Abstract
Frontotemporal lobar degeneration (FTLD) is clinically, pathologically and genetically heterogeneous. Three major proteins are implicated in its pathogenesis. About half of cases are characterized by depositions of the microtubule associated protein, tau (FTLD-tau). In most of the remaining cases, deposits of the transactive response (TAR) DNA-binding protein with Mw of 43 kDa, known as TDP-43 (FTLD-TDP), are seen. Lastly, about 5–10 % of cases are characterized by abnormal accumulations of a third protein, fused in sarcoma (FTLD-FUS). Depending on the protein concerned, the signature accumulations can take the form of inclusion bodies (neuronal cytoplasmic inclusions and neuronal intranuclear inclusions) or dystrophic neurites, in the cerebral cortex, hippocampus and subcortex. In some instances, glial cells are also affected by inclusion body formation. In motor neurone disease (MND), TDP-43 or FUS inclusions can present within motor neurons of the brain stem and spinal cord. This present paper attempts to critically examine the role of such proteins in the pathogenesis of FTLD and MND as to whether they might exert a direct pathogenetic effect (gain of function), or simply act as relatively innocent witnesses to a more fundamental loss of function effect. We conclude that although there is strong evidence for both gain and loss of function effects in respect of each of the proteins concerned, in reality, it is likely that each is a single face of either side of the coin, and that both will play separate, though complementary, roles in driving the damage which ultimately leads to the downfall of neurons and clinical expression of disease.
Keywords: Frontotemporal lobar degeneration, Motor neurone disease, Microtubule associated protein, Tau, TDP-43, FUS, Gain of function, Loss of function
Introduction
Frontotemporal lobar degeneration (FTLD) is clinically, pathologically and genetically heterogeneous. The prototypical clinical syndromes are behavioural variant frontotemporal dementia (bvFTD), a disorder of behaviour and executive impairments, progressive non-fluent aphasia (PNFA), a disorder of expressive language, and semantic dementia (SD), a disorder of conceptual knowledge [54]. A proportion of patients with any of these syndromes of FTLD can develop the amyotrophic form of motor neurone disease (MND) [53, 78], further emphasising clinical heterogeneity within FTLD, and highlighting the long known association with, and suspected pathogenetic links between, FTLD and MND.
Extensive tissue research over the past decade has not only defined the pathological proteins involved in disease pathology, and has characterized their morphological form and topographical distribution within the brain, but most importantly has enabled a rational nomenclature and classification scheme to be introduced, which can now be employed to characterize virtually all but a handful of cases in a logical and consistent manner [13, 45]. Such a scheme had been lacking for many years, and this led to an unhelpful plethora of confusing, and sometimes conflicting, terminologies.
It was known from the 1980s that one of the major proteins involved in FTLD is the microtubule-associated protein, tau, which can accumulate in both nerve cells and glial cells. In sporadic disease, the signature accumulations take the form of neuronal Pick bodies (known as FTLD-tau PiD), tufted astrocytes (FTLD-tau PSP), or astrocytic plaques (FTLD-tau CBD), whereas in inherited cases, these can present as inclusions similar to any of these or with unique tau pathology, such cases being defined as FTLD-tau MAPT. Collectively, FTLD-tau accounts for about half of all cases of FTLD [5, 70]. The second major protein is the transactive response (TAR) DNA-binding protein with Mw of 43 kDa, known as TDP-43 [2, 55]. Pathologically, this is seen as inclusion bodies [neuronal cytoplasmic inclusions (NCI) and neuronal intranuclear inclusions (NII)] or dystrophic neurites (DN) in the cerebral cortex, hippocampus and subcortex. In many instances, the relative proportions of NCI, NII and DN within the tissue may permit subclassification into histological subtypes, A, B, C and D [45], which can aid diagnostic precision, but not all cases always show clear-cut distinctions, and further studies are needed to fully corroborate these entities [3]. In MND similar TDP-43 inclusions are present within motor neurons of the brain stem and spinal cord [2, 55]. Lastly, about 5–10 % of cases of FTLD [5, 46, 51, 57, 58, 68, 73], and a few familial MND cases [39, 82] are characterized by the abnormal accumulation, as cellular inclusions, of a third protein, fused in sarcoma (FUS). Other ubiquitinated, but as yet unidentified, target proteins characterize FTLD cases with CHMP2B mutations [33], and there may still be other rare cases where the hallmark of FTLD is present as microvacuolar change, but no NCI has been detected [13, 44, 45, 72].
On other fronts, more progress has been made in unravelling the genetic basis of FTLD, with mutations in tau (MAPT) [35, 63, 75] and progranulin (GRN) [6, 20, 50] genes on chromosome 17 being the first to be discovered. Elsewhere, mutations were identified in an extended Danish pedigree with bvFTD in CHMP2B gene on chromosome 3 [71], and in rare families with FTD and inclusion body myositis and Paget’s disease of bone (IBMPFD) in VCP on chromosome 9 [84]. Most recently, a hexanucleotide expansion in C9ORF72 gene has been shown to be the most common cause of both FTD (with or without MND), and MND itself [24, 64]. In addition, it has been suggested that variations in UBAP1 on chromosome 9p21 may act as a genetic risk factor for FTLD [65]. Interestingly mutations in TARDBP [30, 76] and FUS [39, 82] can cause MND, but are only rarely associated with FTLD [8].
