Abstract
Renal cell carcinoma (RCC) remains one of the most resistant tumors to systemic chemotherapy, radiotherapy, and immunotherapy. Despite great progress in understanding the basic biology of RCC, the rate of responses in animal models and clinical trials using interferons (IFNs) has not improved significantly. It is likely that the lack of responses can be due to the tumor's ability to develop tumor escape strategies. Currently, the use of targeted therapies has improved the clinical outcomes of patients with RCC and is associated with an increase of Th1-cytokine responses (IFNγ), indicating the importance of IFNγ in inhibiting tumor proliferation. Thus, the present study was designed to investigate a new mechanism by which IFNγ mediates direct anti-proliferative effects against murine renal cell carcinoma cell lines. When cultured RCC cell lines were exposed to murine recombinant IFNγ, a dose dependent growth inhibition in CL-2 and CL-19 cells was observed; this effect was not observed in Renca cells. Growth inhibition in CL-2 and CL-19 cell lines was associated with the intracellular induction of nitric oxide synthase (iNOS) protein, resulting in a sustained elevation of nitric oxide (NO) and citrulline, and a decrease in arginase activity. The inhibition of cell proliferation appears to be due to an arrest in the cell cycle. The results indicate that in certain RCC cell lines, IFNγ modulates L-arginine metabolism by shifting from arginase to iNOS activity, thereby developing a potent inhibitory mechanism to encumber tumor cell proliferation and survival. Elucidating the cellular events triggered by IFNγ in murine RCC cell lines will permit anti-tumor effects to be exploited in the development of new combination therapies that interfere with L-arginine metabolism to effectively combat RCC in patients.
Keywords: nitric oxide, Interferon-gamma, nitric oxide synthase, renal cell carcinoma, arginase 2, polyamines, L-arginine, cell proliferation.
INTRODUCTION
Renal cell carcinoma (RCC) accounts for 5% of all cancers, leading to an estimate of 13,120 mortalities each year in the United States 1. RCC remains one of the most resistant tumors to immunotherapy, chemotherapy, and radiotherapy 2. The overall survival for all RCC patients has not appreciably improved during the last 25 years, indicating the need for more effective ways to treat this disease. Immunotherapy with interferon alpha (IFNα and/or interleukin-2 (IL-2)) has been used as a first-line therapy to treat RCC, but the documented clinical response rate in patients is only 10-20%, with a median survival of approximately 12 months 3-5. In recent years, the advent of targeted therapies for metastatic RCC has grown substantially 6-9; however, these therapies do not produce complete responses and have several adverse effects, and their mechanisms of action are poorly understood.
There is a complex relationship between immunity, tumorigenesis, and the metabolism of L-arginine 10;11 that can be highly relevant to RCC. Recent studies have demonstrated convincingly that the enzymes arginase 1 (ARG1), arginase 2 (ARG2), and inducible nitric oxide synthase (iNOS) play a crucial role in tumor biology 12-14. ARG1 and ARG2 are collectively referred to as ARG in this manuscript because their activities are similar. L-arginine is metabolized by ARG, producing L-ornithine, as substrate for ornithine decarboxylase (ODC) to synthesize polyamines that are essential for cell growth. In contrast, iNOS metabolizes L-arginine to produce nitric oxide (NO) that kills tumor cells by mechanisms that are partially understood 15-17. Since ARG and iNOS compete for L-arginine, this amino acid is a crucial component whose metabolism modulates cell growth/inhibition and suppresses T-cell function 18. In addition, the balance between iNOS and ARG is competitively regulated by Th1 and Th2 type-cytokines; Th1 cytokines induce iNOS, whereas Th2 cytokines induce ARG 19. One of the possible mechanisms to explain the impairment of antitumor immunity in patients with RCC is the well documented shift from Th1 to Th2 cytokine response 20. This shift may prompt the tumor microenvironment to produce high levels of ARG and polyamines instead of initiating an iNOS-dependent production of NO that could be characterized by the lack of Th1 responses in order to survive 21;22 Recently, we demonstrated that murine RCC cell lines with high levels of ARG2 expression are associated with L-arginine depletion resulting in induced T-cell dysfunction and increased tumor growth 23.
For many years, treatment with IFNγ has shown minimal or no activity in patients with RCC. Since the combination of IFNγ and IFNα treatment reflected a response rate similar to IFNα alone, the latter has been the treatment of election for RCC patients for many years 24. This observation is striking since IFNγ has the most potent immunomodulatory activity among the interferons 25-27, indicating that the mechanisms of action of IFNγ are complex, not well understood, and deserve to be re-visited in the context of tumor resistance and tumor microenvironment.
The present study focuses on the mechanisms by which IFNγ can modulate L-arginine metabolism by shifting ARG-dependent cell proliferation to growth inhibition by inducing iNOS and NO production. Understanding these mechanisms will facilitate the development of new, effective, pharmacological strategies that interfere with L-arginine metabolism to control and eliminate RCC in affected patients.
