Abstract
With the increasing use of individualized medical care (personalized medicine) in treating and managing patients with cancer, the utilization of biomarkers in selecting and tailoring such medical approaches also is increasing and becoming more important. Specifically, many therapies are effective against only a subgroup of a specific type of tumors and exposing patients with different non-responsive subgroups of the same tumor to ineffective therapies, not only exposes these patients needlessly to acute and chronic side effects of the therapy, but also adds to the costs of medical care. For example, the Oncotype Dx test for estrogen receptor positive tumors that are node negative has been used to identify low risk tumors for which surgery alone is an adequate therapy. Biomarkers may be used to aid in multiple aspects of medical care related to cancer, including early detection, diagnosis, risk assessment, as well as in predicting the aggressiveness of cancers (i.e., prognosis) and predicting the therapeutic efficacy of treatments (i.e., prediction). Biomarkers may be also used as surrogate endpoints to aid in evaluating therapies and preventive approaches. Types of biomarkers vary greatly and include histopathologic appearance, stage of the lesion, quantitative morphologic features, size of the lesion, metastatic pattern and extent of metastasis, as well as imaging and molecular features. The types of measurements of biomarkers also vary; for example, molecular features can be measured at the DNA, mRNA or protein levels as well as at regulatory levels (e.g., microRNA). The usefulness of each biomarker is limited by its sensitivity and specificity in fulfilling its role (e.g., in early detection) and the requirements of sensitivity and specificity to accomplish specific tasks are affected by multiple variables. For example, both very high specificity and sensitivity of a test are required to screen a population with a low prevalence of a specific tumor. The goal of this manuscript is to introduce the reader to how biomarkers may be used and the limitations on the uses of biomarkers in translational research.
Keywords: Sensitivity, specificity, early detection, prognosis, risk assessment, surrogate endpoints, diagnosis, receiver operating characteristic, prediction, biomarkers, prevalence, medical costs, side effects, histopathology, molecular features, imaging, prevention, treatment, personalized medicine, individualized medical care
1. Introduction
Over the last 50 years of basic research, numerous genes, proteins, signal transduction pathways and other molecules (e.g., microRNA) have been identified that are differentially present in pre-invasive neoplasia and in cancers compared to normal tissues. In addition to the identification of the genes of the human genome, literally thousands of other modified proteins (e.g., phosphorylated or splice variants) also have been identified whose phenotypic expressions are modified in neoplasia and similar processes such as tissue repair or inflammation.
Translational research is research in which any of these molecules or molecular pathways are translated into being useful clinically, i.e., in practical medical uses that directly affect medical care. Such uses include aids in early detection, determination of clinical outcomes (prognosis), diagnosis, detection of recurrence after therapy, risk assessment, identification of targets for therapy, prediction of responses to therapies (i.e., prediction), monitoring clinical outcomes of therapies (i.e., surrogate endpoints), and imaging diseases processes [1–31]. These areas of translational research are discussed subsequently.
1.1. Early detection
It is very important to identify disease processes as rapidly as possible in order to limit the damage of the disease, to treat the disease at a stage at which the disease can be more easily managed and/or to diagnose the disease when it can be cured. The easiest stage to cure a neoplastic disease is at the stage of pre-invasive neoplasia [32]. If all and subsequent recurrent pre-invasive neoplasia (PINN) can be eliminated from a patient, then PINN will not become invasive and hence the neoplastic process will not become life threatening. For example, with the use of the PAP smear, pre-invasive squamous neoplastic lesions of the cervix (cervical intraepithelial neoplasia [CINs]) can be identified and removed or treated before the lesion invades; thus squamous cell carcinoma (SCC) of the cervix can be prevented in a society which screens and treats all women for high grade CIN. Because of the nature of some forms of PINN, the pre-invasive lesions may recur following successful therapy; however, the time course of the disease usually does not increase so the potential development of invasive disease would be delayed after each removal of PINN.
The goal of the translational pathology of PINN is the reliable identification of the lesion by the least invasive and most accurate methods possible. For example, in screening for CIN, originally the screening test was principally by histopathological examination of the cells removed during the scraping of the squamous columnar junction of the cervix. If CIN2 or CIN3 were detected by cytologic examination, then a colposcopic examination and biopsies of cervical lesions would dictate whether or not there was a need for further treatment or for careful follow-up. With the understanding of the biology of CIN, the over treatment of some CIN2 which might not progress and the viral origin of CIN, the medical evaluation of CIN now may include a measure of the types of human papilloma viruses (HPV) present in the lesion [32]. If strongly pathogenic HPVs are detected (e.g., HPV16, 18, 31, 33 or 45), the likelihood of the progression of CIN to SCC is greatly increased. HPV analysis can now identify 13 high risk types of HPV; an extensive study of HPV screening in India indicated that HPV screening identified more cases of cancer and reduced cancer deaths compared to cytologic screening [33]. Also, the understanding of the etiology of cervical cancer has led to vaccine therapy directed at specific pathologic strains of HPV, e.g., HPV 16 and 18 [34].
The screening for neoplastic processes other than cervix has followed different approaches. For example, radiological imaging of the breast by mammography has led to the detection of small lesions in the breast and hence the early identification of not only invasive mammary carcinoma, but also in situ carcinoma. Even with the success of the early detection of cervical cancer and breast cancer in the industrially developed world, because of the costs of these methods of early detection, such screening has not been widely adopted in developing countries.
A major goal of early detection is a test of high sensitivity and specificity that can be performed on easily obtained bodily fluids/samples such as blood, urine, saliva, or feces. A major feature of an early detection test is its sensitivity and specificity. The sensitivity of the test should permit most cases of the neoplastic process to be identified while it is curable. Thus, an early detection test is of little use if it only can identify a large tumor burden which is primarily correlated with large invasive tumors or metastatic disease. For example, the soluble component erbB-2 (HER2/neu) or p105erbB–2 was found elevated primarily in more advanced cancers of the prostate [35] and breast. Also of great concern is the specificity of a test because the rate of false positives which are inversely related to the incidence of the neoplastic process, are very important, especially when the next step after the positive results is of great expense or morbidity.
Some of the major serum/plasma tests for diseases are listed in Table 1. Each of these tests has problems associated with its use as “screening tests” for the early detection of neoplasia [36]. For example, prostatic specific antigen (PSA) is produced by normal prostatic glandular cells and hence benign prostatic hyperplasia (BPH) also causes an elevation of PSA [37–39]. This especially is a problem because BPH causes elevations of PSA that are in the range 2.5–10 ng/ml – the early abnormal range of PSA in which the PSA marker would be most useful in early detection of prostatic cancer. Nevertheless, the use of PSA as a screening test has been adopted because the diagnosis of prostate cancer is so frequent in men over 50 years of age; as a consequence of an elevated PSA, a 12 core biopsy of the prostate is likely to identify prostate cancer. In contrast, the CA125 test is a relatively sensitive test for ovarian carcinoma; yet CA125 is not frequently used as a screening test for ovarian cancer in an asymptomatic, low risk population of women because of its specificity and the high rate of false positives that result because ovarian carcinoma is relatively uncommon [36].
Table 1.
Serum/plasma biomarkers in neoplasia
| Serum/plasma marker | Source malignant lesion |
|---|---|
| PSA | Prostatic adenocarcinoma |
| CA125 (MUC 16) | Female – ovarian, epithelial cancer; GI cancers; endometrial cancers |
| CA19.9 | Pancreatic carcinoma |
| CA15-3, CA27.29 (MUC 1) | Prognosis of breast cancer; response to RX of breast cancer |
| CEA, CEACAM-1 | Gastrointestinal carcinoma, pancreatic carcinoma |
| AFP | Hepatocellular carcinoma, germ cell, nonseminoma |
| hCG | Germ cell – seminomas; choriocarcinoma |
| p105erbB–2 | Breast, prostate, ovarian cancers |
| Long DNA | Many types of cancer, e.g., in feces, colorectal cancer |
| TPA* | Many types of cancers |
| Circulating tumor cells (CTC) | All cases of cancer |
The above issue is demonstrated as follows: If a test is 100% sensitive and 90% specific, but a tumor occurs in only one in 1000 patients, in testing 1000 patients the test will identify the one patient with tumor (true true positives (TP) positive e.g., ), but also over 10% or 111 patients without tumor (false positives e.g., ). In this case the issue on using the test in screening becomes the cost and morbidity associated with follow-up of each of the 111 false positive cases with a more specific test. Thus, the cost, morbidity of follow-up testing, and acceptability of testing as a screening test should be balanced with decreased morbidity and mortality of the disease based on using the screening test [36].
