/0014-5793(94)80501-6. [DOI] [PubMed] [Google Scholar]
- 106.Uchida T, Ishiguro K, Ohnuma J, Takamatsu M, Yonekura S, Imahori K. Precursor of cdk5 activator, the 23 kDa subunit of tau protein kinase II: its sequence and developmental change in brain. FEBS Lett. 1994 Nov 21;355(1):35–40. doi: 10.1016/0014-5793(94)01163-x. [DOI] [PubMed] [Google Scholar]
- 107.Tandon A, Yu H, Wang L, Rogaeva E, Sato C, Chishti MA, et al. Brain levels of CDK5 activator p25 are not increased in Alzheimer’s or other neurodegenerative diseases with neurofibrillary tangles. J Neurochem. 2003 Aug;86(3):572–81. doi: 10.1046/j.1471-4159.2003.01865.x. [DOI] [PubMed] [Google Scholar]
- 108.Nguyen KC, Rosales JL, Barboza M, Lee KY. Controversies over p25 in Alzheimer’s disease. J Alzheimers Dis. 2002 Apr;4(2):123–6. doi: 10.3233/jad-2002-4207. [DOI] [PubMed] [Google Scholar]
- 109.Otth C, Concha II, Arendt T, Stieler J, Schliebs R, Gonzalez-Billault C, et al. AbetaPP induces cdk5-dependent tau hyperphosphorylation in transgenic mice Tg2576. J Alzheimers Dis. 2002 Oct;4(5):417–30. doi: 10.3233/jad-2002-4508. [DOI] [PubMed] [Google Scholar]
- 110.Yoo BC, Lubec G. p25 protein in neurodegeneration. Nature. 2001 Jun 14;411(6839):763–4. doi: 10.1038/35081146. [DOI] [PubMed] [Google Scholar]
- 111.Takashima A, Murayama M, Yasutake K, Takahashi H, Yokoyama M, Ishiguro K. Involvement of cyclin dependent kinase5 activator p25 on tau phosphorylation in mouse brain. Neurosci Lett. 2001 Jun 22;306(1–2):37–40. doi: 10.1016/s0304-3940(01)01864-x. [DOI] [PubMed] [Google Scholar]
- 112.Hashiguchi M, Saito T, Hisanaga S, Hashiguchi T. Truncation of CDK5 activator p35 induces intensive phosphorylation of Ser202/Thr205 of human tau. J Biol Chem. 2002 Nov 15;277(46):44525–30. doi: 10.1074/jbc.M207426200. [DOI] [PubMed] [Google Scholar]
- 113.Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999 Dec 9;402(6762):615–22. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 114.Shelton SB, Krishnamurthy P, Johnson GV. Effects of cyclin-dependent kinase-5 activity on apoptosis and tau phosphorylation in immortalized mouse brain cortical cells. J Neurosci Res. 2004 Apr 1;76(1):110–20. doi: 10.1002/jnr.20051. [DOI] [PubMed] [Google Scholar]
- 115.Jamsa A, Backstrom A, Gustafsson E, Dehvari N, Hiller G, Cowburn RF, et al. Glutamate treatment and p25 transfection increase Cdk5 mediated tau phosphorylation in SH-SY5Y cells. Biochem Biophys Res Commun. 2006 Jun 23;345(1):324–31. doi: 10.1016/j.bbrc.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 116.Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, et al. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci U S A. 2000 Mar 14;97(6):2910–5. doi: 10.1073/pnas.040577797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bian F, Nath R, Sobocinski G, Booher RN, Lipinski WJ, Callahan MJ, et al. Axonopathy, tau abnormalities, and dyskinesia, but no neurofibrillary tangles in p25-transgenic mice. J Comp Neurol. 2002 May 6;446(3):257–66. doi: 10.1002/cne.10186. [DOI] [PubMed] [Google Scholar]
- 118.Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003 Oct 30;40(3):471–83. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- 119.Noble W, Olm V, Takata K, Casey E, Mary O, Meyerson J, et al. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron. 2003 May 22;38(4):555–65. doi: 10.1016/s0896-6273(03)00259-9. [DOI] [PubMed] [Google Scholar]
- 120.Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Titani K, et al. Proline-directed and non-proline-directed phosphorylation of PHF-tau. J Biol Chem. 1995 Jan 13;270(2):823–9. doi: 10.1074/jbc.270.2.823. [DOI] [PubMed] [Google Scholar]
- 121.de Azevedo WF, Leclerc S, Meijer L, Havlicek L, Strnad M, Kim SH. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur J Biochem. 1997 Jan 15;243(1–2):518–26. doi: 10.1111/j.1432-1033.1997.0518a.x. [DOI] [PubMed] [Google Scholar]
