Abstract
Loss of intestinal barrier function after burn injury allows movement of intraluminal contents across the mucosa, which can lead to the development of distant organ injury and multiple organ failure. Tight junction function is highly regulated by membrane-associated proteins including occludin and zonula occludens protein 1 (ZO-1), which can be modulated by systemic inflammation. We hypothesized that (1) burn injury leads to gut barrier injury, and (2) phosphodiesterase inhibition will attenuate these burn-induced changes. Male balb/c mice undergoing a 30% steam burn were randomized to resuscitation with normal saline or normal saline + pentoxifylline (PTX; 12.5 mg/kg). Intestinal injury was assessed by histological diagnosis and TNF-α levels using enzyme-linked immunosorbent assay. Intestinal permeability was assessed by measuring the plasma concentration of fluorescein isothiocyanate–dextran after intraluminal injection in the distal ileum. Occludin and ZO-1 levels were analyzed by immunoblotting and immunohistochemistry. Thirty percent total body surface area (TBSA) burn results in a significant increase in intestinal permeability. Treatment with PTX after burn attenuates intestinal permeability to sham levels. Burn injury resulted in a marked decrease in the levels of tight junction proteins occludin and ZO-1 at 6 and 24 h. The use of PTX after burn significantly decreases the breakdown of occludin and ZO-1. Pentoxifylline also attenuates the burn-induced increase in plasma and intestinal TNF-α. Confocal microscopy demonstrates that PTX attenuates the burn-induced reorganization of occludin and ZO-1 away from the tight junction. Pentoxifylline attenuates burn-induced intestinal permeability and decreases the breakdown and reorganization of intestinal occludin and ZO-1. Therefore, phosphodiesterase inhibition may be a useful adjunct strategy in the attenuation of burn-induced gut barrier injury.
Keywords: Pentoxifylline, occludin, ZO-1, TNF-α, intestinal permeability, confocal microscopy, histological injury, shock
INTRODUCTION
Severe burn injury can result in a systemic inflammatory response syndrome (SIRS) leading to distant organ injury and multiple organ failure (MOF). Due to the high mortality associated with MOF, intensive research efforts have been ongoing, investigating ways to modulate the inflammatory response after trauma and burn. It has been hypothesized that the gut may play a central role in the development of SIRS after shock (1). Bacterial translocation across the intestinal mucosa and into the portal vein was initially believed to be the inciting event in distant organ injury (2). However, studies were unable to document the presence of bacteria or endotoxin in the portal vein (3). Recent research indicates that gut-derived proinflammatory mediators, which travel through the intestinal lymphatics, may be responsible for amplifying the systemic response (4).
An intact intestinal epithelium is required to maintain an effective barrier against luminal bacteria and endotoxin that normally inhabits the gut. The tight junction is a highly regulated structure that defines the overall permeability of the intestinal epithelium. Disruption of the tight junction results in increased intestinal permeability, allowing luminal products to access normally protected layers of the intestine. The intestinal tight junction is maintained, in part, by the structural proteins occludin and zonula occludens protein 1 (ZO-1).
Occludin is a 65-kd protein containing four membrane-spanning segments that extend into the extracellular space, connecting adjacent cells at the tight junction (5). The cytoplasmic extension of occludin interacts with the tight junction protein ZO-1 and links it to the actin cytoskeleton (6). Proinflammatory stimuli have been shown to increase permeability at the tight junction and are associated with downregulation and altered localization of occludin and ZO-1 (7, 8). Burn injury has previously been shown to decrease intestinal occludin protein levels, whereas the effects of burn on ZO-1 levels and the cellular localization of these tight junction proteins are unknown (9).
Phosphodiesterase inhibition with pentoxifylline (PTX) has previously been shown to decrease histological gut injury and intestinal proinflammatory cytokine synthesis in models of hemorrhagic shock (10, 11). We have also observed that phosphodiesterase inhibition prevents the breakdown of the tight junction proteins occludin and ZO-1 in an in vitro model of immunostimulated intestinal epithelial cells (unpublished data). In this study, we further our investigation into the effects of phosphodiesterase inhibition on tight junction structural proteins. We postulate that phosphodiesterase inhibition with PTX will decrease gut barrier injury by attenuating the breakdown and altered localization of the tight junction proteins occludin and ZO-1 in a murine model of severe cutaneous burn injury.
MATERIALS AND METHODS
These experiments were approved by the University of California Animal Subjects Committee and are in accordance with guidelines established by the National Institutes for Health.
Experimental model
Male balb/c mice (20–24 g) were purchased from Jackson Laboratory (Sacramento, Calif). A 12-h light-dark cycle was instituted. Animals were anesthetized using inhaled isoflurane. The dorsal fur was removed using an electric clipper. A template was made to estimate a 30% total body surface area. Animals were then placed in the template and were subjected to a steam burn for 7 seconds. After burn, animals were randomized to receive an i.p. injection of PTX (12.5 mg/kg; Sigma, St Louis, Mo) dissolved in 500 μL normal saline (NS) or NS alone. All animals received a subcutaneous injection of 1.5 mL NS with buprenorphine in a nonburned area for fluid resuscitation and analgesia. Sham animals underwent induction of anesthesia, clipping of dorsal fur, i.p. injection of NS, and subcutaneous injection of buprenorphine in NS but were not subjected to burn. After the experiment, animals were returned to their cages and allowed to recover from anesthesia. They were provided food and water ad libitum.
