Abstract
The availability of human tissues to support biomedical research is critical to advance translational research focused on identifying and characterizing approaches to individualized (personalized) medical care. Providing such tissues relies on three acceptable models – a tissue banking model, a prospective collection model and a combination of these two models. An unacceptable model is the “catch as catch can” model in which tissues are collected, processed and stored without goals or a plan or without standard operating procedures, i.e., portions of tissues are collected as available and processed and stored when time permits. In the tissue banking model, aliquots of tissues are collected according to SOPs. Usually specific sizes and types of tissues are collected and processed (e.g., 0.1 gm of breast cancer frozen in OCT). Using the banking model, tissues may be collected that may not be used and/or do not meet specific needs of investigators; however, at the time of an investigator request, tissues are readily available as is clinical information including clinical outcomes. In the model of prospective collection, tissues are collected based upon investigator requests including specific requirements of investigators. For example, the investigator may request that two 0.15 gm matching aliquots of breast cancer be minced while fresh, put in RPMI media with and without fetal calf serum, cooled to 4°C and shipped to the investigator on wet ice. Thus, the tissues collected prospectively meet investigator needs, all collected specimens are utilized and storage of specimens is minimized; however, investigators must wait until specimens are collected, and if needed, for clinical outcome. The operation of any tissue repository requires well trained and dedicated personnel. A quality assurance program is required which provides quality control information on the diagnosis of a specimen that is matched specifically to the specimen provided to an investigator instead of an overall diagnosis of the specimen via a surgical pathology report. This is necessary because a specific specimen may not match the diagnosis of the case due to many factors such as necrosis, unsuspected tumor invasion of apparently normal tissue, and areas of fibrosis which are mistaken grossly for tumor. Aliquots for quality control (QC) may or may not be collected at the time of collection and in some cases, QC may not occur until specimens are distributed to investigators. In establishing a tumor repository, multiple issues need to be considered. These include the available resources, long term support, space and equipment. The needs of the potential users need to be identified as to the types of tissues and services needed and the annotation expected. Other specific issues to be considered include collection of specimens potentially infected with blood borne pathogens (e.g., hepatitis B), charge back mechanisms, informatics needs and support, and investigator requirements (e.g., recognition of repository contributions in publications). In general, the repository should not perform the research of the investigators, but should provide the infrastructure necessary to support the research of the investigator. Thus, the goals of the repository must be established. Similarly, ethical and regulatory issues must be evaluated. In general, tissue repositories need ethical (e.g., IRB) and privacy (e.g., HIPAA) review. Also, safety issues need to be considered as well as how biohazards will be addressed by investigator-users. Considerations involving the transfer of specimens to other organization usually require a material transfer agreement (MTA). A MTA should address biohazards as well as indemnification. Thus, many issues must be considered and addressed in order to establish and operate successfully a biorepository.
Keywords: Tissue repositories, tissue banking, prospective collections, quality control, quality assurance, repository science, bias, clinical trials, epidemiology, annotation, material transfer agreement, shipping, informatics, safety, biohazards, security, difficult requests, caBIG, vocabulary, common data elements, chemical hazards, cost recovery, HIPAA, informed consent, demographics, clinical information, training, audits, good manufacturing practice, storage, specimen identification, services
1. Introduction
The progress of modern research in medical areas such as cancer and diffuse lung disease ultimately requires access by researchers to high quality specimens of human tissue including bodily fluids. Multiple organizations are now involved in collecting, processing, storage and distribution of human tissues in order to aid investigators in biomedical research. Guidelines as to Best Practices [1–3] in this area recently have been developed, but some of these may be general and may not always include all of the details and knowledge needed by individuals who are new to this field or investigators who use human tissues. While limitations of space also prevent this manuscript from being very comprehensive, our goal is to discuss issues that may aid those new to this area in rapidly acquiring knowledge that may help them in this important area. Those requiring more information are referred to separate manuscripts on the topics briefly reviewed herein [1–16]. In addition, investigators utilizing human tissue in research will find this chapter very helpful, because it presents an in-depth review of issues involving human tissue that may directly impact their research and improve their access to human tissues.
2. Models of tissue collection
There are multiple approaches to obtaining human tissues and bodily fluids to support biomedical research. The most disorganized of these is best described as “catch as catch can” in which surgeons, pathologists or other medical personnel provide tissue specimens, as available, to biomedical researchers. Such specimens characteristically are collected only when time permits and when the request is remembered. Typically these specimens do not have any quality control nor have they been collected, processed, or stored using quality assurance (e.g., standard operating procedures); thus the quality of such specimens may be poor and the diagnosis may be inaccurate. More worrisome is that such specimens may be obtained without oversight of IRBs or Privacy Boards and may violate HIPAA regulations and/or IRB requirements [4].
A more organized approach to obtaining research-grade human tissues is from a tissue organization that follows a “banking model”. In the banking model, a standard operating procedure (SOP) is followed to obtain, process and store human tissues. Typically such tissues are frozen or are collected and processed as paraffin blocks. Fresh, unfrozen samples typically are not available from a tissue bank nor are “specially processed” tissues. Thus the major disadvantage of the banking model is that the specimens may not meet investigator needs as to size, % of tumor, processing and storage [6–8], and needed uninvolved and/or normal tissues (e.g., buffy coat) or fresh tissue may not be available. Advantages of the “banking model” are that large numbers of specimens may be immediately accessible to a researcher and corresponding clinical and demographic information including clinical outcome may be readily available.
The clinical trial model is a subtype of the banking model in that a bank contains the remnants of the tissues/bodily fluids collected from one or more clinical trials. The problems with a more general banking model may be exacerbated in this model because the original informed consent of the clinical trial may not permit different usages and thus, the institution’s IRB may limit the utilization of such specimens for a wide range of different types of research. Similarly, the remnants of the clinical study may be limited in size and number and the way that the tissues were processed for the clinical trial may differ from the processing needs of investigators.
In the “prospective collection model”, investigators specify the type of tissue specimen they need as well as how the specimens are to be collected, processed and stored. This is the model followed by the Cooperative Human Tissue Network (CHTN) [5–7]. The obvious disadvantage of this model is that large numbers of specimens are not readily available nor are outcome data, because the specimens are collected prospectively and have not matured as to outcome. The advantage of the model is that the investigator gets just what is requested (e.g., fresh tumor minced in media of choice); however, the investigator may have to wait on clinical outcome (years) and for numerous specimens to become available (months).
The combination of the prospective and the banking models- a tissue repository model – includes the advantages of each of the two models. It should be noted here that we use the term “repository” as defined by the International Society of Biological and Environmental Repositories (ISBER) Best Practices [2] as “An entity that receives, stores, processes and/or distributes specimens, as needed. It encompasses the physical location as well as the full range of activities associated with its operation.” The main issue in the operation of such a tissue repository is that there are complex administrative needs as well as increased requirements for more complex bioinformatics systems [5–13].
As more organizations have developed tissue repositories and more experience has been gained as to optimizing such operations, an international organization, The International Society of Biological and Environmental Repositories (ISBER), was formed to support and aid organizations operating repositories. Because published information to support the operations of biological repositories was limited, ISBER published guidelines (best practices) for biological repositories [1, 2]. A new version of ISBER best practices is in final editing. The National Cancer Institute (NCI) also established best practices for tissue repositories focused on neoplastic diseases [3]. These documents are an invaluable resource and provide more details as to specific operations.
3. Variables affecting tissue repositories
3.1. Types of tissues provided
Each tissue repository may be quite variable in the tissues provided ranging from just paraffin sections of one cancer (e.g., breast cancer) to fresh, frozen, and fixed solid tissues to bodily fluids from patients with a wide range of diseases. One of the first decisions in developing a tissue repository is to define its goals including what tissues will be supplied. The potential tissues needed by investigators could be determined by surveying the needs of the users likely to be served by the tissue repository. The design of the tissue repository, including space, equipment, personnel and supplies will depend upon the types of processed tissues provided to investigators as well as the model of the tissue repository. Other issues that need to be considered are whether or not tissues will be collected from patients with known fluid borne human pathogens such as hepatitis B and C, HIV and hanta virus. Similarly, tissue repositories may choose to avoid other contagious diseases such as tuberculosis, herpetic infections and antibiotic resistant bacteria.
3.2. Population
Tissues are usually collected from local medical facilities; thus, the tissue repository must estimate its needs for types and subtypes of tissue. Specifically, requests for types of tumors and other tumor characteristics may vary with the research institution based upon the type of research being performed by local investigators. Specific populations of patients may be needed for specific types of research. In addition, different mixtures of populations can introduce bias into studies. For example, in a study of prostate cancers, the controls should not include young men < 30 years of age and/or women. The local geographic area may have a small African-American (AA) population, but many samples of a specific cancer may be needed from AAs by an investigator. In such situations, arrangements for obtaining tissues from a geographic area with a high population of the needed subgroups may be necessary. Usually, tissue repositories are already in operation at appropriate sites with such populations; if not, development of new collaborative sites for the collection of tissues for research may be necessary. Such development may, however, represent a large expenditure of effort and resources. It is our experience that many such collaborative relationships fail, costing time and resources, so these relationships should be approached with careful planning and great care. Also a rapid evaluation of the organization’s progress in meeting documented goals should be performed and a rapid suspension of its funding should occur if acceptable progress toward these goals is not met.
