Abstract
Background
Allogeneic mesenchymal stem (stromal) cells (MSC) are a promising therapy for various pathological conditions. Genetically-modified pig MSC have been demonstrated to downregulate the human T cell response to pig antigens in vitro. Before genetically-modified pig MSC can be used clinically, however, evidence needs to be provided to indicate whether they will survive in a human (xenogeneic) host.
Literature Search and Results
A literature search through the end of 2011 identified 94 reports of the in vivo cross-species administration of MSC in a variety of experimental models. The majority (n=89) involved the use of human MSC in various other species, with an occasional study using pig, rat, or guinea pig MSC. When human MSC were used, they were largely derived from the bone marrow, adipose tissue, or umbilical cord blood. The routes of administration were varied, though almost half of the studies utilized the intravenous route. In 88 experiments (93.6%) there was evidence that the MSC engrafted and functioned across the species barrier, and in only 6 cases (6.4%) was there evidence of failure to function. Importantly, MSC function was confirmed in several different cross-species models. For example, human MSC functioned in no fewer than 7 different recipient species.
Conclusions
The data provided by this literature search strengthen the hypothesis that pig MSC will function satisfactorily in a different species, e.g., humans. The data also suggest that our own in vitro observations on the efficacy of pig MSC in downregulating the strength of the human T cell response to pig antigens will likely be reproduced in vivo in preclinical large animal models and in clinical trials.
Keywords: Mesenchymal stem (stromal) cells, Pig, genetically-engineered, In vivo, Xenotransplantation
INTRODUCTION
Allogeneic mesenchymal stem (stromal) cells (MSC) may be therapeutic in several pathologic conditions [1,2], e.g., steroid-refractory acute graft-versus-host disease [3,4], autoimmune disorders [5], and islet transplantation [6–8]. Encouraging results have been obtained in animal models of ischemic myocardial injury [9], pulmonary hypertension [10], sepsis [11], renal ischemia-reperfusion [12], spinal injury [13], and diabetes [14]. MSC have anti-inflammatory, anti-proliferative, angiogenic, and immunomodulatory functions, and may also be a vehicle for gene therapy and drug delivery [15]. Their therapeutic potential in inhibiting the immune response following organ transplantation is being studied [16–18].
Their immunomodulatory effects have been studied in vivo in various preclinical [19] and clinical models [3, 4]. MSC suppress the proliferation of CD4+T cells [19], prevent maturation of dendritic cells [20], induce T regulatory cells [19], and produce soluble factors, such as prostaglandin E2 (PGE2), transforming growth factor-1 (TGF-1), interleukin-10 (IL-10), hepatocyte growth factor (HGF), and indoleamine 2,3-dioxygenase (IDO), all of which have immunomodulatory effects. MSC may therefore prove of value as cytotherapeutic agents. Increasing data suggest that both cell-cell contact and secretion of soluble cytokines play roles, with cell contact perhaps being more important [21,22].
Currently, MSC from humans are defined based upon 3 minimal criteria - (i) plastic adherence, (ii) trilineage differentiation, (iii) surface expression of CD73, CD90, CD105 and absence of expression of CD45, CD34, CD14 or CD11b, CD79α or CD19, and human leukocyte antigen (HLA)-DR. MSC from animal origin have been defined as cells that fulfill the first two criteria [23].
Kolf et al provide a useful comparison of human and mouse MSC phenotype. The phenotype of mouse MSC includes positivity for CD29, CD44, CD105, CD106, and negativity for CD11b, CD31, CD34, CD45, and CD117. Determining the phenotype of MSC from large animal species is limited by a lack of species-specific antibodies, and so anti-human antibodies have been used for common MSC-selective markers [24]. Boxall and Jones provide a valuable comparison of MSC phenotype between several species, including the human, mouse, and pig. Rho et al also reported on the phenotype of pig MSC [25]. To date, the accepted phenotype of pig MSC includes positivity for CD29, CD44, CD49, CD90, CD105, and SLA-I, and negativity for CD11b, CD14, CD31, CD34, CD45, CD73, CD117, CD133, and SLA-II [26].
Clinical allogeneic MSC therapy depends on effective ex vivo expansion of the cells, and is therefore dependent on the availability of relatively large volumes of bone marrow or adipose tissue and/or extended culture time. However, ex vivo expansion of MSC can be associated with a risk of chromosomal instability [27,28], reduction in cytokine production, and loss of multipotentiality [29] with each passage, and with risks of senescence [30,31] and malignant change [28,32,33]. Furthermore, during expansion, pro-inflammatory (instead of anti-inflammatory) forms of MSC can develop [34], and might account for some of the conflicting observations in MSC immunobiology that have been reported. A logical option that might avoid untoward complications of this nature would be to use low-passage MSC. Human MSC usually reach replicative senescence following 25 cell divisions [31], potentially limiting their expansion for therapeutic purposes.
There may be considerable potential in obtaining the MSC from animal tissues. MSC can be obtained from genetically-modified pigs, e.g., α1,3-galactosyltransferase gene-knockout (GTKO) pigs that additionally express a human complement-regulatory gene and/or an immunosuppressive gene, which would protect the MSC against the human humoral and cellular immune responses. The frequency of MSC is low in adult bone marrow aspirates; the fibroblastoid colony-forming unit frequency from bone marrow has been found to be approximately 0.01% of nucleated cells. Adipose tissue has the highest frequency of MSC, approximately 2×104 fibroblastoid colony-forming units can be obtained initially from 10g of fat tissue, suggesting that approximately 2×108 bone marrow cells are equivalent to 10g of fat tissue [22]. We have previously reported that the human T cell response to GTKO pig MSC is comparable to human MSC [35,36].
Before genetically-modified pig MSC can be used clinically, evidence needs to be provided to indicate whether pig MSC will survive in a human (xenogeneic) host, how efficiently they will suppress the human (xenogeneic) immune response, and whether any adverse effects can be anticipated. To gain some insight into the fate and effect of MSC that are administered across species barriers, we have reviewed the available literature. We have focused our attention on the efficacy of MSC to function across species barriers. We have not considered other aspects of the clinical use of MSC, such as their safety and the selection of suitable candidates for this form of therapy in relation to xenotransplantation.
LITERATURE SEARCH
We have searched the literature to determine what experience exists in the use of MSC derived from a different species from the recipient species into which the MSC are administered. We did not include studies that were solely in vitro. As of the end of 2011, we found a total of 94 reports of the in vivo cross-species administration of MSC (Table 1). The majority (n=89) involved the use of human MSC in various other species, with an occasional study using pig (n=3), rat (n=1) or guinea pig (n=1) MSC. When mice were the recipients of MSC, these included NOD/SCID (n=24), SCID (n=7), nude (n=13), autoimmune pathogenic (n=6), and unmodified (n=13) mice (Table 1).
TABLE 1.
REPORTS IN THE LITERATURE OF CROSS-SPECIES ADMINISTRATION OF MESENCHYMAL STEM CELLS
| Donor Species | Recipient Species | Studies (n) | Reference # |
|---|---|---|---|
| human | NOD/SCID mouse | 24 | 38–40,55,56,58,59,73,79,82,84,88,92–94,98–100,104,112,123,131–133 |
| nude mouse | 13 | 61,63,66–68,80,86,105,111,120,121,124,127 | |
| wild-type mouse | 12 | 48,52,57,101,102,106,108–110,113,125,134 | |
| autoimmune mouse | 6 | 69,70,72,103,108,122 | |
| SCID mouse | 5 | 60,76,78,101,126 | |
| rat | 18 | 41,47,49,50,52,54,62,65,75,77,85,89,114–119 | |
| hamster | 2 | 87,90 | |
| sheep | 3 | 81,83,129 | |
| dog | 2 | 91,128 | |
| pig | 2 | 42,51 | |
| rabbit | 2 | 37,64 | |
| pig | SCID mouse | 2 | 95,96 |
| rat | 1 | 97 | |
| rat | wild type mouse | 1 | 74 |
| guinea pig | rat | 1 | 130 |
|
| |||
| TOTAL | 94 | ||
Cross-species MSC were used in a variety of in vivo experimental models (Table 2). The routes of administration were varied (Table 3), though almost half of the studies utilized the intravenous route.
TABLE 2.