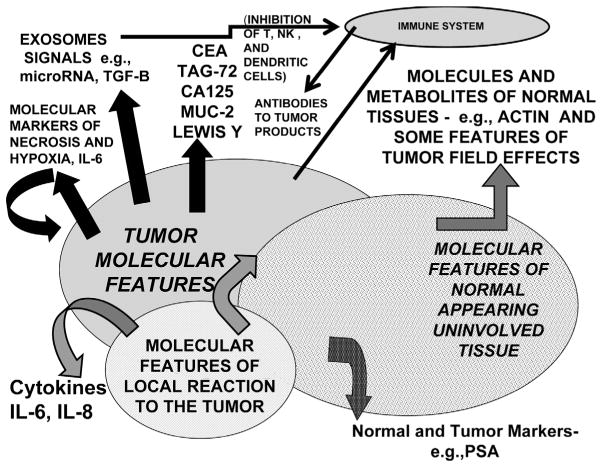
Another limitation of many biomarkers for the early detection of a specific neoplastic process is that many biomarkers are not specific for one type of tumor. For example, increases in serum levels of interleukin 6 (il-6), il-8 or CEA may occur in patients with many types of cancer (e.g., pancreatic or colorectal cancers) as well as some inflammatory processes. Thus an elevated level of a biomarker may indicate a potential tumor in multiple sites (e.g., lung and colon). This may be more likely when the elevation of the screening biomarker is secondary to a general immunological or tissue reaction to a tumor, rather than to a product released specifically by a tumor (38, Fig. 1). For example, acute phase reactants may be elevated secondary to the body’s reaction to a tumor rather than by being produced specifically by the tumor.
Fig. 1.
Demonstrates the complex interaction between a tumor, its surround and the immune system. It shows how various biomarkers may be associated with the presence of a tumor without coming directly or uniquely from a tumor. For example, PSA is produced at higher levels in the cells of normal glandular epithelia of the prostate than in cells of prostate cancer.
The challenge of early detection is to find a marker(s) that are both sensitive and specific and that can be detected, based on biomarker levels in bodily fluids or image/fine needle aspiration, when a neoplastic process is curable (i.e., when the neoplastic process is small or pre-invasive). Except for prostatic specific antigen, most biomarkers that have been reported are not tumor or organ specific. Because a single marker that is both sensitive and specific as well as organ specific has proved to be very difficult to identify for most organ systems or specific tumors, a combination of biomarkers may need to be used in early detection of specific tumors. In this combination of biomarkers, some biomarkers might add sensitivity and others specificity.
1.2. Detection of recurrence or metastatic disease
While not very useful in screening for initial disease, most of the molecules listed in Table 1 are very useful to detect the recurrence of tumors which have been surgically removed or treated with radiation. For example, when the prostate or the source of PSA has been removed either by surgery, successful radiation or hormonal therapy, all of which act on normal as well as neoplastic prostate tissue, PSA should drop to the minimum detectable range; thus, a subsequent consistent increase in PSA is indicative of recurrence or of metastatic disease. Similarly, a continued elevation of CA125 following removal of both ovaries or a subsequent rapid increase in CA125 is usually indicative of recurrence, metastatic ovarian carcinoma or rarely a different primary cancer, that may also produce CA125, for example, pancreatic cancer.
1.3. Diagnosis
The use of biomarkers in diagnosis is closely related to the use of biomarkers in early detection and/or to detect recurrence. The only difference between early detection and diagnosis may be the stage of the disease being detected or diagnosed and the accuracy of the detection. Thus, a method that is 100% sensitive and 100% specific for the early detection of a neoplastic process is actually a diagnostic method. The actual diagnosis of neoplastic lesions may be based upon measurement of biomarkers in either the tumor tissue or in bodily fluids. For example, biomarkers may be very useful in aiding the diagnosis of neoplasia when they are specifically expressed in the tissue of the neoplastic process. Depending upon the biomarker, such biomarkers may be identified by histochemical assays, immunological assays or by microchemical methods.
Over the last three decades, the use of immunohistochemistry to identify biomarkers useful in the diagnosis of neoplastic processes has revolutionized the practice of pathology with a major shift from subtyping tumors using histochemistry to using immunohistochemistry which is usually more specific and more sensitive than histochemistry. For example, melanomas were once diagnosed by using the Fontana-Masson histochemical stain which identifies by staining with a silver solution cells with Argentaffin characteristics. In contrast, currently many immunohistochemical stains which are more specific for melanomas such as MELAN A, HMB45 or S100 are used to diagnose melanomas.
Some biomarkers such as number of mitoses have also been used to separate malignant (e.g., leiomyosar-comas) from benign tumors (e.g., leiomyomas). Such a pathological separation is beyond a marker of prognosis which usually applies to separation of more aggressive from less aggressive cancers as will be discussed subsequently. Several immunohistochemical markers can substitute for counting mitoses (Ki67, PCNA) or may aid in counting mitoses.
In some cases with limited material (small biopsies with a few foci of atypical cells), biomarkers may be used as an aid to separate malignant from benign lesions. For example, in prostate and breast, the presence of basal cells may separate malignant from benign glands because malignant glands typically do not have both luminal and basal cells. In breast, S100 and smooth muscle actin may be used to identify basal myoepithelial cells. In the case of the prostate, basal cells are stained with a basal cytokeratin (34βE12) or p63. Similarly, new markers such as α-methylacyl-CoA-racemase (AMACR) may be expressed in a proportion of malignant cells of the prostate [42] but not in normal or uninvolved prostate cells.
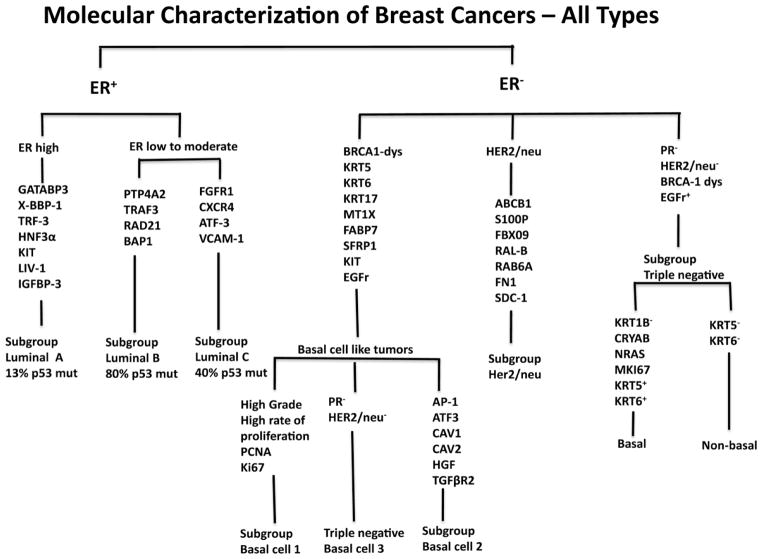
Recently, biomarkers have been increasingly used to subdivide or subtype tumors such as breast carcinomas. By the original classification of breast carcinomas by histopathological analysis several main subtypes were characterized including ductal, lobular, medullary, tubular and papillary. The molecular analysis of breast cancer has further subdivided the above lesions based on molecular expression [43–50]. The luminal type of breast cancer has been characterized primarily by ER+ cells and this group was subdivided into Luminal Type A, B and C. In contrast, breast cancer with ER− cells has been subclassified as of basal type (1 and 2), HER2/neu (erbB-2) subtype, or a triple negative subtype. Of interest, there is significant overlap between basal type (BT) and triple negative breast cancers (TN) with perhaps 28% of TN as being non-basal subtype and 23% of the basal type being non-TN. There also is a strong overlap of both groups with some type of dysregulation in BRCA-1 [48–50]. See Fig. 2. Of interest only medullary carcinoma is associated with primarily one molecular phenotype, the basal cell subtype.
