Summary
Previous studies have raised the possibility that Wnt signaling may regulate both neural progenitor maintenance and neuronal differentiation within a single population. Here we investigate the role of Wnt/β-catenin activity in the zebrafish hypothalamus and find that the pathway is first required for the proliferation of unspecified hypothalamic progenitors in the embryo. At later stages, including adulthood, sequential activation and inhibition of Wnt activity is required for the differentiation of neural progenitors and negatively regulates radial glia differentiation. The presence of Wnt activity is conserved in hypothalamic progenitors of the adult mouse, where it plays a conserved role in inhibiting the differentiation of radial glia. This study establishes the vertebrate hypothalamus as a model for Wnt-regulated post-embryonic neural progenitor differentiation, and defines specific roles for Wnt signaling in neurogenesis.
Introduction
While neurogenesis was originally defined as a phenomenon exclusive to developing brains, it has now been identified in the adult CNS of mammals (Ming and Song, 2011) and non-mammalian vertebrates (Kizil et al., 2011). The regulation of post-embryonic neurogenesis is thus a critical modulator of CNS homeostasis and plasticity, and the molecular mechanisms underlying this process are of obvious intense interest. One of the best-characterized signaling pathways involved in developmental neurogenesis is the regulation of target gene transcription by Wnt/β-catenin signaling. For example, the hippocampus of null mutants for Wnt3a (Lee et al., 2000), LRP6 (Zhou et al., 2004) and Lef1 (Galceran et al., 2000), contains a smaller dentate gyrus, with reduced production of granule neurons and abnormalities in radial glial scaffolding due to proliferation and patterning defects. These results suggest that Wnt activity may be required for the proliferation and normal differentiation of embryonic neural progenitors at early stages.
Recently, several studies with conditional approaches have revealed new aspects of Wnt signaling in post-embryonic neurogenesis. One prevailing model suggests that Wnt activity is required to keep neural progenitors undifferentiated (Machon et al., 2007; Mutch et al., 2010). However, other data indicate that ectopic Wnt activity can both inhibit and promote neuronal differentiation (Tang et al., 2010). Consistent with these conflicting outcomes, hippocampal progenitors persist as GFAP+ radial stem-like cells when Wnt function is lost (Kuwabara et al., 2009), while activation of the pathway in the rostral migratory stream, by Apc deletion, results in developmental arrest of Ascl1+ transit amplifying cells (Imura et al., 2010). Together, all these studies suggest an untested unifying model in which Wnt activity is required for an early step of the neurogenesis pathway, but must be inactivated for differentiation to proceed.
We have previously shown that Wnt signaling through Lef1 is required for neurogenesis in the embryonic zebrafish hypothalamus (Lee et al., 2006). Subsequently, we found that both Wnt signaling and neurogenesis continue in the zebrafish hypothalamus through adult stages (Wang et al., 2009). Compared with other forebrain regions, the hypothalamus is relatively unstudied as a model of post-embryonic neurogenesis. While the hypothalamus has been identified as a region with proliferation and neurogenesis in adult mammals (Kokoeva et al., 2007; Lee et al., 2012; Migaud et al., 2010; Perez-Martin et al., 2010a), the regulation and function of this regional activity are poorly understood. Hypothalamic neurogenesis could be significant in the regulation of multiple autonomic and endocrine pathways, as already demonstrated with feeding behavior (Kokoeva et al., 2005). The presence and gene expression profiles of specific neuronal lineages, such as Dlx+ GABAergic precursors, are similar to those of other brain regions (Yee et al., 2009). In addition, the hypothalamus contains persistent radial glial tanycytes that are essential for endocrine function and can also serve as a neural progenitor population (Lee et al., 2012; Rodriguez et al., 2005). However the regulation of tanycyte differentiation has remained uncharacterized.
Here we identify Wnt-responsive cells in the embryonic and post-embryonic zebrafish hypothalamus, and find evidence for pathway activation first in unspecified progenitors, and again later in post-mitotic neural progenitors. Consistent with the profile of pathway activity, we find that Wnt signaling is first required for proliferative expansion of unspecified progenitors in the embryo and later regulates the differentiation of GABAergic and serotonergic lineages. Significantly, our data indicate that Wnt signaling must be inactivated in order for neuronal differentiation to occur. In contrast, Wnt activity is not required for the differentiation of radial glia, and ectopic pathway activation disrupts the generation of these cells. Finally, we show that Wnt activity is present in ventricular and parenchymal cells of the adult mouse mediobasal hypothalamus that express neural progenitor markers. In this system, we find that the role of Wnt signaling in radial glial development is evolutionarily conserved. Together, these data lead to a general model for Wnt function in post-embryonic hypothalamic progenitor differentiation.
Results
Wnt activity is present in unspecified hypothalamic progenitors at early embryonic stages
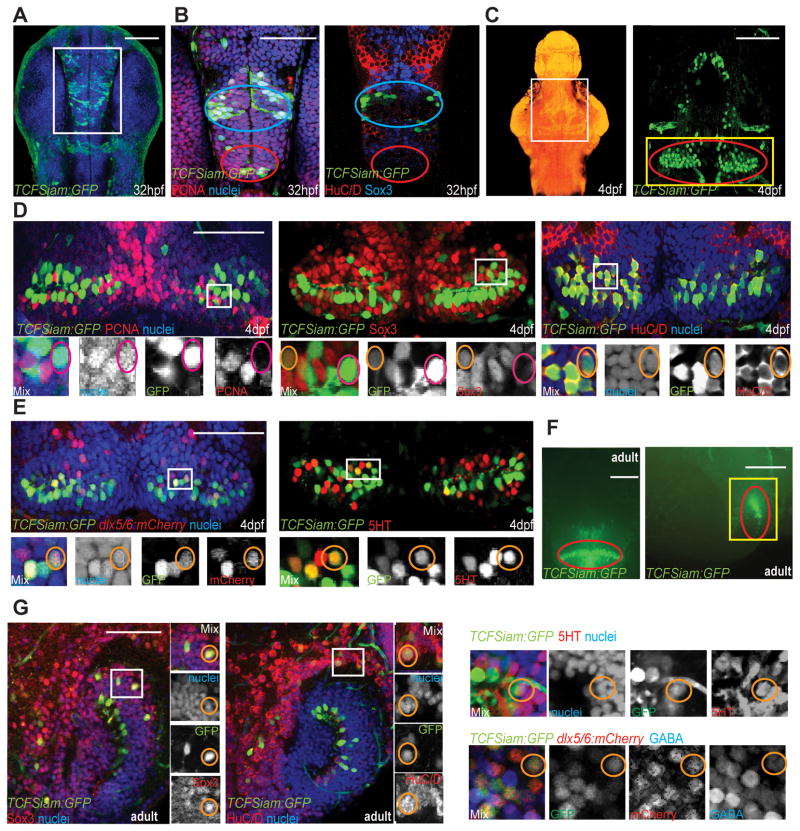
We previously showed that Wnt signaling and the transcriptional mediator Lef1 regulate embryonic hypothalamic neurogenesis (Lee et al., 2006). In subsequent work, we found that Wnt pathway activity is present in the hypothalamus throughout the life of the animal (Wang et al., 2009). To more completely characterize Wnt-responsive cells in the zebrafish hypothalamus here we employed a combination of transcriptional reporter lines. We used both TOP:GFP, a Wnt reporter containing 4 TCF binding sites upstream of a destabilized GFP transgene (Dorsky et al., 2002) as well as TCFSiam:GFP, a Wnt reporter containing the Xenopus siamois promoter upstream of eGFP (Moro et al., 2012), to label Wnt-responsive cells and their progeny. At 32 hours post-fertilization (hpf), we found that Wnt-responsive cells were present in the presumptive lateral and posterior recess regions (Fig. 1A). At this embryonic stage, almost all TCFSiam:GFP+ and TOP:GFP+ cells were PCNA+ (Fig. 1B, Table 1). In contrast, few TCFSiam:GFP+ and TOP:GFP+ cells expressed Sox3 (Fig. 1B, Table 1), a marker and direct activator of neural progenitor gene expression (Bergsland et al., 2011), and co-localization of both reporters with the neuronal marker HuC/D was very low (Fig. 1B, Table 1). Together, these data show that the majority of Wnt-responsive cells and their progeny in the 32hpf zebrafish hypothalamus are proliferating unspecified progenitors.
Figure 1.