EXPERIMENTAL MODELS IN WHICH THE EFFECTS OF CROSS-SPECIES MESENCHYMAL STEM CELLS HAVE BEEN STUDIED
| Model | Studies (n) | Reference # |
|---|---|---|
| 1) Unmodified (healthy) | 18 | 42,80,81,83,91,94,97,104,107,108,114,119,120,124,125,129,133,134 |
| 2) Irradiation injury | 7 | 58,59,82,84,88,99,131 |
| 3) Malignance model | 11 | 38,40,66–68,76,105,109,111,113,123 |
| 4) Acute myocardial infarction | 6 | 52,54,85,90,95,100 |
| 5) Acute liver dysfunction | 5 | 39,60,61,86,126 |
| 6) Autoimmune disease | 6 | 69–72,103,122 |
| 7) Acute cerebral infarction | 5 | 49,50,75,89,115 |
| 8) Transplantation | 9 | 73,74,92,93,96,98,112,130,132 |
| 9) Diabetes | 4 | 55–57,78 |
| 10) Inflammatory | 4 | 53,106,110,121 |
| 11) Spinal cord injury | 3 | 47,77,128 |
| 12) Acute kidney injury | 3 | 62,79,127 |
| 13) Muscle injury | 3 | 65,87,118 |
| 14) Neuropathic | 3 | 41,48,102 |
| 15) Lumbar disc injury | 2 | 51,117 |
| 16) Bone defect | 2 | 37,63 |
| 17) Acute lung injury | 1 | 101 |
| 18) Retinal injury | 1 | 64 |
| 19) Intracerebral hemorrhage | 1 | 116 |
|
| ||
| TOTAL | 94 | |
TABLE 3.
ROUTES OF ADMINISTRATION OF MESENCHYMAL STEM CELLS
| Route (Site of injection) | Studies (n) | Reference # | |
|---|---|---|---|
| 1) Intravenous | 47 | 38,48–50,53,57–59,62,66,68,70,72–74,76,77,79,80,82,84,89,92–94,96–98,100,103,106–108,111–113,115,120,123–125,127,130–134 | |
| 2) Organ/Tissue | 36 | ||
| heart | 7 | 42,52,54,55,85,91,95 | |
| brain | 7 | 41,75,102,105,109,114,122 | |
| liver | 3 | 86,104,119 | |
| spinal cord | 4 | 47,51,117,128 | |
| muscle | 4 | 65,87,90,121 | |
| spleen | 3 | 39,60,126 | |
| tissue defect area | 3 | 37,63,118 | |
| bone marrow | 2 | 40,88 | |
| eye | 1 | 64 | |
| kidney | 1 | 78 | |
| 3) Intraperitoneal | 9 | 61,69,71,81,83,94,110,116,129 | |
| 4) Subcutaneous | 2 | 67,94 | |
| 5) Intratracheal | 1 | 101 | |
|
| |||
| TOTAL | 94 | ||
Details of all 94 experimental studies are provided in Tables 4–8. No studies included a strict comparison between the results of the administration of allogeneic and xenogeneic MSC, and therefore it has not been possible to make this comparison.
TABLE 4.
STUDIES INVOLVING THE ADMINISTRATION OF HUMAN BONE MARROW MESENCHYMAL STEM CELLS (n=54)
| Recipient | Exp model | Route | # of MSCs | Conclusions | Reference |
|---|---|---|---|---|---|
| Unmodified (healthy) | |||||
| rat | unmodified | liver | 1×106 | Engrafted into liver, with hepatic differentiation. | 119 |
| nude mouse | unmodified | IV | 2×105 | Migrated to BM, spleen, and mesenchymal tissues after Tx. | 120 |
| nude mouse | unmodified | IV | Uncertain | Cartilage formation occurred after 6w. | 124 |
| pig | unmodified | Heart | 15–35×105 | Caused inflammation. | 42 |
| NOD/SCID mouse | unmodified | IV | Uncertain | Potential of transformed cells in hMSC culture and highlight the need for karyotyping. | 133 |
| C57/B6 mouse | unmodified | IV | Uncertain | Undifferentiated MSC are detected in majority of case. | 134 |
| fetal sheep | fetal sheep | IP | 1– 2×108/kg | Maintained multipotential capacity and unique immunologic characteristics after Tx. | 81 |
| fetal sheep | fetal sheep | IP | 2×107 | Engrafted and differentiated into multiple cell types. Survived >1y. | 83 |
| Neurological | |||||
| rat | spinal cord injury | spinal cord | 5×105 | Supported axonal growth after spinal cord injury. | 47 |
| rat | spinal cord injury | (1)IV (2)lumbar puncture (3)local injection |
1×106 | Lumbar puncture is an ideal technique to deliver MSCs which can get better cell engraftment and tissue sparing. | 77 |
| CD1 mouse | spared nerve injury | IV | 2×106 | Reduced pain-like behavior (mechanical allodynia, thermal hyperalgesia). | 48 |
| rat | cerebral ischemia | brain | 5×105 | MSC transfected with the brain-derived neurotrophic factor promoted functional recovery in cerebral ischemia. | 75 |
| rat | cerebral ischemia | IV | 1×106 | Promoted free fatty acid metabolism in cerebral ischemia. | 89 |
| rat | cerebral ischemia | IV | 5×105 | Produced structural/functional recovery. | 49 |
| rat | cerebral ischemia | IV | 3×106 | Induced functional improvement, reduced infarct size, provided neuroprotection. | 50 |
| rat | cerebral ischemia | IV | 1×106 | Elicited functional improvement compared with the control sham group. | 115 |
| C57/B6 mouse | Huntington’s disease | brain | 2×105 | Neural differentiation improvement potential, neurotrophic support capability, anti-apoptotic effect. | 102 |
| Musculoskeletal | |||||
| minipig | lumbar discs injured | Intervertebral discs | 5×105 | Survived in disc >6m. Differentiated toward disc-like cells | 51 |
| rat | degenerative intervertebral discs | intervertebral discs | 1× 106 | Increased the heights and signal intensities of intervertebral disc. | 117 |
| hamster | muscle dystrophy | IM | 5–10×105 | Contributed to both preexisting and new muscle fibers, and mediated capillary formation. | 87 |
| rabbit | bone defect | bone defect | 5×106 | The xenogenic treatment group displayed inferior results in all parameters compared with the autogenous MSC treatment group. | 37 |
| Cardiovascular | |||||
| rat | myocardial infarction | heart | 3×106 | Survived and contributed to improvement in cardiac function. | 85 |
| hamster | cardiomyopathy | IM | 2–4×106 | VEGF is a key therapeutic trophic factor in MSC- mediated myocardial regeneration. | 90 |
| rat | myocardial Infarction | heart | 3×106 | Improved cardiac function and reduced infarctionsize. | 52 |
| NOD/SCID mouse | myocardial infarction | IV | 2×106 | Enhanced cardiac function. | 100 |
| C57BL/6 mouse | inflammatoy cardiomyopathy | IV | 5×105 | Improved acute myocarditis. | 53 |
| rat | myocardial infarction | heart | 1×107 | MSC can improve left ventricular ejection fraction. | 54 |
| dog | pacemaker implantation | heart | 15×104– 1×106 | Provided a means for administering pacemakers that functioned 6w without cellular or humoral rejection. | 91 |
| Irradiation injury | |||||
| NOD/SCID mouse | TBI or ALI | IV | 5×105 | TBI can increase MSC implantation into bone marrow and other tissues | 82 |
| NOD/SCID mouse | TBI and/or ALI | IV | 5×106 | Repaired damaged tissues following irradiation. | 84 |
| NOD/SCID mouse | TBI | BM | 1×106 | Reconstituted hematopoietic microenvironment. Contributed to the maintenance human hematopoiesis. | 88 |
| NOD/SCID mouse | ALI | IV | 5×106 | MSC can prevent AST and ALT increasing after ALI. | 58 |
| NOD/SCID mouse | radiation-induced injury | IV | 5 ×106 | MSC bring fast recovery to small intestine function and structure. | 59 |
| NOD/SCID mouse | radiation injury of intestine | IV | 5×106 | Increased self-renewal of small intestinal epithelium. Accelerated structural recovery. | 131 |
| Malignancy | |||||
| SCID mouse | malignant melanoma | IV | 75×104– 1×106 | Engrafted and incorporated into tumor vessels to participate in angiogenesis | 76 |
| NOD/SCID mouse | chronic erythroleukemia | IV | 1×105 | Reduce the antitumor activities of cytokine- induced killer/natural killer cells in vivo. | 38 |
| NOD/SCID mice | breast cancer | BM | 2×105 | Accelerate human breast tumor growth. | 40 |
| Nude mouse | hepatocellular carcinoma. | SC/IV | 6×106/5×105 | Enhanced tumor growth but significantly inhibited the invasiveness and metastasis. | 111 |
| nude mouse | Kaposi’s sarcoma | IV | 4×106 | Possessed intrinsic antineoplastic properties. | 66 |
| nude mouse | renal cell carcinoma | SC | 5 ×106 | Reduced growth of renal cell carcinoma. Enhanced survival. | 67 |