MATERIALS AND METHODS
Cell lines, reagents and culture conditions
For this study, three murine RCC (mRCC) cell lines were used: SIRCC-1.2 (CL-2) and SIRCC 1.19 (CL-19) cells of induced origin 28 and Renca cells of spontaneous origin, kindly provided by Dr. Robert H. Wiltrout (NCI). Cells were maintained at 37oC in continuous cell culture in RPMI-1640 containing 1,140 µM L-arginine (Lonza Inc., Walkersville, MD) and supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 25 mM HEPES, 4 mM L-glutamine, 100 units/ml of penicillin/streptomycin, 1 mM non-essential amino acids, and 1 mM sodium pyruvate (Lonza, Inc.). Recombinant mouse IFNγ (rmIFNγ) purchased from R&D Systems, Minneapolis, MN was used to determine its effect on iNOS induction and cytostasis on mRCC cells (10U/ml). The inhibitor of iNOS, NG-monomethyl-L-arginine (L-NMMA) (EMD-Calbiochem, Gibbstown, NJ), was also titrated to determine the optimal inhibitory conditions (1mM). The experiments were performed by plating 3 x 105 cells in six-well plates which were allowed to attach for 24 h (this cell concentration allow the cells to survive in culture up to 120 h). Fresh media containing IFNγ and L-NMMA (Time 0) were added to the cells and cultured at 2, 4, 6, 24, 48, and 72 h. At the different time points, supernatants were collected and cells were harvested using 0.05% Trypsin/EDTA (Sigma, St. Louis, MO) to perform the different assays. The protein concentration was determined by using the BCA protein assay kit (Pierce Biotechnology Inc., Rockford, IL). Cell extracts were stored at -70 ºC until used for western blots.
Arginase activity
Freshly prepared cytoplasmic extracts from cultured mRCC cells before and after treatment with IFNγ were tested for ARG activity by the conversion of L-arginine to L-ornithine (nanomoles/106 cells/hour), as described elsewhere 29.
Western blot
Equal amounts of cell extract protein (25 μg/lane) were electrophoresed in 8% Tris-glycine gels (Invitrogen, Carlsbad, CA) and transferred to polyvinylidiene difluoride (PVDF) membranes (Invitrogen). Immunoblotting was performed with antibodies for ARG2 (1:500), iNOS (1:200) (Santa Cruz Biotech, Santa Cruz, CA), and GAPDH (1:10,000) (Fitzgerald Industries International, Acton, MA). Detection was achieved by horseradish peroxidase-conjugated antibodies (1:3000, Santa Cruz) and an enhanced chemiluminescent kit (ECL, GE Healthcare, Piscataway, NJ). ARG2 and iNOS protein bands were visualized on X-OMAT AR films (Kodak, Rochester, NY).
Reverse Transcription-PCR analysis
Total RNA from 1 x 106 cells was extracted using TRIzol (Invitrogen), treated with DNase I (Invitrogen), and reverse transcribed using Superscript II (Invitrogen). PCR amplification was completed using primers for mouse iNOS and β-actin as follow: iNOS: forward, 5'-CTT CCG AAG TTT CTG GCA GCA GCG-3', reverse, 5'-GAG CCT CGT GGC TTT GGG CTC CTC-3', and β-actin forward 5'-CCA GAG CAA GAG AGG TAT CC-3', reverse 5'-CTG TGG TGG TGA AGC TGT AG-3'. The expected sizes of amplified fragments were iNOS 459 bp, and β-actin 436 bp. PCR products were visualized in ethidium bromide 1% agarose gels.
Assessment for cell growth and survival
Cell growth and viability was determined by using the previously described MTT assay 30. Briefly, different cell lines were plated at 1x104/well in 24-well plates and treated with IFNγ. At 24, 48, and 72 h, cells were incubated with MTT (0.5 mg/ml) for 4 h at 37 °C. Then, an equal volume of 0.04 M HCl in isopropanol was added to the wells and incubated for 3 h to dissolve the accumulated crystals of formazan. Absorbance was measured at 570 nm with a reference wavelength of 650 nm using a Benchmark Plus plate reader (Bio-Rad, Hercules, CA). Readings obtained from treated cells were compared with untreated controls in triplicate.
DNA fragmentation
Cell lines were treated with IFNγ or untreated for 24, 48, and 72 h. At each time point, the cells were harvested by trypsinization and washed once with phosphate buffered saline (PBS). Fragmented DNA was isolated using a DNA isolation kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Eluted samples containing DNA were electrophoresed on a 2% agarose gels (Life Technologies, Grand Island, NY) containing 0.5 μg/ml solution of ethidium bromide. DNA bands were visualized under UV and exposed to photography.
Cell cycle analysis
For DNA content analysis, 1 × 106 cells were treated with IFNγ for 24 and 48 h. The cells were harvested by trypsin digestion, collected by centrifugation, and fixed in 70% ethanol /phosphate buffered saline for at least 12 h at 4oC. After 100 μL (1 g/L) of RNase treatment, cells were stained with 0.5 μg/ml propidium iodide. Cells were examined by flow cytometry using a LSR II (Becton-Dickinson, USA). The percentage of cells in each phase of the cell cycle was analyzed using Modfit software (Verity, Software House, Topsham, ME).
Determination of Nitric oxide production
Nitrite accumulation in cell culture medium under defined conditions was used as an indirect determination of NO production and measured by a colorimetric assay based on the Griess reaction. Briefly, 200 μL of the culture supernatants were mixed with 200 μL of Griess reagent [one part 0.1% N-(1-naphtyl) ethylene-diamine dihydrochloride and one part 1% sulfanilamide] in 5% concentrated H3PO4 for 10 min at room temperature. The absorbance was measured at 540 nm in a Benchmark Plus plate reader (Bio-Rad). The readings were compared to a standard curve for sodium nitrite with a lower detection limit of 1μmol/L nitrite. In order to confirm that the formed nitrites were derived from iNOS induction, cells were cultured in the presence of the iNOS inhibitor L-NMMA.
Amino acid detection
High performance liquid chromatography (HPLC) was conducted on deproteinized conditioned media labeled with O-phtaldialdehyde (OPA). Analytes were eluted with 100 mM sodium acetate buffer, pH 5.0, with a linear gradient consisting of methanol (100%) and acetonitrile (100%). The analytes in the samples were calculated on the basis of standard curves of known amounts.