- 122.Meijer L, Kim SH. Chemical inhibitors of cyclin-dependent kinases. Methods Enzymol. 1997;283:113–28. doi: 10.1016/s0076-6879(97)83011-x. [DOI] [PubMed] [Google Scholar]
- 123.Meijer L, Raymond E. Roscovitine and other purines as kinase inhibitors. From starfish oocytes to clinical trials. Acc Chem Res. 2003 Jun;36(6):417–25. doi: 10.1021/ar0201198. [DOI] [PubMed] [Google Scholar]
- 124.Mettey Y, Gompel M, Thomas V, Garnier M, Leost M, Ceballos-Picot I, et al. Aloisines, a new family of CDK/GSK-3 inhibitors. SAR study, crystal structure in complex with CDK2, enzyme selectivity, and cellular effects. J Med Chem. 2003 Jan 16;46(2):222–36. doi: 10.1021/jm020319p. [DOI] [PubMed] [Google Scholar]
- 125.Hoessel R, Leclerc S, Endicott JA, Nobel ME, Lawrie A, Tunnah P, et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1999 May;1(1):60–7. doi: 10.1038/9035. [DOI] [PubMed] [Google Scholar]
- 126.Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997 Apr 18;89(2):297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 127.Trinczek B, Brajenovic M, Ebneth A, Drewes G. MARK4 is a novel microtubule-associated proteins/microtubule affinity-regulating kinase that binds to the cellular microtubule network and to centrosomes. J Biol Chem. 2004 Feb 13;279(7):5915–23. doi: 10.1074/jbc.M304528200. [DOI] [PubMed] [Google Scholar]
- 128.Tassan JP, Le GX. An overview of the KIN1/PAR-1/MARK kinase family. Biol Cell. 2004 Apr;96(3):193–9. doi: 10.1016/j.biolcel.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 129.Drewes G. MARKing tau for tangles and toxicity. Trends Biochem Sci. 2004 Oct;29(10):548–55. doi: 10.1016/j.tibs.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 130.Tassan JP, Le GX. An overview of the KIN1/PAR-1/MARK kinase family. Biol Cell. 2004 Apr;96(3):193–9. doi: 10.1016/j.biolcel.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 131.Biernat J, Wu YZ, Timm T, Zheng-Fischhofer Q, Mandelkow E, Meijer L, et al. Protein kinase MARK/PAR-1 is required for neurite outgrowth and establishment of neuronal polarity. Mol Biol Cell. 2002 Nov;13(11):4013–28. doi: 10.1091/mbc.02-03-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mandelkow EM, Thies E, Trinczek B, Biernat J, Mandelkow E. MARK/PAR1 kinase is a regulator of microtubule-dependent transport in axons. J Cell Biol. 2004 Oct 11;167(1):99–110. doi: 10.1083/jcb.200401085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002 Jan;103(1):26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- 134.Chin JY, Knowles RB, Schneider A, Drewes G, Mandelkow EM, Hyman BT. Microtubule-affinity regulating kinase (MARK) is tightly associated with neurofibrillary tangles in Alzheimer brain: a fluorescence resonance energy transfer study. J Neuropathol Exp Neurol. 2000 Nov;59(11):966–71. doi: 10.1093/jnen/59.11.966. [DOI] [PubMed] [Google Scholar]
- 135.Eckermann K, Mocanu MM, Khlistunova I, Biernat J, Nissen A, Hofmann A, et al. The beta-propensity of Tau determines aggregation and synaptic loss in inducible mouse models of tauopathy. J Biol Chem. 2007 Oct 26;282(43):31755–65. doi: 10.1074/jbc.M705282200. [DOI] [PubMed] [Google Scholar]
- 136.Meijer L, Thunnissen AM, White AW, Garnier M, Nikolic M, Tsai LH, et al. Inhibition of cyclin-dependent kinases, GSK-3beta and CK1 by hymenialdisine, a marine sponge constituent. Chem Biol. 2000 Jan;7(1):51–63. doi: 10.1016/s1074-5521(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 137.Timm T, Balusamy K, Li X, Biernat J, Mandelkow E, Mandelkow EM. Glycogen synthase kinase (GSK) 3beta directly phosphorylates Serine 212 in the regulatory loop and inhibits microtubule affinity-regulating kinase (MARK) 2. J Biol Chem. 2008 Jul 4;283(27):18873–82. doi: 10.1074/jbc.M706596200. [DOI] [PubMed] [Google Scholar]
