Abstract
We report the first case of Enterococcus cecorum empyema thoracis and spontaneous bacterial peritonitis in a 44-year-old man with underlying cirrhosis. The patient responded to cefotaxime (MIC, 0.25 μg/ml) treatment and drainage of the empyema. Susceptibility of E. cecorum to expanded-spectrum cephalosporins could be due to its production of types of penicillin-binding proteins similar to those produced by Streptococcus species rather than to those produced by Enterococcus species (as predicted by phylogenetic analysis of the 16S rRNA gene sequences).
CASE REPORT
A 44-year-old Chinese man was admitted to a hospital because of fever, shortness of breath, and generalized abdominal pain for 2 days. He had Wilson's disease complicated by cirrhosis, esophageal varices, portal hypertension, recurrent ascites, recurrent spontaneous bacterial peritonitis, right indirect inguinal hernia, and hemorrhoids. Examination showed an oral temperature of 37.7°C, right pleural effusion, and generalized abdominal tenderness and guarding but minimal ascites. Chest radiograph showed right pleural effusion. The hemoglobin level was 10.6 g/dl, the total white cell count was 13.7 × 109/liter, and the platelet count was 73 × 109/liter. The serum bilirubin level was 76 μmol/liter, the albumin level was 24 g/liter, the alkaline phosphatase level was 249 U/liter, and the alanine aminotransferase level was 40 U/liter. The serum urea and creatinine levels were 15.8 mmol/liter and 111 μmol/liter, respectively. The prothrombin time and activated partial thromboplastin time were 16.6 s (normal range, 11.3 to 13.2 s) and 55.8 s (normal range, 27.6 to 37.6 s), respectively. Blood culture and percutaneous thoracocentesis procedures were performed, and empirical intravenous cefotaxime was administered. Abdominal paracentesis was not performed because of minimal ascites. Examination of the turbid pleural fluid revealed a white cell count of 765 × 106/liter with 82% neutrophils and 18% lymphocytes in the presence of cell clumps, a protein level of 5 g/liter, a lactate dehydrogenase level of 209 U/liter, and a pH of 8.0. Gram smearing of the pleural fluid revealed the presence of numerous polymorphs. Aerobic and anaerobic cultures of the pleural fluid yielded pure growth of a gram-positive coccus. Blood culture results were negative. The patient responded to cefotaxime treatment and adequate drainage of the empyema and was discharged after 10 days of hospitalization.
Enterococcus cecorum, one of the 30 species of the genus Enterococcus, was first described in 1983 as Streptococcus cecorum (3). Since then, it has been identified as part of the intestinal floras of various animals, including pigs, cattle, chickens, ducks, cats, dogs, and canaries (4). In contrast to the common recovery of E. cecorum from animals, human infections associated with E. cecorum have been rarely reported, with only three cases described in the English literature (MEDLINE search [1996 to 2003]) (2, 6, 7). Phenotypically, E. cecorum is often described as more Streptococcus-like than other Enterococcus species in that it prefers incubation in atmospheric conditions with CO2 enrichment, fails to grow on Enterococcus-selective medium and in 6.5% NaCl, and grows poorly on bile-esculin agar (10). However, speciation of E. cecorum is difficult, especially when commercial kits are used. In the three cases of human infections, the three isolates were identified by whole-cell protein analysis, cellular fatty acid analysis, 16S rRNA gene sequencing, and/or tRNA gene PCR and capillary electrophoresis in addition to the use of conventional phenotypic tests. In this article, we describe the first case of empyema thoracis associated with E. cecorum. The epidemiology of E. cecorum infections, as well as its susceptibility to cefotaxime in relation to its phylogenetic position as deduced by 16S rRNA gene sequence analysis, are discussed.
Clinical and microbiological data.