Identification of Wnt-responsive cells in the zebrafish hypothalamus. (A) Ventral view of TCFSiam:GFP. White box marks the area shown in (B). (B) Co-staining with PCNA, Sox3, and Hu. Blue ovals label the presumptive lateral recess, and red ovals label the presumptive posterior recess. (C) Maximum intensity confocal Z-projection of TCFSiam:GFP at 4dpf. In nuclear stained ventral view of brain on left, white box marks hypothalamic area shown on right. Yellow box marks the area shown in (D, E), and red oval marks the posterior recess. (D) Co-staining of TCFSiam:GFP with PCNA, Sox3, and HuC/D. (E) Co-staining of TCFSiam:GFP with dlx5/6:gfp and serotonin. (F) Dissecting microscope ventral and sagittal views of TCFSiam:GFP in the adult hypothalamus. Yellow box marks the area shown in (G), and red oval labels the presumptive posterior recess. (G) Co-staining of TCFSiam:GFP with Sox3, Hu, 5HT, dlx5/6:mCherry, and GABA in the adult posterior hypothalamus. Single optical sections from ventral views are shown in all panels, unless otherwise indicated. Small orange circles label cells with colocalization, and small magenta circles label cells without colocalization. Scale bars: (A–E, G) 80μm, (F) 250μm. See also Figure S1.
Table 1.
Coexpression percentage of cell type markers with reporters. Cells were counted from maximum intensity confocal Z-projections of at least 3 individual brains for each set of markers. The entire hypothalamus (32hpf) or posterior recess of whole (4dpf) and half (adult) brains, defined by anatomical landmarks, was counted. Error=±SD. See also Figure S2.
| PCNA | Sox3 | HuC/D | dlx5/6:mCherry | GABA | 5HT | |
|---|---|---|---|---|---|---|
| 32hpf | ||||||
| TOP:GFP | 97.3±1.5 | 13.1±3.5 | 1.3±0.5 | - | - | - |
| TCFsiam:GFP | 96.2±1.2 | 7.2±2.3 | 2.1±0.8 | - | - | - |
|
| ||||||
| 4dpf | ||||||
| TOP:GFP | 5.2±1.3 | 89.2±7.1 | 5.2±2.1 | 7.1±3.2 | 1.7±1.1 | 2.9±0.5 |
| TCFsiam:GFP | 9.3±1.7 | 40.3±9.9 | 78.2±9.3 | 48.1±9.9 | 31.2±5.0 | 17.2±4.3 |
| tcf7:GFP | 4.3±2.3 | 68.1±11.7 | 81.2±6.8 | 26.1±7.2 | 16.1±4.1 | 30.1±3.2 |
| dlx5/6:GFP | 9.2±1.2 | 39.2±7.1 | 61.3±5.4 | - | 73.1±6.1 | 0 |
|
| ||||||
| Adult | ||||||
| TOP:GFP | <1 | 98.1±1.1 | 3.1±2.4 | <1 | <1 | 1.1±0.7 |
| TCFsiam:GFP | <1 | 47.3±3.4 | 66.3±5.3 | 37.0±4.9 | 7.1±1.8 | 5.2±1.5 |
| tcf7:GFP | <1 | 59.1±4.2 | 73.1±4.1 | - | 9.1±1.6 | 7.3±2.5 |
Wnt signaling is active in hypothalamic neural progenitorsat post-embryonic stages
At 4 days post-fertilization (dpf), the 3rd ventricle and its associated posterior recess serve as reliable hypothalamic landmarks. At this stage expression of TCFSiam:GFP and TOP:GFP suggested that most Wnt-responsive cells are located in the posterior recess (Red oval in Fig. 1C). At this stage, we found that Wnt-responsive cells were primarily PCNA−/Sox3+ neural progenitors, although a few were PCNA+/Sox3+, indicating a transition through cell cycle exit (Fig. 1D and Table 1). BrdU labeling also showed that the majority of 4dpf Wnt-responsive cells were not proliferating (Fig. S1).
At 4dpf, we used GFP perdurance in the two reporter lines to determine which lineages derive from the Wnt-responsive cells. To confirm the difference in reporter responsiveness, we expressed the hs:dkk1 transgene (Stoick-Cooper et al., 2007) to conditionally inhibit canonical Wnt signaling in both reporter lines and monitored the decrease in GFP expression following heat shock. We found that it took 24 hours to achieve an 80% reduction in TCFSiam:GFP+ cells, compared to only 8 hours for TOP:GFP (Fig. S1). Both Wnt reporters were coexpressed with the pan-neuronal marker HuC/D, the GABAergic lineage markers dlx5/6:mCherry and GABA, and serotonin. However, coexpression was much higher in cells expressing the stable reporter TCFSiam:GFP (Fig. 1D, E, and Table 1), and relatively low in cells expressing the destabilized reporter TOP:GFP (Fig. S2, and Table 1). Because we did not observe any overlap between markers for the GABAergic and serotonergic lineages (Fig. S2 and Table 1), these experiments suggest that Wnt-responsive cells generate at least two different neuronal lineages. Furthermore, the different compositions of the destabilized and stable GFP reporter populations suggest that Wnt activity is rapidly silenced as neuronal differentiation proceeds.
In the adult hypothalamus, analysis of TCFSiam:GFP and TOP:GFP expression confirmed a similar distribution of Wnt-responsive cells in the posterior recess region. We previously reported that TOP:GFP+ cells in the adult hypothalamic lateral recess did not express the proliferation marker PCNA, the post-mitotic neuronal marker HuC/D, or GFAP (Wang et al., 2009). To determine the identity of GFP+ cells in the posterior recess, we performed whole-mount sagittal analysis of this region using 150μm confocal projections taken from the midline. We found that Sox3 continues to be expressed in the majority of Wnt-responsive cells in the posterior recess (Fig. 1G and Table 1). The expression of neuronal markers still distinguished the destabilized and stable reporters, as many TCFSiam:GFP+ cells, but very few TOP:GFP+ cells, co-expressed HuC/D (Fig. 1G, Fig. S2, and Table 1). In addition, we found that adult hypothalamic Wnt-responsive cells continue to contribute to the GABAergic and serotonergic lineages identified in 4dpf hypothalamus (Fig. 1G and Table 1).
Together, these analyses show that in the posterior recess region of the 4dpf and adult zebrafish hypothalamus, Wnt activity is present in Sox3+ GABAergic and serotonergic progenitors as they become postmitotic, but is then lost as they undergo further differentiation.
lef1 is required for post-embryonic hypothalamic neurogenesis
By analyzing the expression of multiple pathway components, we found that along with the Wnt8b ligand, the two transcriptional mediators Lef1 and Tcf7 were the best candidates to mediate Wnt signaling in the posterior hypothalamus. In particular, expression of Lef1 protein and of a tcf7:GFP reporter (Nagayoshi et al., 2008) mirrored that of TCFSiam:GFP (Fig. S3 and Table 1). While Lef1 and tcf7:GFP were co-expressed in some cells (Fig. S3), non-overlapping expression in other cells suggested non-redundant roles in the hypothalamus.
Identified mutations in individual zebrafish Tcf genes produce relatively minor defects (Muncan et al., 2007; Nagayoshi et al., 2008), allowing us to investigate the role of Wnt activity in neurogenesis in the context of grossly normal morphology. We chose to focus on lef1 for several reasons. First, zebrafish tcf7 null mutants are viable and fertile, suggesting a minor required function in development (Nagayoshi et al., 2008). Second, mouse Lef1 mutants exhibit a smaller dentate gyrus with neurogenesis defects in the hippocampus (Galceran et al., 2000; Zhou et al., 2004), but hypothalamic phenotypes have not been explored. Finally, in zebrafish lef1 morpholino knockdown produces neurogenesis defects in the hypothalamic GABAergic lineage (Lee et al., 2006).