- 138.Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron. 1996 Dec;17(6):1201–7. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- 139.Drewes G, Mandelkow EM, Baumann K, Goris J, Merlevede W, Mandelkow E. Dephosphorylation of tau protein and Alzheimer paired helical filaments by calcineurin and phosphatase-2A. FEBS Lett. 1993 Dec 28;336(3):425–32. doi: 10.1016/0014-5793(93)80850-t. [DOI] [PubMed] [Google Scholar]
- 140.Planel E, Yasutake K, Fujita SC, Ishiguro K. Inhibition of protein phosphatase 2A overrides tau protein kinase I/glycogen synthase kinase 3 beta and cyclin-dependent kinase 5 inhibition and results in tau hyperphosphorylation in the hippocampus of starved mouse. J Biol Chem. 2001 Sep 7;276(36):34298–306. doi: 10.1074/jbc.M102780200. [DOI] [PubMed] [Google Scholar]
- 141.Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem. 1995 Aug;65(2):732–8. doi: 10.1046/j.1471-4159.1995.65020732.x. [DOI] [PubMed] [Google Scholar]
- 142.Sontag E, Hladik C, Montgomery L, Luangpirom A, Mudrak I, Ogris E, et al. Downregulation of protein phosphatase 2A carboxyl methylation and methyltransferase may contribute to Alzheimer disease pathogenesis. J Neuropathol Exp Neurol. 2004 Oct;63(10):1080–91. doi: 10.1093/jnen/63.10.1080. [DOI] [PubMed] [Google Scholar]
- 143.Pei JJ, Gong CX, An WL, Winblad B, Cowburn RF, Grundke-Iqbal I, et al. Okadaic-acid-induced inhibition of protein phosphatase 2A produces activation of mitogen-activated protein kinases ERK1/2, MEK1/2, and p70 S6, similar to that in Alzheimer’s disease. Am J Pathol. 2003 Sep;163(3):845–58. doi: 10.1016/S0002-9440(10)63445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kins S, Crameri A, Evans DR, Hemmings BA, Nitsch RM, Gotz J. Reduced protein phosphatase 2A activity induces hyperphosphorylation and altered compartmentalization of tau in transgenic mice. J Biol Chem. 2001 Oct 12;276(41):38193–200. doi: 10.1074/jbc.M102621200. [DOI] [PubMed] [Google Scholar]
- 145.Li L, Sengupta A, Haque N, Grundke-Iqbal I, Iqbal K. Memantine inhibits and reverses the Alzheimer type abnormal hyperphosphorylation of tau and associated neurodegeneration. FEBS Lett. 2004 May 21;566(1–3):261–9. doi: 10.1016/j.febslet.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 146.Lu PJ, Wulf G, Zhou XZ, Davies P, Lu KP. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature. 1999 Jun 24;399(6738):784–8. doi: 10.1038/21650. [DOI] [PubMed] [Google Scholar]
- 147.Liou YC, Sun A, Ryo A, Zhou XZ, Yu ZX, Huang HK, et al. Role of the prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature. 2003 Jul 31;424(6948):556–61. doi: 10.1038/nature01832. [DOI] [PubMed] [Google Scholar]
- 148.Chun W, Johnson GV. The role of tau phosphorylation and cleavage in neuronal cell death. Front Biosci. 2007;12:733–56. doi: 10.2741/2097. [DOI] [PubMed] [Google Scholar]
- 149.Schneider A, Biernat J, von BM, Mandelkow E, Mandelkow EM. Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry. 1999 Mar 23;38(12):3549–58. doi: 10.1021/bi981874p. [DOI] [PubMed] [Google Scholar]
- 150.Wainwright M, Crossley KB. Methylene Blue--a therapeutic dye for all seasons? J Chemother. 2002 Oct;14(5):431–43. doi: 10.1179/joc.2002.14.5.431. [DOI] [PubMed] [Google Scholar]
- 151.Oz M, Lorke DE, Petroianu GA. Methylene blue and Alzheimer’s disease. Biochem Pharmacol. 2009 Oct 15;78(8):927–32. doi: 10.1016/j.bcp.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 152.Wischik CM, Edwards PC, Lai RY, Roth M, Harrington CR. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc Natl Acad Sci U S A. 1996 Oct 1;93(20):11213–8. doi: 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wishik CM, Bentham P, Wischik DJ, Seng KM. Tau aggregation inhibitor (TAI) therapy with rember arrestsdisease progression in mild and moderate Alzheimer’s disease over 50 weeks. Alzheimer’s & Dementia. 2008:4. [Google Scholar]
- 154.Necula M, Chirita CN, Kuret J. Cyanine dye N744 inhibits tau fibrillization by blocking filament extension: implications for the treatment of tauopathic neurodegenerative diseases. Biochemistry. 2005 Aug 2;44(30):10227–37. doi: 10.1021/bi050387o. [DOI] [PubMed] [Google Scholar]