Cytokine measurement
Cytokines were measured in tissue culture supernatants released from cells at the different time points using Th1/Th2 panel Bio-Plex cytokine assay (Bio-Rad Laboratories, Hercules, CA) as described previously following the manufacturer's instructions 31. The samples were analyzed for IL-2, IL-4, IL-5, IL-10, IL-12 (p70), GM-CSF, IFNγ, TNFα, IL1β, IL6, and VEGF on a Bio-Rad 96-well plate reader using the Bio-Plex Suspension Array System and Bio-Plex Manager software (Bio-Rad).
Statistical analysis
Statistical analyses were completed in SAS v9.1 (SAS Institute, Cary, NC). For each cell line, nitrite concentration, proliferation, and ARG activity data were analyzed by mixed models for unequal variances. Two-way ANOVA models were used with time and experimental conditions (basal/IFNγ, control/IFNγ, and IFNγ+LNMMA) as factors. After IFNγ treatment, each experimental condition was tested at each time point. Multiple testing was conducted at the 5% level using the Tukey procedure. Least squares means and corresponding standard errors were obtained.
RESULTS
iNOS protein expression and NO production differs in mRCC cell lines
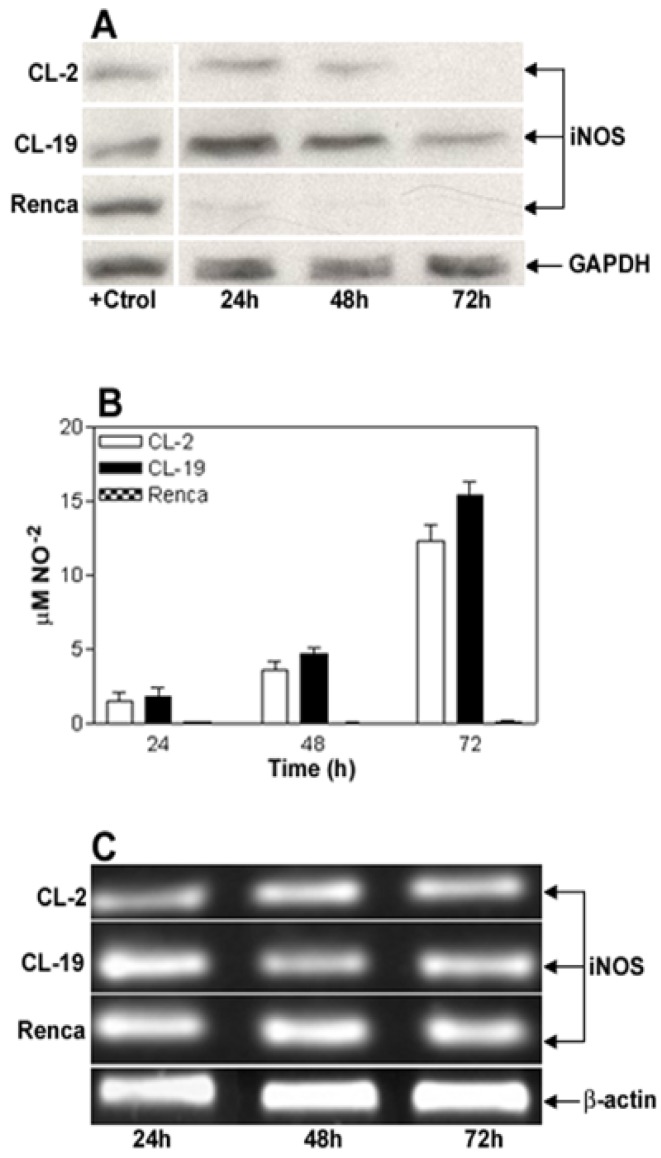
The basal expression level of iNOS protein and nitrite production in RCC cell lines CL-2, CL-19, and Renca, cultured in complete RPMI media for 24, 48, and 72 h was determined. As shown in Figure 1A, iNOS protein is detected at moderate levels in CL-2 and CL-19 during the time course of this experiment. In contrast, a complete absence of iNOS protein expression was observed in Renca cells. When supernatants were tested for NO release, a minimal and continued synthesis of nitrites ranging from 1.4 ± 0.64 μM at 24 h to 12.2 ± 1.12 μM at 72 h in the CL-2 cells and from 1.7 ± 0.63 μM up to 15.4 ± 0.86 μM in CL-19 cells was observed. Although low levels of iNOS are expressed in CL-2 and CL-19 in the absence of IFNγ, we were unable to detect nitrites released by iNOS activity at the level of detection available to us, before 24 h. However, production of NO was not observed in Renca cells at any tested time point (Figure 1B). The absence of iNOS protein expression in Renca cells prompted us to test iNOS mRNA expression by reverse-transcriptase PCR in the aforementioned cell lines. As shown in Figure 1C, iNOS mRNA gene expression was similar in all three cell lines at the different time points of the experiments.
Figure 1.
Characterization of iNOS expression and iNOS activity in murine RCC cell lines cultured in normal conditions. (A) Representative Western blot analysis for iNOS (130 kDa) and GAPDH (35 kDa) in CL-2, CL-19 and Renca cells cultured in normal conditions for 24, 48, and 72 h. Twenty five μg of total protein was loaded per line. RAW 264.7 cells stimulated with LPS plus murine IFNγ were used as positive control (+Ctrl) for iNOS protein. (B) Nitrite production in the 3 cell lines was determined by Greiss reaction in culture media supernatants collected after 24, 48, and 72 h in culture. Results are the mean ± SE of triplicates of five different separate experiments. (C) Reverse transcription-polymerase chain analysis of iNOs mRNA expression. The result of a representative experiment is shown; similar results were obtained in at least three experiments. The expected sizes of amplified fragments were 459 bp for iNOS and 436 bp for β-actin.