Tissue procurement
At 2, 6, or 24 h after burn, animals were once again anesthetized with isoflurane. Animals were exsanguinated using an axillary artery transaction, and blood was collected into Eppendorf tubes and placed on ice. Serum was centrifuged at 10,000g for 10 min, and the plasma was removed and stored at −70°C. The distal ileum was immediately harvested through a midline laparotomy, snap frozen in liquid nitrogen, and stored at −70°C for later analysis. Samples of ileum were also preserved in both formalin and optimal cutting temperature embedding media (Sakura Finetek, Torrance, Calif) for later histological analysis.
Histopathologic evaluation
Segments of distal ileum (n ≥ 3 per group) were stored in 10% phosphate-buffered saline (PBS)–buffered formalin and embedded in paraffin blocks using an automated processor. Seven-micrometer sections were cut and placed onto glass slides and stained with hematoxylineosin (Richard-Allan Scientific, Waltham, Mass). A pathologist (P.W.) blinded to the experimental groups evaluated each sample for presence of intestinal injury. Images were obtained using an Olympus IX70 light microscope (Olympus, Melville, NY) at 20× magnification with Q-imaging software (Q-imaging, Surrey, British Columbia, Canada).
In vivo intestinal permeability assay
An in vivo intestinal permeability assay was performed based on the method previously described by Chen et al. (12). Four hours after burn, animals from each experimental group (n ≥ 5) were anesthetized with isoflurane. A midline laparotomy was performed, and a 5-cm segment of distal ileum was isolated between silk ties. Care was taken to ensure adequate blood supply to the isolated segment of intestine. Two hundred microliters of PBS solution containing 25 mg of 4.4-kd fluorescein isothiocyanate (FITC)–dextran (Sigma) was injected intraluminally. The intestine was returned to the abdominal cavity, and the skin was closed. Thirty minutes after injection of FITC-dextran, blood was obtained via cardiac puncture and placed on ice. Blood was centrifuged at 10,000g for 10 min, and the plasma was removed. The plasma was then analyzed for FITC-dextran concentration using a SpectraMax M5 fluorescence spectrophotometer (Molecular Devices, Sunnyvale, Calif). A standard curve was obtained by diluting serial concentrations of FITC-dextran in mouse serum.
TNF-α levels
Plasma and intestinal TNF-α levels (n ≥ 4 per group) were measured 2 h after burn injury using a commercially available sandwich enzyme immunoassay technique (enzyme-linked immunosorbent assay [ELISA]; R&D Systems, Minneapolis, Minn). Intestinal TNF-α was measured from whole-cell protein extracts from the distal ileum. TNF-α levels were measured in picogram per milliliter.
Immunoblotting
Samples of distal ileum harvested at 2, 6, and 24 h after burn (n ≥ 4 per group) were homogenized in 500 μL ice-cold tissue protein extraction reagent with 1% protease inhibitor and 1% phosphatase inhibitor (Pierce Biotechnology, Rockford, Ill). Homogenates were centrifuged at 10,000g for 5 min. Supernatants were collected, and the protein concentration of each sample was determined using the bicinchoninic acid protein assay (Pierce) using a microplate reader protocol. Samples containing 10 μg of protein were suspended in sodium dodecyl sulfate (SDS) sample buffer (Invitrogen, Carlsbad, Calif) and were boiled at 100°C for 5 min. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis using 6% (ZO-1) or 8% to 16% (occludin, β actin) Trisglycine polyacrylamide gradient gels and subsequently transferred to nitrocellulose membranes (Invitrogen). The membranes were blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline (TBS)/Tween 20 for 1 h. Primary antibodies specific for ZO-1 (1:500; Zymed, Carlsbad, Calif), occludin (1:500; Santa Cruz Biotechnology, Santa Cruz, Calif), or β actin (1:500; Cell Signaling, Danvers, Mass) were incubated with the membranes overnight at 4°C in 5% BSA with Tris-buffered saline/Tween 20. Membranes were washed and incubated for 1 h at room temperature with the secondary antibody horseradish peroxidase–linked antirabbit IgG (1:2000; Cell Signaling) prepared in blocking solution. After thorough washing, the Pierce Supersignal West Pico Chemiluminescent Kit was applied for antibody detection with x-ray film (Amersham Biosciences, Piscataway, NJ). Mean pixel density was estimated using UN-SCAN-IT Gel Digitizing software (Silk Scientific, Orem, Utah). Data are expressed as the relative band density from Western blots. Relative band density was calculated by dividing the pixel density of each sample by the mean pixel density of sham samples.
Confocal microscopy
Samples of distal ileum from each experimental group (n ≥ 3 per group) were fixed in OCT and stored at −70°C for later analysis. Ten-micrometer sections were cryosectioned using the Reichert-Jung Cryocut 1800 (Reichert Microscopes, Depew, NY). Sections of ileum were fixed onto the glass slides with 3.7% paraformaldehyde (Electron Microscopy Series, Hatfield, Pa) for 10 min. Glass slides were washed with PBS, then cells were permeabilized with 0.01% Triton X-100 (Sigma) in PBS for 1 min. After washing with PBS, sections were blocked for 1 h using 3% BSA. Sections were incubated overnight with the primary antibody (1:100) in 1% BSA. After washing with PBS, the sections were treated with the secondary antibody, Alexa Fluor 488 (1:100; Invitrogen), in 1% BSA for 1 h. Cover slips were placed onto glass slides after the addition of Prolong Fade (Invitrogen). Glass slides were allowed to cure overnight in the dark. Images were viewed using an Olympus Fluoroview laser scanning confocal microscope (Olympus) at 60× magnification with a 2.3× zoom.