- 155.Bulic B, Pickhardt M, Schmidt B, Mandelkow EM, Waldmann H, Mandelkow E. Development of tau aggregation inhibitors for Alzheimer’s disease. Angew Chem Int Ed Engl. 2009;48(10):1740–52. doi: 10.1002/anie.200802621. [DOI] [PubMed] [Google Scholar]
- 156.Pickhardt M, von BM, Gazova Z, Hascher A, Biernat J, Mandelkow EM, et al. Screening for inhibitors of tau polymerization. Curr Alzheimer Res. 2005 Apr;2(2):219–26. doi: 10.2174/1567205053585891. [DOI] [PubMed] [Google Scholar]
- 157.Pickhardt M, Larbig G, Khlistunova I, Coksezen A, Meyer B, Mandelkow EM, et al. Phenylthiazolyl-hydrazide and its derivatives are potent inhibitors of tau aggregation and toxicity in vitro and in cells. Biochemistry. 2007 Sep 4;46(35):10016–23. doi: 10.1021/bi700878g. [DOI] [PubMed] [Google Scholar]
- 158.Khlistunova I, Biernat J, Wang Y, Pickhardt M, von BM, Gazova Z, et al. Inducible expression of Tau repeat domain in cell models of tauopathy: aggregation is toxic to cells but can be reversed by inhibitor drugs. J Biol Chem. 2006 Jan 13;281(2):1205–14. doi: 10.1074/jbc.M507753200. [DOI] [PubMed] [Google Scholar]
- 159.Larbig G, Pickhardt M, Lloyd DG, Schmidt B, Mandelkow E. Screening for inhibitors of tau protein aggregation into Alzheimer paired helical filaments: a ligand based approach results in successful scaffold hopping. Curr Alzheimer Res. 2007 Jul;4(3):315–23. doi: 10.2174/156720507781077250. [DOI] [PubMed] [Google Scholar]
- 160.Bulic B, Pickhardt M, Khlistunova I, Biernat J, Mandelkow EM, Mandelkow E, et al. Rhodanine-based tau aggregation inhibitors in cell models of tauopathy. Angew Chem Int Ed Engl. 2007;46(48):9215–9. doi: 10.1002/anie.200704051. [DOI] [PubMed] [Google Scholar]
- 161.Taniguchi S, Suzuki N, Masuda M, Hisanaga S, Iwatsubo T, Goedert M, et al. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J Biol Chem. 2005 Mar 4;280(9):7614–23. doi: 10.1074/jbc.M408714200. [DOI] [PubMed] [Google Scholar]
- 162.Lindwall G, Cole RD. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem. 1984 Apr 25;259(8):5301–5. [PubMed] [Google Scholar]
- 163.Hempen B, Brion JP. Reduction of acetylated alpha-tubulin immunoreactivity in neurofibrillary tangle-bearing neurons in Alzheimer’s disease. J Neuropathol Exp Neurol. 1996 Sep;55(9):964–72. doi: 10.1097/00005072-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 164.Spires-Jones TL, Stoothoff WH, de CA, Jones PB, Hyman BT. Tau pathophysiology in neurodegeneration: a tangled issue. Trends Neurosci. 2009 Mar;32(3):150–9. doi: 10.1016/j.tins.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 165.Michaelis ML, Dobrowsky RT, Li G. Tau neurofibrillary pathology and microtubule stability. J Mol Neurosci. 2002 Dec;19(3):289–93. doi: 10.1385/JMN:19:3:289. [DOI] [PubMed] [Google Scholar]
- 166.Zhang B, Maiti A, Shively S, Lakhani F, McDonald-Jones G, Bruce J, et al. Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc Natl Acad Sci U S A. 2005 Jan 4;102(1):227–31. doi: 10.1073/pnas.0406361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brunden KR, Trojanowski JQ, Lee VM. Advances in tau-focused drug discovery for Alzheimer’s disease and related tauopathies. Nat Rev Drug Discov. 2009 Oct;8(10):783–93. doi: 10.1038/nrd2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhanel M, Spruss T, et al. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest. 2002 Nov;110(9):1309–18. doi: 10.1172/JCI15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sparreboom A, van AJ, Mayer U, Schinkel AH, Smit JW, Meijer DK, et al. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):2031–5. doi: 10.1073/pnas.94.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van AJ, Mayer U, van TO, Beijnen JH. The functional role of P-glycoprotein in the blood-brain barrier. J Pharm Sci. 1997 Aug;86(8):881–4. doi: 10.1021/js9701364. [DOI] [PubMed] [Google Scholar]