IFNγ increases iNOS protein levels and NO production in CL-2 and CL-19 but not in Renca cells
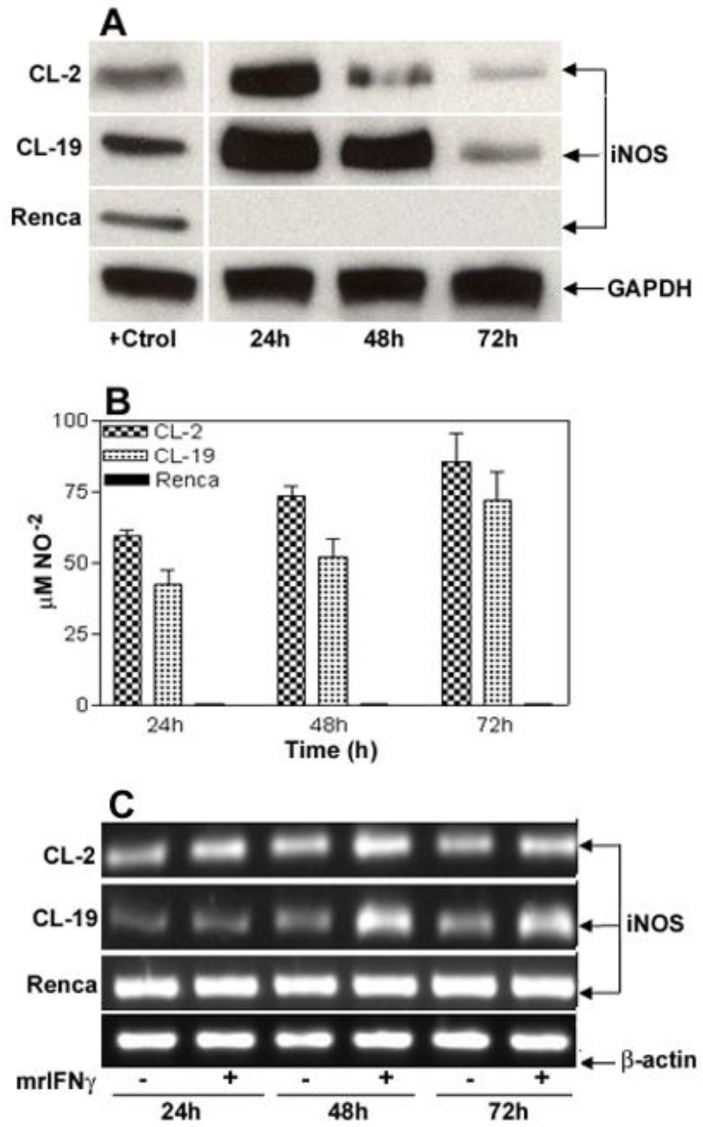
To determine the level of toxicity of IFNγ on the cell lines, kinetic experiments using concentrations of IFNγ ranging from 2.5 to 500 U/ml were performed (data not shown). In this system, it was observed that 10 U/ml of IFNγ was the optimal concentration to induce iNOS expression and to stimulate NO production without a measurable toxic effect on the cells. IFNγ concentrations higher than 15 U/ml were toxic to CL-2 and CL-19 cell lines, where a complete detachment of the cell monolayer was observed as early as 12 h (data not shown). Immunoblot analysis showed a maximal increase in the expression of immunoreactive iNOS protein in CL-2 and CL-19 cells at 24 hours, post-treatment. iNOS levels started to decrease at 48 h and reached basal levels at 72 h (Figure 2A). Interestingly, IFNγ did not induce iNOS protein or cytostasis in Renca cells. This finding prompted us to examine iNOS gene expression pre- and post- IFNγ treatment. Strikingly, iNOS mRNA was expressed in Renca cells at the same levels before and after treatment; this finding suggests a post-transcriptional regulation of iNOS expression. In contrast, IFNγ treatment markedly enhanced levels of iNOS mRNA in CL-2 and CL-19 cell lines (Figure 2B). The increased iNOS mRNA and protein expressions in CL-2 and CL-19 cells are associated with the production of high levels of NO ranging from 59.7 ± 2.0 μM at 24 h to 85.6 ± 9.6 μM at 72 h in CL-2 cells and from 42.4 ± 5.3 μM at 24 h to 71.9 ± 9.9 μM at 72 h in CL-19 cells (Table 1). As expected, production of NO was not observed in Renca cells throughout the course of the experiments. Minimum iNOS protein expression was observed in untreated cells (Figure 1A) that were used as control against treated cells.
Figure 2.
IFNγ-induced iNOS expression and iNOS activity in murine RCC cell lines. For reference, Figure 1A can be used as untreated control for these experiments. (A) Time course of iNOS protein expression by Western blot after stimulation of cells for 24, 48, and 72 h with 10 U/ml of murine IFNγ. (B) Nitrite production in culture supernatants following stimulation with IFNγ. Values for nitrite production are expressed by mean ± SE for three different experiments performed five different times. (C) Pattern of 24, 48, and 72 h iNOS mRNA induction (RT-PCR) in response to IFNγ stimulation. Each Western blot and RT-PCR presented is from single experiment that is representative of three to five separate experiments.
Table 1.