| NOD/SCID mouse | multiple lung metastases | IV | 75×104 | Tracked to multiple lung metastases. | 123 |
| nude mouse | cancer metastasis | IV | uncertain | Reduced lung metastasis. Inhibited growth of human cancer by inducing apoptosis | 68 |
| Liver or kidney injury | |||||
| SCID mouse | hepatic injury | spleen | 1×106 | Engrafted into the host liver parenchyma, and differentiated into hepatocyte-like cells expressing human albumin and α-1-anti-trypsin. | 60 |
| NOD/SCID mouse | hepatic injury | spleen or liver | 5×105 – 1×106 | MSC in certain circumstances might be harmful due to their fibrogenic potential. | 39 |
| NOD/SCID mouse | acute kidney injury | IV | 5×105 | Reduced proximal tubular epithelial cell injury and ameliorated the deficit in renal function. | 79 |
| Nude mouse | glomerulonep hropathy | IV | 5×105 | Found in renal glomeruli. Differentiated into mesangial cells after glomerular injury. | 127 |
| rat | acute renal failure | IV | uncertain | Ameliorated acute renal failure by differentiation into renal tubular epithelial-like cells. | 62 |
| Diabetes mellitus | |||||
| SCID mouse | diabetes (STZ) | kidney | 3×106 | MSCs transfected with three genes: PDX-1, NeuroD1 and Ngn3 can be induced to express insulin sufficient to reduce blood glucose. | 78 |
| NOD/SCID mouse | diabetes (STZ) | heart | 2.5×106 | Enhanced insulin secretion and perhaps improved the renal pathology | 55 |
| NOD/SCID mouse | diabetic (STZ) | IV | 42×106/kg | Safe and effective for blood glucose stabilization. | 56 |
| Transplantation | |||||
| NOD/SCID mouse | CD34+ human HSC Tx (TBI) | IV | 1–2×106 | CoTx with CD34+ HSCs enhanced myelopoiesis and megakaryocytopoiesis. | 73 |
| NOD/SCID mouse | HSC Tx | IV | 1–16 ×106 | CoTx with HSCs enhanced engraftment as the dose of MSCs increased. | 92 |
| Autoimmune disease | |||||
| MRL/lpr mouse | autoimmune diseases | IP | 1×106 | Significantly inhibited autoimmune progression. | 69 |
| C57/B6 mouse | autoimmune myasthenia gravis | IV | 1×106 | Homed specifically to spleen tissue. Improved functional deficits of autoimmune myasthenia gravis. | 70 |
Ad = adipose; ALI = additional local irradiation; BM = bone marrow; GVHD = graft-versus-host disease; HSC = hematopoietic stem cells; IM = intramuscular; IP = intraperitoneal; IV = intravenous; LP = lumbar puncture; MSC = mesenchymal stromal (stem) cells; SC = subcutaneous; STZ = streptozotocin; TBI = total body irradiation; Tx = transplantation; UCB = umbilical cord blood;
TABLE 8.
STUDIES INVOLVING THE ADMINISTRATION OF MESENCHYMAL STEM CELLS CO-TRANSPLANTED WITH A SPECIES-SPECIFIC XENOGRAFT (n=9)
| MSC Species | Recipient | Exp model | Route | Conclusions | Reference |
|---|---|---|---|---|---|
| human | NOD/SCID mouse | HSC Tx | IV | CoTx with CD34+ HSCs enhanced myelopoiesis and megakaryocytopoiesis. | 73 |
| human | NOD/SCID mouse | HSC Tx (TBI) | IV | CoTx with HSCs enhanced engraftment as dose of MSCs increased. | 92 |
| human | NOD/SCID mouse | UCB cell Tx | IV | Promoted hematopoietic engraftment. Limited GVHD. | 93 |
| human | NOD/SCID mouse | HSC Tx (TBI) | IV | Enhanced engraftment of human HSCs. | 98 |
| human | NOD/SCID mouse | UCB cell Tx | IV | Promoted hematopoietic reconstitution. | 132 |
| human | NOD/SCID mouse | HSCs Tx (TBI) | IV | Enhanced engraftment of HSCs. | 112 |
| rat | C57BL/6 mouse | skin Tx (TBI) | IV | Prolonged skin graft survival. | 74 |
| guinea pig | Rat | liver Tx | IV | Possible immunomodulation of hyperacute rejection. | 130 |
| pig | NOD/SCID mouse | BM Tx (TBI) | IV or BM | CoTx with HSCs improved short-term engraftment | 96 |
When human MSC were used (n=89), they were generally derived from the bone marrow (n=54) (Table 4), adipose tissue (n=7) (Table 5), or umbilical cord blood (n=20) (Table 6), with occasional use of MSC from embryonic stem cells (n=4), placenta, liver, and pancreas. Whenever pig, rat, or guinea pig MSC were used (n=5), they were usually bone marrow-derived (Table 7)
TABLE 5.
STUDIES INVOLVING THE ADMINISTRATION OF HUMAN ADIPOSE MESENCHYMAL STEM CELLS (n=7)
| Recipient | Exp model | Route | # of MSCs | Conclusions | Reference |
|---|---|---|---|---|---|
| NOD/SCID Mouse | unmodified | IV | 1×106 | Migrated to multiple tissues. | 94 |
| MRL/lpr mouse | unmodified | IV | 5×105 | Ameliorated systemic lupus erythematosus. Restored immune homeostasis. | 71 |
| SCID mouse | unmodified | IV | 5×106 –25×107/kg | Even at the high numbers (2.5×108 cells/kg), no side effects. | 107 |
| C57BL/6 mouse | unmodified | IV | 5×105 | Reduced inflammatory immune response. Improved, Th1/Th2 balance. | 108 |
| Nude mouse | skull defect | local injection | 15×104 | Ossified calvarial defect without need for pre-differentiation. | 63 |
| Rabbit | retinal defect | retinal defect area | 1×105 | Engrafted in retinal defect. Accelerated healing process. Ameliorated injury recovery. | 64 |
| nude mouse | hepatic injury | IP | 15×105 | Hepatocyte differentiation in vitro. Liver regeneration in vivo. | 61 |
TABLE 6.
STUDIES INVOLVING THE ADMINISTRATION OF HUMAN UMBILICAL CORD MESENCHYMAL STEM CELLS (n=20)
| Recipient | Exp model | Route | # of MSCs | Conclusions | Reference |
|---|---|---|---|---|---|
| Liver injury | |||||
| nude mouse | hepatic injury | liver | 3×106 | Enhanced recovery of CCl4-injured liver. | 86 |
| SCID mouse | hepatic injury | spleen | 1×106 | Engrafted. Expressed human albumin and alpha fetoprotein. | 126 |
| Transplantation | |||||
| NOD/SCID mouse | HSCs Tx | IV | 1×106 | Promoted hematopoietic engraftment. Limited GVHD. | 93 |
| NOD/SCID mouse | HSCs Tx (TBI) | IV | 25×105 | Enhanced engraftment of human HSCs. | 98 |
| NOD/SCID mouse | HSCs Tx (TBI) | IV | 25×105 | Enhanced engraftment of HSCs. | 112 |
| Autoimmune and inflammatory | |||||
| SJL mouse | spontaneous myopathy | IV | 1×106 | Engrafted in muscle. | 72 |
| BALB/c mouse | colitis | IV | 1×106 | Homed to inflamed colon. Ameliorated colitis. | 106 |
| rat | Parkinson’s disease | brain | 1×105 | Co-Tx with fibroblasts abrogated therapeutic efficacy and had damaging effects. | 41 |
| nude mouse | Buerger’s disease | skeletal muscle | 1×106 | Beneficial effect on ischemic limb disease. | 121 |
| transgenic mouse | Alzheimer’s disease | intracerebral | 1×105 | Ameliorated pathophysiology. Reversed cognitive decline. | 122 |
| Neurological | |||||
| ICR mouse | ataxic model | IV | 2×106 | Alleviated cerebellar atrophy. Decreased apoptotis. | 103 |
| rat | intracerebral hemorrhage | IP | 2×105 | Accelerated neurological functional recovery. | 116 |
| dog | spinal cords injured | spinal cord | 1×106 or 2×106 | Enhanced remyelination. | 127 |
| Irradiation injury | |||||
| NOD/SCID mouse | TBI | IP | 10×106 | Inhibited GVHD. | 99 |
| NOD/SCID mouse. | TBI | IV | 1×105 | Promoted hematopoietic reconstitution. Improved survival. | 132 |
| Malignancy | |||||
| C57BL/6 mouse | glioma-bearing | brain | 1×105 | Inhibited tumor growth. Prolonged survival. | 109 |
| C57BL/6 mouse | lung carcinoma | IV | 1×106 | Inhibited lung metastases. | 113 |
| Other | |||||
| nude mouse | unmodified | IV | 5×104 | Homed and survived in BM. | 80 |
| ICR mouse | acute lung injury | Intratracheal | 1×105 | Attenuated E. coli-induced acute lung injury. Down- modulated inflammatory process. | 101 |
| db/db mouse | diabetic wound | IV/local injection | 2×106 | Improved wound healing. | 57 |
TABLE 7.