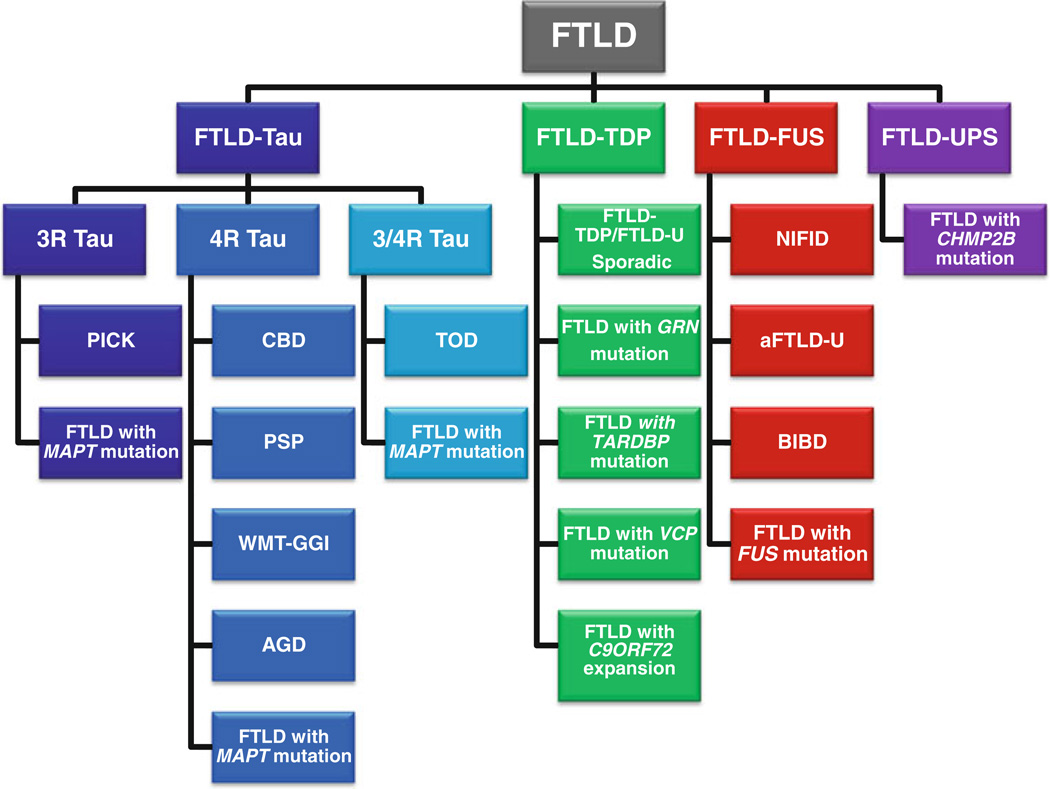
Although FTLD is clinically, genetically, and neuropathologically heterogeneous, these recent advances allow for a logical classification according to molecular pathology and genetics [13, 14, 45] (see Fig. 1). While this heterogeneity may at first sight appear ‘random’, some clinical and/or genetic entities have been associated with particular pathological TDP-43 subtypes. For example, cases of FTD+MND generally show TDP-43 type B histology [13, 52], and familial cases are often associated with mutations in C9ORF72 [24, 64]. Cases of SD most often show TDP-43 type C histology, and are usually sporadic [5]. Cases of PNFA commonly display TDP-43 type A histology [5, 13], and familial cases with this histology are often those which are associated with GRN mutations [5]. Cases of IBMPFD are associated with VCP mutation and show TDP-43 type D histology [56]. BvFTD, however, remains a ‘mixed bag’ with multiple histologies (FTLD-tau, FTLD-TDP and FTLD-FUS), and multiple genes (MAPT, GRN, C9ORF72), contributing to similar, but not always identical, clinical presentations. For example, cases of FTLD-FUS stand out according to their very early onset and the presence of bizarre stereotypic behaviours [68, 73], whereas cases with C9ORF72 mutation may display pronounced psychoses [74].
Fig. 1.
The molecular and genetic classification of FTLD. Three distinct neuropathologic categories may be identified based on the molecular pathology of the misfolded protein within the inclusion: FTLD-Tau, FTLD-TDP, and FTLD-FUS; the molecular pathology of a fourth category, FTLD with epitopes of the ubiquitin–proteasome system (FTLD-UPS), remains indeterminate. 3R, 4R, 3R/4R the predominant tau isoform within the inclusion; PICK, Pick’s disease; FTLD with microtubule-associated protein tau (MAPT) mutation with inclusions of 3R, 4R, or 3R and 4R tau protein; CBD, corticobasal degeneration; PSP, progressive supranuclear palsy; AGD, argyrophilic grain disease; TOD, tangle only dementia; WMT-GGI, white matter tauopathy with globular glial inclusions; FTLD-U, FTLD with ubiquitin inclusions, now called FTLD-TDP; FTLD with progranulin (GRN) mutation; FTLD with TAR DNA-binding protein 43 (TARDBP) gene mutation; FTLD with valosin-containing protein (VCP) mutation; FTLD with C9ORF72 expansion, chromosome 9-linked FTLD with C9ORF72 hexanucleotide repeat expansion; NIFID neuronal intermediate filament inclusion disease; aFTLD-U atypical FTLD with ubiquitin inclusions; BIBD basophilic inclusion body disease; FTLD with fused in sarcoma (FUS) mutation; FTLD with charged multivesicular body protein 2B (CHMP2B) mutation. There may still be unclassified entities within each molecular pathology grouping (modified from [12])
Pathological and biochemical studies of TDP-43 and FUS show that patients with FTLD, and others with FTD+MND, as well as those with MND alone, may share a unifying pathogenetic basis for their disease, or at least have many molecular properties in common. However, it is still not clear whether the differing clinical, histological and genetic forms of FTLD represent variations on a common disease theme, or are, in fact, separate disorders in their own right, which coincidentally damage the same or overlapping key brain structures. Although the aggregated proteins that form NCI, NII or DN within the brain can be useful for diagnostic purposes, it is also unclear whether these are the real culprits of disease pathogenesis, directly triggering or causing damage to neurons (and glial cells), or are mere witnesses to, or products of, the disease process, with their presence, perhaps reflecting a compensatory or protective response within cells to, or against, potentially toxic forerunners. Nonetheless, their sequestration into ‘pathological aggregates’ may lead to loss of normal content of these key proteins, and with that their cellular functions.
This present paper attempts to critically examine the role of such proteins in relationship to whether they might evidence a direct pathogenetic effect (gain of function), or simply act as relatively innocent witnesses to a more fundamental loss of function effect.
Tau: gain of function
Certain genetic forms of FTLD-tau are known to change the types of tau isoforms expressed [35, 63, 75], with recent studies showing differential gene expression in cells overexpressing different tau isoforms [15]. Compared with 3-repeat tau, 4-repeat tau increases transcripts involved in neurite outgrowth and cell death, and decreases transcripts involved in neuronal survival. Changes in tau isoform expression may therefore precipitate a toxic gain of function over time.
Tau: loss of function
A substantive, and specific, focal loss of cortical projection neurons is a unifying feature of all cases of FTLD. While only around half of patients with FTLD have hyperphosphorylated tau inclusions within neurons and/or glial cells [70], substantial research has been focused on the underlying mechanisms involved. A major function of tau is microtubule binding, thereby stabilizing the axonal cytoskeleton and regulating axonal transport [49]. It is the loss of this normal function of tau, with bundling into tangle- or Pick body-type structures, that is considered key to FTLD-tau.
However, tau knockout mice are viable without overt phenotype when young, as microtubule-associated protein (MAP)-1 can perform the same functions, at least during this developmental period [37, 49]. Only very old tau knockout mice display some behavioural and motor deficits [49]. Additional functions, identified using tau knockout models, include the regulation of microtubule acetylation through the inhibition of histone deacetylase (HDAC)-6, with HDAC-6 activity being regulated by tau levels [18, 61]. A nuclear function has also been proposed for tau associated with histone deacetylase activity where it regulates BAF57 levels, a component of the neuron-restrictive silencing factor repressor complex (repressed genes are associated with hypoacetylation) [23]. Interestingly, knock in models of human tau have shown an increase in the numbers of neurons [69], perhaps via this mechanism.