Fig. 2.
Demonstrates the molecular subtyping of breast carcinoma. The abbreviations used are defined subsequently. (GATABP-3) GATA binding protein 3; (X-BBP-1) box binding protein 1; (TRF-3) trefoil factor 3; (HNF3α) hepatocyte nuclear factor – 3α; (PTP4A2) protein tyrosine phosphatase type IVA member; (TRAF3) Tumor necrosis factor receptor associated factor 3; (BAP-1) BRCA associated protein 1; (KPT5, -6 or -17) keratin 5, 6 or 17; (MTIX) metaiothionein 1x; (FABP7) fatty acid binding protein 7; (SFRP1) frizzled related protein 1; (ATF3) activating transcription factor 3; (CAV1 or 2) caveolin 1 and 2; (HGF) hepatocyte growth factor; (TGFβR2) transforming growth factor β receptor II; (ABCB1) multidrug resistance protein 1; (S100P) S100 calcium binding protein P; (FBX09) fatty acid syntase; (RALB) GTP binding protein; (RAB6A) member of RAS oncogene family; (FN1) fibronectin 1; (SDC1) syndecan 1. In the future, the complex cancer such as lung or renal carcinomas also are likely to be subdivided molecularly.
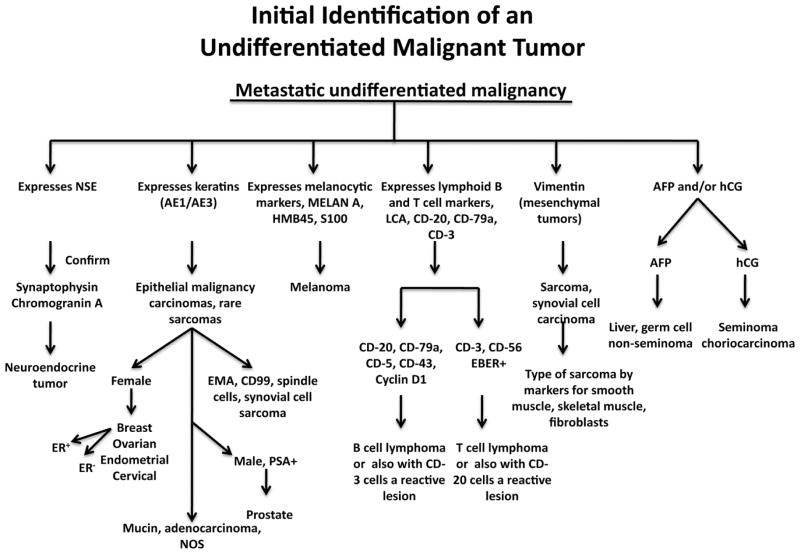
Another use of biomarkers in diagnosis is the identification by biomarker expression of the sources of metastases when a primary cancer has not been identified. In some cases, the marker, such as prostatic specific antigen (PSA), is very accurate in identifying metastatic foci of prostate cancers in tissues. In other cases, the demographics of the patient, the pattern of metastases (e.g., in bone) plus the expression of biomarker is useful. For example, an undifferentiated epithelial-like cancer in the axillary lymph nodes of a woman is a melanoma if the cancer expresses MELAN A, but is likely a breast cancer if the lesion does not express MELAN A, HMB45, or S100, but instead expresses generalized mixtures of high and low weight keratins such as AE1/AE3. Alternatively, the lesion may be a lymphoma if it expresses CD3 or CD20. A general molecular approach to characterizing a metastatic lesion is depicted in Fig. 3.
Fig. 3.
Demonstrates how molecular analysis aids in identifying poorly differentiated malignant tumors, especially metastatic cancers, whose primary cancer is unknown. Typically the pathologist may suspect which type of tumor is the primary cancer and only a few of these molecular markers are used to confirm the initial impression.
Thus, to determine the source of a metastatic lesion, the pathologist and oncologist must rely on the history of the patient as to prior malignant lesions. If none, then the demographics of the patient and the pattern of metastases are important in identifying the primary lesion. For example, metastases only to local lymph nodes and especially to a sentinel node is usually indicative of the location of a lesion draining to these nodes. Finally, each type of cancer has a typical pattern of metastatic spread. For example, gastrointestinal carcinomas metastasize to local lymph nodes and then to the liver, while prostate cancer metastasizes to local lymph nodes and then to bones, especially the vertebral column. After the clues provided by the sex and age of the patient and the pattern of metastases, the pathologist and oncologist frequently must rely on molecular markers to confirm the primary tumor.
Biochemical markers in serum/plasma can also be used in diagnosis of neoplasia. For example, small adrenal cortical tumors producing primarily cortisol and the clinical picture of pure Cushing’s syndrome are likely benign; however, an adrenal cortical tumor producing excessive androgens is likely malignant. Similarly, a small paraganglioma of the adrenal gland producing primarily norepinephrine is more likely to be benign than a similar paraganglioma of the same site producing primarily dopamine; paragangliomas at other sites (e.g., paravertebral) may have a greater rate of malignancy. Also, the identification of a paraprotein of 10 g/L in serum may be indicative of a plasmacytoma or multiple myeloma, but a paraprotein of < 10 g/L may be indicative of a process that may not progress to a plasmacytic malignancy or to a lymphoma.
1.4. Surrogate endpoint biomarkers
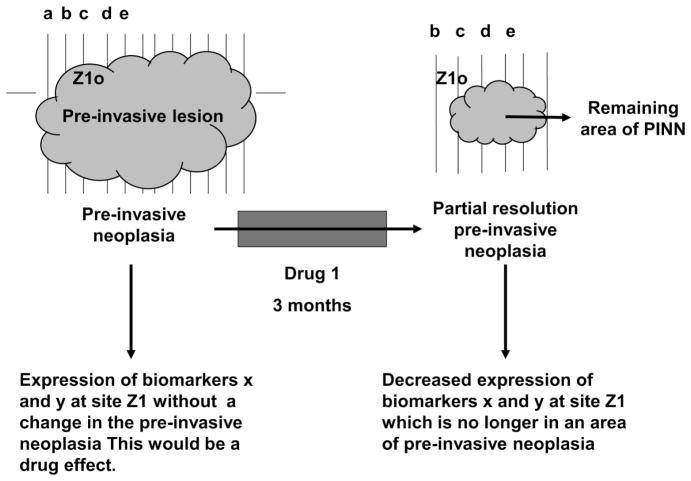
Evaluation of some clinical endpoints such as the prevention of malignancy by a chemopreventive agent may take several years and involve thousands of patients in order to complete the analysis. Thus, such testing can be prohibitively expensive when many agents must be evaluated. If a biomarker(s) could be identified for which changes in the biomarker(s) correlated exactly with a preventive response of an agent, then changes in such biomarker(s) could be used as surrogates to the clinical response; such markers are called surrogate endpoint biomarkers (SEBs). If a biomarker always changed many months to years before a preventive response, then the biomarker could be used as a SEB, i.e., as an inexpensive screening method to determine the effectiveness of preventive agents; then if SEBs indicated that a preventive agent was likely to be successful, the preventive agent could be evaluated by treating a population in which changes in SEBs could be correlated with partial or complete resolution of PINN. Thus, exhaustive clinical trials could be undertaken only for those agents that were most promising in initial trials. It is important to understand that the SEBs should correlate with clinical prevention of the lesion (e.g., complete or partial resolution of a preinvasive neoplastic lesion) and not be only correlated with administration of the clinical drug. Consider Fig. 4.
Fig. 4.