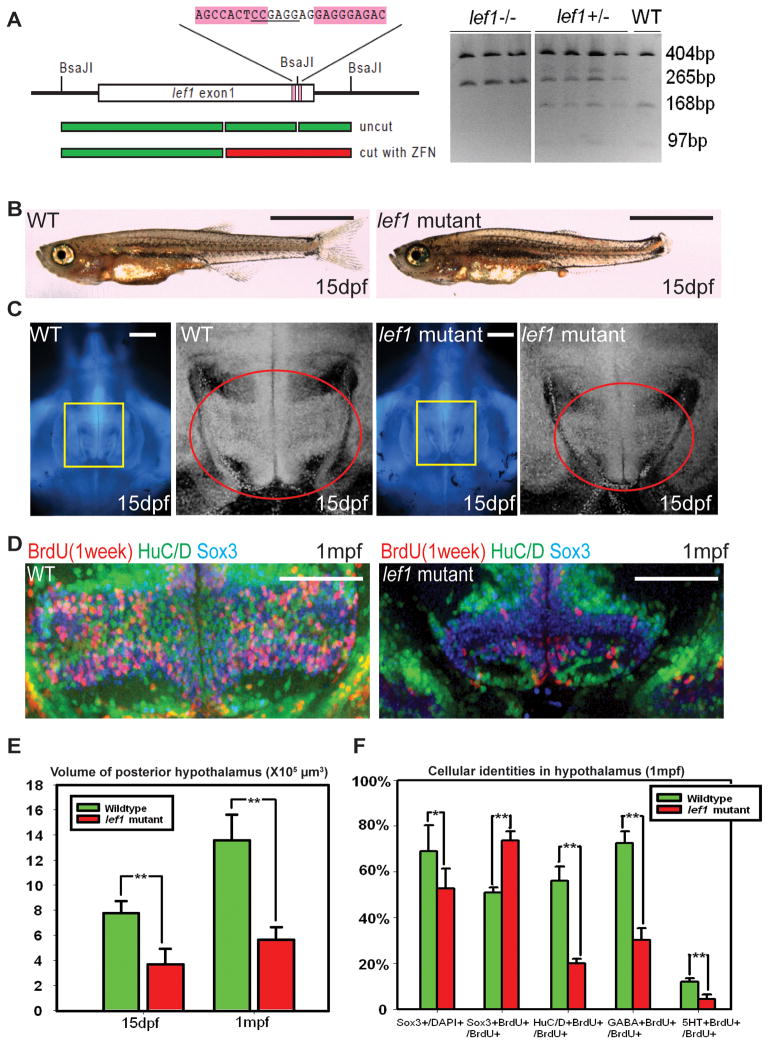
To explore the later functions of lef1 in the hypothalamus, we generated a lef1 mutant using zinc finger nuclease-mediated gene targeting (Meng et al., 2008). Genomic (Fig. 2A) and immunohistochemical (not shown) analyses indicated that this allele is a functional null, and we observed similar phenotypes to two other lef1 null alleles generated by ENU mutagenesis (McGraw et al., 2011; Valdivia et al., 2011). At 15dpf, lef1 mutants can be identified by their smaller fins. While mutant fish exhibit similar body mass and brain size as their wild-type siblings (Fig. 2B, C), we observed a significant decrease in the size of the posterior hypothalamus. Using confocal volume reconstruction, we found the mutant posterior hypothalamus is 50% of wild-type size (Fig. 2C, E), suggesting a specific proliferation defect in this tissue.
Figure 2.
lef1 is required for proliferation and neurogenesis in the post-embryonic hypothalamus. (A) lef1 ZFN target region and genotyping. (B) Whole fish and (C) brain size comparisons of lef1 mutant to wild-type at 15dpf. Boxed region in left panel is enlarged on right, posterior recess is circled. (D) Expression of HuC/D, Sox3, and 7-day BrdU labeling in posterior recess of wild-type and lef1 mutant hypothalamus. (E) Quantification of posterior hypothalamus size. (F) Tracing of proliferating cells. lef1 mutants have a smaller Sox3+ progenitor pool, but a higher percentage of BrdU+ cells express Sox3 and fewer produce HuC/D+,GABA+, or 5HT+ neurons. Single optical sections from ventral views are shown in panels (C) and (D). Scale bars: (B) 2mm. (C) 200μm. (D) 100μm. Brain volumes were calculated using Amira software. Cell counts were collected from maximum intensity Z-projections through the posterior recess of 3 individual samples for each genotype and calculated using Volocity software. *: p<0.05, **: p<0.005. Error=±SD. See also Figure S3.
At 1 month post-fertilization (mpf), the lef1 mutant posterior hypothalamus is only 40% of wild-type size (Fig. 2E), and 1 week BrdU tracing showed few proliferating cells in this region, with most of the remaining BrdU+ cells located near the midline (Fig. 2D). In addition, the HuC/D+ neuronal population in the paraventricular posterior recess region is almost completely absent in lef1 mutants (Fig. 2D). While the Sox3+ neural progenitor pool is also smaller, BrdU labeling suggested that the remaining proliferating cells are arrested in the Sox3+ state and unable to progress into HuC/D+ neurons of either the GABAergic or serotonergic lineages (Fig. 2D, F).
While our previous data suggested a more significant role for lef1 in early hypothalamic development, morpholino-based studies can be subject to off-target effects or targeting of maternally deposited pre-mRNA. Our analysis of zygotic mutants suggests that at post-embryonic stages through adulthood, cells in the lef1 mutant hypothalamus fail to proliferate or differentiate normally and are arrested as Sox3+ neural progenitors.
Wnt signaling is required for hypothalamic neurogenesis throughout life
The phenotype of lef1 mutants suggested a continuous requirement for Wnt signaling in hypothalamic neurogenesis, but the lack of conditional mutagenesis approaches in zebrafish precluded us from fully testing this hypothesis. We therefore used transgenic lines to perform conditional gain- and loss-of-function assays at early embryonic (24–32hpf), later embryonic (3–4dpf), and adult (6mpf) stages. We used three different heat-shock lines to manipulate Wnt/β-catenin signaling: hs:wnt8a to activate the pathway (Weidinger et al., 2005), and hs:dkk1 (Stoick-Cooper et al., 2007) and hs:axin1 to inhibit the pathway. Neither heat shock alone nor transgene expression caused significant apoptosis, except for a small elevation following hs:axin1 expression from 3–4dpf (Fig. S4). In addition, activation and inhibition of Wnt signaling caused the predicted effects on the target gene axin2, as assayed by in situ hybridization and RT-PCR (Fig. S4).
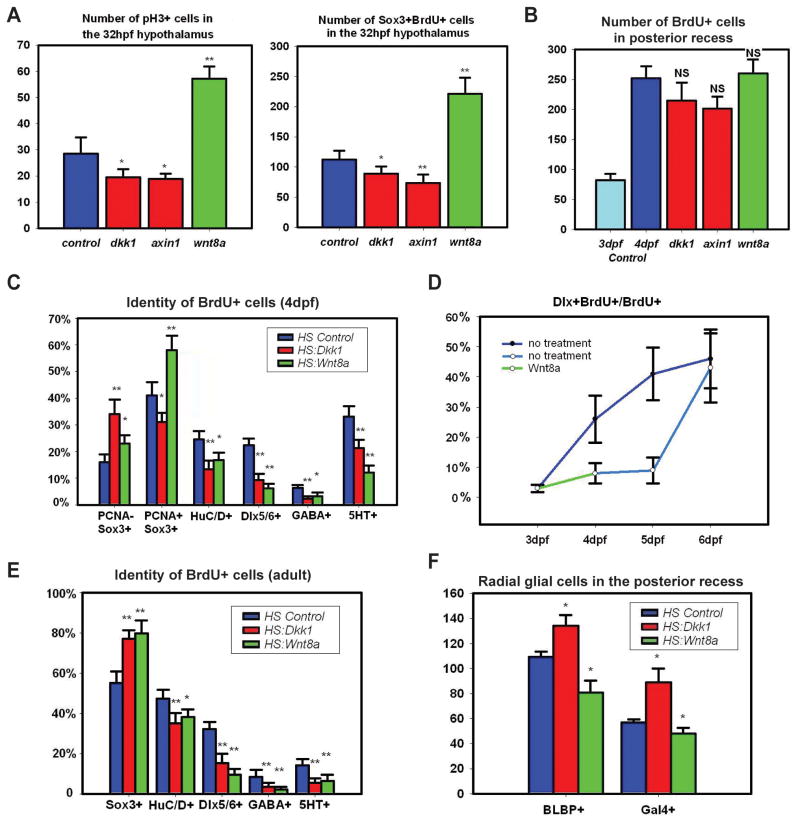
Because our data suggested a role for Wnt signaling in unspecified proliferating progenitors at 32hpf, we first analyzed the effects on proliferation following heat shock. A two-hour BrdU pulse was performed at 22hpf, followed by heat shock at 24hpf, and fixation at 32hpf. We found that the number of pH3+ cells in the hypothalamic region was significantly increased following Wnt activation and decreased following Wnt inhibition (Figs. 3A, S4), suggesting that Wnt signaling regulates overall proliferation levels. Following Wnt activation the hypothalamus was also noticeably larger with a folded ventricle and pH3+ cells outside the midline (Fig. S4). We found similar effects on the number of BrdU+/Sox3+ neural progenitors (Fig. 3A), suggesting canonical Wnt signaling is also necessary and sufficient for the expansion of this population. In contrast, we did not observe any precocious expression of neuronal differentiation markers such as Dlx genes or HuC/D following heat shock (not shown). These data are consistent with existing models of Wnt as a mitogen in progenitor cells (Megason and McMahon, 2002).
Figure 3.
Wnt signaling regulates hypothalamic progenitor proliferation and differentiation. (A) pH3+ and BrdU+/Sox3+ cell numbers in the 32hpf hypothalamus following 2 hour labeling and Wnt pathway inhibition or activation at 24hpf. (B) BrdU+ cell numbers in the 4dpf posterior recess following 2 hour labeling and Wnt pathway inhibition or activation at 3dpf. (C) BrdU+ cell fates in the 4dpf hypothalamus following labeling and Wnt pathway inhibition or activation at 3dpf. (D) Percentage of BrdU+ cells expressing dlx5/6:gfp following Wnt pathway activation at 3dpf. (E) BrdU+ cell fates in the adult hypothalamus following 2 day labeling and Wnt pathway inhibition or activation for 15 days. (F) Number of Gal4+ and BLBP+ cells in the 4dpf posterior recess following Wnt pathway inhibition or activation at 3dpf. All cell counts were collected from ventral maximum intensity confocal Z-projections through 5 individual brains. The entire hypothalamus was counted at 32hpf, the entire posterior recess was counted at 4dpf, and a hemisphere of the posterior recess was counted in adults. *: p<0.05, **: p<0.005. Error=±SD. See also Figure S4.