TDP-43: gain of function
TDP-43 is the major protein component of the abnormal inclusions in FTLD-TDP and most non-SOD1 ALS [2, 55]. TDP-43 is a multifunctional hnRNP protein involved in regulation of RNA splicing, translation, miRNA processing, and mRNA transport and stability [11]. There is evidence for TDP-43 autoregulation, participation in stress granule formation, and a protease-resistant prion-like domain in the C-terminal region [4, 17, 21, 28]. TDP-43 has more than 6,000 RNA targets [62].
Mutations in TARDBP occur predominantly in the C-terminal region, most causing ALS [30, 76], but some rarely causing FTLD [8]. Various cell, fly, and rodent models have shown evidence for either loss or gain of function, or both, in the pathogenesis of TDP-43 protein-opathy, but none has completely modelled human disease. Evidence for a toxic gain of function in model systems has included the following: in rodent models, overexpression of both wild type (WT) [86] and mutant TDP-43 are neurotoxic in a dose-dependent manner, and in some rodent models, C-terminal fragments (CTFs) correlate with disease progression; in cultured rodent neurons, one study showed that CTFs impair neurite outgrowth that is rescued by full-length TDP-43, while in another, neurotoxicity correlated with the amount of cytoplasmic TDP-43 expression; Drosophila studies have shown neurotoxicity with both expression of full-length WT and mutant TDP-43 [7, 42, 77, 80, 85, 87, 88, 91]. TARDBP mutations also increase stress granule formation in response to cellular stress, increase cleavage of TDP-43 and formation of CTFs, and increase the production of low molecular weight prion-like protease-resistant fragments [7, 17, 26, 31, 43, 47, 60, 66, 90]. The evidence for toxic gain of function due to overexpressed or mutant TDP-43 or cytoplasmic TDP-43/CTFs is compelling, but given the numerous crucial functions carried out by normal TDP-43, it is more than likely that a loss of function will also contribute to the pathogenesis of TDP-43 proteinopathy. Indeed, both a loss and a gain of function will most probably play a role in these diseases.
TDP-43: loss of function
TDP-43 protein expression is tightly controlled within narrow limits by an auto-regulatory mechanism, and both over- and under-expression of TDP-43 result in impaired neuronal viability [38, 41, 89]. However, the precise mechanisms leading to cell death are not known. While TDP-43 loss-of-function mechanisms may contribute to neurodegeneration, especially in cases with mutations in the TARDBP gene, alternative etiologies may contribute to pathogenesis in sporadic cases. Cellular stress is an attractive precipitating factor, because it is a feature of most TDP-43 proteinopathies, including primary diseases where the principal molecular pathology is FTLD-TDP (including both sporadic forms of FTLD-TDP, and familial forms with GRN, C9ORF72, TARDBP, or VCP mutation), and those disorders such as Alzheimer’s disease, Dementia with Lewy bodies, and Parkinson’s disease where it is a secondary disease process or co-morbidity. Cellular stress likely causes redistribution of TDP-43 to the cytoplasm where its intrinsic self-aggregating property leads to inclusion body formation or trafficking to stress granules. Cytoplasmic, and less commonly nuclear, aggregation is accompanied by several post-translational modifications including phosphorylation, ubiquitination, and cleavage. These inclusion bodies may act as TDP-43 ‘sinks’ and hinder translocation to the nucleus where TDP normally regulates mRNA processing. Alternatively, these inclusion bodies may have the effect of ‘mopping up’ soluble TDP-43 resulting in an overall depletion of nuclear and usable TDP-43 causing an increase in cellular stress and resulting in neurodegeneration.
Some cellular and animal models indicate that mutant TARDBP does not necessarily result in inclusion body formation, and so both under- and over-expression of TDP-43 is sufficient to cause neurodegeneration [86], but inclusion body formation is likely to contribute additionally to neurodegeneration.
FUS: gain of function
FUS is a ubiquitously expressed multifunctional DNA/RNA-binding protein that can bind to a large number of RNA targets [32, 79]. It is mainly localized to the nucleus [1], but under physiological conditions continuously shuttles between the nuclear and cytoplasmic compartments [92]. In about 5–10 % of FTLD patients (subsumed as FTLD-FUS) [5, 46, 51, 57, 58], and in familial forms of ALS associated with mutations in the FUS gene [39, 82], abnormal accumulation of FUS into cytoplasmic inclusions are the defining hallmark lesion.
The majority of FUS mutations have been shown to disrupt a region characterized as a non-classical nuclear localization sequence, and this disruption leads to impaired transportin-mediated nuclear import of FUS, with redistribution of the protein to the cytoplasm [27, 36]. However, the mechanisms leading to cytoplasmic FUS accumulation in FTLD-FUS in the absence of FUS mutations, and the processes of FUS-associated neurodegeneration, are not yet known. Model systems addressing the fundamental questions on the underlying mechanisms are just emerging with some inconsistent findings. However, there is evidence supporting the idea that neurodegeneration might be triggered through a neurotoxic/gain of function effect of cytoplasmic FUS rather than by a loss of function.
In human pathology, inclusion bearing cells often retain their physiological nuclear FUS staining, arguing against a loss of nuclear function mechanism [57, 58]. In yeast, FUS toxicity is closely related to its cytoplasmic localization [19]. In transgenic worm, fly and rat models, expression of cytoplasmic FUS is sufficient to induce motor defects and premature death, though no depletion of nuclear FUS is observed [16, 34, 81]. Moreover, the severity of phenotype nicely correlates with the level of cytoplasmic FUS, thereby supporting the idea of a neurotoxic effect of cytoplasmic FUS. This might be mediated by either abnormal interaction with cytoplasmic RNA targets or protein binding partners, resulting in disturbance of RNA metabolism, or by a gain of novel function of disease-associated FUS isoforms unrelated to its native function.
FUS: loss of function
Although the specific mechanisms of FUS-associated neurodegeneration are not known, several lines of evidence support a role for loss of FUS physiological function. FUS mutations that cause ALS primarily affect the C terminus that includes the nuclear localization signal. The degree to which these mutations interfere with transportin-mediated nuclear import correlates with the severity of clinical disease and neuropathology [27, 36]. Cases of FTLD-FUS, which are not associated with FUS mutations, also demonstrate reduced nuclear FUS staining of inclusion bearing neurons [51, 57, 58]. This strongly suggests that neurodegeneration is directly related to cellular redistribution of FUS, with one logical explanation being a reduced ability of FUS to perform its normal nuclear functions. Although the results from animal models have been inconsistent, knockdown of FUS or its homologues has been shown to result in abnormal development, reduced viability, motor deficits and abnormal neuronal morphology, with some of these deficits being rescued by expression of FUS transgenes [40, 67, 83]. Evidence against a toxic-gain-of-function mechanism includes the absence of abnormal molecular species in FTLD-FUS [57, 58], the lack of neurodegeneration in some anatomical regions with abundant cytoplasmic FUS inclusions [46] and the absence of FUS aggregates in some models of toxicity [40]. The fact that all FET proteins [22, 59], along with transportin-1 [9, 22], co-accumulate in the cellular inclusions of FTLD-FUS, indicate that dysregulation of several other RNA-binding proteins [29] is also associated with FTLD.