Statistical analysis
Values are expressed as the mean ± SEM of n observations, where n represents the number of animals in each experimental group. Each assay was performed in duplicate where appropriate. The statistical significance among groups was determined using ANOVA with Bonferroni correction. A P < 0.05 was considered statistically significant.
RESULTS
Histopathologic evaluation
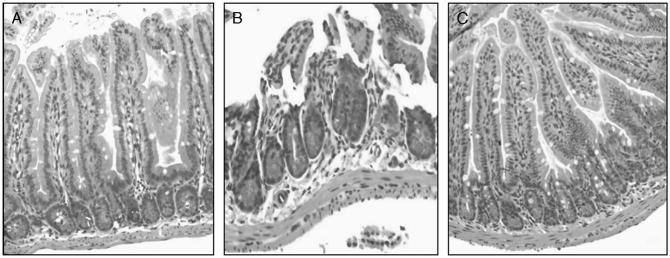
Samples of distal ileum harvested at 6 h after burn were stained with hematoxylineosin and were reviewed by a pathologist blinded to the experimental conditions (Fig. 1). The histopathologic appearance of the distal ileum of burned animals given NS demonstrated marked blunting and necrosis of the intestinal villi. Burned animals treated with PTX exhibited a histological appearance similar to sham, with minimal damage to the villi and no necrosis.
Fig. 1. Histological gut injury after burn.
Samples of distal ileum were harvested 6 h after 30% steam burn (n ≥ 3 per group). A, Normal-appearing distal ileum from sham animals. B, Distal ileum from burned animals that received NS. Destruction of villi and epithelial necrosis is visualized. C, Distal ileum from burned animals treated with PTX. Note the similar appearance to sham animals with normal-appearing villi. Images viewed at 20× magnification.
In vivo intestinal permeability assay
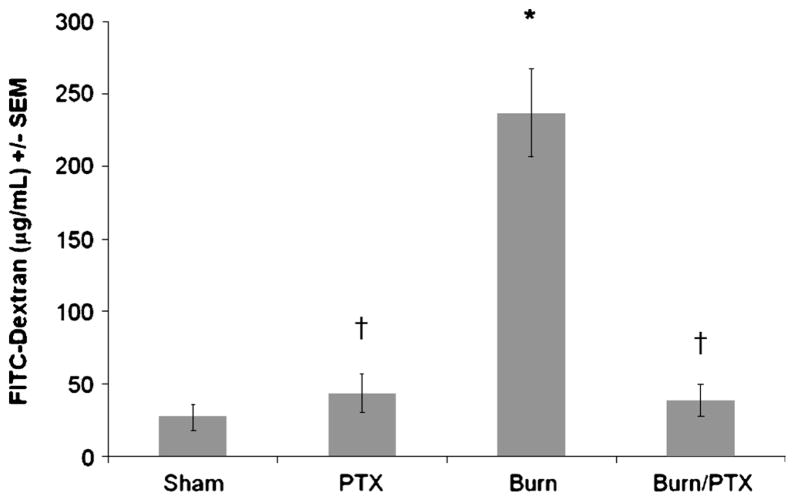
Intestinal permeability was assessed 4 h after 30% TBSA burn by measuring the systemic plasma concentration of intraluminally injected 4.4-kd FITC-dextran (Fig. 2). Compared with sham, there is a significant increase in intestinal permeability in burned animals receiving NS (237 ± 31 vs. 27 ± 9 μg/mL; P < 0.001). Treatment with PTX after burn reduces intestinal permeability to sham levels (38 ± 11 μg/mL; P < 0.001 versus burn). There was no increase in intestinal permeability in sham animals that received PTX (43 ± 13 μg/mL).
Fig. 2. Phosphodiesterase inhibition with PTX attenuates burn-induced intestinal permeability.
Intestinal permeability assessed by measuring FITC-dextran in the systemic circulation after intraluminal injection of 4.4-kd FITC-dextran 4 h after 30% TBSA burn (n ≥ 5 per group). There is a significant increase in intestinal permeability in burned animals treated with NS. Treatment with PTX decreases intestinal permeability to sham levels. *P < 0.001 versus sham; †P < 0.001 versus burn.