Our analysis of Wnt activity at 4dpf suggested a potential role for the pathway in neuronal differentiation. We therefore analyzed the effects on proliferation and differentiation following heat shock at this stage. A two-hour BrdU pulse was performed at 70hpf, followed by multiple heat shocks every 8 hours, and fixation at 96hpf. In control embryos, we found that the number of BrdU+ cells in the posterior recess increased approximately 3-fold over 24 hours, but we observed no statistically significant changes in overall BrdU labeling following expression of hs:wnt8a, hs:dkk1, or hs:axin1 (Fig. 3B). We then analyzed the BrdU+ population to determine whether Wnt signaling was necessary or sufficient to promote differentiation of neural progenitors. Surprisingly, we found that both activation and inhibition of Wnt signaling caused a higher percentage of BrdU+ cells to remain as undifferentiated Sox3+ progenitors, and a lower percentage to differentiate into GABAergic and serotonergic progeny (Figs. 3C, S4). The proportion of BrdU+/Sox3+ cells that were still mitotic (PCNA+) at 4dpf was increased following Wnt activation and decreased following Wnt inhibition (Fig. 3C), indicating a continuing role for the pathway in maintaining the mitotic state. However a decrease in overall dlx5/6:gfp expression (Fig. S4) following Wnt activation, coupled with the lack of an overall increase in BrdU+ cells (most of which are Sox3+), strongly suggest a specific effect on differentiation. We therefore conclude that both activation and inhibition of Wnt signaling lead to a significant failure of progenitors to differentiate, independent from any effect on cell cycle exit.
Considering our observation of Wnt activity only prior to differentiation, these data suggested that Wnt signaling is required for progenitors to commit to neurogenesis but must be inhibited for the process to continue. We therefore tested whether progenitors would eventually differentiate following transient Wnt activation. The same experimental paradigm described above was used, except BrdU+ cells were followed for two additional days. In this case, we found that the block in differentiation was indeed temporary, as by 6dpf the number of BrdU+ GABAergic precursors had recovered to control levels (Fig. 3D).
To test whether the function of Wnt signaling in neurogenesis was conserved at adult stages, we analyzed 6-month-old zebrafish using hs:wnt8a and hs:dkk1. Because of the slower rate of proliferation at this stage, fish were incubated in BrdU for 2 days, followed by 15 daily heat-shocks. We then traced the BrdU+ cells in the posterior recess region and found that as at 4dpf, an increased percentage remained as Sox3+ neural progenitors, and fewer differentiated into mature neurons following both Wnt activation and inhibition (Figs. 3E, S4). These data suggest a constant post-embryonic requirement for sequential activation and inhibition of Wnt signaling in the differentiation of hypothalamic neural progenitors.
Wnt signaling inhibits hypothalamic radial gliogenesis
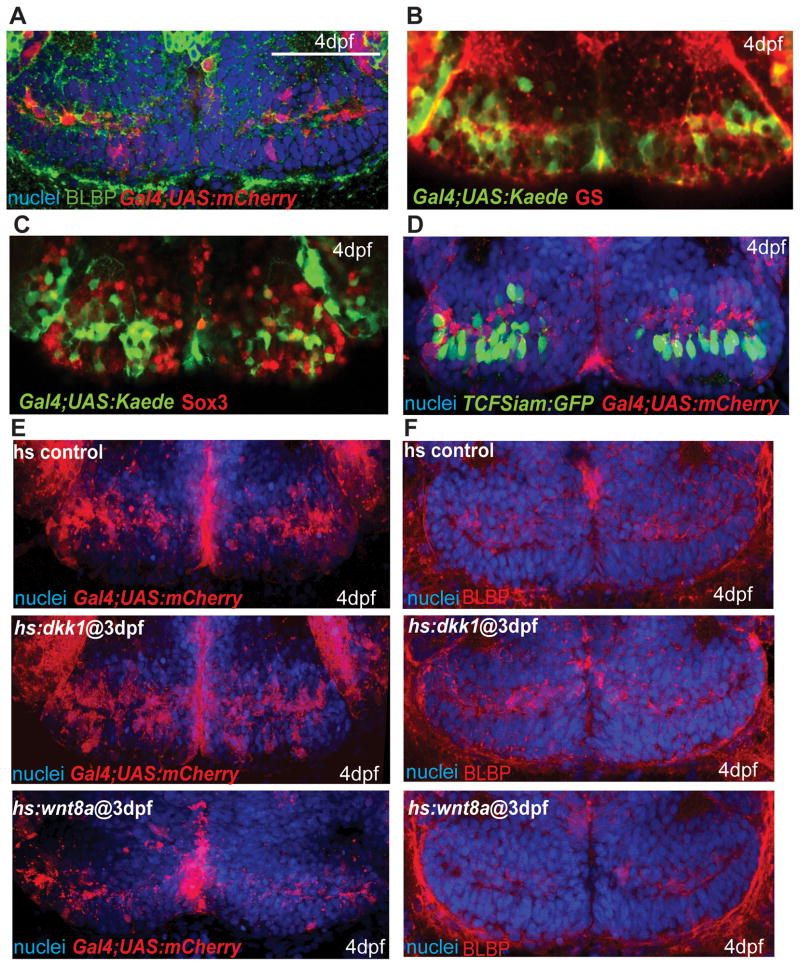
Previous reports have implicated negative regulation of Wnt signaling in the maintenance of radial glia (Wang et al., 2011), a cell type that has been identified as a potential neural progenitor in the hypothalamus (Lee et al., 2012; Xu et al., 2005). We therefore asked whether the Wnt pathway was active and functional in this cell population in zebrafish. While we did not observe any expression of GFAP in the posterior recess (not shown), we took advantage of a Gal4 insertion that labels radial glia expressing Brain lipid-binding protein (BLBP) and Glutamine synthetase, (GS, Fig. 4A, B). The level of Sox3 immunoreactivity in labeled cells was variable (Fig. 4C), suggesting that a subset of them might be neural progenitors. However, few Gal4+ cells showed evidence of Wnt reporter expression at 4dpf (Fig. 4D).
Figure 4.
Wnt signaling inhibits the formation of radial glia in the posterior recess at 4dpf. (A) Co-expression of mCherry driven by the Gal4 zc1066a insertion with the radial glial marker BLBP. (B) Co-expression of Gal4-driven Kaede with the radial glial marker glutamine synthetase. (C) Co-staining for Sox3 and Gal4-driven Kaede. Most Gal4+ cells have low or absent Sox3 expression. (D) Co-staining for TCFSiam:GFP and Gal4-driven mCherry. Few Gal4+ cells show Wnt reporter activity. (E–F) Gal4 zc1066a-driven mCherry (E) and BLBP (F) expression in the posterior recess of 4dpf embryos following Wnt pathway inhibition or activation at 3dpf. Single ventral confocal optical sections are shown in all panels. Scale bar: 80μm.
To test the role of Wnt signaling in radial glial development, we performed functional assays using hs:dkk1 and hs:wnt8a. 3dpf embryos were heat shocked every 8 hours and fixed at 4dpf. We observed a higher number of Gal4+ and BLBP+ cells following Wnt inhibition and a lower number following Wnt activation (Figs. 3F, 4E, F), suggesting that Wnt activity is not required for the formation of radial glia, and ectopic Wnt activity results in a loss of this cell type.