Nitrite concentration (μM) in RCC cell lines of NO-2 before and after treatment with IFNγ. 1 Means of 6 different experiments; 2 SE; * p-values for significance (p=0.0001) between untreated and treated cells with IFNγ for each cell line.
| CL-2 | CL-19 | Renca | ||||
|---|---|---|---|---|---|---|
| Time | -IFNγ | +IFNγ | -IFNγ | +IFNγ | -IFNγ | +IFNγ |
| 24h | 1.41 ± 0.642 | 59.7 ± 2.01 | 1.7 ± 0.63 | 42.4 ± 5.29 | 0 | 0 |
| 48h | 3.5 ± 0.61 | 73.3 ± 3.79 | 4.6 ± 0.36 | 52.0 ± 6.69 | 0 | 0 |
| 72h | 12.2 ± 1.12* | 85.6 ± 9.62* | 15.4 ± 0.86* | 71.9 ± 9.93* | 0 | 0 |
IFNγ induces NO-mediated cell death in CL-2 and CL-19 cell lines that is abrogated by the iNOS inhibitor L-NMMA
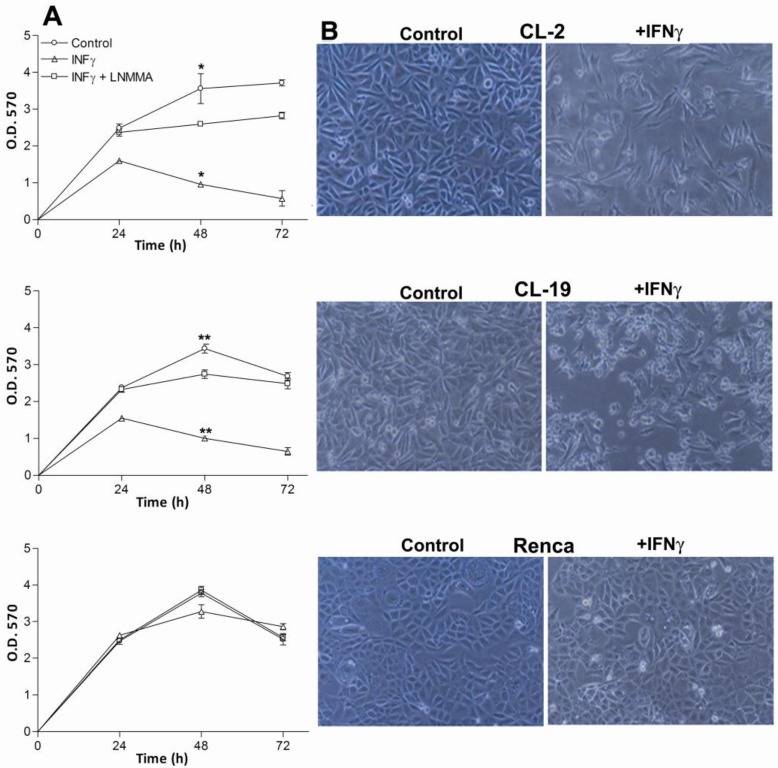
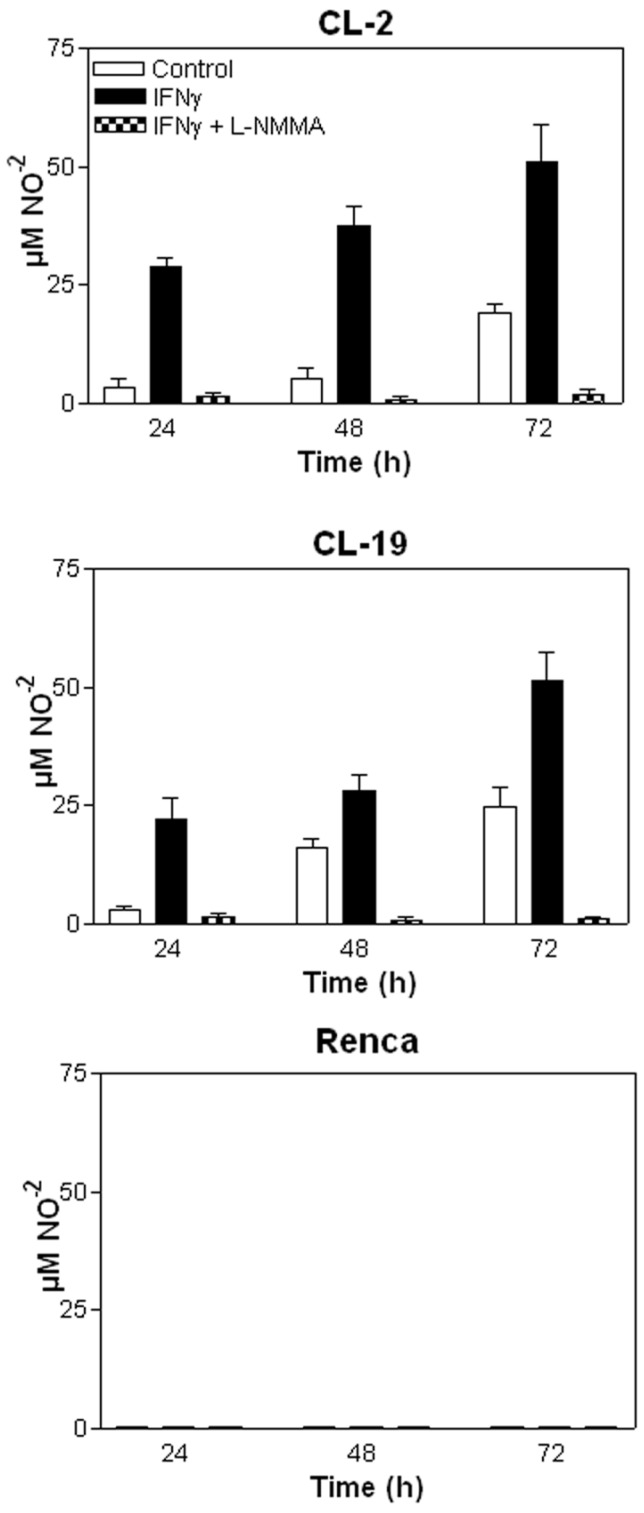
The effectiveness of IFNγ in inhibiting the proliferation of mRCC cell lines was assessed using MTT assays. As seen in Figure 3A, the addition of 10U/ml of IFNγ to mRCC cell lines significantly inhibited cell proliferation of CL-2 (p= 0.008) and CL-19 (p= 0.0001) after 48 h in culture when compared to untreated cells. Not surprisingly, no significant difference in proliferation (p= 0.091) was observed in Renca cells. As seen in Figure 3B, differences in cell morphology and cell growth in CL-2 and CL-19 treated cells were observed at 48 hours post-treatment; these effects were absent in Renca cells at the same time-point. To test whether IFNγ inhibited cell proliferation and induced cell death through the endogenous production of NO, L-NMMA, a non-selective inhibitor of all NOS isoforms, was added to the cultures. Confirming the cell death resulting from NO production, L-NMMA completely reversed the killing effects of IFNγ treatment (Figure 3A). NO production was also measured directly from the supernatants and showed that LNMMA completely inhibited the production of nitrites after stimulation with IFNγ in CL-2 and CL-19 cell lines (*** p<0.0001) as seen in Figure 4.The results are most easily interpreted to indicate that the endogenous production of NO by IFNγ treatment has a potent inhibitory effect on tumor cell survival and proliferation. Only endogenous NO inhibited cell proliferation and survival because control experiments, where nitrites were added exogenously to the media, showed no effect on cell survival (data not shown).