Plasma TNF-α levels
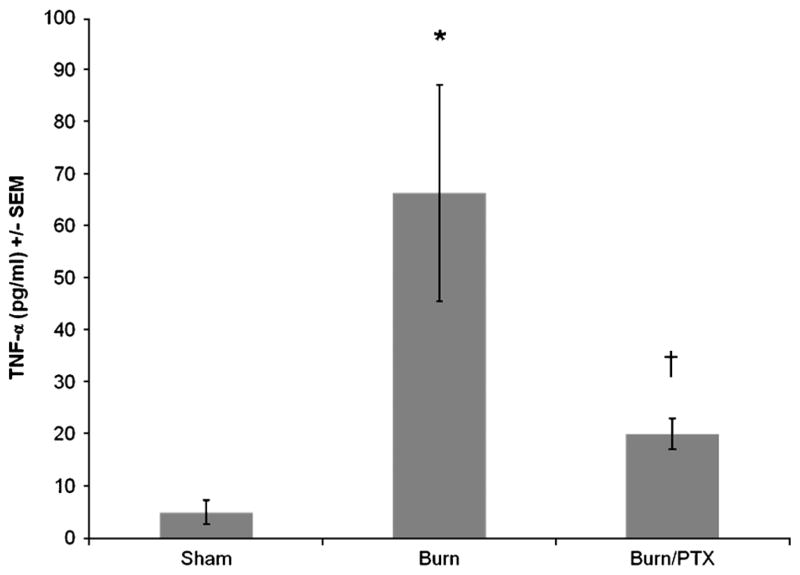
TNF-α is a proximal mediatory of the inflammatory cascade that increases rapidly after proinflammatory stimuli. Therefore, plasma TNF-α levels were measured 2 h after burn (Fig. 3). Burned animals treated with NS had a rapid increase in plasma TNF-α levels (66.4 ± 20.8 pg/mL; P < 0.02 versus sham). Treatment with PTX after burn resulted in a 70% decrease in plasma TNF-α compared with burned animals treated with NS (P < 0.05), with TNF-α levels similar to sham.
Fig. 3. Effects of PTX on plasma TNF-α levels 2 h after burn.
TNF-α concentration determined using ELISA (n ≥ 4 per group). Data are presented as percentage of TNF-α levels in animals receiving NS after burn. Pentoxifyl-line resulted in 70% decrease in plasma TNF-α levels compared with animals treated with NS. *P < 0.02 versus sham; †P < 0.05 versus burn.
Intestinal TNF-α levels
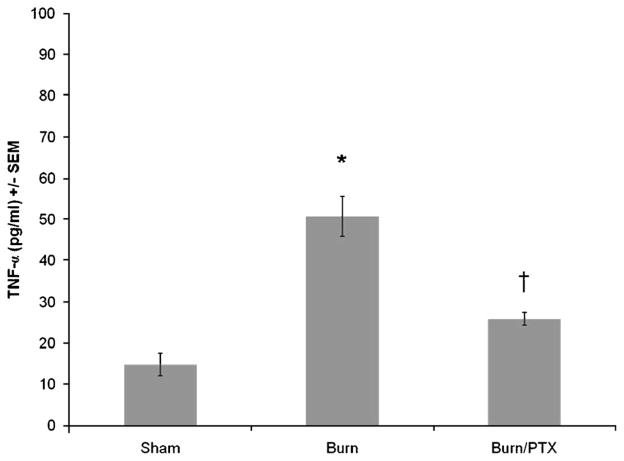
TNF-α from distal ileum extracts was assessed 2 h after burn to analyze the local inflammatory conditions affecting the intestinal tight junction (Fig. 4). Intestinal TNF-α levels were significantly increased in burned animals given NS (50.9 ± 4.9 pg/mL; P < 0.001 versus sham). Treatment with PTX decreased intestinal TNF-α levels by 35% compared with burn alone (26.0 ± 1.6 pg/mL; P < 0.001 versus burn). Minimal TNF-α was measured from sham animals (14.8 ± 2.8 pg/mL).
Fig. 4. Pentoxifylline decreases burn-induced increase in intestinal TNF-α levels 2 h after burn.
TNF-α concentration from distal ileum intestinal extracts determined using ELISA (n ≥ 4 per group). Treatment with PTX after burn resulted in a 45% decrease in intestinal TNF-α levels compared with burned animals treated with NS. *P < 0.001 versus sham; †P < 0.001 versus burn.
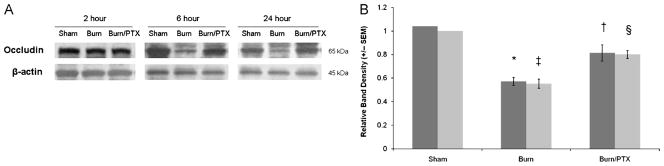
Intestinal occludin levels
Occludin is a transmembrane protein that links adjacent cells at the tight junction and is involved in regulating permeability. Occludin protein levels from the distal ileum were measured at 2, 6, and 24 h after burn (Fig. 5). In burned animals treated with NS, occludin levels decreased 43% compared with sham (P < 0.05) at 6 h after burn. This decrease in occludin protein remained at 24 h (P < 0.02). Treatment with PTX after burn resulted in a 19% decrease in occludin protein compared with sham at 6 h, which was also seen at 24 h. There was no significant decrease in occludin protein levels at 2 h in either group.
Fig. 5. Intestinal occludin levels after burn.
A, Representative Western blots for occludin at 2, 6, and 24 h after burn. B, Graph represents relative band densities from Western blots of distal ileum after burn (n ≥ 4 per group). Results from the 6-h group in dark gray and the 24-h group in light gray. Occludin levels decreased approximately 50% in burned animals given NS. Decreased intestinal occludin protein breakdown was seen in burned animals treated with PTX. *P < 0.05 versus sham; †P < 0.05 versus burn at 6 h; ‡P < 0.002 versus sham; §P < 0.02 versus burn at 24 h.