STUDIES INVOLVING THE ADMINISTRATION OF NONHUMAN MESENCHYMAL STEM CELLS INTO RODENTS (n=5)
| MSC Species | Recipient | Exp model | Route | Conclusions | Reference |
|---|---|---|---|---|---|
| Pig | NOD/SCID mouse | myocardial infarction | Heart | Improved left ventricular ejection fraction. | 95 |
| Pig | NOD/SCID mouse | BM Tx (TBI) | IV | Co-Tx with HSCs improved short-term engraftment. | 96 |
| Pig | Rat | unmodified | IV | Differentiated along a neural lineage. | 97 |
| Rat | NOD/SCID mouse | skin Tx (TBI) | IV | Skin graft survival prolonged. | 74 |
| Guinea pig | Rat | liver Tx | IV | Possible immunomodulation of hyperacute rejection. | 130 |
A small number of studies (n=9; included in Tables 4–7) involved the administration of MSCs together with some form of organ or cell transplantation (Table 8). The majority of these studies involved cotransplantation of MSC with hematopoietic stem cells (HSC).
RESULTS AND DISCUSSION
In 88 of the 94 reports (93.6%) there was evidence that the MSC engrafted and/or functioned across the species barrier. In only 6 reports (6.4%) was there evidence of failure to function or of a detrimental effect of MSC.
Niemeyer et al found that, on the basis of histological, radiological and biomechanical studies, xenogeneic MSC seemed to be associated with inferior results when compared with autogenous MSC. After application of xenogeneic MSC to a critical-size bone defect model, significantly less bone formation was observed histologically when compared with autogenous MSC [37].
Li et al reported that pre-injected MSC reduced the anti-tumor activities of CIK/NK cells in vivo. The probable mechanism is that MSC and CIK/NK cells have a greater opportunity to interact if they are injected simultaneously [38].
Baertschiger et al found that when human bone marrow-derived MSC were transplanted into an injured or regenerating liver, they differentiated into myofibroblasts with development of fibrous tissue. These results indicated that MSC in certain circumstances might be harmful due to their fibrogenic potential, and that this possibility should be considered before administering MSC as cell therapy [39].
Liu et al demonstrated that human bone marrow-derived MSC may accelerate human breast tumor growth in NOD/SCID mice by generating cytokines that affect the cancer stem cell population [40].
Pereira et al reported that co-transplantation of human umbilical cord-derived MSC with fibroblasts exacerbated neurodegeneration in a rat model of Parkinson’s disease [41].
Lyngbaek et al found that transplantation of human bone marrow-derived MSC into a pig heart resulted in a rapid inflammatory response and cell degradation, which conflicted with previous studies that indicated a special immunoprivileged status for MSC [42].
When cotransplantation with donor-specific HSC was carried out, or MSC administration was combined with a skin or liver graft, there was almost uniform evidence of MSC function improving the outcome, e.g., improved engraftment of the HSC. There is, therefore, overwhelming evidence from in vivo studies in several different experimental models involving MSC from several species indicating that MSC function across species barriers.
Importantly, MSC function was confirmed in several different cross-species models (Figure 1). For example, human MSC functioned in no fewer than 7 different recipient species and MSC from 4 different species(human, pig, rat, guinea pig) were demonstrated to function across species barriers (Table 1).
Figure 1.
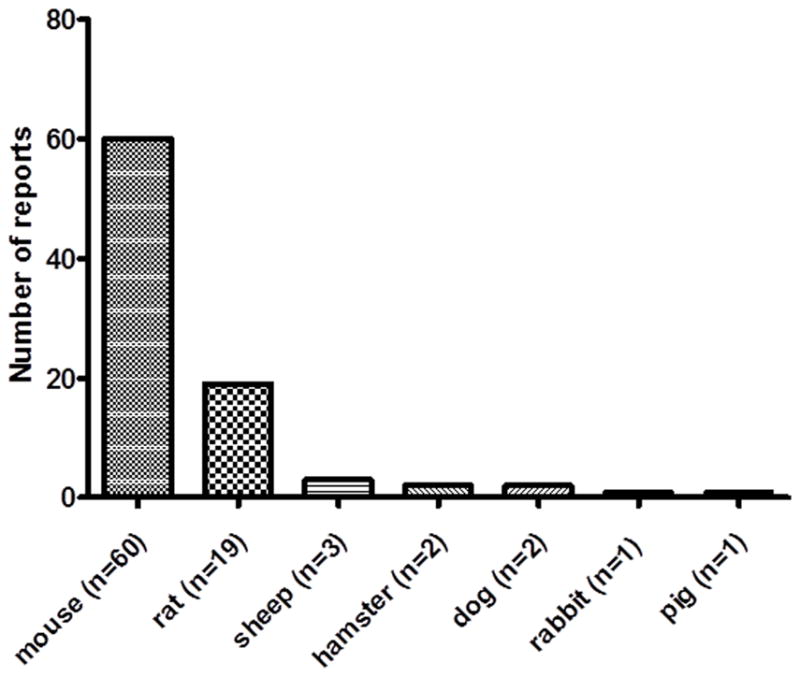
Recipient species in which cross-species MSC have been reported to engraft and/or function (total reports = 88). For example, human MSC functioned in no fewer than 7 different recipient species (see Table 1). Furthermore, MSC from 4 different donor species (human, pig, rat, guinea pig) were demonstrated to function across species barriers, i.e., in recipients of different species (see Table 1)
The number of MSC administered in these various experimental models varied considerably (from 1x105 to 16x106 or 2x108/kg) (Tables 4–6), and it would be unwise to attempt to determine from these data the optimum number of MSC that should be administered to ensure efficacy. The number of MSC administered (the dose) varied depending on the nature of the experiment and the condition for which the MSC were being administered, i.e., the disease model. Doses are unlikely to be the same in one model, e.g., spinal injury, as in another, e.g., diabetes or suppression of rejection of an allograft. In some conditions, MSC are administered locally at the site of a lesion, whereas in other conditions systemic administration is necessary. Dosing may therefore need to vary widely. In view of the great variety of models and species, current data do not allow an accurate conclusion to be drawn as to the ‘optimal’ number of MSC that should be administered.
Bone marrow was the original source of MSC, but there is increasing evidence that MSC can be harvested from a variety of sources, e.g., fat, umbilical cord blood, placenta. Adipose tissue probably has greater promise because the MSC can be harvested in very large numbers from this source, thus reducing the need for long-term culture in vitro. All the evidence is that adipose-derived MSC have similar characteristics (e.g., differentiation ability, phenotype, and immunomodulatory capacity) to bone marrow-derived MSC.
According to the literature, nearly half of all studies have selected the intravenous route, but this depends on the aim of the study. For example, for spinal cord and lumbar disc injury models, local injection has been preferred. In contrast, as would be expected, for models of malignancy, systemic administration has been selected.
MSC from pigs are an attractive option as pig MSC may have advantages over human MSC. (i) It is easier to obtain very large volumes of bone marrow or, particularly, adipose tissue from pigs than humans, and unlimited numbers of source pigs can be made available for this purpose. Pig adipose-derived MSC also have the advantage over bone marrow-derived MSC in having a high frequency of fibroblastoid colony-forming units [43], and thus are a richer source of MSC (with less need for proliferation). (ii) As pig MSC would be plentiful, there would be no need for extensive ex vivo expansion, thus lowering the risks of replicative senescence or malignant change. (iii) Bone marrow- and adipose-derived pig MSC have been demonstrated to have similar immunomodulatory functions [34–36]. (iv) In association with xenografts, e.g., pig islets, pig MSC can be obtained from the same pig source and/or from identical cloned pigs, thus negating exposure to third-party, i.e., allogeneic, MSC. (v) Allogeneic human MSC are known to be hypo-immunogenic, but are usually eliminated by NK cells in a Major Histocompatibility Complex (MHC)-unrestricted manner [44]; NK cytotoxicity is also likely to play a role in the destruction of pig MSC. However, further genetic modifications in pigs, e.g., expression of HLA-E/β2 microglobulin and/or of HLA-G molecules, may decrease the cytotoxicity of human NK cells [45], and may therefore protect the MSC from NK cytotoxicity.