Figure 3.
Effect of IFNγ and LNMMA on CL-2, CL-19 and Renca cell proliferation. (A) Three hundred thousand cells from CL-2, CL-19 and Renca were seeded in six well plates and cultured in presence or absence of IFNγ (10U/ml) or iNOS inhibitor LNMMA (1mM). Cell proliferation was measured at 24, 48, and 72 h using the MTT assay. The results are expressed as O.D (570) means ± SE of triplicate determinations from five independent experiments. Significant differences in proliferation were observed between control and IFNγ treated CL-2 (* p= 0.0084) and CL-19 (**p=0.0001) cells respectively. (B) Morphological changes and inhibition of cell proliferation in the CL-2, CL-19 and Renca cells before and after treatment with IFNγ. Microscopic images of mRCC cells after treatment with IFNγ were taken at 48h. Photographs were taken under phase contrast at 100x magnification.
Figure 4.
Nitrite (NO-2) production measured in mRCC cells after treatment with IFNγ and IFNγ plus LNMMA. Average nitrite concentration between untreated and treated cells with IFNγ over time in (A) CL-2 (* p= 0.003) and (B) CL-19 (** p= 0.037) at 48 and 72 h respectively. Significant reduction of nitrite production (*** p<0.0001) in CL-2 and CL-19 cells after the addition of iNOS inhibitor LNMMA. (C) Nitrite values in Renca cells after IFNγ treatment. Results are the mean ± SE of triplicates of five different separate experiments.
Effects of IFNγ on DNA fragmentation and cell cycle in the cell lines
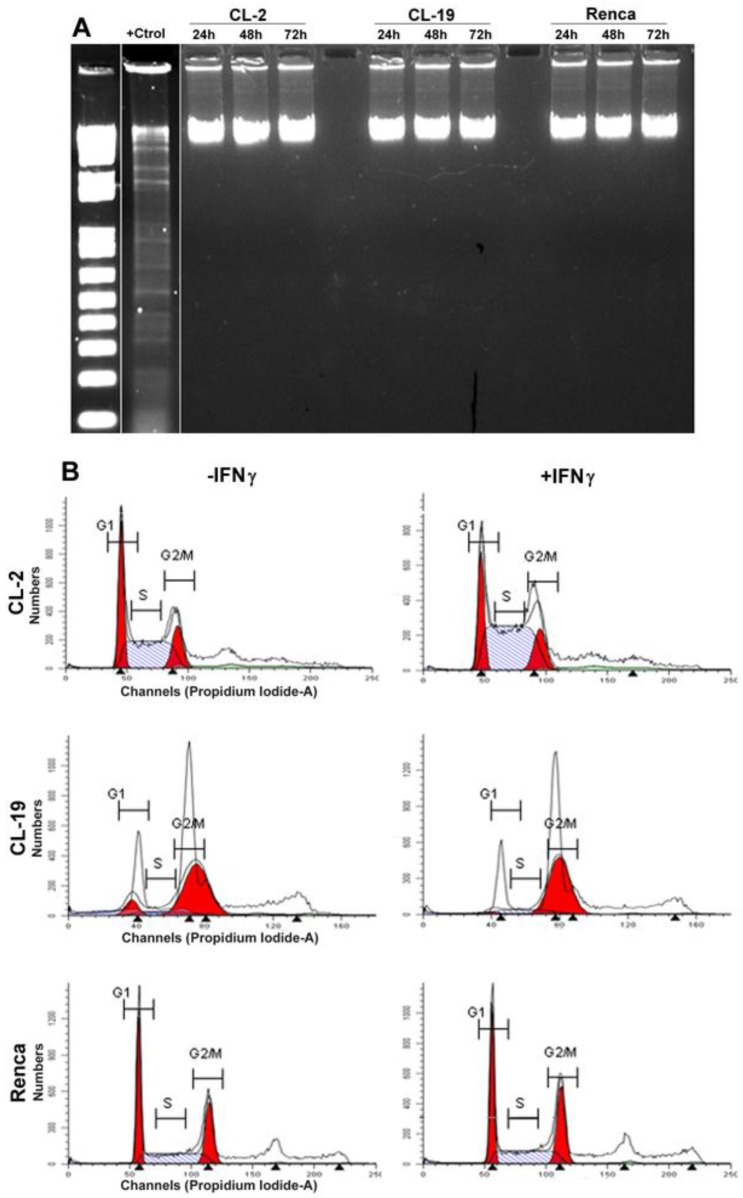
Apoptosis is characterized by DNA fragmentation; therefore, DNA was isolated from the three cell lines after treatment with IFNγ at 24, 48, and 72 h and tested for its integrity. The results shown in Figure 5A indicate that the cytostatic effects of endogenous NO production do not result in an increase in DNA fragmentation in any of the cell lines. An analyses of the cell cycle profile by flow cytometry (Figure 5B) also indicated that IFNγ treatment did not induce apoptosis in CL-2 and CL19 cells; however, CL-2 and CL-19 cells accumulated at G2/M (4N DNA content). Therefore, growth inhibition of these cell-lines by IFNγ may be a result from the cell-cycle arresting at G2/M. No changes in cell cycle were observed in response to IFNγ in Renca cells.