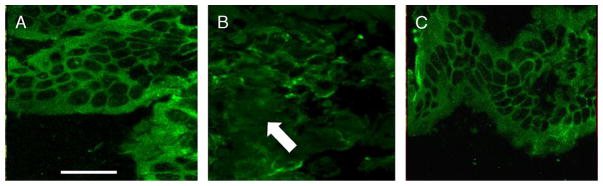
Cellular localization of intestinal occludin
Confocal microscopy images of distal ileum from sham animals show a smooth, continuous pattern of occludin staining in areas of cell-cell contact at the periphery of the cell (Fig. 6A). Burn animals exhibit a more disordered pattern of occludin staining. The continuous pattern is lost, and occludin no longer clearly delineates areas of cell-cell interaction (Fig. 6B). Burned animals treated with PTX have a pattern of occludin localization similar to control (Fig. 6C). Occludin staining in the distal ileum from these animals is much smoother, with a more ordered distribution at the periphery of the cell.
Fig. 6. Confocal microscopy images of distal ileum stained for occludin (n ≥ 3 per group).
A, Representative image from sham animal showing smooth, even distribution of occludin at areas of cell-cell contact. B, Burn injury alters the localization of occludin, with a more disordered appearance (arrow) and decreased staining at the periphery of the cell. C, Pentoxifylline improves the burn-induced alteration in occludin staining, with a more organized distribution at areas of cell-cell contact. Size bar = 20 μm.
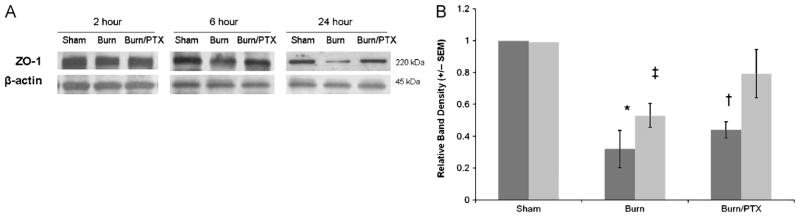
Intestinal ZO-1 levels
Zonula occludens protein 1 is a 220-kd tight junction protein that links the transmembrane protein occludin to the actin cytoskeleton within the apical portion of the cell. Zonula occludens protein 1 protein levels were analyzed from sample of distal ileum at 2, 6, and 24 h after burn (Fig. 7). At 6 h after burn, ZO-1 levels decreased 68% (P < 0.05) in NS-treated animals, whereas ZO-1 levels decreased by 47% (P < 0.02) in PTX-treated animals when compared with sham. At 24 h after burn, ZO-1 levels decreased by only 21% in PTX-treated animals, whereas a significant decrease remained in animals given NS (P < 0.01 versus sham).
Fig. 7. Intestinal ZO-1 levels after burn.
A, Representative Western blots for ZO-1 at 2, 6, and 24 h after 30% TBSA burn. B, Graph represents relative band densities from Western blots of ZO-1 protein at 6 h (dark gray) and 24 h (light gray) after burn (n ≥ 4 per group). Treatment with PTX after burn results in a 12% and 26% improvement in gut ZO-1 levels compared with NS at 6 and 24 h, respectively. *P < 0.05 versus sham; †P < 0.02 versus sham; ‡P < 0.01 versus sham.
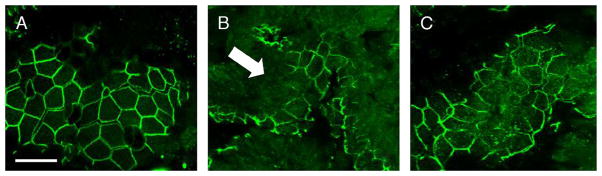
Cellular localization of intestinal ZO-1
Burn-induced changes in localization are also seen in samples of distal ileum stained for ZO-1. An organized, smooth pattern of staining at the periphery of the cell is seen once again in the sham animals (Fig. 8A). Severe burn treated with NS, however, results in altered localization of ZO-1 away from the tight junction. There is clearly a disordered pattern of ZO-1 staining, with destruction of the architecture, and loss of staining at areas of cell-cell contact (Fig. 8B). The intestinal epithelial ZO-1 distribution in burned animals treated with PTX shows improvement compared with animals given NS (Fig. 8C). There is an organized pattern of staining seen at the periphery of the cell with fewer areas of ZO-1 breakdown.
Fig. 8. Confocal microscopy images of intestinal ZO-1 localization after burn (n ≥ 3 per group).
A, Section of distal ileum from sham animal stained for ZO-1. Distribution of ZO-1 is clearly seen at the periphery of the intestinal epithelial cell, with an even distribution at areas of cell-cell contact. B, ZO-1 distribution in distal ileum of burned animals is altered (arrow), with loss of staining at the periphery of the cell. C, Treatment with PTX results in an improved appearance of ZO-1 staining. Zonula occludens protein 1 staining has a smooth appearance with localization at the periphery of the intestinal epithelial cell, with fewer areas of breakdown. Size bar = 20 μm.