Wnt activity is present in the adult mouse hypothalamus
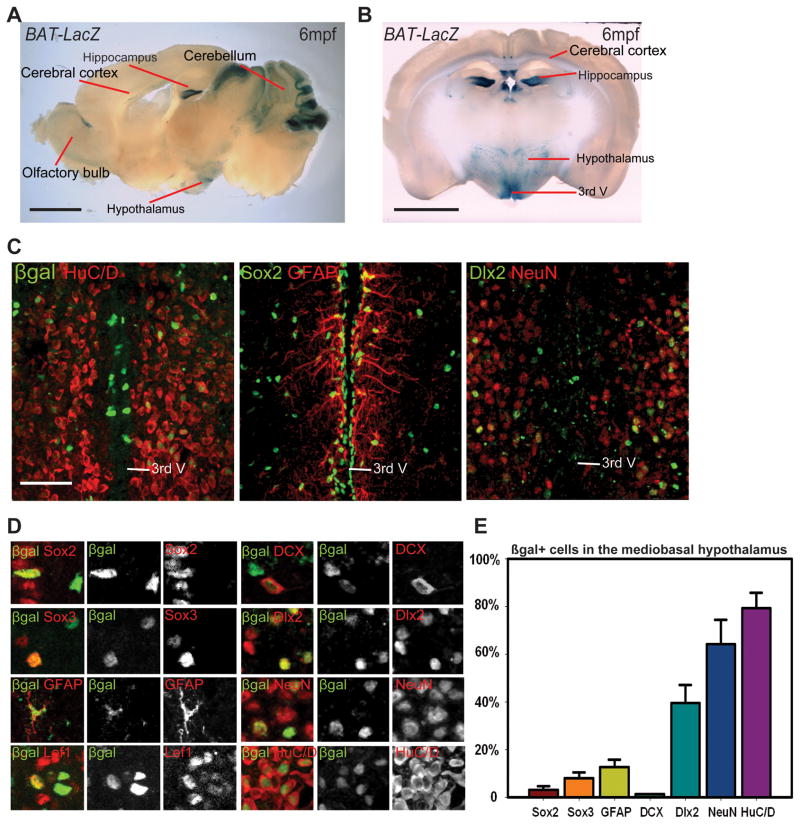
In contrast to zebrafish, which have widespread proliferation throughout the adult CNS (Chapouton et al., 2007), adult neurogenesis is much more limited in mammals. However, proliferating progenitors have been identified in the rodent hypothalamus, where adult neurogenesis can influence feeding behavior (Kokoeva et al., 2005; Lee et al., 2012; Pierce and Xu, 2010). To determine whether the presence of Wnt signaling is conserved in the adult mouse hypothalamus, we used the reporter BAT-LacZ (Maretto et al., 2003) to identify Wnt-responsive cells. From sagittal sections, we observed abundant β-gal+ cells in the adult cerebral cortex, hippocampus, olfactory epithelium, and cerebellum, as well as the posterior hypothalamus (Fig. 5A). From coronal sections through the hypothalamus, we determined that the paraventricular and arcuate nuclei have the highest density of Wnt-responsive cells in this region (Fig. 5B).
Figure 5.
The adult mouse hypothalamus has a Wnt-responsive cell population. (A) Sagittal and (B) coronal sections of adult Bat-LacZ mouse brains. (C) β-gal+ cells are distributed in both the ventricular zone (where Sox2+ and GFAP+ cells reside) and the parenchymal zone (where Sox2, HuC/D+, NeuN+, and Dlx2+ cells reside) Coronal 40μm cryosections are shown. (D) Colocalization of β-gal with specific markers in the hypothalamus. (E) Percentage of marker co-expression within the β-gal+ population. Single confocal optical sections are shown in panels (C) and (D). Scale bars: (A–B) 2mm. (C) 80μm. Cell counts were collected from the mediobasal hypothalamus, using six 40μm cryosections each from three mice. Error=±SD.
To determine the identity of hypothalamic Wnt-responsive cells we performed double immunohistochemistry for β-galactosidase and markers of either progenitors or differentiated cell types. We found that β-gal+ cells exist in the ventricular and parenchymal zones of the mediobasal hypothalamus (Fig. 5C). In the ventricular zone, β-gal+ cells were primarily Sox2+ ependymal cells and Sox2+, Sox3+ and GFAP+ subependymal cells (Fig. 5C–E). While the majority of β-gal+ cells in the parenchymal zone were HuC/D+ and NeuN+ (Fig. 5C–E), some expressed Doublecortin, a marker of differentiating neurons, and many expressed the GABAergic precursor marker Dlx2 (Fig. 5C–E). Together, these results suggest that neural progenitors in the adult mammalian hypothalamus could be Wnt-responsive, as in zebrafish.
β-catenin negatively regulates the production of ventricular hypothalamic tanycytes in the mouse
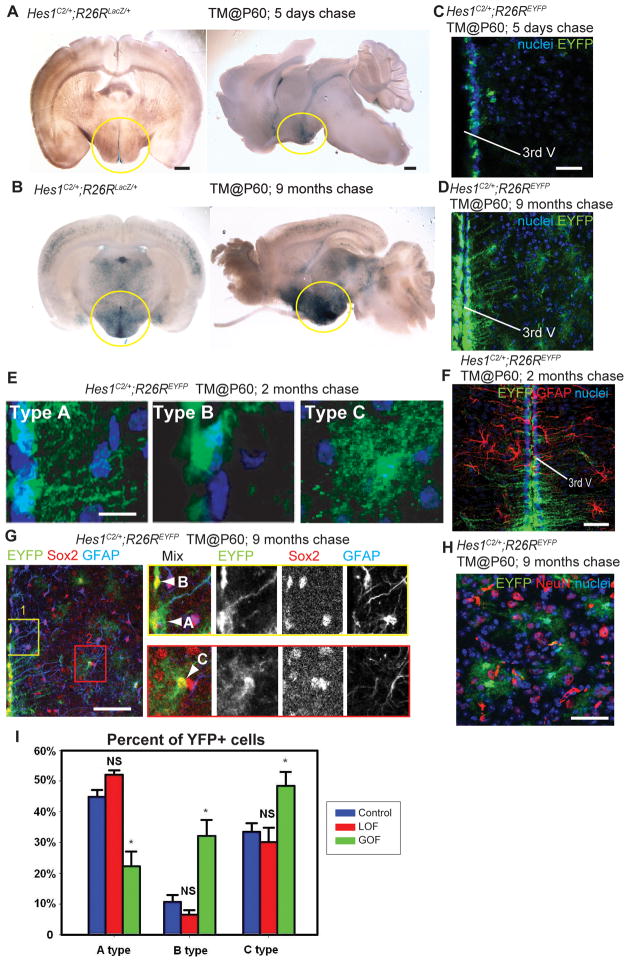
To determine the function of Wnt signaling in adult mouse hypothalamic progenitors, we took a conditional genetic approach to remove and hyperactivate β-catenin. Because the expression of Notch pathway effectors has been observed in both the adult zebrafish and mouse hypothalamic ventricular zones (Chapouton et al., 2011; Lee et al., 2012), we chose to trace and manipulate cells expressing the Notch effector gene Hes1. We observed that knock-in mice expressing tamoxifen-dependent CreERT2 recombinase from the Hes1 locus (Hes1C2) (Kopinke et al., 2011) drive efficient and inducible recombination in hypothalamic ventricular progenitors (Fig. 6A, B). Lineage tracing experiments with R26RLacZ reporter mice (Soriano, 1999) showed that Hes1+ cells are present in the ventricular zone of the adult hypothalamus 5 days after tamoxifen administration (Fig. 6A), followed by expansion into the parenchymal zone 9 months post tamoxifen (Fig. 6B).
Figure 6.
Wnt signaling inhibits the production of tanycytes from adult Hes1+ progenitors. (A, B) Coronal and sagittal sections of adult Hes1C2/+; R26RLacZ/+ mouse brains, 5 days and 9 months after TM administration at P60. (C, D) Coronal cryosections through the hypothalamus of adult Hes1C2/+; R26REYFP/+ mouse brains, 5 days and 9 months after TM administration at P60. (E) The three types of EYFP+ cells observed at 2 months post TM. (F) Expression of EYFP and GFAP in Hes1C2/+; R26REYFP/+ mice 2 months post TM. (G–H) Marker analysis of Hes1C2/+; R26REYFP/+ mice 9 months post TM. Boxes in (G) show enlarged ventricular (yellow) and parenchymal (red) zones in which cell types are indicated by arrowheads. All EYFP+ cells are Sox2+, and some ventricular Type A cells are also GFAP+. (H) Expression of EYFP and NeuN+ in the parenchymal zone, where no colabeled cells are observed. (I) Percentage of Type A, B, and C EYFP+ cells 2 months following β-catenin inactivation or activation. Single confocal optical sections are shown in panels (C)– (H). Scale bars: (A–B) 1mm. (C, D. F, G, H) 80μm. (E) 10μm. Cell counts were collected from the mediobasal hypothalamus, using six 40μm sections from three mice for each genotype. *: p<0.05. Error=±SD.
To further characterize the Hes1-expressing population, we utilized the R26REYFP reporter (Srinivas et al., 2001) in mice that received tamoxifen (TM) at P60. The majority of EYFP+ cells after a 5-day chase were located at the ventricular surface, having a cellular morphology without radial processes (Fig. 6C). After a 2-month or 9-month chase, EYFP+ cells comprised three classes. The most predominant class (“Type A”) was located at the ventricular surface and had long radial processes (Fig. 6D, E). All of these cells were Sox2+ (Fig. 6G) and BLBP+ (not shown), but only the most dorsal were GFAP+ (Fig. 6F, G). This combination of morphology and marker expression closely matches a profile previously described for radial glial tanycytes (Lee et al., 2012; Perez-Martin et al., 2010b; Rodriguez et al., 2005). A second class (“Type B”) was similar to the ventricular cells observed after a 5-day chase, lacking radial processes (Fig. 6E, G). These cells were all Sox2+ (Fig. 6G), BLBP+ (not shown) and GFAP− (Fig. 6G). A third class (“Type C”) was located in the parenchymal zone, and was Sox2+ with stellate morphology (Fig. 6E, G). Based on these characteristics, we conclude that Type C cells are most likely astrocytes. Notably, we never observed any Sox10 (not shown) HuC/D (not shown) or NeuN (Fig. 6H) expression in Type C cells, or in the entire Hes1 lineage. Together, these data indicate that Hes1+ progenitors do not produce neurons in the adult mouse hypothalamus, but do produce multiple classes of glia.