In Fig. 4, note biomarkers x and y may change at site Z1 without the resolution of the area of pre-invasive neoplasia. This would correlate with an effect of the drug and not a pattern indicative of a surrogate endpoint. Similarly, areas of pre-invasive neoplasia may “move” spontaneously, with or without resolution of the extent of the PINN. In this case, there is partial resolution of the area of PINN. The movements of PINN lesions over a period of months without even partial resolution of the PINN is why the use of “controls” is so important in studies to identify responses in SEBs to drugs used in the prevention of neoplasia [51].
An example of such a preventive therapeutic approach is the testing of “preventive agents” (i.e., agents that prevent a malignant lesion from developing in a high risk population or which reverses pre-invasive neoplastic lesions which have developed in an at risk population). For example, a preventive agent may cause the resolution or prevention of dysplastic leukoplakia in abusers of tobacco [15,51], hence the prevention of the development of squamous cell carcinoma of the oral cavity.
1.5. Determination of clinical outcome or prognosis
Besides the diagnosis or subtype of a neoplastic process, many efforts have been made to determine the clinical aggressiveness of malignant lesions. These efforts to categorize the aggressiveness of cancers began with determining the stage and grade of cancers. This was the original basis for the staging of cancers; however, with the utilization of molecular analysis, currently stage is not always the controlling variable in determining clinical outcome [52]. In the case of prostate cancer, Gleason developed a score based on histopathologic features that ranged from 1 to 5 for a single focus of adenocarcinoma or 2 to 10 based upon both the primary and secondary patterns of cancer growth. Low scoring cancers (Gleason combined score ≤ 6) usually have indolent courses, high scoring cancers (score ≥ 8) usually have aggressive outcomes while intermediate scoring cancers, Gleason 7, cannot be easily predicted as to their aggressiveness. Similarly in breast cancer, the modified Bloom and Richardson grading method as well as cytomorphometric analysis have both proved useful in identifying aggressive subtypes of ductal carcinomas [53]. For many cancers, the grade of the cells of the cancer is quite important in outcome.
As discussed, biomarkers have recently been identified that are used in subtyping carcinomas based upon the extent of expression of multiple biomarkers. Specifically, the ER+ Type 1 subtype of breast cancer has been identified as having an excellent outcome; in contrast, breast cancers that do not express estrogen receptor, progesterone receptor and p185erbB–2 have a poor outcome even though a cancer with high p185erbB–2, if untreated, has a poor outcome. For treated lesions, the lack of expression of p185erbB–2 takes away the option of therapy with Herceptin [43–47]. Also, our results in colorectal cancer in proximal carcinomas in Caucasian-Americans indicate those cancers expressing Bcl-2 (high), p27Kip–1 (high) and no nuclear accumulation of p53 have relatively good outcomes [16,20,28].
Epigenetic changes also may affect the aggressiveness of neoplastic processes. As we begin to understand more about epigenetic changes and the development of cancer, increased molecular changes associated with epigenetics have been associated with subsets of cancers that are more or less aggressive. Some epigenetic changes such as methylation of CpG islands associated with the aggressiveness of cancers depend on increased expression of DNA methyl transferase in prostate cancer and increased histone methyl transferase such as EZH2 in breast and prostate cancers [54].
The characteristics of most malignant processes may vary with race and ethnic background. For example, the incidence and aggressiveness of prostate cancer is increased in African-Americans (AA) compared to Caucasian Americans (CA), but the incidence in Hispanic Americans (HA) is less than CA. The usefulness of using specific biomarkers for predicting the aggressiveness of tumors also may vary with race. For example, in colon adenocarcinoma, the nuclear accumulation of p53 as determined by immunohistochemistry is a strong prognostic biomarker for proximal colonic carcinoma, but p53 is not a useful prognostic biomarker in AAs [28]; for African-Americans, malignant cells in colorectal cancer with high grade are a prognostic indicator of very poor outcome [5]. Similarly, biomarkers of clinical outcome of breast cancer have been reported to vary with race [9,10,55].
1.6. Risk assessment
Patients whose genotypes (germ line DNA) carry specific mutations are at risk for developing specific types of cancers. If a risk of developing a cancer is high, then screening methods using biomarkers may be useful even when these same screening methods are a waste of resources for patients with lower risk. For example, children and adults who are from families with multiple endocrine neoplasia Type 2 (MEN2) and carry a mutation in the RET oncogene may be monitored for the occurrence of medullary carcinoma of the thyroid by periodically measuring calcitonin. In contrast, adults carrying a germ line mutation in enzymes that repair DNA mismatches (e.g., MLH2 and MSH6) have an increased risk for several neoplastic processes, especially adenocarcinomas of the proximal colon as do patients that carry a mutated copy of the APC gene. Besides germ line mutations which alone carry an increased risk for the development of specific neoplastic processes, certain other inherited genes result in an increased risk of developing specific cancers when combined with environmental exposures. For example, in a heavy smoker who has a glutathione S-transferase polymorphism in which GSTM1 is reduced has an increased risk for lung cancer [56]. Similar variations in acetylation ability secondary to variants of N-acetyltransferase (NAT) not only affect drug metabolism and hence the effectiveness and toxicity of specific drugs, but also increase the risk for some cancers, especially urothelial cancers of the bladder [57].
Similarly, epigenetic changes in the methylation of specific genes at CpG islands have major effects in the development of early neoplastic changes. Frequently, the methylated CpG islands involve important regulatory elements of suppressor genes such as p16 and of those genes involved in mismatch repair such as MLH1, in apoptosis, cell adhesion (E-cadherin) and in cellular migration (TIMPs); such patterns of methylation result in increased risk for specific cancers.
1.7. Targets for novel therapies
As our understanding of various pathways of cellular signaling and function have expanded and our knowledge of how these pathways are involved in the development and progression of neoplasia, important steps in these pathways are now targeted as primary or secondary forms of therapy for specific neoplastic processes. One of the initial targets was p185erbB–2 or HER2/neu. A monoclonal antibody was developed against p185erbB–2 (Trastuzumab or Herceptin) and it was found to inhibit effectively the functioning of p185erbB–2 and to be effective against breast cancers (and subsequently other cancers) that over-express genes coding for p185erbB–2. Therapy with Herceptin is also now approved for treating breast cancers which overexpresses p185erbB–2.
The EGFr pathway can similarly be targeted by antibodies to EGFr (e.g., Cetuximab). Because almost all SCCs of the oral cavity express large amounts of EGFr on their cell surface membranes, Cetuximab has proved very effective in combined therapy with radiation in squamous lesions of the oral cavity [58]. Antibodies to EGFr have also been evaluated as therapeutic agents for cancers of the lung. They have proved primarily effective in only a small proportion of lung adenocarcinomas (especially bronchoalveolar type, now designated a form of intraepithelial neoplasia leading to adenocarcinoma of the lung) that have activating mutations or overexpression of EGFr [59,60]. In addition, multiple monoclonal antibodies have been developed to target specific molecules or processes e.g., inflammation, thought to provide a stimulating input to various cancers or to the development of the vasculature of cancers (Table 2) and these and many additional such antibodies are now in various stages of testing for a wide range of tumors [7,8,12,14].
Table 2.
Monoclonal antibodies used to target signal pathways in cancer
| Molecular target | Name | Commercial name | Cancers targeted | Comments |
|---|---|---|---|---|
| EGFr | Cetuximab | Erbitux | Oral, non-small cell lung | Non-small cell carcinoma of the lung with EGFr activation. Oral cancer together with radiation |
| Her-2/neu | Trastuzumab | Herceptin | Breast | |
| VEGF | Bevacizumab | Avastin | Colorectal; breast | |
| CD20 | Rituximab | Rituxan | Lymphoid | |
| CD20 | Ibritumomab tiuxetan* Rituximab |
Zevalen | B cell lymphoma | |
| CD26 | Tositumomab** | Bexxar | B cell lymphoma | |
| CD33 | Gemtuzumab ozogamicin (monoclonal anti-body combined with caicheamicin, an antitumor antibiotic) | Mylotary | Acute myelogenous leukemia | |
| CD52 | Alemtuzumab | Campath | Chronic lymphoid leukemia |
Frequently labeled with Y-90;
Labeled with I-131.