Figure 5.
Effect of IFNγ treated cells on apoptosis and cell cycle. (A) Cells were plated for 1 day and then cultured in media containing IFNγ. DNA was extracted and subjected to DNA fragmentation ladder analysis. Data are representative of three independent experiments with similar results. (B) Analysis of cell cycle progression in untreated and treated mRCC cell lines with IFNγ. After 24, 48, and 72 h cells were analyzed for DNA content by flowcytometry. A representative experiment performed at 48 h indicate the distribution of the cells in G1, S, and G2/M phases of the cell cycle.
Effect of IFNγ treatment on arginase activity
The differential expression of ARG2 previously observed 23 led us to test whether IFNγ treatment affected ARG activity and L-arginine concentrations. Cytoplasmic extracts of untreated and treated cells with IFNγ were isolated at 24, 48, and 72 h and tested for ARG activity. The three cell lines express similar basal ARG activity at 24 h. After 48 h, there was a slight increase in CL-2 and Renca cells, while a marked increase in CL-19 cells was observed, as illustrated in Figure 6. Immunoblot analysis for ARG2 protein confirmed these results (data not shown). The increase in basal ARG activity within the culture times is observed only in the absence of media changes during the time course of the experiments. Changes were not observed in both untreated and treated cells with IFNγ. Further, treatment with IFNγ produced a significant decrease in ARG activity in CL-2 (p= 0.048) and CL-19 (p= 0.003) cells after 48 h, an effect that was only observed in Renca cells (p=0.031) after 72 h in culture (Figure 6). Collectively, these data indicate that IFNγ interferes with ARG activity in the three cell lines, perhaps by inhibiting transcription of the ARG2 gene.
Figure 6.
The effects of IFNγ on the induction of arginase activity in the mRCC cell lines. Cells were treated with 10 U/ml of IFNγ for 24, 48, and 72 h and tested for arginase activity (conversion of L-argine to L-ornitine by arginase). As compared to untreated cells (Ctrl) significant reduction of ARG activity is observed: CL-2 (* p= 0.02) CL-19 (** p= 0.0008) and Renca (*** p= 0.03) at 72 h. The data represent the mean ± SE of triplicate determinations from five separate experiments.
Effect of IFNγ in mRCC cell lines on L-arginine concentration and its effect on the production of NO and citrulline
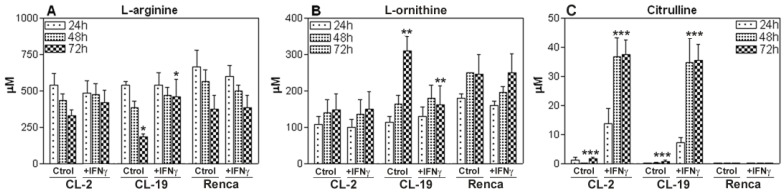
Since L-arginine is the common substrate for iNOS and ARG, we wanted to explore the effect of IFNγ treatment on L-arginine consumption by HPLC. The standard concentration of L-arginine in the cell culture media was 1,140 μM for all experiments. As seen in Figure 6A, in the first 24 h, the depletion of L-arginine was similar in the three cell lines. The CL-19 cell line, which expressed the highest levels of ARG activity, significantly depleted the media of L-arginine concentrations by 6-fold (450 μM) at 24 h, 7-fold (296 μM) at 48h, and 9-fold (110 μM) at 72 h; however, no major changes in L-arginine content were observed in CL-2 and Renca cells at 48 and 72 h in culture. The addition of IFNγ to the cultures significantly (p= 0.0002) decreased the rate of depletion of L-arginine in CL-19 at 72h when compared to untreated cells at the same time point (Figure 7A). The levels of L-arginine in CL-2 and Renca cells remained unchanged after the treatment. Concurrent with the depletion of L-arginine in the three cell lines, there was an increase in L-ornithine production that was abrogated (p= 0.018) after 72 h of IFNγ treatment in CL-19 cells when compared to CL-2 and Renca as illustrated in Figure 7B. As iNOS catalyzes the conversion of L-arginine to equimolar quantities of NO and citrulline, the samples were analyzed for citrulline after treatment with IFNγ. Citrulline concentrations increased significantly in a time-dependent manner in CL-2 (p= 0.0001) and CL-19 (p= 0.0013) at 48 h, whereas no increase was observed in Renca cells (Figure 7C). The data obtained for citrulline were nearly identical to the values obtained for nitrites seen in Figure 4, after IFNγ treatment. Together, these results suggest that IFNγ shifts the L-arginine metabolic pathway towards NO and citrulline production, consequently inhibiting RCC cell proliferation.
Figure 7.