DISCUSSION
Burn injury accounts for more than 40,000 admissions per year in the United States, with a 6% mortality in patients requiring burn center admission (13). Although there is clearly cutaneous damage after burn, injury to multiple organ systems also occurs due to systemic inflammation. Dermal injury may act as an ongoing stimulus for the inflammatory response leading to production of proinflammatory mediators, which can lead to acute respiratory distress syndrome, MOF, and death. Therefore, investigating strategies to limit the SIRS response may lead to improved outcomes for seriously burned patients.
The gastrointestinal tract has been recognized as a key mediator in the SIRS to burns (14). After thermal injuries, the injured gut releases biologic factors into the mesenteric lymph that activates neutrophils, causes endothelial cell injury, and increases endothelial cell permeability (15). It is believed that the systemic circulation of these gut-derived factors can lead to distant organ injury (16). In fact, studies have shown that ligation of the mesenteric lymphatics after shock leads to a decrease in both neutrophil activation and acute lung injury (17).
Increased intestinal permeability likely plays an important role in the immune response to shock. The intestine is an important immunologic organ, with approximately 60% of the total T-cell population located in the small intestine (18). The distal small bowel and colon are normally colonized by approximately 109 bacteria. The host is normally protected from developing bacteremia and sepsis by an intact intestinal epithelium, which prevents systemic spread of bacteria and endotoxin. However, increased intestinal permeability has been documented after burn and may be the inciting incident that ultimately leads to SIRS and MOF.
Intestinal permeability is regulated by the tight junction, located at points of cell-cell contact at the apical portion of the epithelial cell. Tight junction barrier function is highly regulated by both structural and function proteins that control paracellular permeability. Occludin and ZO-1 play a critical role in tight junction structure and have been shown to decrease in sepsis and inflammatory bowel disease (19, 20). Proinflammatory cytokines such as TNF-α and IL-1β are also known to induce changes in occludin and ZO-1 expression and localization within the cell, which can lead to increased permeability (7, 8).
In this series of experiments, we show that the use of the phosphodiesterase inhibitor PTX after severe burn injury results in decreased systemic and intestinal TNF-α levels and decreased breakdown of intestinal tight junction structural proteins. This study is the first to show a decrease in ZO-1 protein levels and altered cellular localization of both occludin and ZO-1 after burn. In addition, we are the first to show that PTX attenuates burn-induced intestinal permeability, decreases the breakdown of intestinal ZO-1 and occludin, and prevents reorganization of these proteins away from the tight junction. Our results also indicate that treatment with PTX also prevents burn-induced histological gut injury.
Pentoxifylline is a nonspecific phosphodiesterase inhibitor that has been studied as an immunomodulator after shock due to effects on inflammatory signaling. Pentoxifylline has been used in the clinical setting as a treatment for peripheral vascular disease because of its ability to improve microcirculation (21). In our laboratory, we have extensively studied the effects of PTX on distant organ injury and inflammatory signaling in models of hemorrhagic shock and sepsis. We have previously shown that treatment with PTX decreases proinflammatory mediator synthesis, including TNF-α. Our studies also indicate that PTX attenuates lung, gut, and liver injury after shock (10, 11, 22–27).
These results shed light onto the time course of tight junction breakdown after burn. We found no significant change in occludin and ZO-1 levels at 2 h after burn. By 6 h, there was a significant decrease in occludin and ZO-1 in burned animals, which was attenuated in animals treated with PTX. This time point for breakdown of the tight junction is supported by a study by Horton (28), who found a decrease in intestinal blood flow and an increase in intestinal permeability that peaked at 5 h after burn. Our data also indicated that the breakdown in occludin and ZO-1 persists at 24 h after burn. After burn injury, the injured skin likely serves as a proinflammatory stimulus that persists until excision of the burned tissue. Pentoxifylline may exert its effects through its ability to modulate inflammatory signaling, thus decreasing distant organ injury caused by the burn.
Phosphodiesterase inhibition with PTX significantly reduced the burn-induced increase in plasma TNF-α at 2 h. TNF-α is a proximal mediator of inflammation which peaks early after burn and regulates the subsequent synthesis of several cytokines and adhesion molecules (29, 30). TNF-α is known to cause downregulation of occludin and ZO-1; therefore, attenuation of circulating TNF-α levels may be 1 mechanism by which PTX modulates tight junction breakdown. Pentoxifyl-line may also prevent tight junction failure through its ability to decrease activation of the proinflammatory transcription factor nuclear factor κB (NF-κB) (26, 31). Nuclear factor κB inhibition can have significant effects, as several proinflammatory mediators (including TNF-α and IL-1β) contain NF-κB binding sites in their promoter regions. Al-Sadi (8) and Ma et al. (32) have shown that NF-κB activation is required for the TNF-α–induced downregulation of intestinal epithelial occludin and ZO-1 levels and increases in intestinal permeability. These findings indicate the importance of NF-κB activation in regulating occludin and ZO-1 and give insight into a key mechanism by which PTX is able to prevent tight junction breakdown.
Pentoxifylline may also prevent changes in occludin and ZO-1 by inhibiting signaling within the intestinal epithelial cell. The intestinal tight junction is regulated by several signaling pathways involving calcium, cyclic adenosine monophosphate, protein kinase A (PKA), and protein kinase C (PKC) (33). Protein kinase A activation has been shown to modulate ZO-1 localization to the tight junction (34). As a phosphodiesterase inhibitor, PTX is known to increase intra-cellular cyclic adenosine monophosphate and has been shown to exert its effects in both PKA-dependent and -independent mechanisms. Protein kinase C has also been shown to regulate intestinal permeability and tight junction protein assembly (35). Pentoxifylline has been shown to inhibit PKC activation and may prevent immunomodulatory signaling to the tight junction (36).