To investigate the role of Wnt/β-catenin signaling in the differentiation of Hes1+ progenitors, we crossed the Hes1C2 line with Catnblox(ex2–6) (Brault et al., 2001) and Catnblox(ex3) (Harada et al., 1999) mice allowing for β-catenin loss- and gain-of-function experiments, respectively. In addition, all mice carried one copy of the R26REYFP reporter allele, enabling us to determine the fate of recombined Hes1+ cells. All mice were genotyped and treated with 2 mg TM at P60, and EYFP-expressing cells in the mediobasal hypothalamus were analyzed 2 months later. This dose of TM was used to avoid lethal effects of recombination in the intestinal Hes1+lineage (Kopinke et al., 2011).
Following both gain and loss of β-catenin function, we observed numerous EYFP+ cells in the ventricular and parenchymal zones, as in controls. Analysis of HuC/D and NeuN expression indicated that no EYFP+ cells of any genotype were neurons (not shown). Because we used a low TM dose, recombination was mosaic and each animal had a variable number of EYFP+ cells, thus we were unable to statistically analyze the size of the lineage. Despite this, we did not observe a qualitative difference in number of EYFP+ cells between different genotypes, suggesting there was no major effect on proliferation during the 2-month period. When we analyzed the proportion of Type A, B, and C EYFP+ cells, we found that β-catenin activity was not required for radial glial differentiation (Fig. 6I). In contrast, β-catenin activation led to a significant decrease in the proportion of radial glia, with a corresponding increase in the proportion of Type B ventricular cells without processes, and Type C astrocyte-like parenchymal cells (Fig. 6I). These results suggest that Wnt/β-catenin activity negatively regulates the production of mouse ventricular hypothalamic tanycytes, consistent with our findings in zebrafish.
Discussion
Wnt signaling regulates hypothalamic progenitor differentiation in zebrafish and mouse
In this study, we have shown that both the post-embryonic zebrafish and adult mouse hypothalamus contain Wnt-responsive cells (Fig. 7). In zebrafish, hypothalamic Wnt-responsive cells are unspecified progenitors at early embryonic stages and Sox3+ neural progenitors at post-embryonic stages, contributing to GABAergic and serotonergic neuronal linages. In the adult mouse, both ventricular and parenchymal cells expressing neural progenitor and GABAergic precursor markers also exhibit Wnt activity. In addition, we have identified radial glial tanycyte lineages in both species, marked by a Gal4 transgene in zebrafish and by Hes1 in mouse. Our functional analyses demonstrate that Wnt/β-catenin signaling regulates progenitor proliferation in the early zebrafish embryo, but primarily regulates neuronal differentiation at post-embryonic stages. In contrast, in both zebrafish and mouse Wnt signaling is not required for the formation of radial glia, and ectopic Wnt activity disrupts the formation of these cells. Together, this work suggests a conserved role for Wnt signaling in post-embryonic hypothalamic progenitor differentiation in vertebrates.
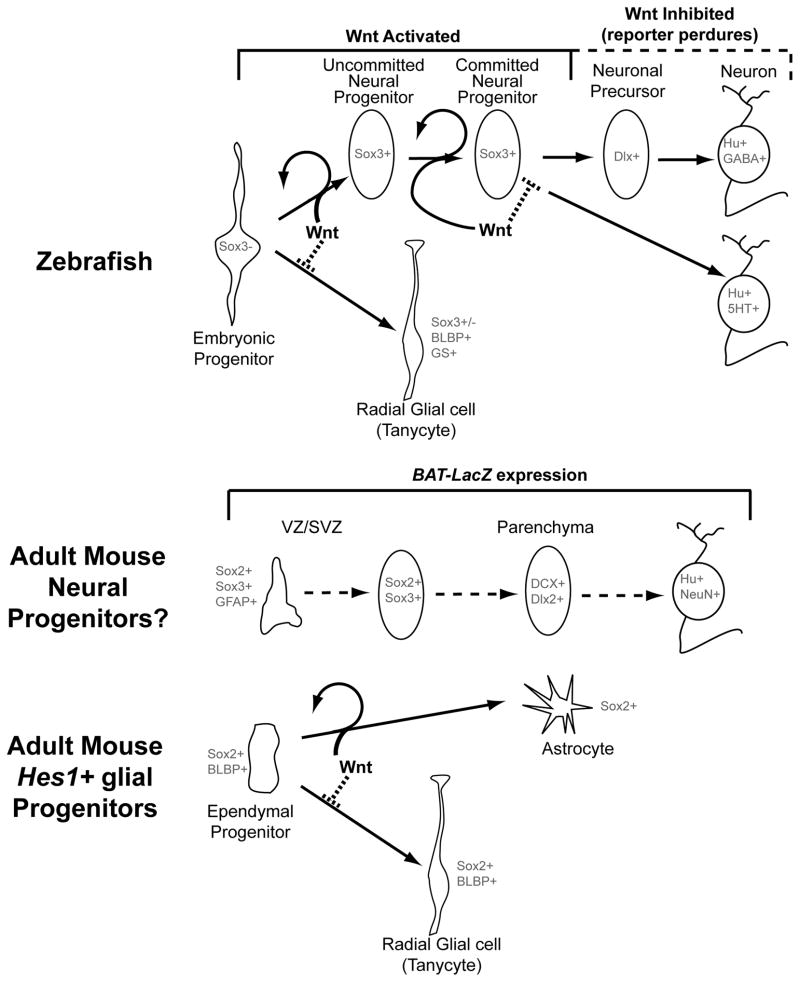
Figure 7.
Model for the role of Wnt signaling in hypothalamic progenitor differentiation. In zebrafish (above), Wnt signaling is active in unspecified embryonic progenitors and Sox3+ post-embryonic neural progenitors, but is lost as these cells undergo neurogenesis. Wnt signaling promotes mitotic activity in progenitors, and is also required for their ability to undergo neuronal differentiation, which underlies the transition from an “uncommitted” to a “committed” progenitor state. Finally, Wnt signaling must be inhibited for differentiation to proceed. In contrast the formation of radial glia is inhibited by Wnt activity. In the adult mouse (below), Wnt signaling is active in ventricular/subventricular zone cells (VZ/SVZ) that express neural progenitor markers, and in parenchymal zone cells that express neuronal and neuronal precursor markers. Ventricular Hes1+ progenitors do not require Wnt activity to make radial glial tanycytes, and ectopic pathway activity inhibits radial glial formation at the expense of other fates.
Sequential activation and inhibition of Wnt signaling is required for GABAergic neurogenesis
Surprisingly, we found that both activation and inhibition of Wnt signaling led to arrest in the Sox3+ progenitor state, and failure of further differentiation. Our data support a model proposed previously in retinal development (Agathocleous et al., 2009), in which Wnt signaling must be active in order for uncommitted progenitors to acquire the competence to differentiate (Fig. 7), but must then be inhibited for cells to complete the differentiation process. Consistent with this model, we find that Wnt signaling is active in postmitotic progenitors before they express Dlx genes, but is absent as they differentiate (Fig. 7). A recent study examining constitutive activation of Wnt signaling in adult neural stem cells also supports this model (Imura et al., 2010). In our study, BrdU tracing only allowed us to follow proliferating cells, leaving open the possibility that once committed progenitors have already received the necessary Wnt signal, pathway inhibition will promote their differentiation.
Consistent with other work suggesting that Wnts generally act as mitogens (Megason and McMahon, 2002), we found that Wnt activity is associated with progenitor proliferation at embryonic and post-embryonic stages. However we also showed a clear role for Wnt activity in post-mitotic neural progenitors, as Sox3+/PCNA- cells still fail to differentiate following Wnt pathway inhibition (Fig. 3C). Even following Wnt pathway activation, our data support a specific effect on neuronal differentiation in addition to any proliferative expansion (Fig. S4). Within a single cell population, the function of Wnt signaling may thus change over time in a context-dependent manner. Our data suggest that while Wnt activation promotes the mitotic expansion of progenitors, it also acts to hold cells in the progenitor state once they have become specified. In different cell populations, the specified state may or may not be proliferative, leading to seemingly conflicting phenotypes.