3.3. Services provided to investigators
The services provided to investigators also affect the resources required by the repository. Potential services beyond providing tissues might include delivery of tissues to local investigators, extracting DNA and/or RNA from tissues, culturing cells from tissues, developing xenograft tumors and extensive analysis of the quality of tissues [5–9]. Providing multiple services may distract the tissue repository from its primary goals. Also, such services may be difficult to stop once provided, even if a service subsequently compromises the primary goals of the tissue repository. If services are performed for an investigator, the tissue repository should be fully compensated on a cost recovery basis for the efforts and resources devoted to such services.
3.4. Labeling of specimens
The correct labeling of specimens of tissue is very important to ensure correct identification of specimens used to support biomedical research. The method of labeling should a) prevent mislabeling secondary to errors by personnel, b) minimize the label separating from the specimen, and c) prevent problems with the identification of the specimen (e.g., illegible handwriting). In general, this can be accomplished most efficiently by the use of barcodes. Barcodes link specimens to an informatics system, typically containing pertinent specimen data, including clinical, demographic and historical information on the individual who was the source of the specimen as well as data on the specimen (e.g., size, number of aliquots, storage site, specimen assignment to specific investigator or project). It should be understood that a barcode is only a “number” or multidimensional link and this link contains little other information; it is only the link of the barcode to software that actually identifies information regarding the bar-coded specimen. Thus, without matching software at a second site, the second site can only identify the specimen link and would need access to the software if electronic access to more extensive information is required.
3.5. Collection variables and limitations
When a tissue repository is first designed/developed or a new project is envisioned, the collection, processing and storage variables that may affect operations should be identified. Variables such as use of neoadjuvant therapy prior to surgery may limit the usefulness of specific tissues and it is important that investigators using such affected tissues understand these potential limitations. For example, many patients with breast cancer or sarcoma are treated with a form of chemotherapy or radiation therapy before surgery, i.e., neoadjuvant therapy [15]. Such therapy may affect or even completely destroy selective populations of neoplastic or other cells. This may result in residual tissue containing neoplastic cells that do not represent the original disease. Similarly, metastatic lesions may be destroyed by initial therapy so that correct staging of the disease is no longer possible; thus, it is important to identify patients who have received neoadjuvant therapy. If such tissue is distributed to investigators, all users should understand the limitations of these specimens.
As new screening methods and improved medical care are more widely applied to the U.S. Population (e.g., mammography), the size of tumors are rapidly declining. Currently, many of the operations for diseases of the breast at UAB are for ductal carcinoma in situ (DCIS). Also, invasive cancers are becoming smaller so that as the average tumor decreases to less than 1 cm, few samples to support biomedical research are available because most of these small specimens are used for diagnosis. Similarly, samples of tissue to support research on other specific tissues are not available. These include lesions that are only biopsied (e.g., small cell undifferentiated carcinoma of the lung, large pancreatic cancers) as well as skin diseases that are either totally submitted for pathologic examination (e.g., primary melanomas) or are only biopsied (e.g., inflammatory lesions of the skin). In addition, with the increased accuracy of radiologic evaluations, metastatic lesions (e.g., bone) frequently are not biopsied. Thus, tissue repositories must resort to other approaches to support biomedical investigations. These approaches include expanding the use of paraffin blocks in research [17, 18] and more novel approaches such as nitrocellulose blotting of biopsies [19].
3.6. Time between surgery and tissue storage
Investigators sometimes request that specimens of human tissue be very rapidly processed and frozen after surgery. While a few aliquots of tissue can be collected, processed and frozen within 15 minutes of surgical removal, this usually requires special assigned personnel and specific resources as well as an efficiently operating pathology gross room [16]. Most tissue repositories do not have the resources including personnel to collect and process specific tissue that rapidly; nevertheless, it is important to minimize and record the times between the removal of operative specimens or the times of transfer of these specimens from the operating room to the tissue repository. A good general rule for tissue repositories is that if it cannot control a parameter, a record of the variability of such parameters for each specimen should still be maintained. It should be noted that for a large specimen, dissection, processing and labeling multiple aliquots from this single specimen may require in excess of 1 hour. We recommend that after removal, specimens be maintained at about 4°C (wet ice), but not frozen, until processed. Note, artificial, e.g., “blue ice,” may be cooled to below 0°C and hence can freeze tissue with which it comes into direct contact; therefore, its use either should be avoided or its temperature should be controlled carefully.
The time until storage should be as short as pragmatic; however, delays in processing may occur when a very large specimen or multiple specimens must be processed simultaneously. In this situation, one or two aliquots from each specimen can be snap frozen in liquid nitrogen vapor or on dry ice and other aliquots can be collected and frozen later. Processing times and the time to storage should be recorded.
Human tissues frequently undergo periods of warm ischemia when blood vessels are tied off or compromised during surgery. Thus, because catabolic enzymes are functioning optimally at body temperature, many more labile molecules have degraded by the time the tissues associated with surgical resections are removed from the patient. The scientific importance of very rapid collection and processing of human specimens (< 30 minutes) is controversial for many studies. Avoiding warm ischemia is limited to biopsies and bodily fluids. Most clinical biopsies are not available to support research unless obtained as part of a specific research protocol. Spreuessel et al. [20] reported that 80% of genes changed less than 2 fold within 30 minutes after removal from the body. Others have reported that in analysis of human tissue specimens using spotted arrays less than 14% of the 2400 human genes evaluated changed by more than 50%. Specifically, the mRNA for most genes showed rather small increases when specimens were maintained in vitro for 5 minutes post-surgery were compared with those maintained for 60 minutes post surgery [21]. In addition, Dash et al. [22] noted that less than 1% of genes changed within one hour after removal of prostate tissue from the body. Thus, very rapid processing of tissue after removal from the body (< 30 minutes) to maintain non-degraded mRNA may not be justified. This is supported by studies of ribosomal RNA which have demonstrated that rRNA remains stable at 4° or at room temperature for several hours after tissue is removed surgically [23].
Post-translationally modified proteins such as phosphoproteins actually may be one of the most unstable types of molecules in tissue specimens. Specifically, Baker et al. [24] reported that 9 of 13 rapidly processed biopsies of adenocarcinomas of the esophagus expressed pAkt; however, pAkt was identified in none of the matching surgically resected specimens that had been exposed to warm ischemia. In addition, using a xenograft model, they reported that the half life of Akt was 180 minutes, but the half life of pAkt was only 20 minutes. In contrast, pAkt in formalin fixed, paraffin embedded tissue from radical prostatectomies has been used as a prognostic biomarker [25]; however, one would not be surprised if specific molecules had different profiles of stability in different tissues. Thus, very rapid processing of specific tissue to maintain phosphoproteins may be necessary for some types of tissue and/or research. Investigators should evaluate the stability in tissue of the molecular features they are studying and should be aware that such stability may vary among specific tissues.
3.7. Storage of specimens
Ultra-cold storage systems to house tissue specimens may not be readily available to investigators. A tissue repository may temporarily store tissue specimens for investigators, but the tissue repository should charge for such storage.
The optimal method for long term (≥6 months) storage of tissues to support biomedical research remains controversial. Data are available to demonstrate that unprocessed tissue specimens should not be stored at temperatures of −20°C for greater than one month; however, such storage for even shorter periods is probably unwise. In addition, tissues should not be stored for any period in self-defrost freezers or cryostats [26, 27]. Not much information is available as to whether or not storage of human tissue specimens (solid or bodily fluids) in the vapor phase of liquid nitrogen freezers is better than storage at −80°C. It has been reported that bias resulted in one study due to differences in tissue from cases and from controls because these tissues were collected by varying methods and were stored at −80°C for different intervals [28,29]; however, because other variables (sites and methods of collection) were also different, the bias in the study might have been introduced by variables other than the temperature – time of storage. The unpublished data of the CHTN indicate that laboratories of the CHTN could not detect differences in mRNA or in proteins between storage of equivalent aliquots of the same human tumors in vapor phase of liquid nitrogen versus at −80°C for longer than 5 years.
If the goal of a study is to culture cells in vitro or grow tumors as xenografts, variations in specimen preparation and storage are limited to several approaches. Cells can be grown in culture if the cells are processed fresh and cultured or if purified cells are stored in media plus 10% DMSO or other media to prevent the formation of ice crystals at the temperature of liquid nitrogen vapor. The chance of a successful culture from a cellular sample increases with the number of cells; thus, a short-term culture to increase the number of cells stored would be useful to promote viability of cells in future cultures developed from frozen specimens. Note that such cell cultures would likely be mixed (e.g., tumor cells plus inflammatory cells and fibroblasts/fibrocytes). Multiple texts on primary cell cultures are available and these should be consulted before culturing cells obtained from samples of solid tissue. If solid tissue is frozen without additives (e.g., neat) or in OCT, it is unlikely that viable cells can be obtained subsequently because the ice crystals will form and will lyse most cells within the tissue. There are a few anecdotal reports that indicate that chopped up fragments of solid tissue can be frozen in media plus 10% DMSO and viable cells subsequently can be isolated from them. Also, some tissue repositories transplant human cells directly into immunocompromised mice and subsequently try to develop viable cell lines or cultures.