Treatment with the phosphodiesterase inhibitor PTX prevented the burn-induced decrease in intestinal occludin and ZO-1 protein levels and prevented changes in the cellular localization of these proteins. Importantly, we also observed a decrease in burn-induced intestinal permeability after treatment with PTX. Phosphodiesterase inhibition may serve as a beneficial adjunct in the treatment of severely burned patients because of its ability to attenuate gut barrier injury and potentially limit the SIRS response.
Footnotes
Presented at the International Shock Congress; June 30, 2008; Cologne, Germany.
References
- 1.Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg. 1999;178:449–453. doi: 10.1016/s0002-9610(99)00231-7. [DOI] [PubMed] [Google Scholar]
- 2.Deitch EA, Bridges W, Baker J, Ma JW, Ma L, Grisham MB, Granger DN, Specian RD, Berg R. Hemorrhagic shock-induced bacterial translocation is reduced by xanthine oxidase inhibition or inactivation. Surgery. 1988;104:191–198. [PubMed] [Google Scholar]
- 3.Moore FA, Moore EE, Poggetti R, McAnena OJ, Peterson VM, Abernathy CM, Parsons PE. Gut bacterial translocation via the portal vein: a clinical perspective with major torso trauma. J Trauma. 1991;31:629–636. doi: 10.1097/00005373-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228:518–527. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita SA, Tsukita SH. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 7.Chavez AM, Menconi MJ, Hodin RA, Fink MP. Cytokine-induced intestinal epithelial hyperpermeability: role of nitric oxide. Crit Care Med. 1999;27:2246–2251. doi: 10.1097/00003246-199910000-00030. [DOI] [PubMed] [Google Scholar]
- 8.Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–4649. doi: 10.4049/jimmunol.178.7.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samonte VA, Goto M, Ravindranath TM, Fazal N, Holloway VM, Goyal A, Gamelli RL, Sayeed MM. Exacerbation of intestinal permeability in rats after a two-hit injury: burn and Enterococcus faecalis infection. Crit Care Med. 2004;32:2267–2273. doi: 10.1097/01.ccm.0000145579.66001.05. [DOI] [PubMed] [Google Scholar]
- 10.Coimbra R, Porcides R, Loomis W, Melbostad H, Lall R, Deree J, Wolf P, Hoyt DB. HSPTX protects against hemorrhagic shock resuscitation-induced tissue injury: an attractive alternative to Ringer’s lactate. J Trauma. 2006;60:41–51. doi: 10.1097/01.ta.0000197417.03460.0a. [DOI] [PubMed] [Google Scholar]
- 11.Deree J, de Campos T, Shenvi E, Loomis W, Hoyt DB, Coimbra R. Hypertonic saline and pentoxifylline attenuates gut injury after hemorrhagic shock: the kinder, gentler resuscitation. J Trauma. 2007;62:818–828. doi: 10.1097/TA.0b013e31802d9745. [DOI] [PubMed] [Google Scholar]
- 12.Chen LW, Wang JS, Hwang B, Chen JS, Hsu CM. Reversal of the effect of albumin on gut barrier function in burn by the inhibition of inducible isoform of nitric oxide synthase. Arch Surg. 2003;138:1219–1225. doi: 10.1001/archsurg.138.11.1219. [DOI] [PubMed] [Google Scholar]
- 13.American Burn Association. Burn Incidence and Treatment in the US: 2007 Fact Sheet. Chicago, IL: ABA; 2007. [Google Scholar]
- 14.Gosain A, Gamelli RL. Role of the gastrointestinal tract in burn sepsis. J Burn Care Rehabil. 2005;26:85–91. doi: 10.1097/01.bcr.0000150212.21651.79. [DOI] [PubMed] [Google Scholar]
- 15.Deitch EA, Shi HP, Lu Q, Feketeova E, Skurnick J, Xu DZ. Mesenteric lymph from burned rats induces endothelial cell injury and activates neutrophils. Crit Care Med. 2002;32:533–538. doi: 10.1097/01.CCM.0000109773.00644.F4. [DOI] [PubMed] [Google Scholar]
- 16.Cavriani G, Domingos HV, Soares AL, Trezena AG, Ligeiro-Oliveira AP, Oliveira-Filho RM, Sudo-Hayashi LS, Tavares de Lima W. Lymphatic system as a path underlying the spread of lung and gut injury after intestinal ischemia/reperfusion in rats. Shock. 2005;23:330–336. doi: 10.1097/01.shk.0000157303.76749.9b. [DOI] [PubMed] [Google Scholar]
- 17.Watkins AC, Caputo FJ, Badami C, Barlos D, Xu da Z, Lu Q, Feketeova E, Deitch EA. Mesenteric lymph duct ligation attenuates lung injury and neutrophil activation after intraperitoneal injection of endotoxin in rats. J Trauma. 2008;64:126–130. doi: 10.1097/TA.0b013e3181574a8a. [DOI] [PubMed] [Google Scholar]
- 18.Mowat AM, Viney JL. The anatomic basis of intestinal immunity. Immunol Rev. 1997;156:145–166. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 19.Han X, Fink MP, Yang R, Delude RL. Increased iNOS activity is essential for intestinal epithelial tight junction dysfunction in endotoxemic mice. Shock. 2004;21:261–270. doi: 10.1097/01.shk.0000112346.38599.10. [DOI] [PubMed] [Google Scholar]