- 171.Bauer B, Hartz AM, Fricker G, Miller DS. Modulation of p-glycoprotein transport function at the blood-brain barrier. Exp Biol Med (Maywood) 2005 Feb;230(2):118–27. doi: 10.1177/153537020523000206. [DOI] [PubMed] [Google Scholar]
- 172.Begley GS, Horvath AR, Taylor JC, Higgins CF. Cytoplasmic domains of the transporter associated with antigen processing and P-glycoprotein interact with subunits of the proteasome. Mol Immunol. 2005 Jan;42(1):137–41. doi: 10.1016/j.molimm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 173.Fromm MF. Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol Sci. 2004 Aug;25(8):423–9. doi: 10.1016/j.tips.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 174.Hubensack M, Muller C, Hocherl P, Fellner S, Spruss T, Bernhardt G, et al. Effect of the ABCB1 modulators elacridar and tariquidar on the distribution of paclitaxel in nude mice. J Cancer Res Clin Oncol. 2008 May;134(5):597–607. doi: 10.1007/s00432-007-0323-9. [DOI] [PubMed] [Google Scholar]
- 175.Brunden KR, Zhang B, Carroll J, Yao Y, Potuzak JS, Hogan AM, et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J Neurosci. 2010 Oct 13;30(41):13861–6. doi: 10.1523/JNEUROSCI.3059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gozes I, Divinski I. The femtomolar-acting NAP interacts with microtubules: Novel aspects of astrocyte protection. J Alzheimers Dis. 2004 Dec;6(6 Suppl):S37–S41. doi: 10.3233/jad-2004-6s605. [DOI] [PubMed] [Google Scholar]
- 177.Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem. 1999 Mar;72(3):1283–93. doi: 10.1046/j.1471-4159.1999.0721283.x. [DOI] [PubMed] [Google Scholar]
- 178.Zamostiano R, Pinhasov A, Gelber E, Steingart RA, Seroussi E, Giladi E, et al. Cloning and characterization of the human activity-dependent neuroprotective protein. J Biol Chem. 2001 Jan 5;276(1):708–14. doi: 10.1074/jbc.M007416200. [DOI] [PubMed] [Google Scholar]
- 179.Gozes I. Activity-dependent neuroprotective protein: from gene to drug candidate. Pharmacol Ther. 2007 May;114(2):146–54. doi: 10.1016/j.pharmthera.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 180.Gozes I, Morimoto BH, Tiong J, Fox A, Sutherland K, Dangoor D, et al. NAP: research and development of a peptide derived from activity-dependent neuroprotective protein (ADNP) CNS Drug Rev. 2005;11(4):353–68. doi: 10.1111/j.1527-3458.2005.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Divinski I, Holtser-Cochav M, Vulih-Schultzman I, Steingart RA, Gozes I. Peptide neuroprotection through specific interaction with brain tubulin. J Neurochem. 2006 Aug;98(3):973–84. doi: 10.1111/j.1471-4159.2006.03936.x. [DOI] [PubMed] [Google Scholar]
- 182.Matsuoka Y, Gray AJ, Hirata-Fukae C, Minami SS, Waterhouse EG, Mattson MP, et al. Intranasal NAP administration reduces accumulation of amyloid peptide and tau hyperphosphorylation in a transgenic mouse model of Alzheimer’s disease at early pathological stage. J Mol Neurosci. 2007;31(2):165–70. doi: 10.1385/jmn/31:02:165. [DOI] [PubMed] [Google Scholar]
- 183.Matsuoka Y, Jouroukhin Y, Gray AJ, Ma L, Hirata-Fukae C, Li HF, et al. A neuronal microtubule-interacting agent, NAPVSIPQ, reduces tau pathology and enhances cognitive function in a mouse model of Alzheimer’s disease. J Pharmacol Exp Ther. 2008 Apr;325(1):146–53. doi: 10.1124/jpet.107.130526. [DOI] [PubMed] [Google Scholar]
- 184.Shiryaev N, Jouroukhin Y, Giladi E, Polyzoidou E, Grigoriadis NC, Rosenmann H, et al. NAP protects memory, increases soluble tau and reduces tau hyperphosphorylation in a tauopathy model. Neurobiol Dis. 2009 May;34(2):381–8. doi: 10.1016/j.nbd.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 185.Geerts H. AL-108 and AL-208, formulations of the neuroprotective NAP fragment of activity-dependent neuroprotective protein, for cognitive disorders. Curr Opin Investig Drugs. 2008 Jul;9(7):800–11. [PubMed] [Google Scholar]