Other molecules, many of which are designated small (inhibitory) molecules (e.g., < 50D in molecular weight) have been developed to target the functional aspects of endogenous molecules or pathways thought to stimulate the growth of cancers. For example, gefitinib (Iressa) was developed to target the phosphokinase functions of EGFr; however, when tested as to its effectiveness against non-small cell lung cancers (NSCLC), like cetuximab, it required a tyrosine-kinase mutation in EGFr and/or some dysregulation or overexpression of EGFr expression for the therapy to be successful. However, a similar phosphokinase inhibitor of EGFr, erlotinib (Tarceva) has been approved for treatment of NSCLC.
Another small molecule developed to target a transduction system is Gleevec which targets the tyrosine-kinase domain of the fusion protein bcr:abl and the ATP binding pocket of KIT and PDGFRA. CML which frequently has the fusion protein bcr:abl is usually responsive to imatinib or the related kinase inhibitor dasatinib. Also, neoplastic lesions, especially gastrointestinal stromal tumors (GIST), with mutations in KIT or PDGFRA are usually at least transiently responsive to imatinib.
As described above, targeting of growth factors or their receptors with monoclonal antibodies or using small molecules to inhibit signal transduction pathways of growth factors aids in therapy of some neoplastic processes. Table 3 lists small inhibitory molecules that target signal transduction pathways.
Table 3.
Small molecules/specific molecules used to target aspects of signal pathways in cancer
| Signal transduction pathway target | Therapeutic small molecule | Commercial name | Molecular target | Cancer targeted | Comments |
|---|---|---|---|---|---|
| EGFr | Gefitinib | Iressa | Tyrosine kinase – EGFr | Bronchoalveolar carcinoma in Japanese women | Only activated EGFr; not on market |
| EGFr | Erlotinib | Tarceva | Tyrosine kinase – EGFr | Non-small cell lung/pancreas | In combination with gemcitabine for pancreas |
| Kit, PDGFR, Bcr:abl | Imatinib | Gleevec | Tyrosine kinase of c kit and PDGFR, fusion protein, bcr:abl | GIST, CML | Response is frequently transient |
| Bcr:abl, SRC | Dasatinib | Sprycel | Tyrosine kinase | CML | Works on some patients resistant to imatinib |
| VEGFR – 2 & 3, FLT-3, Kit, PDGFR-β, Fms, craf, braf | Sorafenib | Nexavar | Tyrosine kinase of target pathway | Renal | |
| PDGFR-α & β, VEG-FR1, 2, 3, Kit, FLT-3, CSF-1, RET | sunitinib | Sutent | Multiple kinases of pathway | GIST, renal | |
| GnRH | Abarelix | Plenaxis | Antagonist to GnRH receptor | Breast and prostate | |
| CD25 | denileukin; diftitox | Ontak | CD25 component of il-2 receptor | Cutaneous T cell lymphoma | |
| CXCR1 | Repertaxin | Repertaxin | il-8 receptor | All | Reduces inflammation mediated by il-8 |
Thus, translational research to identify therapeutic approaches which target signal transduction pathways which modulate specific neoplastic processes has been successful for multiple types of tumors. This area of translational research is rapidly expanding and is extremely promising.
1.8. Patterns of molecular expression to predict therapeutic responsiveness (prediction)
An important goal of translational pathology is to be able to use molecular features of a type of neoplasia to predict its responsiveness to specific therapies. Obviously, if the therapy targets a known biological feature, the presence of that biological feature is usually needed for successful therapy. For example, proliferation may be turned on and targeted to be turned off or apoptosis may be turned off and targeted to turn on. Similarly, if a specific molecule or pathway is targeted by a therapy, the presence of that molecule at a specific level may be necessary for the therapy to be successful. Sometimes the molecule targeted must be “turned-on” or constitutively active for the therapy to be successful, such as EGFr in the therapy for NSCLC; however, rarely some therapy such as therapy with the TRA-8 antibody to the death receptor, DR5, is not based in this case on target levels of DR5 [12,14]. Thus, some types of therapy may work independent of high levels of the molecules which are thought to be targets of the therapy. In such cases, it would be useful to be able to identify molecular features which can be correlated with the potential effectiveness of a therapy. This area of translational research is very early in its development, but it is potentially very important.
1.9. Imaging
Techniques which image neoplastic lesions have improved greatly over the last three decades, especially in the modalities of imaging and the sensitivity of imaging, including the minimum size of lesions that can be imaged. Imaging, including x-ray computerized tomography (CT), magnetic resonance imaging (MRI), ultrasound (US), positron emission tomography (PET), single-photon emission computerized tomography (SPET), and bioluminescence and fluorescence optical imaging, can be used successfully in identifying primary or metastatic lesions (i.e., staging of disease) and to separate benign from malignant lesions. These imaging models, e.g., ultrasound, can be used to detect neoplastic nodules and hence guide fine needles for aspiration and thus to aid in early detection/diagnosis [11]. Other uses of imaging methods include determining the effectiveness of specific therapies, evaluating the pharmokinetics of therapeutic drugs, predicting drug resistance, determining tumor vascularity of neoplastic lesions, evaluating novel therapies in animal models and aids in evaluating gene therapy [7,8,61,62]. Imaging can be used to evaluate neoplastic processes including the evaluation of the extent of tumor metabolism using labeled glucose transport analogues. Also agents for imaging that correlate with proliferation and apoptosis are under development. In general, imaging, although a relatively new area of translational research, is a major growth area, as many of the clinical targets for therapy can also be targeted for imaging.
2. Challenges and advances in translational pathology
Several areas of translational pathology use immunohistochemistry in evaluations. For example, the determination of estrogen receptor and progesterone receptors in the diagnosis/prognosis of breast cancers as well as erbB-2 expression to verify the target for Herceptin therapy can be measured by this technique. The collection and processing of tissues can have a great effect on immunohistochemistry [63]; yet the effects on immunohistochemistry of collection and processing of tissues are inadequately understood. Specifically both the fixation and the processing of tissues to paraffin blocks may greatly affect immunohistochemistry [64, 65]. In addition, standardized methods of performing immunohistochemistry have not been accepted [66]. For example, some antigens (e.g., pAkt) rapidly decay when exposed to ischemia while the phenotypic expression of other proteins (e.g., heat shock proteins) may be increased by warm ischemia (reviewed in [67]).
Over the years there has been a great concern that the degradation of mRNA will be limiting in the use of human tissues to identify molecular features of neoplastic lesions. Our current knowledge indicates that the more unstable types of mRNA are most likely degraded during the period of warm ischemia, i.e., between compromise of the vascular supply to an area of tissue and the removal of the tissue. One of the advances in evaluating translational pathology is the use of amplicons of < 100 bp in the analysis of gene expression using real time quantitative PCR [68,69]. Specifically, our results and those of others indicate that the use of small amplicons permits the successful utilization of partially degraded mRNA in studies of gene expression in tissues that have extended periods of warm ischemia. As the speed of complete sequencing of genes increases as its costs decrease, full sequencing is likely to replace PCR in analysis. Of concern will be the quality of the mRNA and the effects of partial degradation of mRNA on the results of sequencing. These issues are now being evaluated.
In summary, evaluating the translational pathology of neoplastic processes has been greatly slowed by a lack of understanding of the effects of handling of human tissues on molecular methods as well as attention to the quality of human tissues used in assays. In addition, the methodology used in analysis has not been standardized. This creates problems in the literature, especially for molecules for which there are multiple splice variants and different patterns of staining depending if antibodies are to the C or N tail of the antigen.
Acknowledgments
Supported in part by the Early Detection Research Network (EDRN) (5U24 CA86359), Department of Defense, “Biomarkers in the Detection of Prostate Cancer in African-Americans” (PC093309), the Breast (5P50CA089019) and Pancreatic (2P50CA101955) SPORES at UAB, the Susan G. Komen Breast Cancer Foundation (BCTR0600484), the Skin Disease Research Center at UAB (5P30AR50948) to William E. Grizzle, and (POP138306) to Upender Manne.