- 20.Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yada-Langui MM, Anjos-Valotta EA, Sannomiya P, Rocha e Silva M, Coimbra R. Resuscitation affects microcirculatory polymorphonuclear leukocyte behavior after hemorrhagic shock: role of hypertonic saline and pentoxifylline. Exp Biol Med. 2004;229:684–693. doi: 10.1177/153537020422900713. [DOI] [PubMed] [Google Scholar]
- 22.Coimbra R, Melbostad H, Hoyt DB. Effects of phosphodiesterase inhibition on the inflammatory response after shock: role of pentoxifylline. J Trauma. 2004;56:442–449. doi: 10.1097/01.TA.0000096642.54111.E8. [DOI] [PubMed] [Google Scholar]
- 23.Coimbra R, Loomis W, Melbostad H, Tobar M, Porcides RD, Lall R, Holbrook T, Hoyt DB. Role of hypertonic saline and Pentoxifylline on neutrophil activation and tumor necrosis factor-α synthesis: a novel resuscitation strategy. J Trauma. 2005;59:257–265. doi: 10.1097/01.ta.0000174678.12523.9f. [DOI] [PubMed] [Google Scholar]
- 24.Deree J, Martins JO, Leedom A, Lamon B, Putnam JG, de Campos T, Hoyt DB, Wolf P, Coimbra R. Hypertonic saline and pentoxifylline reduces hemorrhagic shock resuscitation-induced pulmonary inflammation through attenuation of neutrophil degranulation and proinflammatory mediator synthesis. J Trauma. 2007;62:104–111. doi: 10.1097/TA.0b013e31802d96cb. [DOI] [PubMed] [Google Scholar]
- 25.Deree J, Martins J, de Campos T, Putnam JG, Loomis W, Wolf P, Coimbra R. Pentoxifylline attenuates lung injury and modulates transcription factor activity in hemorrhagic shock. J Surg Res. 2007;143:99–108. doi: 10.1016/j.jss.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 26.Coimbra R, Melbostad H, Loomis W, Porcides RD, Wolf P, Tobar M, Hoyt DB. LPS-induced acute lung injury is attenuated by phosphodiesterase inhibition: effects on proinflammatory mediators, metalloproteinases, NF-κB, and ICAM-1 expression. J Trauma. 2006;60:115–125. doi: 10.1097/01.ta.0000200075.12489.74. [DOI] [PubMed] [Google Scholar]
- 27.Coimbra R, Porcides RD, Melbostad H, Loomis W, Tobar M, Hoyt DB, Wolf P. Nonspecific phosphodiesterase inhibition attenuates liver injury in acute endotoxemia. Surg Infect. 2005;6:73–85. doi: 10.1089/sur.2005.6.73. [DOI] [PubMed] [Google Scholar]
- 28.Horton JW. Bacterial translocation after burn injury: the contribution of ischemia and permeability changes. Shock. 2004;1:286–290. [PubMed] [Google Scholar]
- 29.Wu X, Woodside KJ, Song J, Wolf SE. Burn-induced gut mucosal homeostasis in TCRδ receptor–deficient mice. Shock. 2004;21:52–57. doi: 10.1097/01.shk.0000104268.15342.8f. [DOI] [PubMed] [Google Scholar]
- 30.Plackett TP, Colantoni A, Heinrich SA, Messingham KA, Gamelli RL, Kovacs EJ. The early acute phase response after burn injury in mice. J Burn Care Res. 2007;28:167–172. doi: 10.1097/BCR.0b013E31802CB84F. [DOI] [PubMed] [Google Scholar]
- 31.Coimbra R, Melbostad H, Loomis W, Tobar M, Hoyt DB. Phosphodiesterase inhibition decreases nuclear factor-kappa B activation and shifts the cytokine response toward anti-inflammatory activity in acute endotoxemia. J Trauma. 2005;59:575–582. [PubMed] [Google Scholar]
- 32.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-α–induced increase in intestinal epithelial tight junction permeability requires NF-κB activation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422–G430. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 33.Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, Chiba H. Tight junctions and human disease. Med Electron Microsc. 2003;36:147–156. doi: 10.1007/s00795-003-0219-y. [DOI] [PubMed] [Google Scholar]
- 34.Kohler K, Louvard D, Zahraoui A. Rab13 regulates PKA signaling during tight junction assembly. J Cell Biol. 2004;165:175–180. doi: 10.1083/jcb.200312118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cario E, Berken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1 –associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127:224–238. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Chen YM, Tu CJ, Hung KY, Wu KD, Tsai TJ, Hsieh BS. Inhibition by pentoxifylline of TNF-alpha–stimulated fractalkine production in vascular smooth muscle cells: evidence for mediation by NF-kappa B down-regulation. Br J Pharmacol. 2003;138:950–958. doi: 10.1038/sj.bjp.0705088. [DOI] [PMC free article] [PubMed] [Google Scholar]