3.8. Records of collecting, processing and storage
Detailed and accurate records concerning the variables of collection, processing and storage of tissues are critical to the quality assurance program of a tissue repository and these should be an important component of an informatics program. Of special importance are “times” by which the history of each specimen can be reconstructed. Many operative times may not be readily available (e.g., time vessels are tied or compromised). These times may be important because they might permit estimates of the times of “warm ischemia”, i.e., the times between ligation or compromise of vessels to tissues and removal of the tissues from the body. Such times usually are not included in the surgical record; however, warm ischemic time might be estimated based upon other times in the operative record. Changing operative reporting is challenging. Review of operative reports to estimate such times requires a large amount of effort and would add greatly to the costs of specimens. In addition, the time and temperature of processing and the time to freezing (or media) are important to record. For tissues prepared as paraffin blocks, the time to fixation, the fixative (e.g., 10% neutral buffered formalin) and the length of fixation are important variables to record. Also the chemical components and time in each step of the tissue processor as well as the type of tissue processor is important to record if paraffin blocks are a product of the tissue repository [30–32]. The parameters do not usually change over a year, so such information can be contained in a “long term record”. When such variables are identified, fields for them should be included in the informatics system (See Quality Assurance and Informatics sections).
3.9. Paraffin blocks
Some tissue repositories supply paraffin blocks, tissue microarrays or sections from paraffin blocks. The paraffin blocks may be part of the quality control blocks prepared by the tissue repository or sometimes tissue microarrays and paraffin sections may be obtained from archival diagnostic blocks. For diagnostic blocks, most will have been fixed in 10% NBF [30–32]. Blocks constructed by the tissue repository can have variable fixation, but it is our experience that most investigators request paraffin blocks for which the initial fixation was 10% NBF so that the results from such blocks can be compared with paraffin blocks obtained elsewhere, especially diagnostic archives. Because the practice of medicine is changing (e.g., reduced times of fixation, different methods of tissue processing, use of neoadjuvant therapy and variable forms of adjuvant therapy), paraffin blocks representative of various diseases may not be able to be replaced. Thus, repositories should consider maintaining paraffin blocks by providing only aliquots (sections or cores) from them. In addition, paraffin blocks are being used to construct tissue microarrays (TMAs). TMAs represent an approach to study many different tissues via immunohistochemistry or in situ hybridization in one experimental run. Some tissue repositories are now constructing TMAs and providing sections from TMAs to investigators as a means, in part, to conserve paraffin blocks.
4. Quality assurance
Quality assurance (QA) is an important component of any aspect of biomedical research [1–3,5–8,14]. QA is especially important to the infrastructure used to support research including facilities which collect, process, store and provide tissues for biomedical research.
Quality Assurance/Management is an area of administration which focuses on operational improvements in the global aspects of all activities of an organization in order to ensure that procedures or products are of a defined quality. Quality Control (QC) is a sum of technical activities that measures the attributes and performance of a process, or product, against defined standards and verifies that the defined standards are met fully. Thus, QC is one component of an overall QA program.
The personnel of a quality assurance program should report directly to upper levels of administration concerning all QA issues. QA personnel should help in the development of SOPs and ensure that all SOPs are followed. When problems in collection, processing, storage or shipping are identified and/or if any specimens of poor quality are identified, QA personnel should lead efforts to identify and correct these problems [14].
4.1. Standard operating procedures
The use of standard operating procedures (SOPs) that are detailed documented methods of each of the activities of a tissue repository is necessary to ensure that all methods are performed uniformly. The SOP should be prepared in great detail so that someone who has not performed the procedure previously can perform the method just as accurately as an employee experienced in the method. Changes in SOPs should be made only by authorized supervisors. Most SOPs are currently computerized and changes should be made so that the date and the supervisor making the change are incorporated into the SOP. Also, the previous dated version of the SOP should be maintained as a historical record in order to ensure that differences in the collection, processing, storage and/or distribution of current products caused by changes in SOPs can be determined. The SOP should be reviewed at least annually, and revised as necessary. The SOPs should be organized into a procedure manual which is easily available to all personnel. SOPs must be readily available at the bench so employees can follow them exactly.
4.2. Good manufacturing practices (GMP)
Documents describing GMP provide guidelines that can be adopted and used by tissue repository organizations to aid in meeting its goals of high quality operations and products. The International Organization for Standardization (ISO), an international federation of national standards organizations, has published ISO9001. The purpose of this document is to provide all types of organizations with useful, internationally recognized standards for operating a quality management system. ISO standards are an aid to ensuring GMP; they are more detailed than typical GMP descriptions and are accepted internationally. Tissue facilities can utilize ISO9001 in developing and monitoring their QA/QC programs. Also, tissue repositories can obtain certifications in ISO9001 or other ISO standards.
4.3. Audits
Audits are written periodic evaluations of operating procedures and infrastructure. QA personnel are responsible for designing, overseeing and evaluating audits of operations to determine the adherence of all employees to QA requirements. Tissue repository organizations should conduct regular audits such as the example listed subsequently. Audits can be as simple as a monthly review of the logs of daily records of temperatures of refrigerators. They may be as complex such as a six month review of the storage locations of a sample of specimens. QA personnel perform these audits and document any problems. Reports of all audits are submitted to upper management, who are responsible for quality assurance, as well as directly to the chief executive officer of the tissue repository.
The QA program of a tissue repository should describe how, why and when specific audits are conducted. For example, specific audits and records might include the following:
Monitoring the adherence to specific SOPs,
Storage of specimens (sites and availability),
Equipment maintenance and repair,
Equipment monitoring (e.g., determining the cutting thickness of microtomes),
Training records and adherence of staff to required training (e.g., training in biohazards); many aspects of training may be required by local or federal government (e.g., safety, IRB, HIPAA) so records of training are monitored frequently by external offices,
Quality of tissues processed by SOPs.
4.4. Surveys
Tissue repository organizations should consider determining the satisfaction of users/investigators with the tissue they have been supplied during the preceding calendar year. A survey could be completed “on line” in electronic format, or a paper survey could be sent to all users. The results of surveys should be evaluated carefully by Quality Assurance personnel. Investigators reporting unsatisfactory results should be contacted and their problems discussed and corrected if possible.
If an organization provides tissues to extramural investigators, the shipment of frozen specimens should not be sent until the recipient is notified and is available to receive the shipment. In general, shipments should be sent on Monday through Thursday unless special arrangements are made. Also, any problems with a shipment should be determined. This can be accomplished by including a self addressed postcard with each shipment. This card can survey standard issues with shipping, and it serves to document receipt of the shipment in addition to problems with the shipment (e.g., not enough dry ice).
4.5. Quality control of tissues
Quality control of tissue requires monitoring the quality and diagnoses of the actual tissues provided for research. The form of QC described was introduced by Dr. Grizzle at UAB in 1983 and incorporated into CHTN operations in 1987. Many tissues, especially tumors, are heterogeneous; thus, specimens of tumors vary as to the proportion of neoplastic cells, necrosis, fibrosis (desomoplasia), mucin production, or inflammatory and stromal cells. Because fibrosis in and adjacent to tumors may be intermixed with the tumor and some tumors may diffusely infiltrate normal appearing tissues, just knowing the general diagnosis of the tissue from a patient is not adequate quality control for the specific tissues provided for research. This is especially true for infiltrative tumors such as breast, prostate, pancreas, and sarcomas as well as tumors with typically large proportions of necrosis such as renal cell carcinomas and metastases to liver of colorectal cancer.
The minimum quality control examination that a tissue repository organization should provide to users is the microscopic examination by a pathologist of an aliquot of tissue that is very representative of the specific tissue that is supplied for research [1,2,14]; optimally, a quality control examination is made on a mirror image piece of tissue to that supplied for research (Fig. 1). For a large tumor, there may be multiple aliquots for which sub-aliquots are supplied to different investigators. For example, Fig. 1 represents the QC of the second of 3 specimens collected to support research. This specimen is subdivided into two aliquots, 2A and 2B, for which 2AB is processed to a paraffin block for histopathologic examinations for quality control as a mirror image for both aliquot 2A and aliquot 2B.
Fig. 1.
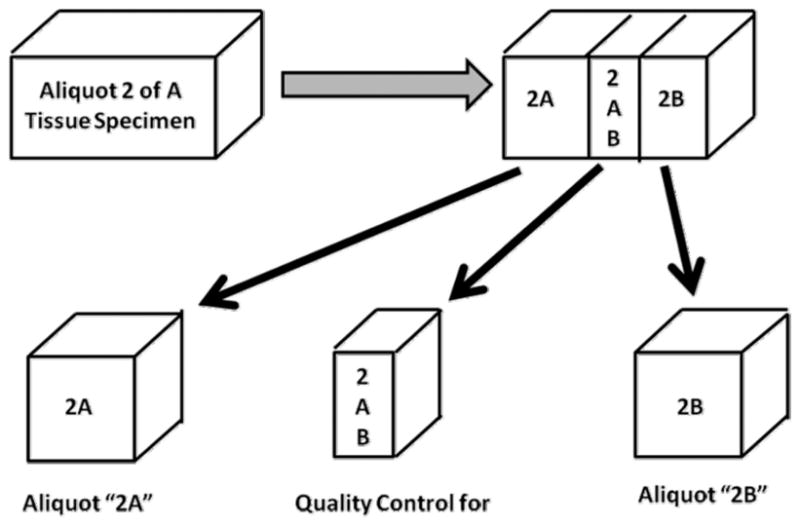
Tissue Specimens Supplied for Research.