Hes1+ ventricular zone progenitors do not produce neurons in the adult mouse hypothalamus
Ventricular zones have been identified as the primary adult neural stem cell niches in the vertebrate brain (Garcia-Verdugo et al., 2002). Using Hes1C2 lineage tracing, we observed a significant number of cells expanding outside the ventricular zone, but these cells did not become neurons. Adult hypothalamic neurogenesis in mammals has only been characterized relatively recently (Yuan and Arias-Carrion, 2011), partly because very few neurons are produced at any time. Consistent with the idea that the mammalian hypothalamus contains a functionally significant level of adult neurogenesis during normal homeostasis (Lee et al., 2012; Yuan and Arias-Carrion, 2011), we observed numerous Dlx2+ cells in the parenchymal zone. While recent work has identified ventricular Nestin+ tanycytes of the hypothalamic medial eminence as neural progenitors in the postnatal mouse (Lee et al., 2012), it is not clear whether they contribute to homeostatic adult neurogenesis as the lineage was only analyzed until P75. Furthermore, this study described additional neurogenesis that did not arise from Nestin+ cells. It is possible, therefore, that a progenitor niche separate from the ventricular zone contributes to neurogenesis, or that Nestin−/Hes1− neural progenitors exist in the ventricular zone and subsequently migrate to the parenchyma (Migaud et al., 2010). To test the specific role of Wnt signaling in adult mouse hypothalamic neurogenesis, it will be necessary to employ conditional approaches using markers identified by our BAT-LacZ analysis, such as Sox2, GFAP, and Dlx2, in addition to Nestin.
The role of Wnt signaling in hypothalamic radial glia
Our data show that tanycyte formation and maintenance does not require Wnt signaling. In contrast, we find that Wnt pathway activation leads to decreased tanycyte numbers at the expense of neurons in zebrafish, and other glial cell types in mouse. Other work has revealed a similar role for β-catenin in Bergmann glia, which are transformed into astrocyte-like cells following Apc mutation (Wang et al., 2011). Interestingly, zebrafish do not contain true astrocytes but instead have radial astroglial-like cells throughout their brain (Grupp et al., 2010). Combined with the known neurogenic capacity of radial glia in the adult zebrafish brain (Kroehne et al., 2011), this raises the interesting possibility that the alternative fates promoted by Wnt signaling differ between zebrafish and mouse due to the different developmental potential of glial progenitors.
In summary, this work resolves some of the seemingly conflicting conclusions regarding the role of Wnt signaling in neurogenesis, from studies in different systems using different manipulations. We believe that many of these results can be explained by a dual role for the pathway, first in expansion of unspecified progenitors, then later as a required inducer of neuronal differentiation that must subsequently be inhibited for neurogenesis to occur. Furthermore, we have established the vertebrate hypothalamus as a model system for Wnt function in post-embryonic progenitor differentiation, and opened the field to future studies examining the role of this process in animal behavior.
Experimental Procedures
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Utah.
Zebrafish
Zebrafish embryos were obtained from natural spawning of wild-type (AB*), transgenic, and mutant adult fish. A detailed description of the strains used can be found in the Supplemental Experimental Procedures. Adult brains were dissected from anesthetized fish fixed in 4% paraformadehyde for 2 days. Fish received BrdU pulses by immersion in E3 media with 10mM BrdU, and received heat shock by immersion in prewarmed E3 media.
Mice
A detailed listing of mouse strains used can be found in the Supplemental Experimental Procedures. Tamoxifen (Sigma T-5648) was dissolved in corn oil, and administered by oral gavage at doses of 10 mg (Hes1 lineage) or 2 mg (β-catenin loss and gain of function experiments) per mouse between 6–8 weeks of age.
X-gal staining, in situ hybridization and immunohistochemistry
For X-gal staining in mice, fixed whole brains were sliced at 500μm thickness, and staining was performed as described previously (Seymour et al., 2004) followed by clearing in 100% glycerol. Mouse immunostaining and analysis were performed on 40μm cryosections as described previously (Kopinke et al., 2011). Zebrafish in situ hybridization and immunohistochemistry were performed as described previously (Oxtoby and Jowett, 1993), except that whole mount brains were first dissected (4dpf and adult) and sliced longitudinally (adult), followed by collagenase treatment. All mRNA probes have been described previously (Lee et al., 2006; Wang et al., 2009). A detailed list of antibodies used can be found in the Supplemental Experimental Procedures.
Cryosectioning and microscopy
Cryosections were cut at a thickness of 12μm for embryonic zebrafish and 40μm for adult mouse brains. All analysis of zebrafish brains was performed on whole-mount samples, except for Lef1 immunohistochemistry. Fluorescent images of whole mount zebrafish brains were taken using an Olympus FV1000 confocal microscope and an Olympus SZX12 fluorescent dissecting microscope. Maximum intensity Z-projections were used for all quantitative analyses. Bright field images were obtained using an Olympus BX51WI compound microscope.
Data Analysis
Colocalization analysis, volume measurements, statistical calculations, and graphs were generated with Image J, Volocity5.4, Amira 5.3.3, Microsoft Excel, and Sigma Plot 10.0. All analyses in zebrafish were perfumed on whole-mount brains. For analysis at 32hpf, the entire hypothalamus was counted using the third ventricle and nuclear staining as landmarks. For analysis at 4dpf-1mpf, a rectangle defining the entire posterior recess was counted, using the ventricle and nuclear staining as landmarks. For analysis of adults, a rectangle defining the posterior recess in a mid-sagittal view was counted, using the ventricle and nuclear staining as landmarks. All analyses in mouse were performed on cryosections using morphological landmarks to define the mediobasal hypothalamus. Error bars represent standard deviations from at least 3 samples (N is listed in each figure legend), and comparisons of data sets were performed with one-tailed homoscedastic or heteroscedastic t-tests.
Quantitative RT-PCR
Total RNA was isolated using an RNeasy extraction kit (Qiagen) followed by DNase treatment. cDNA was synthesized by SuperScript II reverse transcriptase (Invitrogen). mRNA levels were normalized to an average of beta-actin. Quantitative realtime PCR using the Sybr Green reagent was performed with an ABI7900, using primers listed below:
axin2: F: TGAAGCGGGAACAGGAAAC; R: AGGAGCAAAGGCAGAGAA.
beta-actin: F: AGGATGCGGAAACTGGAAAG; R: GAGGAGGGCAAAGTGGTAAA.
Isolation of lef1 mutants using engineered zinc-finger nuclease
To introduce mutations in the lef1 gene, a target site was chosen within the exon 1 using ZiFiT (http://zifit.partners.org/ZiFiT/). ZFNs were prepared using a detailed protocol posted elsewhere (http://wiki.zfin.org/), in which three-finger array libraries were constructed using OPEN pools (Maeder et al., 2009), but modified to be selectable in bacterial one-hybrid (B1H) system (Maeder et al., 2008). Selected three-finger array sequences were converted to ZFNs by fusion with the FokI nuclease domain. Synthetic mRNAs encoding the ZFNs were injected into one-cell stage zebrafish embryos. Mutations were identified by loss of BsaJI restriction sequence located in the target site. Genomic DNA was extracted from the individual 24 hpf embryos and amplified with the following primers:
F: TTGGAGGTGTGCTACTCACG; R: CACTCTCTCCAGCCCAACAT.
To isolate germ-line transmitted lef1 mutations, ZFN-injected embryos were raised to adulthood and progeny were analyzed using PCR and BsaJI digestion.
Supplementary Material
Highlights.
Sequential Wnt activation and inhibition mediates hypothalamic neurogenesis.
Wnt signaling is required for zebrafish hypothalamic neurogenesis throughout life.
Wnt activity negatively regulates the formation of hypothalamic radial glia.
Similar Wnt activity and function are present in the adult mouse hypothalamus.