All clinical data were collected prospectively as described in a previous publication by Woo et al. (13). The bacterium was identified by standard conventional biochemical methods (9). Lancefield serogrouping was performed using Streptex (Murex Biotech Ltd., Dartford, United Kingdom). All tests were performed in triplicate with freshly prepared medium on separate occasions. In addition, a Vitek System (GPI) (bioMerieux Vitek, Hazelwood, Mo.), an API system (20 STREP) (bioMerieux Vitek), and an ATB expression system (ID32 STREP) were used for the identification of the bacterial isolate. Enterococcus faecalis (ATCC 29212) was used as a control. The pleural fluid isolate was a gram-positive, non-spore-forming coccus arranged in chains. It grew on sheep blood agar as nonhemolytic, gray colonies 1 mm in diameter after 24 h of incubation at 37°C in 5% CO2 and pinpoint colonies after 24 h of incubation at 37°C in ambient air. It also grew well on chocolate and MacConkey agars and in an anaerobic environment but slowly in 40% bile, on bile esculin agar, or in 6.5% NaCl. It was catalase negative and resistant to optochin and bacitracin. It is nongroupable with Lancefield group A, B, C, D, F, or G antisera. It is nonmotile at both 25 and 37°C. The biochemical profile of the isolate is shown in Table 1. Positive results were obtained for a Voges-Proskauer test, alkaline phosphatase, β-glucuronidase, and α-galactosidase and for utilization of trehalose, raffinose, maltose, ribose, cyclodextrin, methyl-β-d-glucopyranoside, melibiose, melezitose, lactose, salicin, sucrose, inulin, cellobiose, d-mannose, starch, and tagatose. It did not produce pyrrolidonylarylamidase. Investigations using a Vitek system (GPI), an API system (20 STREP), and an ATB Expression system (ID32 STREP) yielded no identification.
TABLE 1.
Biochemical profile of the pleural fluid isolate by conventional biochemical tests and Vitek GPI, API 20 STREP, and ATB ID32 STREP systems
| Biochemical reaction or enzyme or test | Results by indicated testa
|
|||
|---|---|---|---|---|
| Conventional | Vitek GPI | API 20 STREP | ATB ID32 STREP | |
| Catalase | − | − | ||
| Resistance to: | ||||
| Bacitracin | + | + | ||
| Optochin | + | + | ||
| Growth in: | ||||
| 6% NaCl | + | + | ||
| 10% bile | + | + | ||
| 40% bile | + | + | ||
| Esculin hydrolysis | + | + | + | |
| Hippurate hydrolysis | − | − | ||
| Arginine hydrolysis | − | − | − | − |
| Lysine decarboxylase | − | |||
| Omithine decarboxylase | − | |||
| Urease | − | − | − | |
| Voges-Proskauer test | + | + | + | |
| Tetrazolium reduction | − | |||
| Novobiocin | − | − | ||
| Polymyxin B | + | + | ||
| Utilization of: | ||||
| Hemicellulase | + | |||
| Dextrose | + | |||
| Lactose | + | + | + | + |
| Mannitol | − | − | − | − |
| Raffinose | + | + | + | + |
| Salicin | + | + | ||
| Sorbitol | − | − | − | − |
| Sucrose | + | + | + | |
| Trehalose | + | + | + | + |
| Arabinose | − | − | − | − |
| Pyruvate | − | − | ||
| Pullulan | − | − | ||
| Inulin | + | + | + | |
| Melibiose | + | + | + | |
| Melezitose | + | + | ||
| Cellobiose | + | + | ||
| Ribose | + | + | + | + |
| Xylose | − | − | ||
| d-glucose | − | |||
| d-mannose | + | |||
| Maltose | + | |||
| Starch | + | |||
| Glycogen | − | − | ||
| Arabitol | − | |||
| Methyl-β-d-glucopyranoside | + | |||
| Tagatose | + | |||
| Cyclodextrin | + | |||
| Pyrrolidonylarylamidase | − | − | − | |
| α-Galactosidase | + | + | ||
| β-Glucuronidase | + | + | ||
| β-Galactosidase | + | + | ||
| Leucine arylamidase | + | |||
| β-Glucosidase | + | |||
| Alanine-phenylatanine-proline arylamidase | − | |||
| Acetyl-β-glucosaminidase | + | |||
| Glycyl-tryptophane arylamidase | + | |||
| β-Mannosidase | + | |||
| Alkaline phosphatase | + | + | ||
+, positive; −, negative.