Concluding remarks
The last decade has seen an extraordinary development in our understanding of FTLD. In recent years, the genetic causes of most cases of autosomal dominant FTLD have been discovered [6, 20, 24, 63, 64, 75], and the molecular pathologies associated with this group have largely been defined [13, 14, 45]. Thus, it is true to say the clinical, genetic, and neuropathological phenotypes that constitute FTLD have been characterized in large part. What remains to be determined is the elucidation of the mechanisms of neurodegeneration caused by different gene defects and different molecular pathologies. In the present article, evidence has been presented in support of whether the underlying pathogenetic mechanism appertaining to the major pathological proteins of FTLD can be best represented by a ‘gain’ or a ‘loss’ of function effect.
However, it is clear that the situation for each protein is unlikely to be an ‘either–or’ situation, but some combination of both. If it is postulated that there is a ‘gain of function’ of potentially toxic versions, or species, of each protein due to pathophysiological changes which favour aggregation, then it is clear that conversion of normal protein into an abnormal form must involve a loss of content of ‘wild-type’ protein, and along with that, a loss of function. For example, oligomerisation of phospho-tau may induce neurotoxicity prior to full aggregation into fibrillar tau. However, phosphorylation of tau will induce loss of microtubule binding capacity, and along with that reduced tau function. Similarly, phosphorylation of TDP-43 and FUS may induce cytoplasmic aggregation into NCI, and promote sequestration of normal TDP-43 and FUS into the aggregation process through a seeding effect. However, by so doing, loss of nuclear localization will cause loss of normal RNA-binding properties, and reduce transcriptional activity.
There are other issues. For example, how can molecular changes in three distinct proteins lead to three separate pathological cascades, each of which can threaten the viability of neurons in the same regions of brain, and bring about a similar clinical dysfunction in each? Perhaps, the question is better asked as to how changes in these three proteins trigger or promote the same pathological cascade? Where is the common ground that links these molecular changes? Many believe the molecular and pathological changes of Alzheimer’s disease can be linked to formation of soluble and toxic oligomers of beta amyloid protein, which are either overproduced in familial forms of disease, or accumulated in the brain in sporadic disease through deficiencies in amyloid clearance pathways through the extracellular fluid, or failures of enzymatic degradation. Similarly in Parkinson’s disease, oligomerisation of phosphorylated forms of alpha-synuclein can lead to Lewy body formation. It is clear that in FTLD, when aggregated, tau, TDP-43, and probably also FUS, are phosphorylated, and although tau may undergo a similar process of oligomerisation in FTLD, as seen in AD, it is not so clear that TDP-43 and FUS in FTLD follow a similar route to aggregation and inclusion body formation.
What other clues are there? Certain inherited forms of FTLD (i.e. those associated with CHMP2B [71] or VCP [84] mutations) implicate issues in protein trafficking and sorting, and proteasomal failures, or both. Such evidence is supported by genetic associations with variations in UBAP-1 [65]. In addition, mutations in UBQLN2 in familial X-linked ALS, and the association of ubiquilin-2 immunoreactivity with inclusions in ALS unrelated to UBQLN2 mutations, FTLD-TDP, synucleinopathies, multiple system atrophy, polyglutamine disorders, and neuronal intranuclear hyaline inclusion disease [10, 25, 48], suggests that failure of proteasomal degradation of ubiquitinated proteins may play a role in the pathogenesis of diverse degenerative disorders. Cellular stress may cause a redistribution of TDP-43 to the cytoplasm where its intrinsic self-aggregating property leads to inclusion body formation, or results in its trafficking to stress granules. Failure to degrade aggregated proteins is a common theme across many neurodegenerative diseases where inclusion bodies are characteristic. However, this may ‘simply’ represent a neuroprotective response on the part of the ‘diseased’ cell—a way of sequestering potentially harmful molecules and packaging them into a relatively innocuous form. Nonetheless, a consequence of this may involve the depletion of normal protein, or even its conversion into a form or structure compatible with the abnormal form, promoting further aggregation. Large aggregates of protein may simply be too large for the proteasomal pathways to handle and degrade, and intracellular accumulation is the price paid. Notwithstanding this, extreme accumulations of protein may ultimately bring about metabolic collapse through a volume effect, crowding out useful membranes and organelles. Failure to degrade protein aggregates may be a downstream consequence of primary physiological or structural changes in proteins which promote or trigger that tendency to self aggregate, far removed from the principal events that drive the neurodegenerative cascade, though loss of efficiency in protein degradation pathways, associated with mutational events, will only exacerbate this process.
In short, we have provided evidence in this paper arguing as to whether the underlying pathogenetic mechanism(s) appertaining to the major pathological proteins of FTLD can be best represented by ‘gain’ or ‘loss’ of function effects. In reality, it is likely that each is a single face of either side of the coin, and that both will play separate, though complementary, roles in driving the damage which ultimately leads to the downfall of neurons and clinical expression of disease.
Acknowledgments
GH receives a NHMRC Senior Principal Research Fellowship 630434. EHB is supported by National Institute on Aging grant (P30 AG13854). NJC is supported by grants from the National Institute on Aging of the National Institutes of Health (P50 AG05681 and P01 AG03991), the Hope Center for Neurological Disorders, and the Charles F. and Joanne Knight Alzheimer’s Disease Research Centre. MN is supported by the Swiss National Science Foundation (31003A-132864 and CRSII3-136222), the German Federal Ministry of Education and Research (01GI1005B), and the Hans and Ilse Breuer Foundation. IRM is funded by the Canadian Institutes of Health Research Grants (179009 and 74580), and the Pacific Alzheimer’s Research Foundation Center Grant (C06-01). DMAM is supported by grants from the Wellcome Trust and Medical Research Council, and the Manchester Brain Bank receives funding from Alzheimers Research UK and Alzheimers Society through the Brains for Dementia Research Initiative.
Footnotes
This work was presented at a Round Table Discussion Session at the 8th International Conference on Fronto-temporal Dementias, held in Manchester, UK, 6–8 September 2012, and was supported by Springer.
Contributor Information
Glenda Halliday, Neuroscience Research Australia, University of New South Wales, Sydney, Australia.
Eileen H. Bigio, Alzheimer Disease Center, Department of Pathology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA
Nigel J. Cairns, Department of Neurology, Washington University School of Medicine, St. Louis, Missouri, USA
Manuela Neumann, Department of Neuropathology, German Center for Neurodegenerative Diseases Tuebingen, University of Tuebingen, Tuebingen, Germany.