The data provided by this literature search strengthen the hypothesis that pig MSC will function effectively in a different species, e.g., humans. The data suggest that our own in vitro observations on the efficacy of pig MSC in downregulating the strength of the human T cell response to pig antigens will be reproduced in in vivo preclinical large animal models and in clinical trials. MSC from genetically-engineered pigs may therefore provide an alternative to human MSC, at least in the context of xenotransplantation [46].
There are a relatively large number of clinical trials continuing at the present time, but to our knowledge these all involve autologous or allogeneic MSC. They relate to the treatment of various pathological conditions, such as graft-versus-host disease (GVHD) and autoimmune disorders [1–4], but also include their application in organ transplantation. However, to our knowledge, none of the clinical trials has involved cross-species MSC. To our knowledge, clinical trials of autologous or allogeneic MSC have not been associated with major complications, but a discussion of the safety aspects of pig MSC is beyond the confines of this review.
In conclusion, MSC from one species can clearly differentiate and promote tissue recovery when transplanted into another species, resulting in improved function. In the unmodified host, MSC migrate and engraft in multiple tissues (bone marrow, spleen, liver, muscle), maintain multi-potential capacity, and have unique immunologic characteristics that allow persistence in a xenogeneic environment. Importantly, xenogeneic MSC would, of course, need to be fully protected themselves from the human immune response, and this could be achieved by genetic engineering of pigs [22,35,36]. The data we have reviewed suggest that cross-species MSC might prove valuable in numerous different disease states, e.g., GVHD, diabetes, myocardial infarction, organ transplantation, etc.
To summarize, in various experimental models the following effects have been documented across a species barrier:
Neurological – support axonal growth, induce functional improvement, reduce brain infarct size, and reduce pain-like behavior [47–50].
Musculoskeletal - MSC survive in the intervertebral discs and differentiate toward disc-like cells [51].
Cardiovascular – reduce myocardial infarct size and improve ventricular function, as well as improve acute myocarditis [52–54].
Diabetes mellitus - enhance insulin secretion, islet graft survival, and wound healing [55–57].
Tissue injury - (i) repair radiation damage, e.g., to liver and intestine [58,59], (ii) engraft in the host liver parenchyma, differentiate into hepatocyte-like cells, and enhance hepatic recovery [60,61], (iii) differentiate into renal tubular epithelial-like cells, and improve renal function [62], (iv) ossifiy calvarial defects [63], (v) accelerate healing of retinal defects [64], and (vi) have a beneficial effect on ischemic limb disease [65].
Malignancy - MSC possess intrinsic anti-neoplastic properties, inhibiting tumor growth and metastasis [66–68].
Autoimmune/inflammatory disease - inhibit progression of autoimmune disease and restore immune homeostasis [69] in myasthenia gravis [70], systemic lupus erythematosus [71], GVHD, and colitis [72].
Co-transplantation - enhance engraftment of HSC, and prolong skin graft Survival [73,74].
Acknowledgments
Jiang Li is funded by the China Scholarship Council. Mohamed Ezzelarab is supported in part by the Shelley Patrick Fellowship of the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh. Studies on xenotransplantation at the Thomas E. Starzl Transplantation Institute are supported in part by NIH grant # 1U19AI090959-01 and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA.
ABBREVIATIONS
- GTKO
α1,3-galactosyltransferase gene-knockout
- GVHD
graft-versus-host disease
- HLA
human leukocyte antigen
- HSC
hematopoietic stem cells
- MSC
mesenchymal stem (stromal) cells
- NK
natural killer
- NOD/SCID
nonobese diabetic/severe combined immunodeficient
Footnotes
CONFLICT OF INTEREST
The authors have no financial or other conflict of interest.
References
- 1.ABDI R, FIORINA P, ADRA CN, ATKINSON M, SAYEGH MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759–1767. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SORDI V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87:S42–45. doi: 10.1097/TP.0b013e3181a28533. [DOI] [PubMed] [Google Scholar]
- 3.LE BLANC K, RASMUSSON I, SUNDBERG B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 4.LE BLANC K, FRASSONI F, BALL L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 5.MACDONALD GI, AUGELLO A, DEBARIC Role of mesenchymal stem cells in reestablishing immunologic tolerance in autoimmune rheumatic diseases. Arthritis Rheum. 2011;63:2547–2557. doi: 10.1002/art.30474. [DOI] [PubMed] [Google Scholar]
- 6.RACKHAM CL, CHAGASTELLES PC, NARDI NB, et al. Co-transplantation of mesenchymal stem cells maintains islet organisation and morphology in mice. Diabetologia. 2011;54:1127–1135. doi: 10.1007/s00125-011-2053-4. [DOI] [PubMed] [Google Scholar]
- 7.BERMAN DM, WILLMAN MA, HAN D, et al. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes. 2010;59:2558–2568. doi: 10.2337/db10-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LU Y, JIN X, CHEN Y, et al. Mesenchymal stem cells protect islets from hypoxia/reoxygenation-induced injury. Cell Biochem Funct. 2010;28:637–643. doi: 10.1002/cbf.1701. [DOI] [PubMed] [Google Scholar]
- 9.JACKSON KA, MAJKA SM, WANG H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BABER SR, DENG W, MASTER RG, et al. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1120–1128. doi: 10.1152/ajpheart.00173.2006. [DOI] [PubMed] [Google Scholar]
- 11.GONZALEZ-REY E, ANDERSON P, GONZALEZ MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 12.LIN F, CORDES K, LI L, et al. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003;14:1188–1199. doi: 10.1097/01.asn.0000061595.28546.a0. [DOI] [PubMed] [Google Scholar]
- 13.MCDONALD JW, LIU XZ, QU Y, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 14.ITAKURA S, ASARI S, RAWSON J, et al. Mesenchymal stem cells facilitate the induction of mixed hematopoietic chimerism and islet allograft tolerance without GVHD in the rat. Am J Transplant. 2007;7:336–346. doi: 10.1111/j.1600-6143.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 15.PROCKOP DJ. Marrow stromal cells as stem cells for continual renewal of nonhematopoietic tissues and as potential vectors for gene therapy. J Cell Biochem Suppl. 1998;30–31:284–285. [PubMed] [Google Scholar]
- 16.PERICO N, CASIRAGHI F, INTRONA M, et al. Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol. 2011;6:412–422. doi: 10.2215/CJN.04950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CROP MJ, BAAN CC, KOREVAAR SS, et al. Donor-derived mesenchymal stem cells suppress alloreactivity of kidney transplant patients. Transplantation. 2009;87:896–906. doi: 10.1097/TP.0b013e31819b3d72. [DOI] [PubMed] [Google Scholar]
- 18.POPP FC, RENNER P, EGGENHOFER E, et al. Mesenchymal stem cells as immunomodulators after liver transplantation. Liver Transpl. 2009;15:1192–1198. doi: 10.1002/lt.21862. [DOI] [PubMed] [Google Scholar]
- 19.BARTHOLOMEW A, STURGEON C, SIATSKAS M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 20.ZHANG W, GE W, LI C, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 21.YI T, SONG SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res. 2012;35:213–221. doi: 10.1007/s12272-012-0202-z. [DOI] [PubMed] [Google Scholar]
- 22.KUMAR G, HARA H, LONG C, et al. Adipose-derived mesenchymal stromal cells from genetically modified pigs: immunogenicity and immune modulatory properties. Cytotherapy. 2012;14:494–504. doi: 10.3109/14653249.2011.651529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DOMINICI M, LE BLANC K, MUELLER I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 24.KOLF CM, CHO E, TUAN RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.RHO GJ, KUMAR BM, BALASUBRAMANIAN SS. Porcine mesenchymal stem cells--current technological status and future perspective. Front Biosci. 2009;14:3942–3961. doi: 10.2741/3503. [DOI] [PubMed] [Google Scholar]