Antibiotic susceptibility.
Antimicrobial susceptibility was investigated by E-testing, and the results were interpreted according to National Committee for Clinical Laboratory Standards criteria, with Staphylococcus aureus (ATCC 29213) and Streptococcus pneumoniae (ATCC 49619) as controls. The MICs of penicillin, cefotaxime, and vancomycin were 0.094, 0.25, and 1.5 μg/ml, respectively.
16S rRNA gene sequencing and phylogenetic characterization.
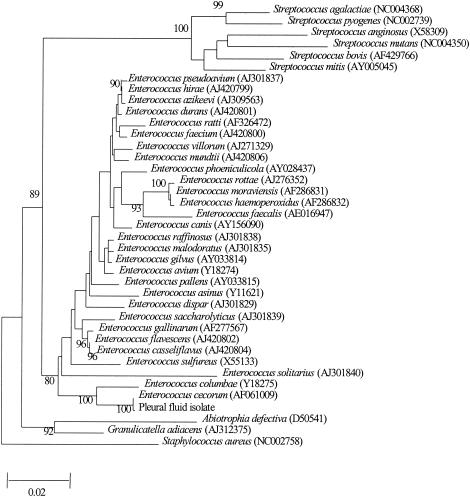
PCR amplification and DNA sequencing of the 16S rRNA gene of the isolate were performed as previously described (15, 16). LPW55 (5′-AGTTTGATCCTGGCTCAG-3′) and LPW205 (5′-CTTGTTACGACTTCACCC-3′) (Gibco BRL, Rockville, Md.) were used as the PCR and sequencing primers. E. faecalis (ATCC 29212) was used as a control. The sequences of the PCR products were compared with known GenBank 16S rRNA gene sequences by multiple sequence alignment using the CLUSTAL W program (11). Phylogenetic relationships of the isolate recovered from the patient with other Enterococcus species, other representative Streptococcus species (S. pyogenes, S. mitis, S. agalactiae, S. intermedius, S. mutans, and S. bovis), Granulicatella adiacens, and Abiotrophia defectiva were determined using PileUp and the neighbor-joining method with GrowTree (Genetics Computer Group, Inc.). A total of 1,235 nucleotide positions of the 16S rRNA genes were included in the analysis. PCR of the 16S rRNA gene of the pleural fluid isolate showed a band at about 1,460 bp. There were two (0.1%) base differences between the 16S rRNA gene sequence of the isolate and that of E. cecorum (GenBank accession no. AF061009), 33 (2.3%) base differences between the 16S rRNA gene sequence of the isolate and that of E. columbae (GenBank accession no. Y18275), and 55 (3.8%) base differences between the 16S rRNA gene sequence of the isolate and that of E. gallinarum (GenBank accession no. AF277567), indicating that the isolate was a strain of E. cecorum (Fig. 1).
FIG. 1.
Phylogenetic tree showing the relationships of the pleural fluid isolate species to other Enterococcus species, other representative Streptococcus species (S. pyogenes, S. mitis, S. agalactiae, S. intermedius, S. mutans, and S. bovis), G. adiacens, and A. defectiva. The tree was inferred from 16S rRNA data by the neighbor-joining method and rooted using the 16S rRNA gene sequence of S. aureus. Bootstrap values were calculated from 1,000 trees. The scale bar indicates the number of substitutions per 50 bases (estimated using the Jukes-Cantor correction). Names and accession numbers are given as cited in the GenBank database.