The Cooperative Human Tissue Network has found, using similar methods of quality control, that about 15% of tissues collected for specific research cannot be used for the research for which the specimens originally were collected [5–8,14]. Typically in the QC examination, the proportion (percent) of the specimen that is tumor or diseased is specified along with the percent necrosis/fibrosis/mucin within the tumor, and the proportion of neoplastic cells (i.e., neoplastic cells versus total nucleated cells) in the tumor. Quality control also can be performed on frozen sections of a specimen embedded in frozen section support medium (e.g., OCT). However, OCT may interfere with some assays and is more difficult to interpret especially if pre-invasive neoplasia is present.
A quality control examination can also include a molecular analysis in which mRNA, DNA and protein are extracted from small aliquots followed by characterization of specific molecules. Molecular quality control may be performed if investigators request this level of QC, but investigators usually are requested to pay for the increased costs associated with molecular quality control. In addition, the quality of a sample of specimens provided by the facility could be evaluated periodically by molecular analysis as part of the overall quality assurance program of the facility. Molecular analysis also may be performed when investigators indicate that there is a problem with specific specimens if additional aliquots of those specimens are still available at the facility.
Sometimes a more extensive quality control examination may be requested for specific projects. As the required quality control examinations become more extensive, there is a “price” that includes increased time and effort by the tissue repository organization and increased “costs” of the specimen to the investigator. Also, the sizes of specimens are decreased by additional sampling.
QC also can be tailored to individual investigators. A “platinum level” of quality control might include microdissection of specimens to increase the proportion of tumor in specimens. In this approach, frozen sections of the whole specimen are made followed by microdissection of both sides of the specimen to enrich the specimen in diseased cells. Also, aliquots from the microdissection could be analyzed molecularly. Such an approach would increase costs of processing specimens, as well as reduce the tissue available as the specimen is exhausted. If the research projects do not require or need such extensive quality control, this form of QC may not be cost effective.
The quality control examination of tissues can be matched to the needs of specific investigators; however, for most tissue specimens and investigators, the approach shown in Fig. 1 should be adequate. If mRNA is to be analyzed, mRNA can be extracted from frozen sections of tissue and can be evaluated to determine its quality (e.g., by RIN values); obviously, if specimens are not to be used in RNA analyses, QC analysis at the mRNA level adds unnecessary costs. Note that using short amplicons (< 100 bps) in real time reverse phase quantitative PCR techniques permits the use of degraded RNA and even RNA extracted from paraffin blocks can be analyzed and give equivalent results to those obtained from frozen tissues [17,18]. The best and most cost effective approach for a QC is to utilize a simple approach (Fig. 1) that can be expanded to the platinum level, but only at the request of and costs to an investigator.
4.6. Quality assurance/control for the collection of bodily fluids
The development of SOPs is a key for obtaining high quality bodily fluids to support biomedical research. Incorporated into these SOPs should be the parameters that have been identified as being important in collection, processing, storing and distributing these fluids. The first aspect of the SOPSs is that all bodily fluid samples should be frozen as rapidly as practicable, but at least for blood and urine within 4 hours of collection. Also, the samples should be maintained about 4°C until processed and or/stored [5–8,14,33]. For samples of blood components, the samples, after clotting if applicable, should not be hemolyzed because hemolysis releases proteolytic enzymes as well as large quantities of proteins, e.g., hemoglobin. Thus, if it can be controlled, the draw needle should not be smaller than 23 gauge and the draw rate should not be too fast. Also, whole blood, prior to fractionization, should not be frozen without additives if hemolysis is to be avoided. Note that not all assays are affected by hemolysis (e.g., immunoassays for some molecules).
All other aspects related to labeling and storage of bodily fluids should be incorporated into the SOPs. Finally, before distributing fluid specimens, the involvement of bias in studies using fluid specimens should be considered [26–29,34,35]. Bias is also discussed in detail subsequently in Section V. ISBER Best Practices [1,2] discusses some of the issues related to fluid specimens in more detail as does Guidelines of the Early Detection Research Network [33].
4.7. Education
The provision of high quality tissues requires using standard operating procedures so that tissues are collected, processed and stored in a consistent manner. All personnel need to be trained in repository operations including carefully following SOPs. Training of all non-administrative/clerical staff in most repository operations is best to permit flexibility. Training of all staff should include safety as well as regulatory (HIPAA) and ethical issues (IRB). Such training must be documented. It is also important to educate users of the repository; therefore, the repository should also serve as an educational resource for users. Specifically, investigators may need information concerning the selection of the optimal tissues to support their research. Investigators must understand how specimen requirements that may be too restrictive and, in some cases, may be unnecessary, may prevent them from obtaining needed quantities of tissues. Such excessive requirements may also increase their costs. Investigators also need to understand the limitations of the specific tissue repository in obtaining tissues. It is important for investigators to understand how tissues differ in their appropriateness to support a specific research project (e.g., smooth muscle from the wall of the uterus which is estrogen sensitive is not equivalent to smooth muscle of the wall of the stomach). Pathologists or other equivalently knowledgeable professionals associated with the tissue repository should review all requests for human tissues. These professionals should provide guidance to investigators concerning the types of tissue they need for their specific research projects and investigators should request such guidance prior to designing their research projects. If a request is difficult to meet because of unnecessary requirements, an educational approach is an opportunity to explain to the investigators limitations in tissue collection and processing.
5. Matching tissue to tissue needs
5.1. Tissue requests
Obtaining optimal tissues becomes more difficult as more requirements are added to a request [5–8,36]. For example, a request for “any breast carcinoma” is less difficult to meet than a request for “well differentiated ductal carcinoma of the breast from a male less than 40 years of age”, because breast cancers are rare in males as are breast cancers from relatively young individuals. Also, as more and more even apparently simple requirements are added, the likelihood of finding a case that meets all requirements decreases. If we added both African-American and male to any requirements, only about 15% of all cases at the University of Alabama at Birmingham would be eligible to even meet the most general of requests. However, an African-American requirement for either sex could be met by 30% of requests. Another investigator requirement that makes a request difficult to meet is a request for very large amounts of tissue (e.g., 5 grams) from tumors that are typically small. Screening for tumors such as breast and prostate carcinoma has resulted in earlier detection and smaller tumor size in resected specimens; also due to their infiltrative growth pattern, some tumors such as breast, prostate and pancreas frequently are diffusely mixed with uninvolved epithelium and connective tissues resulting in a low percentage of neoplastic cells in a tissue sample. These tumor types are also in great demand by investigators. In general, a request from multiple investigators, each requiring a small amount of tumor (e.g., 0.1 gram), will be filled before a request from a single investigator for a large amount of tumor (e.g., 5 grams). Thus, it is to investigators advantages to adapt their methods so that very small samples can be used. Similarly, for tumors in high demand such as breast and prostate which also tend to be small and requests for large numbers of case within one year (e.g., 100) are unlikely to be met. Similarly, requests for large numbers of relatively rare tumors (paragangliomas) or tumors or other tissues that typically are not removed surgically (e.g., normal heart or normal brain) usually cannot be met. As discussed, because of the wide use of newer methods of screening (e.g., the use of prostatic specific antigen – PSA), tissues involved by the disease may typically be smaller and frequently are of lower stage.
Independent of newer and improved approaches to diagnosis, access to tissues not removed during routine surgery (e.g., normal brain, heart or undifferentiated small cell [oat cell] carcinoma) is very limited. Some cardiac and pulmonary tissues are available from diseases treated by transplantation (e.g., cystic fibrosis), but such tissues usually are removed after-hours and rapid retrieval would require an expensive on call group of personnel with each employee on call about 450 hours per month. At UAB, just the on-call costs of our after-hours tissue retrieval program which utilizes three employees are almost $3,000 per month. Also, finding dedicated employees who are willing to work after hours and covering the cost of overtime is a great challenge. Similarly, tissues associated with transplantation including normal tissues from organs deemed not acceptable for transplant can be obtained if an on-call collection system is developed. Nevertheless, access to rare tumors such as synovial cell sarcomas and rare subtypes of epithelial tumors (e.g., medullary carcinoma of the thyroid) will always be very limited as will tumors such as primary melanomas which are completely processed for diagnosis.
5.2. Available resources for collecting tissues
Most tissue repositories try to provide tissues equitably to investigators requesting tissues. Because efforts (time) devoted to collecting and processing specific requests for tissues should be equitable as to services (e.g., tissues) provided to investigators, tissue requests requiring extensive effort (e.g., requests for removal of a vertebral column at autopsy) cannot usually be met by a busy tissue repository; thus, it is important to educate investigators as to those requirements which make their requests difficult or impossible to meet. This includes unreasonably rapid times for processing specimens after their surgical removal. If difficult requests are very important to an investigator, he/she should consider providing additional funding to the tissue repository in order to provide the additional resources required for filling such a request.