- 26.BOXALL SA, JONES E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int. 2012;2012:975871. doi: 10.1155/2012/975871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MIURA M, MIURA Y, PADILLA-NASH HM, et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 28.ROSLAND GV, SVENDSEN A, TORSVIK A, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 29.VACANTI V, KONG E, SUZUKI G, et al. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol. 2005;205:194–201. doi: 10.1002/jcp.20376. [DOI] [PubMed] [Google Scholar]
- 30.STENDERUP K, JUSTESEN J, CLAUSEN C, KASSEM M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 31.WAGNER W, HORN P, CASTOLDI M, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.TOLAR J, NAUTA AJ, OSBORN MJ, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 33.LI Q, HISHA H, TAKAKI T, et al. Transformation potential of bone marrow stromal cells into undifferentiated high-grade pleomorphic sarcoma. J Cancer Res Clin Oncol. 2010;136:829–838. doi: 10.1007/s00432-009-0723-0. [DOI] [PubMed] [Google Scholar]
- 34.WATERMAN RS, TOMCHUCK SL, HENKLE SL, BETANCOURT AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.EZZELARAB M, AYARES D, COOPER DK. The potential of genetically-modified pig mesenchymal stromal cells in xenotransplantation. Xenotransplantation. 2010;17:3–5. doi: 10.1111/j.1399-3089.2009.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.EZZELARAB M, EZZELARAB C, WILHITE T, et al. Genetically-modified pig mesenchymal stromal cells: xenoantigenicity and effect on human T-cell xenoresponses. Xenotransplantation. 2011;18:183–195. doi: 10.1111/j.1399-3089.2011.00635.x. [DOI] [PubMed] [Google Scholar]
- 37.NIEMEYER P, SZALAY K, LUGINBUHL R, SUDKAMP NP, KASTEN P. Transplantation of human mesenchymal stem cells in a non-autogenous setting for bone regeneration in a rabbit critical-size defect model. Acta Biomater. 2010;6:900–908. doi: 10.1016/j.actbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 38.LI Y, QU YH, WU YF, et al. Bone marrow mesenchymal stem cells reduce the antitumor activity of cytokine-induced killer/natural killer cells in K562 NOD/SCID mice. Ann Hematol. 2011;90:873–885. doi: 10.1007/s00277-011-1156-9. [DOI] [PubMed] [Google Scholar]
- 39.BAERTSCHIGER RM, SERRE-BEINIER V, MOREL P, et al. Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One. 2009;4:e6657. doi: 10.1371/journal.pone.0006657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LIU S, GINESTIER C, OU SJ, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.PEREIRA MC, SECCO M, SUZUKI DE, et al. Contamination of mesenchymal stem-cells with fibroblasts accelerates neurodegeneration in an experimental model of Parkinson’s disease. Stem Cell Rev. 2011;7:1006–1017. doi: 10.1007/s12015-011-9256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LYNGBAEK S, RIPA RS, HAACK-SORENSEN M, et al. Serial in vivo imaging of the porcine heart after percutaneous, intramyocardially injected 111In-labeled human mesenchymal stromal cells. Int J Cardiovasc Imaging. 2010;26:273–284. doi: 10.1007/s10554-009-9532-4. [DOI] [PubMed] [Google Scholar]
- 43.KERN S, EICHLER H, STOEVE J, KLUTER H, BIEBACK K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 44.UCCELLI A, MORETTA L, PISTOIA V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 45.FORTE P, PAZMANY L, MATTER-REISSMANN UB, et al. HLA-G inhibits rolling adhesion of activated human NK cells on porcine endothelial cells. J Immunol. 2001;167:6002–6008. doi: 10.4049/jimmunol.167.10.6002. [DOI] [PubMed] [Google Scholar]
- 46.PONCELET AJ, DENIS D, GIANELLO P. Cellular xenotransplantation. Curr Opin Organ Transplant. 2009;14:168–174. doi: 10.1097/mot.0b013e3283292522. [DOI] [PubMed] [Google Scholar]
- 47.NEUHUBER B, TIMOTHY HIMES B, SHUMSKY JS, GALLO G, FISCHER I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73–85. doi: 10.1016/j.brainres.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 48.SINISCALCO D, GIORDANO C, GALDERISI U, et al. Long-lasting effects of human mesenchymal stem cell systemic administration on pain-like behaviors, cellular, and biomolecular modifications in neuropathic mice. Front Integr Neurosci. 2011;5:79. doi: 10.3389/fnint.2011.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.YANG M, WEI X, LI J, et al. Changes in host blood factors and brain glia accompanying the functional recovery after systemic administration of bone marrow stem cells in ischemic stroke rats. Cell Transplant. 2010;19:1073–1084. doi: 10.3727/096368910X503415. [DOI] [PubMed] [Google Scholar]
- 50.WAKABAYASHI K, NAGAI A, SHEIKH AM, et al. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res. 2010;88:1017–1025. doi: 10.1002/jnr.22279. [DOI] [PubMed] [Google Scholar]
- 51.HENRIKSSON HB, SVANVIK T, JONSSON M, et al. Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine (Phila Pa 1976) 2009;34:141–148. doi: 10.1097/BRS.0b013e31818f8c20. [DOI] [PubMed] [Google Scholar]
- 52.PAUL A, SRIVASTAVA S, CHEN G, SHUM-TIM D, PRAKASH S. Functional Assessment of Adipose Stem Cells for Xenotransplantation Using Myocardial Infarction Immunocompetent Models: Comparison with Bone Marrow Stem Cells. Cell Biochem Biophys. 2011 doi: 10.1007/s12013-011-9323-0. [DOI] [PubMed] [Google Scholar]
- 53.VAN LINTHOUT S, SAVVATIS K, MITEVA K, et al. Mesenchymal stem cells improve murine acute coxsackievirus B3-induced myocarditis. Eur Heart J. 2011;32:2168–2178. doi: 10.1093/eurheartj/ehq467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.KIM YJ, HUH YM, CHOE KO, et al. In vivo magnetic resonance imaging of injected mesenchymal stem cells in rat myocardial infarction; simultaneous cell tracking and left ventricular function measurement. Int J Cardiovasc Imaging. 2009;25 (Suppl 1):99–109. doi: 10.1007/s10554-008-9407-0. [DOI] [PubMed] [Google Scholar]
- 55.LEE RH, SEO MJ, REGER RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.HO JH, TSENG TC, MA WH, et al. Multiple intravenous transplantations of mesenchymal stem cells effectively restore long-term blood glucose homeostasis by hepatic engraftment and beta cell differentiation in streptozosin-induced diabetic mice. Cell Transplant. 2011 doi: 10.3727/096368911X603611. [DOI] [PubMed] [Google Scholar]
- 57.TARK KC, HONG JW, KIM YS, et al. Effects of human cord blood mesenchymal stem cells on cutaneous wound healing in leprdb mice. Ann Plast Surg. 2010;65:565–572. doi: 10.1097/SAP.0b013e3181d9aae2. [DOI] [PubMed] [Google Scholar]
- 58.MOUISEDDINE M, FRANCOIS S, SOUIDI M, CHAPEL A. Intravenous human mesenchymal stem cells transplantation in NOD/SCID mice preserve liver integrity of irradiation damage. Methods Mol Biol. 2012;826:179–188. doi: 10.1007/978-1-61779-468-1_15. [DOI] [PubMed] [Google Scholar]
- 59.SEMONT A, MOUISEDDINE M, FRANCOIS A, et al. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2010;17:952–961. doi: 10.1038/cdd.2009.187. [DOI] [PubMed] [Google Scholar]
- 60.TAO XR, LI WL, SU J, et al. Clonal mesenchymal stem cells derived from human bone marrow can differentiate into hepatocyte-like cells in injured livers of SCID mice. J Cell Biochem. 2009;108:693–704. doi: 10.1002/jcb.22306. [DOI] [PubMed] [Google Scholar]
- 61.BANAS A, TERATANI T, YAMAMOTO Y, et al. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009;24:70–77. doi: 10.1111/j.1440-1746.2008.05496.x. [DOI] [PubMed] [Google Scholar]
- 62.QIAN H, YANG H, XU W, et al. Bone marrow mesenchymal stem cells ameliorate rat acute renal failure by differentiation into renal tubular epithelial-like cells. Int J Mol Med. 2008;22:325–332. [PubMed] [Google Scholar]
- 63.LEVI B, JAMES AW, NELSON ER, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 2010;5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.XUQIAN W, KANGHUA L, WEIHONG Y, et al. Intraocular Transplantation of Human Adipose-Derived Mesenchymal Stem Cells in a Rabbit Model of Experimental Retinal Holes. Ophthalmic Res. 2011;46:199–207. doi: 10.1159/000323910. [DOI] [PubMed] [Google Scholar]