We report the first case of empyema thoracis caused by E. cecorum. The patient probably had concomitant spontaneous bacterial peritonitis, as he had abdominal pain, generalized abdominal tenderness, and guarding, although abdominal paracentesis was not performed because of minimal ascites. Including the present case, four cases of E. cecorum infections have been reported in the English literature (2, 6, 7). Three patients were males, and one was female. All four patients had underlying diseases. Three of them had cirrhosis of the liver, which seems to be a major underlying disease predisposing patients to E. cecorum infections. Two patients had bacteremia and two had peritonitis. Overall, one of the four patients died.
The portal of entry for E. cecorum in the present case, as well as in the cases of the other two patients with E. cecorum peritonitis, was probably the gastrointestinal tract. It is well known that decompensated liver cirrhosis with portal hypertension and ascites is associated with the presence of intestinal mucosal edema and local immunosuppression secondary to portal venous congestive vasculopathy. Therefore, the gastrointestinal tracts of patients with liver cirrhosis are often the primary site of infection or the portal of entry for extraintestinal infections. According to one recently published article on spontaneous bacterial empyema in cirrhotic patients, 13 of the 18 patients with positive cultures from pleural fluid had pathogens (Escherichia coli in eight patients, Enterococcus species in three, and Klebsiella pneumoniae in two) that likely originated from the gastrointestinal tract (17). None of these bacteria are conventional pathogens causing pneumonia, bone and joint infections, or skin and soft tissue infections. Interestingly, Laribacter hongkongensis, a recently described organism which has been recovered from fecal specimens of patients with gastroenteritis, was also originally discovered in the blood and empyema pus cultures of a patient with alcoholic liver cirrhosis (8, 14, 18).
Sensitivity of E. cecorum to expanded-spectrum cephalosporins could be due to its production of types of penicillin-binding proteins (PBPs) different from those produced by other Enterococcus species but similar to the PBPs produced by other Streptococcus species. Enterococci, as opposed to streptococci, are intrinsically resistant to the cephalosporins. This differential susceptibility to cephalosporins in these two closely related genera is related to the production of different types of PBPs, of which PBP 5, the major PBP in E. faecalis and E. faecium, is demonstrated to have a low affinity to cephalosporins (1, 5, 12). However, in the present case and in another case reported in the literature (2), E. cecorum has been noted to be susceptible to cefotaxime and ceftriaxone. We speculate that this unexpected finding was due to the presence of types of PBPs in E. cecorum different from those found with E. faecalis and E. faecium. In fact, E. cecorum and E. columbae, with their 16S rRNA gene sequences making up the first branch in the phylogenetic tree among the Enterococcus species (Fig. 1), are probably the ancestors of the other Enterococcus species and are also more closely related to the Streptococcus species than the other Enterococcus species. This is in line with the more Streptococcus-like culture characteristics of E. cecorum (10). Therefore, it is not surprising that the PBPs of E. cecorum, which render E. cecorum susceptible to cefotaxime and ceftriaxone, might be closely related to those of the Streptococcus species.
Nucleotide sequence accession number.
The 16S rRNA gene sequence of the isolate has been deposited in GenBank (accession no. AY365054).
Acknowledgments
This work is partly supported by the University Development Fund, University Research Grant Council, and the Committee for Research and Conference Grant, The University of Hong Kong.