5.3. Clinical information and demographics: Annotation of tissue specimens
The minimum annotation of a tissue specimen includes age, race and sex of the donor and the QC diagnosis of the specific aliquot of tissue provided for research. Beyond this, the extent of annotation required for a specimen aliquot varies with the needs of the investigator and the capability of the tissue repository to provide additional information. For example, if the primary goal of a project of the tissue repository is to support a complex epidemiological study of a specific disease process (e.g., diabetes mellitus, type II), then detailed clinical, familial and social histories of the patient, as well as special testing – beyond the information usually available in a typical chart – might be needed. Much of such data needs to be obtained close to the time tissues are collected when the patients who are the sources of the tissues are readily accessed. If key pieces of information are critical to a study of breast cancer (e.g., stage of the menstrual cycle, use of hormones, prior removal of the ovaries), such information, which may not be available to the repository in all situations, might need to be collected directly from each patient as a specific funded component of the study. In contrast, if tissues were being collected and used to study the biochemistry of tissues of a specific disease, less clinical information might be required to support the study. Thus, the collection of detailed clinical information associated with all specimens from all patients frequently is a waste of resources in that information beyond that in the chart would need to be collected specifically and would be different for each study. Also, many research projects do not require detailed clinical information. When detailed annotation on only part of a specimen collection will be needed for a specific project (10–20% of collected cases), it is more efficient to collect the information only on the patients from whom specific information will be needed. Special resources may be required up-front to support a study requiring extensive data. Similarly, information typically included in charts can be collected when the specimens are collected, when the specimens are requested, or after an investigator has had an opportunity to test the specimen and confirms for the repository that the additional information is actually needed.
Selective tissue repositories are funded to obtain extensive demographic and clinical information upon each patient from whom tissue is collected. The best example of one such type of repository is that associated with Specialized Projects of Research Excellence (SPOREs). If the tissues in such repositories are used only in projects which require such information, the annotation of all specimens may be efficient. Similarly, carefully annotated specimens should not be used in projects for which such annotation is unnecessary.
6. Bias and other unknown variables affecting tissue resources
Bias is an error introduced into a study because of unrecognized systematic experimentally measured differences between cases and controls unrelated to the process/disease being studied. Bias may affect studies because these systematic differences between cases and controls are incorrectly attributed to biologic differences caused by the diseases [26–29]. Bias results in an incorrect conclusion based on the evaluation of the experimental results. The potential for biases to affect studies increases as the number of experimental measurements [26–29] increase. Bias cannot be identified statistically; it usually is detected when the initial conclusions are evaluated on an independent set of samples or data.
Bias is frequently introduced into research projects using human tissues because of the differences in collecting, processing or storing tissues of the cases versus controls or systematic differences between cases and controls based on the populations used in each. Use of SOPs aids in minimizing bias and detailed and accurate records may be helpful to identify causes of bias. Thus, if cases of a specific disease were to be evaluated using a sample of EDTA plasma that had had multiple freeze thaws and was collected via a mobile van, but, in contrast, the associated plasma controls were from citrate tubes, had only one freeze thaw cycle and were collected in a hospital clinic, mass spectrometry and other very sensitive methods might identify the differences due only to the type of plasma, the number of freeze thaw cycles and/or the sites of collection. Thus, investigators might conclude falsely that there were proteomic differences in the plasma from patients with a specific disease and individuals who did not have the disease; a conclusion based upon the bias of the two sample sets. The incorrect conclusions might not be identified until attempts are made to validate the initial experimental results at which time the inability to confirm the initial results might be attributed to bias in the design of the studies. Clearly, the above scenario demonstrates the importance of using standard operating procedures (SOPs), so that tissues are collected, processed and stored similarly. A careful review of records might identify the differences between samples from cases and those from controls and the biased conclusions might not be published until confirmed on a new set of samples collected, processed and stored more uniformly. Some of the potential causes of bias are listed in Table 1.
Table 1.
Examples of potential sources of bias in tissue sets
|
Bias is best avoided by first recording and next identifying systematic differences between cases and controls and matching cases and controls on these systematic differences (the number of African-Americans in cases equals the number of African-Americans in controls). When samples are collected from multiple sites, the number of cases should equal the number of controls from each site.
7. Other administrative issues
7.1. Material transfer agreements
Material transfer agreements (MTAs) facilitate the non-commercial transfer of materials and information between organizations. While these agreements are unidirectional and are designed primarily to protect the originating organization, MTAs also aid to some degree the interests of the recipient by clarifying the requirements related to the material/information being transferred. For tissue repositories, MTAs usually involve the transfer of tissue including bodily fluids and their associated information. There are several components to an MTA that are very important to tissue repositories. These include establishing safety requirements, indemnification agreements, commercial use of specimens and transfer of specimens to third parties. The requirement that all users of tissue specimens be educated in their dangers and in biohazards is important in that it warns all users that universal precautions must be taken with all tissues. Indemnification means that the receiving organization takes over the liability associated with the specimen; thus, if someone cuts their hand and subsequently is infected with hepatitis C, the initiating organization is not liable for the dangers inherent in all specimens of tissue. The prohibition against transfer to a third party is important to prevent the loss of control as to usages of specimens, and other important agreements such as indemnification, biohazard training, commercial uses and issues in intellectual property.
Issues in MTAs are very important and complex. Thus, an organization’s administrative offices that deal with patents and intellectual property should be consulted early in the process of transferring specimens. Of interest, Fig. 2 is the agreement of the Cooperative Human Tissue Network which is a form of an MTA utilized for decades by the CHTN.
Fig. 2.
Cooperative human tissue network agreement for use of tissue.
7.2. Shipping
When tissues are collected at one site and processed or stored at a different site, shipping of specimens is frequently required. Similarly, shipping is necessary when specimens are distributed extramurally to investigators. Usually this shipping utilizes couriers and thus may involve transfer by air. Shipments by air must meet the requirements of the International Air Transport Association (IATA) and meeting these requirements cannot be avoided by using a courier. IA-TA has requirements that are strictly enforced and not following IATA regulations can result in severe fines. If tissue specimens are to be shipped by air, someone in the tissue repository who is responsible for shipping must be trained in shipping according to IATA shipping standards (http://www.iata.org/Pages/default.aspx). Issues that must be addressed in air shipments of human tissues include biohazards as well as chemical hazards especially if tissues are shipped in alcohols or other flammable agents. IATA regulations also may change and the shipper is responsible for meeting new regulations.
8. Regulatory and ethical issues in tissue repositories
8.1. Informed consent and HIPAA authorization
Tissue repositories usually obtain remnant operative tissues that are not needed clinically. Such remnant tissues would otherwise be discarded or destroyed. In addition, samples of bodily fluids frequently may be obtained from consented patients and/or controls specifically to support research. For unneeded diagnostic remnants, the local IRB and Privacy Office determines whether or not the patients who are the source of these tissues should be consented for the use of their specimens in research and if HIPAA authorization should be obtained for the use of the patients’ protected health care information (PHI) in research. Alternatively, the local IRB and Privacy Office may elect to waive the requirement for informed consent and HIPAA authorization for some categories of patients [38,39].
Although when asked in the United States, typically at least 90% of patients agree to have their tissues and PHI used in biomedical research, informed consent is not easy to obtain because the personnel of the tissue repository usually have no clinical or other relationship with these patients. Personnel who do have a clinical relationship with the patients (e.g., surgical nurses or surgeons), have been demonstrated to be too busy to obtain informed consent for use of tissues in research not affecting modes of care. Also, if thousands of patients each year must be consented for one tissue organization, the resources needed to obtain such consents (e.g., money, personnel, etc.) may not be available. In addition, there is no optimal time or place for tissue repository personnel to obtain informed consent [5–8,40]. Obtaining consent upon admission can be problematic because patients may be overwhelmed at admission and may tend to sign a consent without understanding it. In the admission area, the clinic or in the preoperative area, there may be no space or time for personnel of the tissue repository to obtain consent. Also, many clinics operate simultaneously requiring multiple personnel at widely separated sites to be involved in the consent process. In contrast, postoperatively, patients may be on medications which may affect their ability to provide informed consent. In addition, patients may be discharged rapidly after surgery, on the same day of surgery or on weekends so that postoperative consent may be difficult to obtain within these constraints. Thus, there is no perfect time and place to obtain informed consent. If consent is obtained preoperatively, not all consented patients will have tissues collected; thus collecting consent postoperatively is more efficient because only patients from whom tissues are collected are consented [5–8,40]. Obtaining informed consent typically may require about 20 minutes per patient when the time to access the patient is included. Since thousands of patients per year may be sources of tissue, consenting individual patients is very expensive, usually requiring several full time highly trained personnel. Also, bias may be introduced into the tissue collection based on specific tissues that are not collected because consent cannot be obtained.
While obtaining informed consent, HIPAA authorization of patients in order to use their protected health care information in research also can be obtained; however, for tissue repositories, the description of the research may often be too vague, general or unknown to meet HIPAA requirements. Nevertheless, obtaining HIPAA authorization from patients to have their tissues and PHI put into a tissue bank is permitted. Subsequently, tissues and PHI may be provided to investigators either as de-identified or as a limited dataset neither of which requires HIPAA authorization for the investigators using the data.