Ian R. A. Mackenzie, Department of Pathology, University of British Columbia, Vancouver, Canada
David M. A. Mann, Email: david.mann@manchester.ac.uk, Institute of Brain, Behaviour and Mental Health, School of Community Based Medicine, University of Manchester, Manchester, UK; Salford Royal Hospital, University of Manchester, Stott Lane, Salford M6 8HD, UK.
References
- 1.Andersson MK, Stahlberg A, Arvidsson Y, Olofsson A, Semb H, Stenman G, Nilsson O, Aman P. The multifunctional FUS, EWS and TAF15 proto-oncoproteins show cell type specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol. 2008;9:37. doi: 10.1186/1471-2121-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong RA, Ellis W, Hamilton RL, Mackenzie IR, Hedreen J, Gearing M, Montine T, Vonsattel JP, Head E, Lieberman AP, Cairns NJ. Neuropathological heterogeneity in fronto-temporal lobar degeneration with TDP-43 proteinopathy: a quantitative study of 94 cases using principal components analysis. J Neural Transm. 2010;117:227–239. doi: 10.1007/s00702-009-0350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayala YM, De Conti L, Avendano-Vasquez SE, Dhir A, Romano M, D’Ambrogio A, Tollervey J, Ule J, Baralle M, Buratti E, Baralle F. TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO. 2011;30:277–288. doi: 10.1038/emboj.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baborie A, Griffiths TD, Jaros E, Richardson A, Ferrari R, Moreno J, Momeni P, McKeith IG, Burn DJ, Duplessis D, Pal P, Rollinson S, Pickering-Brown SM, Thompson JC, Neary D, Snowden JS, Perry R, Mann DMA. Pathological correlates of frontotemporal lobar degeneration in the elderly. Acta Neuropathol. 2011;12:365–373. doi: 10.1007/s00401-010-0765-z. [DOI] [PubMed] [Google Scholar]
- 6.Baker M, Mackenzie IRA, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 7.Barmada SJ, Skibinski G, Korb E, Rao EJ, Wu JY, Finkbeiner S. Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial amyotrophic lateral sclerosis. J Neurosci. 2010;30:639–649. doi: 10.1523/JNEUROSCI.4988-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borroni B, Bonvicini C, Alberici A, Buratti E, Agosti C, Archetti S, Papetti A, Stuani C, Di Luca M, Gennarelli M, Padovani A. Mutation within TARDBP leads to frontotemporal dementia without motor neuron disease. Hum Mutat. 2009;30:E974–E983. doi: 10.1002/humu.21100. [DOI] [PubMed] [Google Scholar]
- 9.Brellstaff J, Lashley T, Holton JL, Lees AJ, Rossor MN, Bandopadhyay R, Revesz T. Transportin 1: a marker for FTLD-FUS. Acta Neuropathol. 2011;122:591–600. doi: 10.1007/s00401-011-0863-6. [DOI] [PubMed] [Google Scholar]
- 10.Brettschneider J, Van Deerlin VM, Robinson JL, Kwong L, Lee EB, Ali YO, Safren N, Monteiro MJ, Toledo JB, Elman L, McCluskey L, Irwin DJ, Grossman M, Molina-Porcel L, Lee VM-Y, Trojanowski JQ. Pattern of ubiquilin pathology in ALS and FTLD indicates presence of C9ORF72 hexanucleotide expansion. Acta Neuropathol. 2012;123:825–839. doi: 10.1007/s00401-012-0970-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buratti E, Baralle FE. The molecular links between TDP-43 dysfunction and neurodegeneration. Adv Genet. 2009;66:1–34. doi: 10.1016/S0065-2660(09)66001-6. [DOI] [PubMed] [Google Scholar]
- 12.Cairns NJ, Ghoshal N. FUS: a new actor on the frontotemporal lobar degeneration stage. Neurology. 2010;74:354–356. doi: 10.1212/WNL.0b013e3181ce5dd6. [DOI] [PubMed] [Google Scholar]
- 13.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL, 3rd, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM Consortium for Frontotemporal Lobar Degeneration. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL, 3rd, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A, Xu Y, Forman MS, Trojanowski JQ, Lee VM, Mackenzie IR. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007;171:227–240. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen S, Townsend K, Goldberg TE, Davies P, Conejero-Goldberg C. MAPT isoforms: differential transcriptional profiles related to 3R and 4R splice variants. J Alz Dis. 2010;22:1313–1329. doi: 10.3233/JAD-2010-101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Yang M, Deng J, Chen X, Ye Y, Zhu L, Liu J, Ye H, Shen Y, Li Y, Rao EJ, Fushimi K, Zhou X, Bigio EH, Mesulam M, Xu Q, Wu JY. Expression of human FUS protein in Drosophila leads to progressive neurodegeneration. Protein Cell. 2011;2:477–486. doi: 10.1007/s13238-011-1065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colombrita C, Zennaro E, Fallini C, Weber M, Sommacal A, Buratti E, Silani V, Ratti A. TDP-43 is recruited to stress granules in conditions of oxidative insult. J Neurochem. 2009;111:1051–1061. doi: 10.1111/j.1471-4159.2009.06383.x. [DOI] [PubMed] [Google Scholar]
- 18.Cook C, Gebdron TF, Scheffel K, Carlomagno Y, Dunmore J, Deture M, Petrucelli L. Loss of HDAC6, a novel CHIP substrate, alleviates abnormal tau accumulation. Hum Mol Genet. 2012;21:2936–2945. doi: 10.1093/hmg/dds125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couthouis J, Hart MP, Shorter J, DeJesus-Hernandez M, Erion R, Oristano R, Liu AX, Ramos D, Jethava N, Hosangadi D, Epstein J, Chiang A, Diaz Z, Nakaya T, Ibrahim F, Kim HJ, Solski JA, Williams KL, Mojsilovic-Petrovic J, Ingre C, Boylan K, Graff-Radford NR, Dickson DW, Clay-Falcone D, Elman L, McCluskey L, Greene R, Kalb RG, Lee VM, Trojanowski JQ, Ludolph A, Robberecht W, Andersen PM, Nicholson GA, Blair IP, King OD, Bonini NM, Van Deerlin V, Rademakers R, Mourelatos Z, Gitler AD. A yeast functional screen predicts new candidate ALS disease genes. Proc Natl Acad Sci USA. 2011;108:20881–20890. doi: 10.1073/pnas.1109434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, van Duijn C, Peeters K, Sciot R, Santens P, De Pooter T, Mattheijssens M, Van den Broeck M, Cuijt I, Vennekens K, De Deyn PP, Kumar-Singh S, Van Broeckhoven C. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 21.Cushman M, Johnson BS, King OD, Gitler AD, Shorter J. Prion-like disorders: blurring the divide between transmissibility and infectivity. J Cell Sci. 2010;123:1191–1201. doi: 10.1242/jcs.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson YS, Robinson AC, Hu Q, Mishra M, Baborie A, Jaros E, Perry RH, Cairns NJ, Richardson A, Gerhard A, Neary D, Snowden JS, Bigio EH, Mann DM. Nuclear carrier and RNA binding proteins in frontotemporal lobar degeneration associated with fused in sarcoma (FUS) pathological changes. Neuropathol Appl Neurobiol. 