References
- 1.Grizzle WE, Myers RB, Manne U. The Use of Biomarker Expression to Characterize Neoplastic Processes. Biotech Histochem. 1997;72(2):96–104. doi: 10.3109/10520299709082218. [DOI] [PubMed] [Google Scholar]
- 2.Myers RB, Grizzle WE. Changes in Biomarker Expression in the Development of Prostatic Adenocarcinoma. Biotech Histochem. 1997;72(2):86–95. doi: 10.3109/10520299709082217. [DOI] [PubMed] [Google Scholar]
- 3.Grizzle WE, Manne U, Jhala NC, Weiss HL. Molecular Characterization of Colorectal Neoplasia in Translational Research. Arch Pathol Lab Med. 2001;125(1):91–98. doi: 10.5858/2001-125-0091-MCOCNI. [DOI] [PubMed] [Google Scholar]
- 4.Chatla C, Jhala NC, Katkoori VR, Alexander D, Meleth S, Grizzle WE, Manne U. Recurrence and survival predictive value of phenotypic expression of Bcl-2 varies with tumor stage of colorectal adenocarcinoma. Cancer Biomark. 2005;1:241–250. doi: 10.3233/cbm-2005-14-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander D, Jhala N, Chatla C, Steinhauer J, Funkhouser E, Coffey CS, Grizzle WE, Manne U. High-grade tumor differentiation is an indicator of poor prognosis in African Americans with colonic adenocarcinomas. Cancer. 2005;103(10):2163–2170. doi: 10.1002/cncr.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabian CJ, Kimler BF, Anderson J, Tawfik OW, Mayo MS, Burak WE, Jr, O’Shaughnessy JA, Albain KS, Hyams DM, Budd GT, Ganz PA, Sauter ER, Beenken SW, Grizzle WE, Fruehauf JP, Arneson DW, Bacus JW, Lagios MD, Johnson KA, Browne D. Breast cancer chemo-prevention phase I evaluation of biomarker modulation by Arzoxifene, a third generation selective estrogen receptor modulator. Clin Can Research. 2004;10:5403–5417. doi: 10.1158/1078-0432.CCR-04-0171. [DOI] [PubMed] [Google Scholar]
- 7.Kim H, Morgan DE, Buchsbaum DJ, Zeng H, Grizzle WE, Warram JM, Stockard CR, McNally LR, Long JW, Sellers JC, Forero A, Zinn KR. Early therapy evaluation of combined anti-death receptor 5 antibody and gemcitabine in orthotopic pancreatic tumor xenografts by diffusion-weighted magnetic resonance imaging. Cancer Res. 2008;68(20):8369–8376. doi: 10.1158/0008-5472.CAN-08-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H, Morgan DE, Zeng H, Grizzle WE, Warram JM, Stockard CR, Wang D, Zinn KR. Breast tumor xenografts: diffusion-weighted MR imaging to assess early therapy with novel apoptosis-inducing anti-DR5 antibody. Radiology. 2008;248(3):844–851. doi: 10.1148/radiol.2483071740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talley LI, Grizzle WE, Waterbor JW, Brown K, Weiss H, Frost AR. Hormone receptors and proliferation in breast carcinomas of equivalent histologic grades in pre-and post-menopausal women. Int J Cancer. 2002;98(1):118–127. doi: 10.1002/ijc.10171. [DOI] [PubMed] [Google Scholar]
- 10.Talley L, Chhieng DC, Bell WC, Grizzle WE, Frost AR. Immunohistochemical detection of EGFR, p185 (erbB-2), Bcl-2 and p53 in breast carcinomas in pre-menopausal and post-menopausal women. Biotech Histochem. 2008;83(1):5–14. doi: 10.1080/10520290701822436. [DOI] [PubMed] [Google Scholar]
- 11.Jhala N, Jhala D, Vickers SM, Eltoum I, Batra SK, Manne U, Eloubeidi M, Jones JJ, Grizzle WE. Biomarkers in diagnosis of pancreatic carcinoma in fine-needle aspirates: A model for translational research application. Am J Clin Pathol. 2006;126(4):572–579. doi: 10.1309/cev30be088cbdqd9. [DOI] [PubMed] [Google Scholar]
- 12.Buschbaum DJ, Zhou T, Grizzle WE, Oliver PG, Hammond CJ, Carpenter M, LoBuglio AF. Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clinical Cancer Res. 2003;9:3731–3741. [PubMed] [Google Scholar]
- 13.Alvarez RD, Conner MG, Weiss H, Klug PM, Niwas S, Manne U, Bacus J, Kagan V, Sexton KC, Grubbs CJ, Eltoum IE, Grizzle WE. The efficacy of 9-cis retinoic acid (aliretinoin) as a chemopreventive agent for cervical dysplasia: Results of a randomized double blind clinical trial. Cancer Epidemiol Biomarkers Prev. 2003;12(2):114–119. [PubMed] [Google Scholar]
- 14.Buschbaum DJ, Bonner JA, Grizzle WE, Stack-house MA, Capenter M, Hicklin DJ, Bohlen P, Raisch KP. Treatment of pancreatic cancer xenografts with Erbitux (IMC-C225) anti-EGFR antibody, gemcitabine, and radiation. Int J Radia Oncol Biol Phys. 54(4):1180–1193. doi: 10.1016/s0360-3016(02)03788-4. [DOI] [PubMed] [Google Scholar]
- 15.Beenken S, Jr, Hockett R, Grizzle W, Weiss HL, Pickens A, Perloff M, Malone WF, Bland KI. Transforming growth factor α (TGF-α): A surrogate endpoint biomarker? J Am Coll Surg. 2002;195(2):149–158. doi: 10.1016/s1072-7515(02)01219-x. (Not found in PubMed) [DOI] [PubMed] [Google Scholar]
- 16.Grizzle WE, Manne U, Weiss HL, Jhala N, Talley LI. Molecular staging of colorectal cancer in African-American and Caucasian patients using phenotypic expression of p53, Bcl-2, MUC-1 and p27kip–1. Int J Cancer. 2002;97(4):403–409. doi: 10.1002/ijc.1617. [DOI] [PubMed] [Google Scholar]
- 17.Urban D, Irwin W, Kirk M, Markiewicz MA, Myers R, Smith M, Weiss H, Grizzle WE, Barnes S. The Effect of Isolated Soy Protein on Plasma Biomarkers in Elderly Men with Elevated Serum Prostate Specific Antigen. J Urol. 2001;165:294–300. doi: 10.1097/00005392-200101000-00082. [DOI] [PubMed] [Google Scholar]
- 18.Myers RB, Oelschlager DK, Weiss HL, Frost AR, Grizzle WE. Fatty Acid Synthase – An Early Molecular Marker of Progression of Prostatic Adenocarcinoma to Androgen Independence. J Urol, Investigative Urology. 2001;165(3):1027–1032. [PubMed] [Google Scholar]
- 19.Manne U, Weiss H, Grizzle WE. Racial differences in the prognostic usefulness of MUC1 and MUC2 in colorectal adenocarcinomas. Clin Cancer Res. 2000;6(10):4017–4025. [PubMed] [Google Scholar]
- 20.Manne U, Weiss HL, Grizzle WE. Bcl-2 Expression is Associated with Improved Prognosis in Patients with Distal Colorectal Adenocarcinomas. Int J Cancer. 2000;89(5):423–430. doi: 10.1002/1097-0215(20000920)89:5<423::aid-ijc5>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 21.Piyathilake CJ, Frost AR, Manne U, Bell WC, Weiss H, Heimburger DC, Grizzle WE. The Expression of Fatty Acid Synthease (FASE) is an Early Event in the Development and Progression of Squamous Cell Carcinoma of the Lung. Hum Pathol. 2000;31(9):1068–1073. doi: 10.1053/hupa.2000.9842. [DOI] [PubMed] [Google Scholar]
- 22.Saleh MN, Raisch KP, Stackhouse MA, Grizzle WE, Bonner JA, Mayo MS, Kim HG, Meredith RF, Wheeler RH, Buchsbaum DJ. Combined Modality Therapy of A431 Human Epidermoid Cancer Using Anti-EGFr Antibody C225 and Radiation. Cancer Biother Radiopharm. 1999;14(6):451–463. doi: 10.1089/cbr.1999.14.451. (Not found in PubMed) [DOI] [PubMed] [Google Scholar]