Obtaining tissues from various racial and ethnic groups may be problematic based on social, cultural, and religious issues. Similarly, access to health care may affect tissue availability [41].
8.2. Cost recovery
It is illegal in most countries to buy or sell human tissues; however, it is legal and ethical to recover the costs of collecting, processing, storing and distributing tissues to biomedical researchers. Funds obtained as cost recovery charges significantly help in funding a tissue repository. A main consideration is to decide upon the proportion of costs to recover and subsequently, set a cost for each specimen. If there is support via grants or donations so a portion of the costs of a tissue repository can be covered, the costs to investigators of specimens may be reduced, otherwise, complete recovery of costs is necessary. Also, complete recovery should be obtained from for-profit entities. For example, because of its grant support, the CHTN is able to set its costs near that of experimental animals, but complete costs are accessed to for-profit organizations. Cost recovery should be part of a long-term budgeting plan so that a tissue repository can ensure its long-term survival and provide security to its personnel.
9. Safety
There are many potential sources of danger in a tissue repository, including biohazards and chemical hazards as well as hazards associated with physical, electrical and fire injuries [1,2,5–8,42–44]. To minimize these potential sources of injury, a tissue repository must develop a safety program. A safety program of the tissue repository can be stand alone or be a part of a safety program of the institution with which the tissue repository is associated. As part of the safety program, a safety committee and a safety officer are selected to work with the tissue repository. The safety committee and safety officer are responsible for developing the safety program, for ensuring training of personnel and for evaluating incidents related to safety. Their goal is to prevent any injuries, but especially the recurrence of specific injuries. Several articles can be used to help in developing a safety program [5–8,42–44].
9.1. Biohazards
A tissue repository must decide if it will collect tissues from patients who have diseases associated with major human blood borne pathogens (e.g., HIV, hepatitis B and C) or from patients who are at risk for being infected with other specific human pathogens (e.g., i.v. drug abusers or patients with tuberculosis). Many tissue repositories choose not to collect such tissues; nevertheless, any tissue repository might unknowingly supply investigators with infected tissue from a single case. Thus, it is important that a tissue repository require that its personnel as well as all personnel, internal or extramural, using the tissues it supplies be educated in biohazards. All personnel who handle tissues supplied by the tissue repository should agree to treat the tissue with universal precautions. It is also important that investigators/organizationsreceiving tissues sign an indemnification agreement, i.e., an agreement to hold the tissue repository not responsible for any injuries caused by the tissues or tissue products that are provided. Such an agreement is a standard component of a material transfer agreement (MTA). Also, the training of the personnel of the tissue repository in safety must be updated yearly. All personnel of the tissue repository in contact with human tissues should be offered vaccinations for hepatitis B.
9.2. Chemical hazards
Xylene and formaldehyde are two hazardous chemicals to which personnel of a tissue repository are likely to be exposed. These and other chemicals represent potential dangers either via direct contact or via irritating or toxic vapors. A safety program should be developed to minimize such exposure including the correct use of safety equipment. A component of this safety program is yearly education in chemical hazards and the maintenance of an inventory of all chemicals along with their material safety data sheets [43,44].
9.3. Other safety hazards
Protection against physical, fire and electrical hazards are also covered by the safety committee and safety officer. Issues of safety in these areas are handled just like biohazards and chemical hazards. Further information on laboratory safety covering these areas are beyond this chapter, but can be found in several sources [43,44].
10. Informatics
10.1. General issues in informatics
The tissue repository should select an informatics program based upon the goals and size of the repository. The performance of the informatics system should benefit the repository by saving time for its personnel. Data should be added easily to the database with few required fields and easy navigation. Also, retrieval of data and reports should be easy. For larger repositories, the informatics system should support labeling of specimens with barcodes as has been discussed previously. Most importantly, the organization of the database should mirror the business model of the repository; this means that the fields are organized according to the pattern in which specimens are typically collected, processed and stored. The fields of the database should parallel the history of each specimen and the fields should cover all the steps and important times associated with the collection, processing, storage and distribution of specimens.
10.2. Common data elements and vocabulary
The vocabulary of choice of a database which must frequently interact with investigators requesting solid tissues or bodily fluids is different from an informatics system which focuses primarily on banking of specimens and thus uses the diagnostic vocabulary used by pathologists. Specifically, an investigator may request EDTA plasma from African-American patients with any lung cancer; in contrast, pathologists seldom make a diagnosis of “lung cancer”, but rather pathological diagnoses usually are very specific – “well-differentiated squamous cell carcinoma of the lung”. Similarly, race and sex may not be a component of the diagnosis but must be incorporated into a typical request for tissues, and race may not be an available variable in a surgical pathology report. In addition, pathologists rarely deal with the diagnoses of patients from whom only fluids are collected, and even the clinical diagnosis may not be easily available to them as well as to personnel of the tissue repository. Thus, the approach to vocabulary of a tissue repository and hence its informatics system must be more flexible than that of a system relying on “diagnostic vocabulary” of the anatomic pathologist [12, 13].
10.3. Security
Informatics programs that contain patient identifiable health care information and ensure confidentiality must meet HIPAA security requirements including being installed on a secure server behind a firewall. HIPAA requirements are sufficient to ensure confidentiality. They include preventing unauthorized use of the database so that access is only via unique individual strong access codes or passwords. These passwords also should permit each user an approved specific type of access. For example, passwords of some repository personnel might permit them to read only, some would allow only read plus data entry, but other supervisory codes could permit editing of data. Administrative codes might permit modification of the fields of the database.
One of the major HIPAA requirements for an informatics system containing identified patient information is maintenance of an audit trail that records all individuals who access databases with identified patient information. We have found it useful to divide a database into two components that are stored on different servers. One is very limited and has the patient identity, hospital record number, specimen or surgical pathology number, birth date, signed consent forms, and other information including some of the 18 HIPAA pieces of information that might be able to identify an individual. This database is used to provide a unique identifier of the patient and specimen for use as a de-identified code. This is the database for which audit trails must be maintained and access to this relatively small database is limited to only administrative personnel except for new initial entry of data. The second database contains only de-identified data coded by the unique identifier provided by the identified database. This de-identified database is not under HIPAA security requirements, but should be under strict security to prevent unauthorized access; however, audit trails of “read only” are not necessary for a database containing only de-identified data.
10.4. caBIG
The NCI has been developing the caBIG program to provide consistent informatics approaches to cover most research activities, especially most work areas associated with NCI Comprehensive Cancer Centers. The goals include linking the databases of the various Comprehensive Cancer Centers so that investigators and cancer centers can communicate more efficiently with each other via an informatics grid. In the area of human tissues, a program, caTissue Suite, has been developed by caBIG which includes caTissue as an entry informatics program for use by tissue repositories (https://cabig.nci.nih.gov/tools/catissuesuite). This program is available at no cost. Similarly, an informatics program focused on investigator requests has been developed by the Cooperative Human Tissue Network for broad communication among large tissue repositories that serve many investigators. In the future, all NCI funded projects must be able to communicate via the grid of caBIG; however, caTissue Suite utilization is not required, only silver compatibility. caTissue Suite is continually being revised and improved.
10.5. Other NCI requirements
Based upon the National Cancer Institute Best Practices for Biospecimen Resources and the Best Practices of the International Society of Biological and Environmental Repositories [1–3], detailed records will be required to develop a “history” of each human tissue stored in a tissue repository. This will necessitate an informatics program which can incorporate such data. Also, a process of harmonization is likely to be required among tissue repositories so that activities such as collection of informed consent and HIPAA authorization are core elements of the design unless exempted by the local IRB. These changes are likely to be applied eventually to all repositories funded by any agency of the U.S. government.
11. Problems in meeting needs of tissue requests including the support of genomic studies
Access to national tissue repositories to obtain tissues for extensive genomic studies has been found to be problematic because of the nature of tissue banks. Usually each tissue bank has SOPs for banking specimens and the SOPs can be quite variable among banks. For example, an SOP from a bank may collect specimens that are 0.1 gram and at least 10% tumor and quality control may not be performed until a specimen is distributed. Thus, requests for specimens of tumor that are a minimum of 75% tumor and a minimum of 0.2 grams could not easily be met by such a bank, nor may some tumors develop with 75% tumor cellularity; thus, such requests frequently require some type of microdissection. For very demanding requests, prospective collection is an optimal approach, but it may require extensive resources to collect the needed specimens and it will require time to collect the specimens depending upon specimen requirements. In addition, factors such as the type and details of patient consent may prevent tissues from being used in genomic research, especially if the results of the genomic research are made public via published complete individual sequences rather than aggregate data.
Thus, when groups foresee that they will be requesting tissues and their requirements will be very demanding, the collection of such tissues is best done prospectively in collaboration with tissue repositories which are very experienced with the difficulties inherent in the collection of specific difficult to meet requests for tissues.
As discussed, some genomic requirements (75% tumor cells) are not possible to meet for several types of tumors, e.g., medullary carcinoma of the breast, as well as tumors for which only a few specimens may meet these requirements. Specifically, lymphocytic inflammation is a common feature of many tumors. Because lymphocytes are small and infiltrative, they frequently will comprise more than 25% of the overall tumor cellularity and may not be able to be separated from tumor cells unless laser capture microdissection is performed. Of note, requirements to exclude tumors with an inflammatory component may cause bias in a study with such requirements.