REFERENCES
- 1.Chen, H. Y., and J. D. Williams. 1987. Penicillin-binding proteins in Streptococcus faecalis and S. faecium. J. Med. Microbiol. 23:141-147. [DOI] [PubMed] [Google Scholar]
- 2.De Baere, T., G. Claeys, G. Verschraegen, L. A. Devriese, M. Baele, B. Van Vlem, R. Vanholder, C. Dequidt, and M. Vaneechoutte. 2000. Continuous ambulatory peritoneal dialysis peritonitis due to Enterococcus cecorum. J. Clin. Microbiol. 38:3511-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devriese, L. A., G. N. Nutta, J. A. E. Farrow, A. van de Kerckhove, and B. A. Phillips. 1983. Streptococcus cecorum, a new species isolated from chickens. Int. J. Syst. Bacteriol. 33:772-776. [Google Scholar]
- 4.Devriese, L. A., K. Ceyssens, and F. Haesebrouck. 1991. Characteristics of Enterococcus cecorum strains from the intestines of different animal species. Lett. Appl. Microbiol. 12:137-139. [Google Scholar]
- 5.Fontana, R., P. Canepari, M. M. Lieo, and G. Satta. 1990. Mechanisms of resistance of enterococci to beta-lactam antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 9:103-105. [DOI] [PubMed] [Google Scholar]
- 6.Greub, G., L. A. Devriese, B. Pot, J. Dominguez, and J. Bille. 1997. Enterococcus cecorum septicemia in a malnourished adult patient. Eur. J. Clin. Microbiol. Infect. Dis. 16:594-598. [DOI] [PubMed] [Google Scholar]
- 7.Hsueh, P.-R., L.-J. Teng, Y.-C. Chen, P.-C. Yang, S.-W. Ho, and K.-T. Luh. 2000. Recurrent bacteremic peritonitis caused by Enterococcus cecorum in a patient with liver cirrhosis. J. Clin. Microbiol. 38:2450-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau, S. K. P., P. C. Y. Woo, W.-T. Hui, M. W. S. Li, J. L. L. Teng, T.-L. Que, R. W. H. Yung, W.-K. Luk, R. W. M. Lai, and K.-Y. Yuen. 2003. Cefoperazone MacConkey agar for selective isolation of Laribacter hongkongensis. J. Clin. Microbiol. 41:4839-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray, P. R., E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.). 1999. Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 10.Reuter, G. 1992. Culture media for enterococci and group D-streptococci. Int. J. Food. Microbiol. 17:101-111. [DOI] [PubMed] [Google Scholar]
- 11.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williamson, R., S. B. Calderwood, R. C. Moellering, Jr., and A. Tomasz. 1983. Studies on the mechanism of intrinsic resistance to beta-lactam antibiotics in group D streptococci. J. Gen Microbiol. 129:813-822. [DOI] [PubMed] [Google Scholar]
- 13.Woo, P. C. Y., S. S. Y. Wong, P. N. L. Lum, W. T. Hui, and K. Y. Yuen. 2001. Cell-wall-deficient bacteria and culture-negative febrile episodes in bone-marrow-transplant recipients. Lancet 357:675-679. [DOI] [PubMed] [Google Scholar]
- 14.Woo, P. C. Y., P. Kuhnert, A. P. Burnens, J. L. L. Teng, S. K. P. Lau, T. L. Que, H. H. Yau, and K. Y. Yuen. 2003. Laribacter hongkongensis: a potential cause of infectious diarrhea. Diagn. Microbiol. Infect. Dis. 47:551-556. [DOI] [PubMed]
- 15.Woo, P. C. Y., A. M. Y. Fung, S. K. P. Lau, S. S. Y. Wong, and K.-Y. Yuen. 2001. Group G beta-hemolytic streptococcal bacteremia characterized by 16S ribosomal RNA gene sequencing. J. Clin. Microbiol. 39:3147-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo, P. C. Y., D. M. W. Tam, K.-W. Leung, S. K. P. Lau, J. L. L. Teng, M. K. M. Wong, and K.-Y. Yuen. 2002. Streptococcus sinensis sp. nov., a novel Streptococcus species isolated from a patient with infective endocarditis. J. Clin. Microbiol. 40:805-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiol, X., J. M. Castellvi, J. Guardiola, E. Sese, J. Castellote, A. Perello, X. Cervantes, and M. J. Iborra. 1996. Spontaneous bacterial empyema in cirrhotic patients: a prospective study. Hepatology 23:719-723. [DOI] [PubMed] [Google Scholar]
- 18.Yuen, K.-Y., P. C. Y. Woo, J. L. L. Teng, K.-W. Leung, M. K. M. Wong, and S. K. P. Lau. 2001. Laribacter hongkongensis gen. nov., sp. nov., a novel gram-negative bacterium isolated from a cirrhotic patient with bacteremia and empyema. J. Clin. Microbiol. 39:4227-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]