- 23.Beenken S, Sellers M, Huang P, Peters G, Krontiras H, Dixon P, Stockard C, Listinsky C, Grizzle WE. Transforming Growth Factor α (TGFα), Expression in Dysplastic Oral Leukoplakia: Modulation by 13-cis Retinoic Acid. Head Neck. 1999;21(6):566–573. doi: 10.1002/(sici)1097-0347(199909)21:6<566::aid-hed11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 24.Urban D, Myers R, Manne U, Weiss H, Mohler J, Perkins D, Marklewicz M, Lieberman R, Kelloff G, Marshall M, Grizzle W. Evaluation of Biomarker Modulation by Fenreinide in Prostate Cancer Patients. Eur Urol. 1999;35(5–6):429–438. doi: 10.1159/000019875. [DOI] [PubMed] [Google Scholar]
- 25.Rogers BE, Garver RI, Grizzle WE, Buchsbaum DJ. Genetic Induction of Antigens and Receptors as Targets for Cancer Radiotherapy. Tumor Targeting. 1998;3:122–137. (Not found in PubMed) [Google Scholar]
- 26.Saleh MN, Tilden AB, Meredith RF, LoBuglio AF, Grizzle WE. Chimeric Antibodies with Specificity for Tumor Antigens: Demonstration of in situ Localization to Tumors after Antibody Therapy. Biotech Histochem. 1998;73(4):186–197. doi: 10.3109/10520299809141109. [DOI] [PubMed] [Google Scholar]
- 27.Fabian CJ, Kimler BF, Elledge RM, Grizzle WE, Beenken SW, Ward JH. Models for early chemoprevention trials in breast cancer. Hematol Oncol Clin North Am. 1998;12(5):993–1017. doi: 10.1016/s0889-8588(05)70038-1. [DOI] [PubMed] [Google Scholar]
- 28.Manne U, Weiss HL, Myers RB, Danner OK, Moron C, Srivastava S, Grizzle WE. Nuclear Accumulation of p53 in Colorectal Adenocarcinoma: Prognostic Importance Differs with Race and Location of the Tumor. Cancer. 1998;83(12):2456–2467. doi: 10.1002/(sici)1097-0142(19981215)83:12<2456::aid-cncr8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Kim M, Wright M, Deshane J, Accavitti MA, Tilden A, Saleh M, Vaughan WP, Carabasi MH, Rogers MD, Hockett RD, Grizzle WE, Curiel DT. A Novel Gene Therapy Strategy for Elimination of Prostate Carcinoma Cells from Human Bone Marrow. Hum Gene Ther. 1997;8(2):157–170. doi: 10.1089/hum.1997.8.2-157. (Not found in PubMed) [DOI] [PubMed] [Google Scholar]
- 30.Alexander D, Chatla C, Funkhouser E, Meleth S, Grizzle WE, Manne U. Post-surgical disparity in survival between African-Americans and Caucasians with colonic adenocarcinomas. Clin Cancer Res. 2004;101(1):66–76. doi: 10.1002/cncr.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Burford C, Barnes MN, Oelschlager DK, Myers RB, Talley LI, Partridge EE, Grizzle WE. Effects of nonsteroidal anti-inflammatory agents (NSAIDs) on ovarian carcinoma cell lines: preclinical evaluation of NSAIDs as chemopreventive agents. Clin Cancer Res. 2002;8(1):202–209. [PubMed] [Google Scholar]
- 32.Grizzle WE, Srivastava S, Manne U. The biology of incipient, pre-invasive or intraepithelial neoplasia. Cancer Biomark. 2011;9:21–39. doi: 10.3233/CBM-2011-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. NEJM. 2009;360:1385–1394. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 34.Zimet GD. Improving adolescent health: focus on HPV vaccine acceptance. Journal of Adolescent Health. 2005;37(6):517–523. doi: 10.1016/j.jadohealth.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Myers RB, Brown D, Oelschlager DK, Waterbor JW, Marshall ME, Srivastava S, Stockard CR, Urban DA, Grizzle WE. Elevated Serum Levels of p105erbB–2 in Patients with Advanced Stage Prostatic Adenocarcinoma. Int J Cancer. 1996;69(5):398–402. doi: 10.1002/(SICI)1097-0215(19961021)69:5<398::AID-IJC8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Hennessey B, Bast RC, Jr, Gonzalez-Angulo AM, Mills GB. Early detection of cancer: molecular screening. In: Mendelsohn J, Howley PM, Israel MA, Gray JW, Thompson CB, editors. The Molecular Basis of Cancer. 3. Philadelphia, PA: Saunders Elsevier; 2008. pp. 335–347. [Google Scholar]
- 37.Thompson IM, Pauler D, Goodman PJ, Tangen PJ, Lucia MS, Parnes MS, Minasian LM, Ford LM, Lippman LM, Crawford ED, Crowley JJ, Jr, Coltman CA. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤ ng per milliliter. NEJM. 2004;350(22):2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 38.Thompson IM, Ankerst DP, Etzioni R, Wang T. It’s time to abandon an upper limit of normal for prostate specific antigen: assessing the risk of prostate cancer. J Urol. 2008;180:1219–1222. doi: 10.1016/j.juro.2008.07.089. [DOI] [PubMed] [Google Scholar]
- 39.Grizzle WE, Semmes OJ, Bigbee W, Zhu L, Malik G, Oelschlager G, Manne B, Manne U. The need for the review and understanding of SELDI/MALDI mass spectroscopy data prior to analysis. Cancer Informatics. 2005;1(1):86–97. [PMC free article] [PubMed] [Google Scholar]
- 40.Mellerik DM, Osborn M, Weber K. On the nature of serological tissue polypeptide antigen (TPA): monoclonal keratin 8, 18, 19 antibodies react differently with TPA prepared from human cultured carcinoma cells and TPA in human serum. Oncogene. 1990;5:100–117. [PubMed] [Google Scholar]
- 41.Findeisen R, Albrecht S, Richter B, Deutschmann K, Distler W. Comparison of tissue polypeptide antigen (TPA) with cancer antigen 15-3 (CA 15-3) and carcinoembryonic antigen (CEA) in follow-up of breast cancer. Clinical Chemistry and Laboratory Medicine. 1998;36(11):841–846. doi: 10.1515/CCLM.1998.148. [DOI] [PubMed] [Google Scholar]
- 42.Duffield AS, Epstein JI. Detection of cancer in radical prostatectomy specimens with no residual carcinoma in the initial review of slides. AJSP. 2009;33(1):120–125. doi: 10.1097/PAS.0b013e318185723e. [DOI] [PubMed] [Google Scholar]
- 43.Sotiriou C, Desmedt C, Durbecq V, Dal Lago L, Lacroix M, Cardoso F, Piccart M. Genomic and molecular classification of breast cancer. In: Ross JS, Hortobagvi GN, editors. Molecular Oncology of Breast Cancer. Sudbury, MA: Jones and Bartlett Publishers; 2005. pp. 81–95. [Google Scholar]
- 44.Bertucci F, Finetti P, Cervera N, Charafe-Jauffret N, Mamessier E, Adelélaïde J, Debono S, Houvenaeghel G, Maraninchi D, Viens P, Charpin C, Jacquemier J, Birnbaum D. Gene expression of profiling shows medullary breast cancer is a subgroup of basal breast cancers. Cancer Res. 2006;6(9):4636–4644. doi: 10.1158/0008-5472.CAN-06-0031. [DOI] [PubMed] [Google Scholar]
- 45.Sørlie T, Tibshirani R, Parker J, Hastie T, Marron T, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lønning PE, Brown PO, Børresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. PNAS. 2003;100(14):8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hongjuan Z, Langerød A, Youngran J, Nowels KW, Nesland JM, Tibshirani JM, Bukholm IK, Kårensen R, Botstein D, Børresen-Dale AL, Jeffrey SS. Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol Biol Cell. 2004;15:2523–2536. doi: 10.1091/mbc.E03-11-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perou CM, Sørlie T, Elsen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JRJR, Ross, Johnsen H, Akslen LA, Fluge Ø, Pergamenschikov A, Williams C, Zhu SX, Lenning PE, Børresen-Dale A-L, Brown PO, Botstein D. Molecular portraits of human breast tumours (letter) Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 48.Bertucci F, Finetti P, Cervera N, Esterni B, Hermitte F, Viens P, Birnbaum D. How basal are triple-negative breast cancers? Int J Cancer. 2008;123:236–240. doi: 10.1002/ijc.23518. [DOI] [PubMed] [Google Scholar]