12. Challenges and new directions of tissue repositories
The developing challenges to the availability of human tissues to support biomedical research have been discussed previously in this document and include increasing use of neoadjuvant therapy, the decreasing size of tumors, the unavailability of metastatic tissues and of tissues from diseases not treated surgically, requirements to use all of a specific tissue for diagnosis and the use of fine needle aspirates for diagnosis. These challenges can be met partially by the increasing development of methods that can utilize paraffin embedded tissue such as extraction of mRNA from paraffin sections and its use in real time reverse transcriptase quantitative PCR (RT-Q-PCR) [17,18]. In addition, small specimens of tissue can be touched (blotted) on nitrocellulose paper prior to fixation without affecting the diagnostic usefulness of the tissue [19]. These blots can be frozen and then used subsequently for extraction of hundreds of nanograms of RNA for the analysis of potentially hundreds of genes by RT-Q-PCR as well as discovery of proteins associated with diseases. In addition, frozen sections of neoplastic lesions that are processed completely for diagnosis such as DCIS, could be used to exclude microinvasion and increase the availability of tissues for research. Warm autopsy programs can be developed as well as organized efforts to retrieve tissues removed because of transplantation and retrieval of normal tissues not used in transplantation. Also, patients can be requested to donate extra biopsies/fine needle aspirates obtained during their clinical evaluations to support research. For example, UAB has been successful in obtaining extra EUS-FNAs for research from patients evaluated for pancreatic cancer. Such an approach could also be successful for obtaining an extra biopsy from patients suspected of having metastatic disease. All these approaches require greatly increased resources for a tissue repository.
As part of the efforts of tissue repositories to obtain funding, their usefulness to biomedical research and investigators as well as to society needs to be “advertised” and explained [45]. Efforts in this area are being taken so that journal editors are being made more aware of the importance of the use of high quality tissues in research. Similarly, the public needs to be aware of the services provided by ethically operated tissue repositories and “scandals” need to be addressed by the tissue repository community. By participating in organizations such as ISBER (http://www.isber.org/) tissue repository organizations can increase their voices as well as support the acquisition of important operational information useful to tissue repositories and disseminate information on scientific issues concerning the acquisition, processing, storing and dissemination of tissues to support research. In addition, increased funding is necessary to clarify important issues in the science of repositories.
Acknowledgments
Support in part by the Early Detection Research Network (EDRN – 5U24CA086359), the Cooperative Human Tissue Network (CHTN – 5U01CA44968), the Skin Diseases Research Center (P30AR50948-07), the NCI Specialized Programs of Research Excellence (SPORE – Pancreas – P20CA101955), (SPORE –Breast – 5P50CA89019), NIH-NCI CCC Core Support Grant-Tissue Procurement 5P30CA13148-39 and the PAH Transplant and Preparation Center and the Center for Tissue Processing to Support Research in Pulmonary Arterial Hypertension through the Pulmonary Hypertension Breakthrough Initiative (PHBI).
References
- 1.Aamodt RL, Anouna A, Baird P, Beck JC, Bledsoe MA, DeSouza Y, Grizzle WE, Gosh J, Holland NT, Hakimian R, Michels C, Pitt KE, Sexton KC, Shea K, Stark A, Vaught J. Best practices for repositories I: collection, storage and retrieval of human biological materials for research. Cell Preservation Tech. 2005;3(5):48. [Google Scholar]
- 2.Pitt K, Campbell L, Skubitz A, Somiari S, Sexton K, Pugh R, Aamodt R, Baird P, Betsou F, Cohen L, De Souza Y, Gaffney E, Geary P, Grizzle WE, Gunter E, Horsefall D, Kessler J, Michels C, Kaercher E, Morales O, Morente M, Morrin H, Petersen G, Robb J, Seberg O, Thomas J, Thorne H, Walters C, Riegman P. Best practices for repositories: collection, storage, retrieval and distribution of biological materials for research, Second Edition. Cell Preservation Technology. 2008;6(1):5–58. [Google Scholar]
- 3.NCI. National Cancer Institute Best Practices for Biospecimen Resources. 2007 http://biospecimens.cancer.gov/global/pdfs/NCI_Best_Practices_060507.pdf.
- 4.Grizzle WE, Woodruff KH, Trainer TD. The Pathologist’s Role in the Use of Human Tissues in Research – Legal, Ethical, and Other Issues. Arch Pathol Lab Med. 1996;120(10):909–912. [PubMed] [Google Scholar]
- 5.Grizzle WE, Aamodt R, Clausen K, LiVolsi V, Pretlow TG, Qualman S. Providing Human Tissues for Research: How to Establish A Program. Arch Pathol Lab Med. 1998;122(12):1065–1076. [PubMed] [Google Scholar]
- 6.Grizzle WE, Sexton KC. Development of a Facility to Supply Human Tissues to Aid in Medical Research. In: Srivastava S, Henson DE, Gazdar A, editors. Molecular Pathology of Early Cancer. Chapter 24. IOS Press; Amsterdam, Netherlands: 1999. pp. 371–383. [Google Scholar]
- 7.Grizzle WE, Bell W, Sexton KC. Best practices and challenges in collecting and processing human tissues to support biomedical research. AACR 96th Annual Meeting, Education Book; 2005. pp. 305–310. [Google Scholar]
- 8.Bell WC, Sexton KC, Grizzle WE. Organizational issues in providing high-quality human tissues and clinical information for the support of biomedical research, In. In: Grützmann R, Pilarsky C, editors. Methods Mol Biol. Vol. 576. 2010. pp. 1–30. [DOI] [PubMed] [Google Scholar]
- 9.Qualman SJ, France M, Grizzle WE, LiVolsi VA, Moskaluk CA, Ramirez NC, Washington MK. Establishing a tumour bank: banking, informatics and ethics. Br J Cancer. 2004;90(6):1115–1119. doi: 10.1038/sj.bjc.6601678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clausen KC, Grizzle WE, LiVolsi VA, Newton WE. Availability of Human Tissues for Research in Cancer. Science. 1987;237(4810):10–11. [PubMed] [Google Scholar]
- 11.Clausen KP, Grizzle WE, Livolsi V, Newton WA, Aamodt R. The Cooperative Human Tissue Network. Cancer. 1989;63(7):1452–1455. doi: 10.1002/1097-0142(19890401)63:7<1452::aid-cncr2820630736>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Edgerton ME, Morrison C, LiVolsi VA, Moskaluk CAA, Qualman SJ, Washington MK, Grizzle WE. A Standards Based Ontological Approach to Information Handling for Use by Organizations Providing Human Tissue for Research. Cancer Informatics. 2008;6:127–136. [PMC free article] [PubMed] [Google Scholar]
- 13.Edgerton ME, Grizzle WE, Washington MK. The Deployment of a Tissue Request Tracking System for the CHTN: A Case Study in Managing Change In Informatics for Biobanking Operations. BMC Med Inform Decis Mak. 2010 Jun 2;10:32. doi: 10.1186/1472-6947-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grizzle WE, Sexton KC, Bell WC. Quality Assurance in Tissue Resources Supporting Biomedical Research. Cell Preservation Technology. 2008;6(2):113–118. doi: 10.1089/cpt.2008.9993.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhieng DC, Rodriguez-Burford C, Meleth S, Grizzle WE, Ferguson SM. Assessment of biomarker expression in predicting pathologic response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Breast J. 2007;13(5):534–535. doi: 10.1111/j.1524-4741.2007.00483.x. [DOI] [PubMed] [Google Scholar]
- 16.Bell WC, Young ES, Billings PE, Grizzle WE. The efficient operation of the surgical pathology gross room. Biotech Histochem. 2008;83(2):71–82. doi: 10.1080/10520290802127610. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steg A, Wang W, Blanquicett C, Grunda JM, Eltoum IA, Wang K, Buchsbaum DJ, Vickers SM, Russo S, Diasio RB, Frost AR, Grizzle WE, Johnson MR. Multiple gene expression analyses in paraffin-embedded tissues by Taqman low density array: application to Hedgehog and Wnt pathway analysis in ovarian endometrioid adenocarcinoma. J Mol Diagn. 2006;8(1):76–83. doi: 10.2353/jmoldx.2006.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steg A, Vickers SM, Eloubeidi M, Wang W, Eltoum IA, Grizzle WE, Saif MW, Lobuglio AF, Frost AR, Johnson MR. Hedgehog pathway expression in heterogeneous pancreatic adenocarcinoma: implications for the molecular analysis of clinically available biopsies. Diagn Mol Pathol. 2007;16(4):229–237. doi: 10.1097/PDM.0b013e31811edc7e. [DOI] [PubMed] [Google Scholar]