- 186.Wang JZ, Grundke-Iqbal I, Iqbal K. Glycosylation of microtubule-associated protein tau: an abnormal posttranslational modification in Alzheimer’s disease. Nat Med. 1996 Aug;2(8):871–5. doi: 10.1038/nm0896-871. [DOI] [PubMed] [Google Scholar]
- 187.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007 Apr 26;446(7139):1017–22. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 188.Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004 Jul 20;101(29):10804–9. doi: 10.1073/pnas.0400348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008 Aug;4(8):483–90. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 190.Wilhelmus MM, de Waal RM, Verbeek MM. Heat shock proteins and amateur chaperones in amyloid-Beta accumulation and clearance in Alzheimer’s disease. Mol Neurobiol. 2007 Jun;35(3):203–16. doi: 10.1007/s12035-007-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Koren J, III, Jinwal UK, Lee DC, Jones JR, Shults CL, Johnson AG, et al. Chaperone signalling complexes in Alzheimer’s disease. J Cell Mol Med. 2009 Apr;13(4):619–30. doi: 10.1111/j.1582-4934.2008.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Renkawek K, Bosman GJ, de Jong WW. Expression of small heat-shock protein hsp 27 in reactive gliosis in Alzheimer disease and other types of dementia. Acta Neuropathol. 1994;87(5):511–9. doi: 10.1007/BF00294178. [DOI] [PubMed] [Google Scholar]
- 193.Shimura H, Miura-Shimura Y, Kosik KS. Binding of tau to heat shock protein 27 leads to decreased concentration of hyperphosphorylated tau and enhanced cell survival. J Biol Chem. 2004 Apr 23;279(17):17957–62. doi: 10.1074/jbc.M400351200. [DOI] [PubMed] [Google Scholar]
- 194.Hohfeld J, Cyr DM, Patterson C. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep. 2001 Oct;2(10):885–90. doi: 10.1093/embo-reports/kve206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cisse S, Perry G, Lacoste-Royal G, Cabana T, Gauvreau D. Immunochemical identification of ubiquitin and heat-shock proteins in corpora amylacea from normal aged and Alzheimer’s disease brains. Acta Neuropathol. 1993;85(3):233–40. doi: 10.1007/BF00227716. [DOI] [PubMed] [Google Scholar]
- 196.Perez N, Sugar J, Charya S, Johnson G, Merril C, Bierer L, et al. Increased synthesis and accumulation of heat shock 70 proteins in Alzheimer’s disease. Brain Res Mol Brain Res. 1991 Oct;11(3–4):249–54. doi: 10.1016/0169-328x(91)90033-t. [DOI] [PubMed] [Google Scholar]
- 197.Dickey CA, Dunmore J, Lu B, Wang JW, Lee WC, Kamal A, et al. HSP induction mediates selective clearance of tau phosphorylated at proline-directed Ser/Thr sites but not KXGS (MARK) sites. FASEB J. 2006 Apr;20(6):753–5. doi: 10.1096/fj.05-5343fje. [DOI] [PubMed] [Google Scholar]
- 198.Luo W, Dou F, Rodina A, Chip S, Kim J, Zhao Q, et al. Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc Natl Acad Sci U S A. 2007 May 29;104(22):9511–6. doi: 10.1073/pnas.0701055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse [In Process Citation] Nature. 1999 Jul 8;400(6740):173–7. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 200.Solomon B, Koppel R, Hanan E, Katzav T. Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer β-amyloid peptide. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):452–5. doi: 10.1073/pnas.93.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000 Aug;6(8):916–9. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 202.Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003 Jul 8;61(1):46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 203.Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, et al. Abeta species removal after abeta42 immunization. J Neuropathol Exp Neurol. 2006 Nov;65(11):1040–8. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- 204.Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, et al. Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003 May 22;38(4):547–54. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 205.