- 49.Diaz LK, Cryns VL, Symmans WF, Sneige N. Triple negative breast carcinoma and the basal phenotype: From expression profiling to clinical practice. Adv Anat Pathol. 2007;14:419–430. doi: 10.1097/PAP.0b013e3181594733. [DOI] [PubMed] [Google Scholar]
- 50.Reis-Filho JS, Tutt ANJ. Triple negative tumours: a critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 51.Beenken S, Huang P, Sellers M, Peters G, Listinsky G, Stockard C, Hubbard W, Wheeler R, Grizzle WE. Retinoid Modulation of Biomarkers in Oral Leukoplakia/Dysplasia. Cell Biochem. 1994;19:270–277. [PubMed] [Google Scholar]
- 52.Burke HB. Outcome prediction and the future of the TNM staging system. (Editorial) Journal of the National Cancer Institute. 2004;96(19):1409. doi: 10.1093/jnci/djh293. [DOI] [PubMed] [Google Scholar]
- 53.Poulin N, Frost A, Carraro A, Mommers E, Guillaud M, Van Diest PJ, Grizzle PJ, Beenken S. Risk biomarker assessment for breast cancer progression: replication precision of nuclear morphometry. Anal Cell Pathol. 2003;25(3):129–138. doi: 10.1155/2003/262918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varambally S, Dhanasokaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Plenta KJ, Sewall RGAB, Otte AP, Rubin MA, Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. [DOI] [PubMed] [Google Scholar]
- 55.Chhieng DF, Frost AR, Talley LL, Grizzle WE. Biology of Breast Cancer. Molecular and pathologic features of ductal neoplasia of the breast: Racial Considerations. In: Williams CKO, Hunter CP, Falkson C, Olopade O, editors. Breast Cancer in Women of African Descent. Chapter 3. 2005. pp. 39–70. [Google Scholar]; McIlwain CC, Townsend DM, Tew KD. Glutathione S-transferase polymorphisms: cancer incidence and therapy. Oncogene. 2006;25:1639. doi: 10.1038/sj.onc.1209373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hein DW. N-Acetyltransferase genetic and their role in predisposition to aromatic and heterocyclic amine-induced carcinogenesis. Toxicol Lett. 2000:112–113. 349. doi: 10.1016/s0378-4274(99)00226-x. [DOI] [PubMed] [Google Scholar]
- 57.Hein DW. N-Acetyltransferase 2 genetic polymorphism: effects of carcinogen and haplotype on urinary bladder cancer risk. Oncogene. 2006;25:1649. doi: 10.1038/sj.onc.1209374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonner JA, Buchsbaum DJ, Rogers BE, Grizzle WE, Trummell HQ, Curiel DT, Fiveash JB, Ove R, Paisch KP. Adenoviral vector-mediated augmentation of epidermal growth factor receptor (EGFR) enhances the radiosensitization properties of anti-EGFR treatment in prostate cancer cells. Int J Radiat Oncol Biol Phys. 2004;58(3):950–958. doi: 10.1016/j.ijrobp.2003.09.095. [DOI] [PubMed] [Google Scholar]
- 59.Varella-Garcia M, Mitsudomi T, Yatabe T, Kosaka T, Nakajima E, Xavier AC, Skokan M, Zeng C, Franklin WA, Bunn PA, Jr, Hirsch FR. EGFR and HER2 genomic gain in recurrent non-small cell lung cancer after surgery; impact on outcome to treatment with gefitinib and association with EGFR and KRAS mutations in a Japanese cohort. J Thorac Oncol. 2009;4(3):318–325. doi: 10.1097/JTO.0b013e31819667a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helfrich BA, Raben D, Varella-Garcia D, Gustafson D, Chan D, Bernie D, Coldren D, Baron D, Zeng D, Franklin D, Hirsch D, Gazdar A, Minna J, Bunn PA., Jr Antitumor activity of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor gefitinib (ZD1839, Iressa) in non-small cell lung cancer cell lines correlates with gene copy number and EGFR mutations but not EGFR protein levels. Clin Cancer Res. 2006;12(23):7117–7125. doi: 10.1158/1078-0432.CCR-06-0760. [DOI] [PubMed] [Google Scholar]
- 61.Zinn KR, Chaudhuri TR, Krasnykh VN, Buschbaum DJ, Belousova DJ, Grizzle DJ, Curiel DJ, Rogers BE. Gamma camera dual imaging with a somatostatin receptor and thymidine kinase after gene transfer with a bicistronic adenovirus in mice. Radiology. 2002;223(2):417–425. doi: 10.1148/radiol.2232010501. [DOI] [PubMed] [Google Scholar]
- 62.Zinn KR, Buchsbaum DJ, Chaudhuri T, Mountz T, Grizzle T, Rogers BE. Noninvasive Monitoring of Gene Transfer Using a Reporter Receptor Imaged with a High Affinity Peptide Radiolabeled with 99mTc or 188Re. J Nucl Med. 2000;41(5):887–895. [PubMed] [Google Scholar]
- 63.Grizzle W. Special symposium: fixation and tissue processing models. Biotech Histochem. 2009 Jun;22:1–9. doi: 10.3109/10520290903039052. NIHMSID: NIHMS116025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arnold MM, Srivastava S, Fredenburgh S, Stockard CR, Myers CR, Grizzle WE. Effect of Fixation and Tissue Processing on Immunohistochemical Demonstration of Specific Antigens. Biotech Histochem. 1996;71(5):224–230. doi: 10.3109/10520299609117164. [DOI] [PubMed] [Google Scholar]
- 65.Otali D, Stockard C, Oelschlager D, Wan D, Manne D, Watts D, Grizzle WE. The combined effects of formalin fixation and individual steps in tissue processing on immunorecognition. Biotech Histochem. 2009 Jun;26:1–25. doi: 10.3109/10520290903039094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taylor CR. Standardization in immunohistochemistry: the role of antigen retrieval in molecular morphology. Biotech and Histochem. 2006;81:3–12. doi: 10.1080/10520290600667866. [DOI] [PubMed] [Google Scholar]
- 67.Bell WC, Sexton KC, Grizzle WE. Organizational issues in providing high-quality human tissues and clinical information for the support of biomedical research. In: Grützmann R, Pilarsky C, editors. Methods Mol Bilo. Vol. 576. 2010. pp. 1–30. [DOI] [PubMed] [Google Scholar]
- 68.Steg A, Wang W, Blanquicett C, Grunda JM, Eltoum IA, Wang K, Buchsbaum K, Vickers K, Russo S, Diasio RB, Frost AR, Grizzle WE, Johnson MR. Multiple gene expression analyses in paraffin-embedded tissues by Taqman low density array: application to Hedgehog and Wnt pathway analysis in ovarian endometrioid adenocarcinoma. J Mol Diagn. 2006;8(1):76–83. doi: 10.2353/jmoldx.2006.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steg A, Vickers A, Eloubeidi M, Wang W, Eltoum IA, Grizzle WE, Saif MW, Lobuglio AF, Frost AR, Johnson MR. Hedgehog pathway expression in heterogeneous pancreatic adenocarcinoma: implications for the molecular analysis of clinically available biopsies. Diagn Mol Pathol. 2007;16(4):229–237. doi: 10.1097/PDM.0b013e31811edc7e. [DOI] [PubMed] [Google Scholar]