- 65.LAURILA JP, LAATIKAINEN L, CASTELLONE MD, et al. Human embryonic stem cell-derived mesenchymal stromal cell transplantation in a rat hind limb injury model. Cytotherapy. 2009;11:726–737. doi: 10.3109/14653240903067299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.KHAKOO AY, PATI S, ANDERSON SA, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.GAO P, DING Q, WU Z, JIANG H, FANG Z. Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer Lett. 2010;290:157–166. doi: 10.1016/j.canlet.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 68.SUN B, ROH KH, PARK JR, et al. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy. 2009;11:289–298. doi: 10.1080/14653240902807026. 281 p following 298. [DOI] [PubMed] [Google Scholar]
- 69.ZHOU K, ZHANG H, JIN O, et al. Transplantation of human bone marrow mesenchymal stem cell ameliorates the autoimmune pathogenesis in MRL/lpr mice. Cell Mol Immunol. 2008;5:417–424. doi: 10.1038/cmi.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.YU J, ZHENG C, REN X, et al. Intravenous administration of bone marrow mesenchymal stem cells benefits experimental autoimmune myasthenia gravis mice through an immunomodulatory action. Scand J Immunol. 2010;72:242–249. doi: 10.1111/j.1365-3083.2010.02445.x. [DOI] [PubMed] [Google Scholar]
- 71.CHOI EW, SHIN IS, PARK SY, et al. Reversal of serologic, immunologic, and histologic dysfunction in mice with systemic lupus erythematosus by long-term serial adipose tissue-derived mesenchymal stem cell transplantation. Arthritis Rheum. 2012;64:243–253. doi: 10.1002/art.33313. [DOI] [PubMed] [Google Scholar]
- 72.ZUCCONI E, VIEIRA NM, BUENO CR, JR, et al. Preclinical studies with umbilical cord mesenchymal stromal cells in different animal models for muscular dystrophy. J Biomed Biotechnol. 2011;2011:715251. doi: 10.1155/2011/715251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.ANGELOPOULOU M, NOVELLI E, GROVE JE, et al. Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Exp Hematol. 2003;31:413–420. doi: 10.1016/s0301-472x(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 74.MOADSIRI A, POLCHERT D, GENRICH K, et al. Mesenchymal stem cells enhance xenochimerism in NK-depleted hosts. Surgery. 2006;140:315–321. doi: 10.1016/j.surg.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 75.KUROZUMI K, NAKAMURA K, TAMIYA T, et al. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9:189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 76.SUN B, ZHANG S, NI C, et al. Correlation between melanoma angiogenesis and the mesenchymal stem cells and endothelial progenitor cells derived from bone marrow. Stem Cells Dev. 2005;14:292–298. doi: 10.1089/scd.2005.14.292. [DOI] [PubMed] [Google Scholar]
- 77.PAUL C, SAMDANI AF, BETZ RR, FISCHER I, NEUHUBER B. Grafting of human bone marrow stromal cells into spinal cord injury: a comparison of delivery methods. Spine (Phila Pa 1976) 2009;34:328–334. doi: 10.1097/BRS.0b013e31819403ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.ZHAO M, AMIEL SA, AJAMI S, et al. Amelioration of streptozotocin-induced diabetes in mice with cells derived from human marrow stromal cells. PLoS One. 2008;3:e2666. doi: 10.1371/journal.pone.0002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.MORIGI M, INTRONA M, IMBERTI B, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 80.ERICES AA, ALLERS CI, CONGET PA, ROJAS CV, MINGUELL JJ. Human cord blood-derived mesenchymal stem cells home and survive in the marrow of immunodeficient mice after systemic infusion. Cell Transplant. 2003;12:555–561. doi: 10.3727/000000003108747154. [DOI] [PubMed] [Google Scholar]
- 81.LIECHTY KW, MACKENZIE TC, SHAABAN AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 82.MOUISEDDINE M, FRANCOIS S, SEMONT A, et al. Human mesenchymal stem cells home specifically to radiation-injured tissues in a non-obese diabetes/severe combined immunodeficiency mouse model. Br J Radiol. 2007;80(Spec No 1):S49–55. doi: 10.1259/bjr/25927054. [DOI] [PubMed] [Google Scholar]
- 83.MACKENZIE TC, FLAKE AW. Human mesenchymal stem cells persist, demonstrate site-specific multipotential differentiation, and are present in sites of wound healing and tissue regeneration after transplantation into fetal sheep. Blood Cells Mol Dis. 2001;27:601–604. doi: 10.1006/bcmd.2001.0424. [DOI] [PubMed] [Google Scholar]
- 84.FRANCOIS S, BENSIDHOUM M, MOUISEDDINE M, et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 2006;24:1020–1029. doi: 10.1634/stemcells.2005-0260. [DOI] [PubMed] [Google Scholar]
- 85.ATOUI R, ASENJO JF, DUONG M, et al. Marrow stromal cells as universal donor cells for myocardial regenerative therapy: their unique immune tolerance. Ann Thorac Surg. 2008;85:571–579. doi: 10.1016/j.athoracsur.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 86.YAN Y, XU W, QIAN H, et al. Mesenchymal stem cells from human umbilical cords ameliorate mouse hepatic injury in vivo. Liver Int. 2009;29:356–365. doi: 10.1111/j.1478-3231.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- 87.SHABBIR A, ZISA D, LEIKER M, et al. Muscular dystrophy therapy by nonautologous mesenchymal stem cells: muscle regeneration without immunosuppression and inflammation. Transplantation. 2009;87:1275–1282. doi: 10.1097/TP.0b013e3181a1719b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.MUGURUMA Y, YAHATA T, MIYATAKE H, et al. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood. 2006;107:1878–1887. doi: 10.1182/blood-2005-06-2211. [DOI] [PubMed] [Google Scholar]
- 89.PAIK MJ, LI WY, AHN YH, et al. The free fatty acid metabolome in cerebral ischemia following human mesenchymal stem cell transplantation in rats. Clin Chim Acta. 2009;402:25–30. doi: 10.1016/j.cca.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 90.ZISA D, SHABBIR A, SUZUKI G, LEE T. Vascular endothelial growth factor (VEGF) as a key therapeutic trophic factor in bone marrow mesenchymal stem cell-mediated cardiac repair. Biochem Biophys Res Commun. 2009;390:834–838. doi: 10.1016/j.bbrc.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.PLOTNIKOV AN, SHLAPAKOVA I, SZABOLCS MJ, et al. Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation. 2007;116:706–713. doi: 10.1161/CIRCULATIONAHA.107.703231. [DOI] [PubMed] [Google Scholar]
- 92.KIM DH, YOO KH, YIM YS, et al. Cotransplanted bone marrow derived mesenchymal stem cells (MSC) enhanced engraftment of hematopoietic stem cells in a MSC-dose dependent manner in NOD/SCID mice. J Korean Med Sci. 2006;21:1000–1004. doi: 10.3346/jkms.2006.21.6.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.MAITRA B, SZEKELY E, GJINI K, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 94.MEYERROSE TE, DE UGARTE DA, HOFLING AA, et al. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells. 2007;25:220–227. doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.NAKAMURA Y, WANG X, XU C, et al. Xenotransplantation of long-term-cultured swine bone marrow-derived mesenchymal stem cells. Stem Cells. 2007;25:612–620. doi: 10.1634/stemcells.2006-0168. [DOI] [PubMed] [Google Scholar]
- 96.EGUCHI H, KUROIWA Y, MATSUI A, et al. Intra-bone marrow cotransplantation of donor mesenchymal stem cells in pig-to-NOD/SCID mouse bone marrow transplantation facilitates short-term xenogeneic hematopoietic engraftment. Transplant Proc. 2008;40:574–577. doi: 10.1016/j.transproceed.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 97.MEDICETTY S, BLEDSOE AR, FAHRENHOLTZ CB, TROYER D, WEISS ML. Transplantation of pig stem cells into rat brain: proliferation during the first 8 weeks. Exp Neurol. 2004;190:32–41. doi: 10.1016/j.expneurol.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 98.NOH YH, YIM YS, KIM DH, et al. Correlation between chemokines released from umbilical cord blood-derived mesenchymal stem cells and engraftment of hematopoietic stem cells in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. Pediatr Hematol Oncol. 2011;28:682–690. doi: 10.3109/08880018.2011.599477. [DOI] [PubMed] [Google Scholar]
- 99.GREGOIRE-GAUTHIER J, SELLERI S, FONTAINE F, et al. Therapeutic Efficacy of Cord Blood-Derived Mesenchymal Stromal Cells for the Prevention of Acute Graft-Versus-Host Disease in a Xenogenic Mouse Model. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0413. [DOI] [PubMed] [Google Scholar]