2012 doi: 10.1111/j.1365-2990.2012.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Barreda EG, Dawson HN, Vitek MP, Avila J. Tau deficiency leads to the upregulation of BAR-57, a protein involved in neuron-specific gene repression. FEBS Lett. 2010;584:2265–2270. doi: 10.1016/j.febslet.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 24.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R. Expanded GGGGCC Hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng H-X, Chen W, Hong S-T, Boycott KM, Gorrie GH, Siddique N, Yang Y, Fecto F, Shi Y, Zhai H, Jiang H, Hirano M, Rampersaud E, Jansen GH, Donkervoort S, Bigio EH, Brooks BR, Ajroud K, Sufit RL, Haines JL, Mugnaini E, Pericak-Vance M, Siddique T. Mutations in UBQLN2 cause dominant X-linked form of juvenile and adult-onset ALS and ALS/dementia. Nature. 2011;477:211–215. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dewey CM, Cenik B, Sephton CF, Johnson BA, Herz J, Yu G. TDP-43 aggregation in neurodegeneration: are stress granules the key? Brain Res. 2012;1462:16–25. doi: 10.1016/j.brainres.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dormann D, Rodde R, Edbauer D, Bentmann E, Fischer I, Hruscha A, Than ME, Mackenzie IR, Capell A, Scmid B, Neumann M, Haass C. ALS-associated fused in sarcoma (FUS) mutations disrupt transportin-mediated nuclear import. EMBO J. 2010;29:2841–2857. doi: 10.1038/emboj.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuentealba RA, Udan M, Bell S, Wegorzewska I, Shao J, Diamond MI, Weihl CC, Baloh RH. Interaction with polyglutamine aggregates reveals a Q/N-rich domain in TDP-43. J Biol Chem. 2010;285:26304–26314. doi: 10.1074/jbc.M110.125039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao FB, Taylor JP. RNA-binding proteins in neurological disease. Brain Res. 2012;1462:1–2. doi: 10.1016/j.brainres.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 30.Gitcho MA, Baloh RH, Chakraverty S, Mayo K, Norton JB, Levitch D, Hatanpaa KJ, White CL, 3rd, Bigio EH, Caselli R, Baker M, Al-Lozi MT, Morris JC, Pestronk A, Rademakers R, Goate AM, Cairns NJ. TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol. 2008;63:535–538. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo W, Chen Y, Zhou X, Kar A, Ray P, Chen X, Rao EJ, Yang M, Ye H, Zhu L, Liu J, Zhang D, Buratti E, Baralle FE, Bigio EH, Mesulam M, Xu Q, Shen Y, Fushimi K, Wu JY. An ALS-associated mutation affecting TDP-43 enhances protein aggregation, fibril formation and neurotoxicity. Nature Struct Mol Biol. 2011;18:822–830. doi: 10.1038/nsmb.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoell JI, Larsson E, Runge S, Nusbaum JD, Duggimpudi S, Farazi TA, Hafner M, Borkhardt A, Sander C, Tuschl T. RNA targets of wild-type and mutant FET family proteins. Nat Struct Mol Biol. 2011;18:1428–1431. doi: 10.1038/nsmb.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holm IE, Isaacs AM, Mackenzie IR. Absence of FUS-immunoreactive pathology in frontotemporal dementia linked to chromosome 3 (FTD-3) caused by mutation in the CHMP2B gene. Acta Neuropathol. 2009;118:719–720. doi: 10.1007/s00401-009-0593-1. [DOI] [PubMed] [Google Scholar]
- 34.Huang C, Zhou H, Tong J, Chen H, Liu YJ, Wang D, Wei X, Xia XG. FUS transgenic rats develop the phenotypes of amyotrophic lateral sclerosis and frontotemporal lobar degeneration. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1002011. e1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden M, Pickering-Brown SM, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaf E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski JQ, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd P, Hayward N, Kwok JBJ, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann DM, Lynch T, Heutink P. Association of missense and 5′-splice-site mutation in tau with inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 36.Ito D, Seki M, Tsunoda Y, Uchiyama H, Suzuki N. Nuclear transport impairment of amyotrophic lateral sclerosis-linked mutations in FUS/TLS. Ann Neurol. 2011;69:152–162. doi: 10.1002/ana.22246. [DOI] [PubMed] [Google Scholar]
- 37.Ke DK, Suchowerska AK, van der Hoven J, De Silva DM, Wu CW, van Eersel J, Ittner A, Ittner LM. Lessons from tau-deficient mice. Int J Alz Dis. 2012 doi: 10.1155/2012/873270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar-Singh S. Progranulin and TDP-43: mechanistic links and future directions. J Mol Neurosci. 2011;45:561–573. doi: 10.1007/s12031-011-9625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwiatkowski TJ, Bosco DA, LeClerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PA, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, Kenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH., Jr Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 40.Lanson NA, Pandey UB. FUS-related proteinopathies: lessons from animal models. Brain Res. 2012;1462:44–60. doi: 10.1016/j.brainres.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 41.Lee EB, Lee VM, Trojanowski JQ. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nat Rev Neurosci. 2011;13:38–50. doi: 10.1038/nrn3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Ray P, Rao E, Shi C, Guo W, Chen X, Woodruff EA, III, Fushimi K, Wu JY. A Drosophila model for TDP-43 proteinopathy. Proc Natl Acad Sci. 2010;107:3169–3174. doi: 10.1073/pnas.0913602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu-Yesucevitz L, Bilqutay A, Zhang YJ, Vanderweyde T, Citro A, Mehta T, Zaarur N, McKee A, Bowser R, Sherman M, Petrucelli L, Wolozin B. Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS ONE. 2010;5:e13250. doi: 10.1371/journal.pone.0013250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackenzie IRA, Shi J, Shaw CL, Du Plessis D, Neary D, Snowden D, Mann DMA. Dementia lacking distinctive histology (DLDH) revisited. Acta Neuropathol. 2006;112:551–559. doi: 10.1007/s00401-006-0123-3. [DOI] [PubMed] [Google Scholar]
- 45.Mackenzie IRA, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DMA, Lee VM-Y. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mackenzie IRA, Munoz DG, Kusaka H, Yokota O, Ishihara K, Roeber S, Kretzschmar HA, Cairns NJ, Neumann M. Distinct pathological subtypes of FTLD-FUS. Acta Neuropathol. 2011;121:207–218. doi: 10.1007/s00401-010-0764-0. [DOI] [PubMed] [Google Scholar]