Acknowledgments
We thank Michael Klymkowsky (University of Colorado at Boulder) for sharing the Sox3 antibody, Jan Kaslin (Monash University, Australia) for sharing unpublished data, and Sabine Fuhrmann and Gabrielle Kardon (University of Utah) for providing BAT-LacZ mice. FA is supported by EU grant ZF-HEALTH CT-2010-242048. R.I.D. was supported by NIH (NINDS); R21NS055138.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agathocleous M, Iordanova I, Willardsen MI, Xue XY, Vetter ML, Harris WA, Moore KB. A directional Wnt/beta-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development. 2009;136:3289–3299. doi: 10.1242/dev.040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsland M, Ramskold D, Zaouter C, Klum S, Sandberg R, Muhr J. Sequentially acting Sox transcription factors in neural lineage development. Genes & development. 2011;25:2453–2464. doi: 10.1101/gad.176008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Chapouton P, Jagasia R, Bally-Cuif L. Adult neurogenesis in non-mammalian vertebrates. Bioessays. 2007;29:745–757. doi: 10.1002/bies.20615. [DOI] [PubMed] [Google Scholar]
- Chapouton P, Webb KJ, Stigloher C, Alunni A, Adolf B, Hesl B, Topp S, Kremmer E, Bally-Cuif L. Expression of hairy/enhancer of split genes in neural progenitors and neurogenesis domains of the adult zebrafish brain. The Journal of comparative neurology. 2011;519:1748–1769. doi: 10.1002/cne.22599. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Sheldahl LC, Moon RT. A Transgenic Lef1/beta-Catenin-Dependent Reporter Is Expressed in Spatially Restricted Domains throughout Zebrafish Development. Dev Biol. 2002;241:229–237. doi: 10.1006/dbio.2001.0515. [DOI] [PubMed] [Google Scholar]
- Galceran J, Miyashita-Lin EM, Devaney E, Rubenstein JL, Grosschedl R. Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development. 2000;127:469–482. doi: 10.1242/dev.127.3.469. [DOI] [PubMed] [Google Scholar]
- Garcia-Verdugo JM, Ferron S, Flames N, Collado L, Desfilis E, Font E. The proliferative ventricular zone in adult vertebrates: a comparative study using reptiles, birds, and mammals. Brain Res Bull. 2002;57:765–775. doi: 10.1016/s0361-9230(01)00769-9. [DOI] [PubMed] [Google Scholar]
- Grupp L, Wolburg H, Mack AF. Astroglial structures in the zebrafish brain. The Journal of comparative neurology. 2010;518:4277–4287. doi: 10.1002/cne.22481. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura T, Wang X, Noda T, Sofroniew MV, Fushiki S. Adenomatous polyposis coli is essential for both neuronal differentiation and maintenance of adult neural stem cells in subventricular zone and hippocampus. Stem Cells. 2010;28:2053–2064. doi: 10.1002/stem.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizil C, Kaslin J, Kroehne V, Brand M. Adult neurogenesis and brain regeneration in zebrafish. Dev Neurobiol. 2011 doi: 10.1002/dneu.20918. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol. 2007;505:209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- Kopinke D, Brailsford M, Shea JE, Leavitt R, Scaife CL, Murtaugh LC. Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development. 2011;138:431–441. doi: 10.1242/dev.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroehne V, Freudenreich D, Hans S, Kaslin J, Brand M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development. 2011;138:4831–4841. doi: 10.1242/dev.072587. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, Takiar V, Charubhumi V, Balordi F, Takebayashi H, et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nature neuroscience. 2012;15:700–702. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Wu SF, Goering LM, Dorsky RI. Canonical Wnt signaling through Lef1 is required for hypothalamic neurogenesis. Development. 2006;133:4451–4461. doi: 10.1242/dev.02613. [DOI] [PubMed] [Google Scholar]
- Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- Machon O, Backman M, Machonova O, Kozmik Z, Vacik T, Andersen L, Krauss S. A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Developmental Biology. 2007;311:223–237. doi: 10.1016/j.ydbio.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Maeder ML, Thibodeau-Beganny S, Osiak A, Wright DA, Anthony RM, Eichtinger M, Jiang T, Foley JE, Winfrey RJ, Townsend JA, et al. Rapid “open-source” engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol Cell. 2008;31:294–301. doi: 10.1016/j.molcel.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder ML, Thibodeau-Beganny S, Sander JD, Voytas DF, Joung JK. Oligomerized pool engineering (OPEN): an ‘open-source’ protocol for making customized zinc-finger arrays. Nat Protoc. 2009;4:1471–1501. doi: 10.1038/nprot.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw HF, Drerup CM, Culbertson MD, Linbo T, Raible DW, Nechiporuk AV. Lef1 is required for progenitor cell identity in the zebrafish lateral line primordium. Development. 2011;138:3921–3930. doi: 10.1242/dev.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud M, Batailler M, Segura S, Duittoz A, Franceschini I, Pillon D. Emerging new sites for adult neurogenesis in the mammalian brain: a comparative study between the hypothalamus and the classical neurogenic zones. Eur J Neurosci. 2010;32:2042–2052. doi: 10.1111/j.1460-9568.2010.07521.x. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro E, Ozhan-Kizil G, Mongera A, Beis D, Wierzbicki C, Young RM, Bournele D, Domenichini A, Valdivia LE, Lum L, et al. In vivo Wnt signaling tracing through a transgenic biosensor fish reveals novel activity domains. Developmental biology. 2012 doi: 10.1016/j.ydbio.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Muncan V, Faro A, Haramis AP, Hurlstone AF, Wienholds E, van Es J, Korving J, Begthel H, Zivkovic D, Clevers H. T-cell factor 4 (Tcf7l2) maintains proliferative compartments in zebrafish intestine. EMBO Rep. 2007;8:966–973. doi: 10.1038/sj.embor.7401071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutch CA, Schulte JD, Olson E, Chenn A. Beta-catenin signaling negatively regulates intermediate progenitor population numbers in the developing cortex. PloS one. 2010;5:e12376. doi: 10.1371/journal.pone.0012376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayoshi S, Hayashi E, Abe G, Osato N, Asakawa K, Urasaki A, Horikawa K, Ikeo K, Takeda H, Kawakami K. Insertional mutagenesis by the Tol2 transposon-mediated enhancer trap approach generated mutations in two developmental genes: tcf7 and synembryn-like. Development. 2008;135:159–169. doi: 10.1242/dev.009050. [DOI] [PubMed] [Google Scholar]
- Oxtoby E, Jowett T. Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 1993;21:1087–1095. doi: 10.1093/nar/21.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martin M, Cifuentes M, Grondona JM, Lopez-Avalos MD, Gomez-Pinedo U, Garcia-Verdugo JM, Fernandez-Llebrez P. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. Eur J Neurosci. 2010a;31:1533–1548. doi: 10.1111/j.1460-9568.2010.07220.x. [DOI] [PubMed] [Google Scholar]
- Perez-Martin M, Cifuentes M, Grondona JM, Lopez-Avalos MD, Gomez-Pinedo U, Garcia-Verdugo JM, Fernandez-Llebrez P. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. The European journal of neuroscience. 2010b;31:1533–1548. doi: 10.1111/j.1460-9568.2010.07220.x. [DOI] [PubMed] [Google Scholar]
- Pierce AA, Xu AW. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J Neurosci. 2010;30:723–730. doi: 10.1523/JNEUROSCI.2479-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez EM, Blazquez JL, Pastor FE, Pelaez B, Pena P, Peruzzo B, Amat P. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- Seymour PA, Bennett WR, Slack JM. Fission of pancreatic islets during postnatal growth of the mouse. J Anat. 2004;204:103–116. doi: 10.1111/j.1469-7580.2004.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Tang M, Villaescusa JC, Luo SX, Guitarte C, Lei S, Miyamoto Y, Taketo MM, Arenas E, Huang EJ. Interactions of Wnt/beta-catenin signaling and sonic hedgehog regulate the neurogenesis of ventral midbrain dopamine neurons. J Neurosci. 2010;30:9280–9291. doi: 10.1523/JNEUROSCI.0860-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia LE, Young RM, Hawkins TA, Stickney HL, Cavodeassi F, Schwarz Q, Pullin LM, Villegas R, Moro E, Argeton F, et al. Lef1-dependent Wnt/β-catenin signalling drives the proliferative engine that maintains tissue homeostasis during lateral line development. Development. 2011;138 doi: 10.1242/dev.062695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Imura T, Sofroniew MV, Fushiki S. Loss of adenomatous polyposis coli in Bergmann glia disrupts their unique architecture and leads to cell nonautonomous neurodegeneration of cerebellar Purkinje neurons. Glia. 2011;59:857–868. doi: 10.1002/glia.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lee JE, Dorsky RI. Identification of Wnt-responsive cells in the zebrafish hypothalamus. Zebrafish. 2009;6:49–58. doi: 10.1089/zeb.2008.0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger G, Thorpe CJ, Wuennenberg-Stapleton K, Ngai J, Moon RT. The Sp1-related transcription factors sp5 and sp5-like act downstream of Wnt/beta-catenin signaling in mesoderm and neuroectoderm patterning. Curr Biol. 2005;15:489–500. doi: 10.1016/j.cub.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N, Dezawa M, Ide C. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol. 2005;192:251–264. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Yee CL, Wang Y, Anderson S, Ekker M, Rubenstein JL. Arcuate nucleus expression of NKX2.1 and DLX and lineages expressing these transcription factors in neuropeptide Y(+), proopiomelanocortin(+), and tyrosine hydroxylase(+) neurons in neonatal and adult mice. J Comp Neurol. 2009;517:37–50. doi: 10.1002/cne.22132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan TF, Arias-Carrion O. Adult Neurogenesis in the Hypothalamus: Evidence, Functions, and Implications. CNS Neurol Disord Drug Targets. 2011 doi: 10.2174/187152711795563985. [DOI] [PubMed] [Google Scholar]
- Zhou CJ, Zhao C, Pleasure SJ. Wnt signaling mutants have decreased dentate granule cell production and radial glial scaffolding abnormalities. J Neurosci. 2004;24:121–126. doi: 10.1523/JNEUROSCI.4071-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.