- 19.Gaston SM, Soares MA, Siddiqui MM, Vu D, Lee JM, Goldner DL, Brice MJ, Shih JC, Upton MP, Perides G, Baptista J, Lavin PT, Bloch BN, Genega EM, Rubin MA, Lenkinski RE. Tissue-print and print-phoresis as platform technologies for the molecular analysis of human surgical specimens: mapping tumor invasion of the prostate capsule. Nat Med. 2005;11(1):95–101. doi: 10.1038/nm1169. [DOI] [PubMed] [Google Scholar]
- 20.Spruessel A, Steinmann G, Jung M, Lee SA, Carr T, Fentz AJ, Spangenberg J, Zornig C, Juhl HH, David KA. Tissue ischemia time affects gene and protein expression patterns within minutes following surgical tumor excision. Biotechniques. 2004;36(6):1030–1037. doi: 10.2144/04366RR04. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Qi R, Quackenbush J, Dauway E, Lazaridis E, Yeatman T. Effecs of ischemia on gene expression. J Surg Res. 2001;99:222–227. doi: 10.1006/jsre.2001.6195. [DOI] [PubMed] [Google Scholar]
- 22.Dash A, Maine IP, Varambally S, Shen R, Chinnaiyan M, Rubin MA. Changes in differential gene expression because of warm ischemia time of radical prostatectomy specimens. Am J Pathol. 2002;161(5):1743–1748. doi: 10.1016/S0002-9440(10)64451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jewell SD, Srinivasan M, McCart LM, Williams N, Grizzle WE, LiVolsi V, MacLennan G, Sedmak DD. Analysis of the molecular quality of human tissues: An experience from the Cooperative Human Tissue Network. Am J Clin Pathol. 2002;118(5):733–741. doi: 10.1309/VPQL-RT21-X7YH-XDXK. [DOI] [PubMed] [Google Scholar]
- 24.Baker AF, Dragovich T, Ihic NT, William R, Fernogilio-Presiser C, Powis G. Stability of phosphoprotein as a biological marker of tumor signaling. Clin Cancer Res. 2005;11(12):4338–4340. doi: 10.1158/1078-0432.CCR-05-0422. [DOI] [PubMed] [Google Scholar]
- 25.Ayala G, Thompson T, Yang G, Frolov A, Li R, Scardino P, Ohori M, Wheeler T, Harper W. High levels of phosphorylated form of Akt-1 in prostate cancer and non-neoplastic prostate tissues are strong predictors of biochemical recurrence. Clin Cancer Res. 2004;10:6572–6578. doi: 10.1158/1078-0432.CCR-04-0477. [DOI] [PubMed] [Google Scholar]
- 26.Grizzle WE, Semmes OJ, Bigbee W, Zhu L, Malik G, Oelschlager DK, Manne B, Manne U. The need for the review and understanding of SELDI/MALDI mass spectroscopy data prior to analysis. Cancer Informatics. 2005;1(1):86–97. [PMC free article] [PubMed] [Google Scholar]
- 27.Grizzle WE, Semmes OJ, Bigbee WL, Malik G, Miller E, Manne B, Oelschalger DK, Zhu L, Manne U. Use of mass spectrographic methods to identify disease processes. In: Patrinos G, Ansorg W, editors. Molecular Diagnostics. Chapter 17. 2005. pp. 211–222. [Google Scholar]
- 28.McLerran D, Grizzle WE, Feng Z, Thompson IM, Bigbee WL, Cazares LH, Chan DW, Dahlgren J, Diaz J, Kagan J, Lin DW, Malik G, Oelschlager D, Partin A, Randolph TW, Sokoll L, Srivastava S, Srivastava S, Thornquist M, Troyer D, Wright GL, Zhang Z, Zhu L, Semmes OJ. SELDI-TOF MS Whole Serum Proteomic Profiling with IMAC Surface Does Not Reliably Detect Prostate Cancer. Clin Chem. 2008;54(1):53–60. doi: 10.1373/clinchem.2007.091496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLerran D, Grizzle WE, Feng Z, Bigbee WL, Banez LL, Cazares LH, Chan DW, Diaz J, Izbicka E, Kagan J, Malehorn DE, Malik G, Oelschlager D, Partin A, Randolph T, Rosenzweig N, Srivastava S, Srivastava S, Thompson IM, Thornquist M, Troyer D, Yasui Y, Zhang Z, Zhu L, Semmes OJ. Analytical Validation of Serum Proteomic Profiling for Diagnosis of Prostate Cancer: Sources of Sample Bias. Clin Chem. 2008;54(1):44–52. doi: 10.1373/clinchem.2007.091470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grizzle W. Special symposium: fixation and tissue processing models. Biotech Histochem. 2009 Jun;22:1–9. doi: 10.3109/10520290903039052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otali D, Stockard CR, Oelschlager DK, Wan W, Manne U, Watts SA, Grizzle WE. Combined effects of formalin fixation and tissue processing on immunorecognition. Biotech Histochem. 2009;84(5):223–247. doi: 10.3109/10520290903039094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grizzle WE, Fredenburgh J, Myers RB. Fixation of Tissues. In: Bancroft J, Gamble M, editors. Theory and Practice of Histological Techniques. 6. Chapter 4. Churchill Livingstone; Edinburgh, United Kingdom: 2007. pp. 53–74. [Google Scholar]
- 33.Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, Rom W, Sanda M, Sorbara L, Stass S, Wang W, Brenner DE. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009 Jan;8(1):113–117. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grizzle WE, Srivastava S, editors. Translational Pathology of Early Cancer. IOS Press; BV, Amsterdam, The Netherlands: [Google Scholar]
- 35.Grizzle WE, Srivastava S, Manne U. Translational pathology of neoplasia. Cancer Biomark. 2011;9:7–20. doi: 10.3233/CBM-2011-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell WC, Sexton KC, Grizzle WE. How to efficiently obtain human tissues to support specific biomedical research projects. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1676–1679. doi: 10.1158/1055-9965.EPI-08-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ransohoff DF. Promises and limitations of biomarkers. Recent Results Cancer Res. 2009;181:55–59. doi: 10.1007/978-3-540-69297-3_6. Review. [DOI] [PubMed] [Google Scholar]
- 38.Aamodt R, Barnes M, Bledsoe MJ, Bernstein S, Carr S, Goddard A, Grizzle W, Hakimian R, Hammond E, Kathi H, Heath E, Hohmann EL, Hojvat S, Hull S, Johnson OM, Kaneshiro J, Korn D, Kornetsky SZ, Lee BM, LePay D, Levine RJ, Marks E, McQuillan G, Muhlbaier LH, Murphy J, Murphy S, O’Rourke PP, Perotti J, Porter JP, Rasooly R, Rose SL, Selwitz AS, Sobel M, Terry S, Wandall HBM. The PRIM&R Tissue Working Group Report, Section II. 2008. Section II Tools for Investigators, IRBs and Repository Managers. [Google Scholar]
- 39.Aamodt R, Barnes M, Bledsoe MJ, Bernstein S, Carr S, Goddard A, Grizzle W, Hakimian R, Hammond E, Kathi Hanna, Heath E, Hohmann EL, Hojvat S, Hull S, Johnson OM, Kaneshiro J, Korn D, Kornetsky SZ, Lee BM, LePay D, Levine RJ, Marks E, McQuillan G, Muhlbaier LH, Murphy J, Murphy S, O’Rourke PP, Perotti J, Porter JP, Rasooly R, Rose SL, Selwitz AS, Sobel M, Terry S, Wandall HBM. The PRIM&R Human Tissue/Specimen Banking Working Group. 2007. Report of the Public Responsibility in Medicine and Research. [Google Scholar]
- 40.Hewitt R, Watson PH, Dhir R, Aamodt R, Thomas G, Mercola D, Grizzle WE, Morente MM. Timing of consent for the research use of surgically removed tissue: is postoperative consenting acceptable? Cancer. 2009;115(1):4–9. doi: 10.1002/cncr.23999. [DOI] [PubMed] [Google Scholar]
- 41.Carpenter WR, Godley PA, Clark JA, Talcott JA, Finnegan T, Mishel M, Bensen J, Rayford W, Su LJ, Fontham ETH, Mohler JL. Racial differences in trust and regular source of patient care and the implications for prostate cancer screening use. Cancer. 2009;115:5048–5059. doi: 10.1002/cncr.24539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grizzle WE, Fredenburgh J. Avoiding biohazards in medical, veterinary and research laboratories. Biotech Histochem. 2001;76(4):183–206. [PubMed] [Google Scholar]
- 43.Grizzle WE, Bell W, Fredenburgh J. Safety in biomedical and other laboratories. In: Patrinos G, Ansorg W, editors. Molecular Diagnostics. Chapter 33. 2005. pp. 421–428. [Google Scholar]
- 44.Grizzle WE, Bell WC, Fredenburgh J. General Considerations Concerning Safety in Biomedical Research Laboratories. In: Patrinos GP, Ansorg W, editors. Molecular Diagnostics. 2. Chapter 39. 2010. pp. 563–572. [Google Scholar]
- 45.Betsou F, Rimm DL, Watson PH, Womack C, Hubel A, Coleman RA, Horn L, Terry SF, Zeps N, Clark BJ, Miranda LB, Hewitt RE, Elliott GD. What are the biggest challenges and opportunities for biorepositories in the next three to five years? Biopreservation and Biobanking. 2010;8(2):81–88. doi: 10.1089/bio.2010.8210. [DOI] [PubMed] [Google Scholar]