- 100.DAYAN V, YANNARELLI G, BILLIA F, et al. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol. 2011;106:1299–1310. doi: 10.1007/s00395-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 101.KIM ES, CHANG YS, CHOI SJ, et al. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuates Escherichia coli-induced acute lung injury in mice. Respir Res. 2011;12:108. doi: 10.1186/1465-9921-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.LIN YT, CHERN Y, SHEN CK, et al. Human mesenchymal stem cells prolong survival and ameliorate motor deficit through trophic support in Huntington’s disease mouse models. PLoS One. 2011;6:e22924. doi: 10.1371/journal.pone.0022924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.ZHANG MJ, SUN JJ, QIAN L, et al. Human umbilical mesenchymal stem cells enhance the expression of neurotrophic factors and protect ataxic mice. Brain Res. 2011;1402:122–131. doi: 10.1016/j.brainres.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 104.PAN Q, FOURASCHEN SM, KAYA FS, et al. Mobilization of hepatic mesenchymal stem cells from human liver grafts. Liver Transpl. 2011;17:596–609. doi: 10.1002/lt.22260. [DOI] [PubMed] [Google Scholar]
- 105.BAK XY, LAM DH, YANG J, et al. Human embryonic stem cell-derived mesenchymal stem cells as cellular delivery vehicles for prodrug gene therapy of glioblastoma. Hum Gene Ther. 2011;22:1365–1377. doi: 10.1089/hum.2010.212. [DOI] [PubMed] [Google Scholar]
- 106.LIANG L, DONG C, CHEN X, et al. Human umbilical cord mesenchymal stem cells ameliorate mice trinitrobenzene sulfonic acid (TNBS)-induced colitis. Cell Transplant. 2011;20:1395–1408. doi: 10.3727/096368910X557245. [DOI] [PubMed] [Google Scholar]
- 107.RA JC, SHIN IS, KIM SH, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297–1308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 108.CHOI EW, SHIN IS, LEE HW, et al. Transplantation of CTLA4Ig gene-transduced adipose tissue-derived mesenchymal stem cells reduces inflammatory immune response and improves Th1/Th2 balance in experimental autoimmune thyroiditis. J Gene Med. 2011;13:3–16. doi: 10.1002/jgm.1531. [DOI] [PubMed] [Google Scholar]
- 109.RYU CH, PARK SH, PARK SA, et al. Gene therapy of intracranial glioma using interleukin 12-secreting human umbilical cord blood-derived mesenchymal stem cells. Hum Gene Ther. 2011;22:733–743. doi: 10.1089/hum.2010.187. [DOI] [PubMed] [Google Scholar]
- 110.TAN Z, SU ZY, WU RR, et al. Immunomodulative effects of mesenchymal stem cells derived from human embryonic stem cells in vivo and in vitro. J Zhejiang Univ Sci B. 2011;12:18–27. doi: 10.1631/jzus.B1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.LI GC, YE QH, XUE YH, et al. Human mesenchymal stem cells inhibit metastasis of a hepatocellular carcinoma model using the MHCC97–H cell line. Cancer Sci. 2010;101:2546–2553. doi: 10.1111/j.1349-7006.2010.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.YIM YS, NOH YH, KIM DH, et al. Correlation between the immature characteristics of umbilical cord blood-derived mesenchymal stem cells and engraftment of hematopoietic stem cells in NOD/SCID mice. Transplant Proc. 2010;42:2753–2758. doi: 10.1016/j.transproceed.2010.05.146. [DOI] [PubMed] [Google Scholar]
- 113.DI GH, JIANG S, LI FQ, et al. Human umbilical cord mesenchymal stromal cells mitigate chemotherapy-associated tissue injury in a pre-clinical mouse model. Cytotherapy. 2012;14:412–422. doi: 10.3109/14653249.2011.646044. [DOI] [PubMed] [Google Scholar]
- 114.SCHOEBERLEIN A, MUELLER M, REINHART U, et al. Homing of placenta-derived mesenchymal stem cells after perinatal intracerebral transplantation in a rat model. Am J Obstet Gynecol. 2011;205:277 e271–276. doi: 10.1016/j.ajog.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 115.ZHENG W, HONMOU O, MIYATA K, et al. Therapeutic benefits of human mesenchymal stem cells derived from bone marrow after global cerebral ischemia. Brain Res. 2010;1310:8–16. doi: 10.1016/j.brainres.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 116.LIAO W, ZHONG J, YU J, et al. Therapeutic benefit of human umbilical cord derived mesenchymal stromal cells in intracerebral hemorrhage rat: implications of anti-inflammation and angiogenesis. Cell Physiol Biochem. 2009;24:307–316. doi: 10.1159/000233255. [DOI] [PubMed] [Google Scholar]
- 117.JEONG JH, JIN ES, MIN JK, et al. Human mesenchymal stem cells implantation into the degenerated coccygeal disc of the rat. Cytotechnology. 2009;59:55–64. doi: 10.1007/s10616-009-9192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.CHEN X, SONG XH, YIN Z, et al. Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells. 2009;27:1276–1287. doi: 10.1002/stem.61. [DOI] [PubMed] [Google Scholar]
- 119.SATO Y, ARAKI H, KATO J, et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–763. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 120.ALLERS C, SIERRALTA WD, NEUBAUER S, et al. Dynamic of distribution of human bone marrow-derived mesenchymal stem cells after transplantation into adult unconditioned mice. Transplantation. 2004;78:503–508. doi: 10.1097/01.tp.0000128334.93343.b3. [DOI] [PubMed] [Google Scholar]
- 121.KIM SW, HAN H, CHAE GT, et al. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger’s disease and ischemic limb disease animal model. Stem Cells. 2006;24:1620–1626. doi: 10.1634/stemcells.2005-0365. [DOI] [PubMed] [Google Scholar]
- 122.LEE HJ, LEE JK, LEE H, et al. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiol Aging. 2012;33:588–602. doi: 10.1016/j.neurobiolaging.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 123.LOEBINGER MR, KYRTATOS PG, TURMAINE M, et al. Magnetic resonance imaging of mesenchymal stem cells homing to pulmonary metastases using biocompatible magnetic nanoparticles. Cancer Res. 2009;69:8862–8867. doi: 10.1158/0008-5472.CAN-09-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.JIANG X, CUI PC, CHEN WX, ZHANG ZP. In vivo chondrogenesis of induced human marrow mesenchymal stem cells in nude mice. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:766–769. 773. [PubMed] [Google Scholar]
- 125.BARLOW S, BROOKE G, CHATTERJEE K, et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008;17:1095–1107. doi: 10.1089/scd.2007.0154. [DOI] [PubMed] [Google Scholar]
- 126.CAMPARD D, LYSY PA, NAJIMI M, SOKAL EM. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134:833–848. doi: 10.1053/j.gastro.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 127.WONG CY, CHEONG SK, MOK PL, LEONG CF. Differentiation of human mesenchymal stem cells into mesangial cells in post-glomerular injury murine model. Pathology. 2008;40:52–57. doi: 10.1080/00313020701716367. [DOI] [PubMed] [Google Scholar]
- 128.LEE JH, CHUNG WH, KANG EH, et al. Schwann cell-like remyelination following transplantation of human umbilical cord blood (hUCB)-derived mesenchymal stem cells in dogs with acute spinal cord injury. J Neurol Sci. 2011;300:86–96. doi: 10.1016/j.jns.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 129.ERSEK A, PIXLEY JS, GOODRICH AD, et al. Persistent circulating human insulin in sheep transplanted in utero with human mesenchymal stem cells. Exp Hematol. 2010;38:311–320. doi: 10.1016/j.exphem.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.WANG JW, LIU YB, XU B, et al. The study on immunomodulation of donor mesenchymal stem cells on discordant liver xenotransplantation. Zhonghua Wai Ke Za Zhi. 2005;43:1254–1258. [PubMed] [Google Scholar]
- 131.SEMONT A, FRANCOIS S, MOUISEDDINE M, et al. Mesenchymal stem cells increase self-renewal of small intestinal epithelium and accelerate structural recovery after radiation injury. Adv Exp Med Biol. 2006;585:19–30. doi: 10.1007/978-0-387-34133-0_2. [DOI] [PubMed] [Google Scholar]
- 132.ZHOU DH, HUANG SL, HUANG K, et al. Mesenchymal stem cells from human cord blood promote engraftment of human umbilical cord blood-derived CD34+ cells in NOD/SCID mice. Zhonghua Xue Ye Xue Za Zhi. 2005;26:732–735. [PubMed] [Google Scholar]
- 133.WANG Y, HUSO DL, HARRINGTON J, et al. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy. 2005;7:509–519. doi: 10.1080/14653240500363216. [DOI] [PubMed] [Google Scholar]
- 134.NIEMEYER P, VOHRER J, SCHMAL H, et al. Survival of human mesenchymal stromal cells from bone marrow and adipose tissue after xenogenic transplantation in immunocompetent mice. Cytotherapy. 2008;10:784–795. doi: 10.1080/14653240802419302. [DOI] [PubMed] [Google Scholar]