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of A{beta} immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005 Apr 7;64(9):1553–62. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 206.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of A beta(42) immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008 Jul 19;372(9634):216–23. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 207.Morrissette DA, Parachikova A, Green KN, LaFerla FM. Relevance of transgenic mouse models to human Alzheimer disease. J Biol Chem. 2009 Mar 6;284(10):6033–7. doi: 10.1074/jbc.R800030200. [DOI] [PubMed] [Google Scholar]
- 208.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003 Apr;9(4):448–52. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 209.Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, et al. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005 Jan 11;64(1):129–31. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- 210.Boche D, Donald J, Love S, Harris S, Neal JW, Holmes C, et al. Reduction of aggregated Tau in neuronal processes but not in the cell bodies after Abeta42 immunisation in Alzheimer’s disease. Acta Neuropathol. 2010 Jul;120(1):13–20. doi: 10.1007/s00401-010-0705-y. [DOI] [PubMed] [Google Scholar]
- 211.Serrano-Pozo A, William CM, Ferrer I, Uro-Coste E, Delisle MB, Maurage CA, et al. Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology. Brain. 2010 May;133(Pt 5):1312–27. doi: 10.1093/brain/awq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004 Aug 5;43(3):321–32. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 213.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007 Aug 22;27(34):9115–29. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sigurdsson EM, Quartermain D, Boutajangout A. Tau immunotherapy prevents cognitive decline and clears pathological tau in a tangle mouse model. Alzheimer’s & Dementia. 2008;4(4):T191–T192. [Google Scholar]
- 215.Krishnamurthy PK, Rajamohamed Sait HB, Boutajangout A, Sigurdsson EM. Immunotherapy targeting Alzheimer’s phospho-tau epitope within the microtubule binding region of tau clears pathological tau and prevents functional decline in a mouse model of tauopathy. Alzheimer’s and Dementia. 2009 Jul;5(4, Supplement 1):112. [Google Scholar]
- 216.Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci. 2010 Dec 8;30(49):16559–66. doi: 10.1523/JNEUROSCI.4363-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. Alzheimer & Dementia. 2010 doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem. 2011 Jun 3; doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sigurdsson EM. Immunotherapy targeting pathological tau protein in Alzheimer’s disease and related tauopathies. Journal of Alzheimer’s Disease. 2008;15:157–68. doi: 10.3233/jad-2008-15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, et al. Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005 Jun 16;46(6):857–68. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 221.Tampellini D, Magrane J, Takahashi RH, Li F, Lin MT, Almeida CG, et al. Internalized antibodies to the A beta domain of APP reduce neuronal A beta and protect against synaptic alterations. J Biol Chem. 2007 Jun 29;282(26):18895–906. doi: 10.1074/jbc.M700373200. [DOI] [PubMed] [Google Scholar]
- 222.Gomez-Ramos A, Diaz-Hernandez M, Cuadros R, Hernandez F, Avila J. Extracellular tau is toxic to neuronal cells. FEBS Lett. 2006 Sep 4;580(20):4842–50. doi: 10.1016/j.febslet.2006.07.078. [DOI] [PubMed] [Google Scholar]
- 223.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009 May 8;284(19):12845–52. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009 Jul;11(7):909–13. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sigurdsson EM. Tau-focused immunotherapy for Alzheimer’s disease and related tauopathies. Curr Alzheimer Res. 2009;6(5):446–50. doi: 10.2174/156720509789207930. [DOI] [PMC free article] [PubMed] [Google Scholar]