- 47.McDonald KK, Aulas A, Destroismaisons L, Pickles S, Beleac E, Camu W, Rouleau GA, Vande Velde C. TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TOZ-1. Hum Mol Genet. 2011;20:1400–1410. doi: 10.1093/hmg/ddr021. [DOI] [PubMed] [Google Scholar]
- 48.Mori F, Tanji K, Odagiri S, Toyoshima Y, Yoshida M, Ikeda T, Sasaki H, Kakita A, Takahashi H, Wakabayashi K. Ubiquilin immunoreactivity in cytoplasmic and nuclear inclusions in synucleinopathies, polyglutamine diseases, and intranuclear inclusion body disease. Acta Neuropathol. 2012;124:149–151. doi: 10.1007/s00401-012-0999-z. [DOI] [PubMed] [Google Scholar]
- 49.Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011;70:410–426. doi: 10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukherjee O, Pastor P, Cairns NJ, Chakraverty S, Kauwe JS, Shears S, Behrens MI, Budde J, Hinrichs AL, Norton J, Levitch D, Taylor-Reinwald L, Gitcho M, Tu PH, Tenenholz Grinberg L, Liscic RM, Armendariz J, Morris JC, Goate AM. HDDD2 is a familial frontotemporal lobar degeneration with ubiquitin-positive, tau-negative inclusions caused by a missense mutation in the signal peptide of progranulin. Ann Neurol. 2006;60:314–322. doi: 10.1002/ana.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munoz DG, Neumann M, Kusaka H, et al. FUS pathology in basophilic inclusion body disease. Acta Neuropathol. 2009;118:617–627. doi: 10.1007/s00401-009-0598-9. [DOI] [PubMed] [Google Scholar]
- 52.Murray ME, DeJesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford N, Wszolek ZK, Ferman TJ, Josephs KA, Boylan KB, Rademakers R, Dickson DW. Clinical and neuropathological heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol. 2011;122:673–690. doi: 10.1007/s00401-011-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neary D, Snowden JS, Mann DMA, Northen B, Goulding PJ, MacDermott N. Frontal lobe dementia and motor neurone disease. J Neurol Neurosurg Psychiatry. 1990;53:23–32. doi: 10.1136/jnnp.53.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 55.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar H, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 56.Neumann M, Mackenzie IR, Cairns NJ, Boyer PJ, Markesbery WR, Smith CD, Taylor JP, Kretzschmar HA, Kimonis VE, Forman MS. TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J Neuropathol Exp Neurol. 2007;66:152–157. doi: 10.1097/nen.0b013e31803020b9. [DOI] [PubMed] [Google Scholar]
- 57.Neumann M, Roeber S, Kretzchmar HA, Rademakers R, Baker M, Mackenzie IRA. Abundant FUS-immunoreactive pathology in neuronal intermediate filament inclusion disease. Acta Neuropathol. 2009;118:605–616. doi: 10.1007/s00401-009-0581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IRA. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009;132:2922–2931. doi: 10.1093/brain/awp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neumann M, Bentmann M, Dormann D, Jawaid A, DeJesus-Hernandez M, Ansorge O, Roeber S, Kretzschmar HA, Munoz DG, Kusaka H, Yokota O, Ang LC, Bilbao J, Rademakers R, Haass C, Mackenzie IRA. FET proteins TAF15 and EWS are selective markers that distinguish FTLD-FUS from ALS with FUS mutations. Brain. 2011;134:2595–2609. doi: 10.1093/brain/awr201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nonaka T, Kametani F, Aiai T, Akiyama H, Hasegawa M. Truncation and pathogenic mutations facilitate the formation of intracellular aggregates of TDP-43. Hum Mol Genet. 2009;18:3353–3364. doi: 10.1093/hmg/ddp275. [DOI] [PubMed] [Google Scholar]
- 61.Perez M, Santa-Maria I, de Barreda EG, Zhu X, Cuadris R, Cabrero JR, Sanchez-Madrid F, Dawson HN, Vitek MP, Perry G, MAM S, Avila J. Tau-an inhibitor of deacetylase HDAC6 function. J Neurochem. 2009;109:1756–1766. doi: 10.1111/j.1471-4159.2009.06102.x. [DOI] [PubMed] [Google Scholar]
- 62.Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga S, Moran J, Liang TY, Ling S-C, Sun E, Wancewicz E, Mazur C, Kordasiewicz H, Sedaghat Y, Donohue JP, Shiue L, Bennett CF, Yeo GW, Cleveland DW. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci. 2011;14:459–468. doi: 10.1038/nn.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- 64.Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, Laaksovirta H, Schymick JC, van Swieten J, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister J, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita V-M, Kaivorinne A-L, Holtta-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chio A, Restagno G, Borghero G, Sabatelli M, Heckerman D, Rogaeva E, Zinman L, Rothstein J, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ The ITALSGEN Consortium. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked amyotrophic lateral sclerosis-frontotemporal dementia. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rollinson S, Rizzu P, Sikkink S, Baker M, Halliwell N, Snowden J, Traynor B, Ruano D, Cairns N, Rohrer JD, Mead S, Collinge J, Rossor M, Akay E, Guerreiro R, Rademakers R, Morrison KE, Pastor P, Alonso E, Martinez-Lage P, Graf-Radford N, Neary D, Heutink P, Mann DMA, Van Swieten J, Pickering-Brown S. Ubiquitin associated protein 1 is a risk factor for frontotemporal lobar degeneration. Neurobiol Ageing. 2009;30:656–665. doi: 10.1016/j.neurobiolaging.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rutherford NJ, Zhang Y-J, Baker M, Gass JM, Finch NA, Xu Y-F, Stewart H, Kelley BJ, Kuntz K, Crook RJP, Sreedharan J, Vance C, Sorenson E, Lippa C, Bigio EH, Geschwind DH, Knopman DS, Mitsumoto H, Petersen RC, Cashman NR, Hutton M, Shaw CE, Boylan KB, Boeve B, Graff-Radford NR, Wxzolek ZK, Caselli RJ, Dickson DW, Mackenzie IR, Petrucelli L, Rademakers R. Novel mutations in TARDBP (TDP-43) in patients with familial amyotrophic lateral sclerosis. PLoS Genet. 2011;4 doi: 10.1371/journal.pgen.1000193. e1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sasyama H, Shimamura M, Tokuda T, Azuma Y, Yoshida T, Mizuno T, Nakagawa M, Fujikake N, Nagai Y, Yamaguchi M. Knockdown of the Drosophila fused in sarcoma (FUS) homologue causes deficient locomotive behavior and shortening of motoneuron terminal branches. PLoS ONE. 2012;7:e39483. doi: 10.1371/journal.pone.0039483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seelaar H, Klijnsma KY, de Koning I, van der Lugt A, Chiu WZ, Azmani A, Rozemuller AJM, Van Swieten JC. Frequency of ubiquitin and FUS-positive, TDP-43-negative frontotemporal lobar degeneration. J Neurol. 2010;257:747–753. doi: 10.1007/s00415-009-5404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sennvik K, Boekhoorn K, Lasrado R, Terwel D, Verhaeghe S, Korr H, Schmitz C, Tomiyama T, Mori H, Krugers H, Joels M, Ramakers GJ, Lucassen PJ, Van Leuven F. Tau-4R suppresses proliferation and promotes neuronal differentiation in the hippocampus of tau knockin/knockout mice. FASEB J. 2007;21:2149–2161. doi: 10.1096/fj.06-7735com. [DOI] [PubMed] [Google Scholar]
- 70.Shi J, Shaw CL, Richardson AMT, Bailey K, Tian J, Varma AR, Neary D, Snowden JS, Mann DMA. Histopathological changes underlying frontotemporal lobar degeneration with clinicopathological correlation. Acta Neuropathol. 2005;110:501–512. doi: 10.1007/s00401-005-1079-4. [DOI] [PubMed] [Google Scholar]
- 71.Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H, Nielsen JE, Hodges JR, Spillantini MG, Thusgaard T, Brandner S, Brun A, Rossor MN, Gade A, Johannsen P, Sørensen SA, Gydesen S, Fisher EM, Collinge J. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet. 2005;37:806–808. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- 72.Snowden JS, Neary D, Mann DMA. Autopsy proven frontotemporal dementia, due to microvacuolar-type histology, with onset at 21 years of age. J Neurol Neurosurg Psychiatry. 2004;75:1337–1339. doi: 10.1136/jnnp.2003.028498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Snowden JS, Hu Q, Rollinson S, Halliwell N, Robinson A, Davidson YS, Momeni P, Baborie A, Griffiths TD, Jaros E, Perry RH, Richardson A, Neary D, Pickering-Brown SM, Mann DMA. The most common form of FTLD-FUS (aFTLD-U) is associated with a distinct clinical form of frontotemporal dementia, but not related to mutations in the FUS gene. Acta Neuropathol. 2011;122:99–110. doi: 10.1007/s00401-011-0816-0. [DOI] [PubMed] [Google Scholar]
- 74.Snowden JS, Snowden JS, Rollinson S, Thompson JC, Harris J, Stopford CL, Richardson A, Jones M, Gerhard A, Davidson Y, Robinson A, Gibbons L, Hu Q, Halliwell N, DuPlessis D, Neary D, Mann DMA, Pickering-Brown S. Distinct clinical characteristics in patients with frontotemporal dementia and C9ORF72 mutations: a study of demographics, neurology, behaviour, cognition, and histopathology. Brain. 2011;135:693–708. doi: 10.1093/brain/awr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multisystem tauopathy with presenile dementia. Proc Natl Acad Sci. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stallings NR, Puttaparthi K, Luther CM, Burns DK, Elliott JL. Progressive motor weakness in transgenic mice expressing human TDP-43. Neurobiol Dis. 2010;40:404–414. doi: 10.1016/j.nbd.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 78.Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, Murphy J, Shoesmith C, Rosenfeld J, Leigh PN, Bruijn L, Ince P, Figlewicz D. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10:131–146. doi: 10.1080/17482960802654364. [DOI] [PubMed] [Google Scholar]
- 79.Tan AY, Manley JL. The TET family of proteins: functions and roles in disease. J Mol Cell Biol. 2009;1:82–92. doi: 10.1093/jmcb/mjp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsai KJ, Yang CH, Fang YH, Cho KH, Chien WL, Wang WT, Wu TW, Lin CP, Fu WM, Shen CK. Elevated expression of TDP-43 in the forebrain of mice is sufficient to cause neurological and pathological phenotypes mimicking FTLD-U. J Exp Med. 2010;207:1661–1673. doi: 10.1084/jem.20092164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vaccaro A, Tauffenberger A, Aggad D, Rouleau G, Drapeau P, Parker JA. Mutant TDP-43 and FUS cause age-dependent paralysis and neurodegeneration in C. elegans. PLoS One. 2012;7:e31321. doi: 10.1371/journal.pone.0031321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vance C, Rogelj B, Hortobágyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al-Saraj S, Al-Chalabi A, Leigh PN, Blair IP, Nicholson G, de Belleroche J, Gallo JM, Miller CC, Shaw CE. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang JW, Brent JR, Tomilnson A, Shneider NA, McCabe BD. The ALS-associated proteins FUS and TDP-43 function together to affect Drosophila locomotion and life span. J Clin Invest. 2011;121:4118–4126. doi: 10.1172/JCI57883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 85.Wegorzewska I, Baloh RH. TDP-43-based animal models of neurodegeneration: new insights into ALS pathology and pathophysiology. Neurodegener Dis. 2011;8:262–274. doi: 10.1159/000321547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci USA. 2009;106:18809–18814. doi: 10.1073/pnas.0908767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wils H, Kleinberger G, Janssens J, Pereson S, Joris G, Cuijt I, Smits V, Groote CC, Van Broeckhoven C, Kumar-Singh S. TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci. 2010;107:3858–3863. doi: 10.1073/pnas.0912417107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu Y-F, Gendron TF, Zhang Y-J, Lin W-L, D’Alton S, Sheng H, Casey MC, Tong J, Knight J, Yu X, Rademakers R, Boylan K, Hutton M, McGowan E, Dickson DW, Lewis J, Petrucelli L. Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice. J Neurosci. 2010;30:10851–10859. doi: 10.1523/JNEUROSCI.1630-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu Z. Does a loss of TDP-43 function cause neurodegeneration? Mol Neurodegener. 2012;7:27. doi: 10.1186/1750-1326-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang C, Tan W, Whittle C, Qiu L, Cao L, Akbarian S, Xu Z. The C-terminal TDP-43 fragments have a high aggregation propensity and harm neurons by a dominant-negative mechanism. PLoS ONE. 2010;5:e15878. doi: 10.1371/journal.pone.0015878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou H, Huang C, Chen H, Wang D, Landel CP, Xia PY, Bowser R, Liu YJ, Xia XG. Transgenic rat model of neurodegeneration caused by mutation in the TDP gene. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1000887. e1000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zinszner H, Sok J, Immanuel D, Yin Y, Ron D. TLS (FUS) binds RNA in vivo and engages in nucleo-cytoplasmic shuttling. J Cell Sci. 1997;110:1741–1750. doi: 10.1242/jcs.110.15.1741. [DOI] [PubMed] [Google Scholar]