L-arginine, L-ornithine, and citrulline levels. Culture supernatants of CL-2, CL-19 and Renca cells untreated and treated with IFNγ were collected at 24, 48, and 72 h and analyzed by HPLC after deproteinization with methanol and derivatization with OPA. Standards for L-arginine, L-ornithine, and citrulline in methanol were run with each experiment. Asterisks show significant differences between untreated and treated cells. (A) CL-19 L-arginine (* p= 0.0002), (B) CL-19. L-ornithine (** p=0.0183), and (C) CL-2 and CL-19 citrulline (*** p=0.0001). The results are expressed as mean ± SE of duplicate determinations from four independent experiments.
Cytokine Production in mRCC cell lines
In order to gain insights into the biology of mRCC cell lines and to determine their ability to produce cytokines, we tested for IL-2, IL-4, IL-5, IL-10, IL-12 (p70), GM-CSF, IFNγ, TNFα, and VEGF concentrations in the culture supernatants. Figure 8 shows the cytokine expression in the three cell lines at 48 h. We choose 48 h since the maximum production of these cytokines was observed at this time point. Moderate concentrations of IL-1β and IL-6 and high concentrations of VEGF and TGFβ were observed in all three cell lines. It appears that IFNγ treatment had a significant effect in the production of IL-1β and IL-6 (p= 0.001 and p= 0.028) in CL-2 and IL-1β (p= 0.09) in CL-19 cells (Figure 8). This effect was not observed in Renca cells. Although, the amount of VEGF and TGFβ was considerably higher in all three cell lines at basal level, treatment with IFNγ had no significant effect on the concentrations of these cytokines.
Figure 8.
Cytokine profile in supernatants of mRCC cell lines. The cell lines were seeded in 6 well plates and treated with IFNγ (10U/ml) for 24, 48, and 72 h. Supernatants were collected and assayed using a Th1/Th2 panel Bio-Plex cytokine assay. IL-1β, IL-6, VEGF, and TGFβ levels are shown. Treatment with IFNγ has a significant inhibitory effect in CL-2 cells production of IL-1β and IL-6 in CL-2 (* p<0.001; ** p=0.002) and IL-1β in CL-19 (* p=0.009). Data are representative of experiments at 48h of at least 3 independent experiments.
DISCUSSION
In this study, we reported the following: 1) a new mechanism to define the cytostatic role of NO in the inhibition of RCC cell proliferation; 2) the effect of IFNγ in modulating L-arginine metabolism and cytokine production; 3) the resistance of certain RCC cell lines (Renca) to IFNγ treatment; and, 4) new insights into the mechanisms by which RCC tumors may locally and systemically shut down an important effector arm of the immune response. IFNs have been widely used in preclinical models and in immunotherapy trials for RCC 32;33. The activity of monotherapy with IFNα in metastatic RCC has been extensively evaluated 34;35 with disappointing and controversial results. However, trials using a combination of IL-2 and IFNα indicated a clinically useful antitumor activity with a 15% overall response 36;37. In contrast, therapy with the single agent IFNγ has shown discouraging results in patients with RCC. Although preliminary studies suggested the benefits of IFNγ treatment 38, only a 4.4% overall response has been seen in phase III studies indicating that IFNγ alone has no major impact on RCC treatment 39. These results are striking since many IFNγ-mediated properties (e.g. anti-proliferative, apoptotic, and anti-angiogenic activity) have been attributed to this cytokine 40;41.
Our study was aimed to determine the mechanism(s) by which IFNγ is capable of inducing growth inhibition of murine RCC cell lines. Previously, we reported that murine CL-2, CL-19, and Renca cell lines have different levels of ARG2 expression that appear to be associated with the enhancement of cell proliferation 23.The results indicate a need for the tumor cells to use ARG2 to metabolize L-arginine for the synthesis of polyamines to promote cell growth. Conversely, NO is a free radical that, at low concentrations, acts as single signal transducer whereas at higher concentrations can act as a cytotoxic/cytostatic defensive mechanism against pathogens and perhaps tumors 42. In our study, we found that RCC cell lines in the logarithmic phase of proliferation expressed low iNOS protein levels and low concentrations of NO as measured by the production of nitrites (15 -20 μM), whereas ARG2 activity was highly present in the 3 cell lines. These results indicate that low iNOS and high ARG2 activity favor the growth of the cell lines and are supported by previous studies showing that high expression of ARG can inhibit iNOS activity and NO production 43-45. We believe this could be a mechanism used by tumor cells to survive by directly avoiding the cytostatic effects of NO.
Since IFNγ is a potent inducer of iNOS, we wanted to determine in vitro the mechanism(s) by which this cytokine is capable of inhibiting RCC cell proliferation. We successfully demonstrated that iNOS induction within the tumor cells in response to IFNγ caused a robust and sustained accumulation of endogenous NO that resulted in an 80-85% growth inhibition of CL-2 and CL-19 cell lines (Figure 3). This effect was abrogated by the addition of the iNOS inhibitor, L-NMMA, showing that the reduction in cell growth was mediated by NO. It is possible that the NO-mediated cytostatic effects observed in our in vitro model could produce a different outcome in an in vivo model due to the presence of NO-producing cells infiltrating the tumor microenvironment. Here, we found that the cytostatic effect of NO in RCC cell lines was not due to apoptosis as demonstrated by others 46;47, but rather by a cell cycle arrest at the G2/M phase. This possibly could be mediated by the transcriptional modulation of cyclins and/or cyclin-dependent kinases as seen in breast cancer cell lines and T cells 48;49. Since the growth-inhibitory activity of IFNγ appears to be accompanied by the inhibition of ARG activity in CL-2 and CL-19 cells (Figure 5), we are suggesting that this inhibition could be partially responsible for the growth-inhibitory effects of IFNγ in addition to the major cytostatic effects of NO. The importance of ARG and/or NO in the growth-inhibition of RCC cells needs to be explored further by possibly using ARG and iNOS-KO mice. Altogether, our data suggest that ARG2 and iNOS can be modulated in a coordinated fashion by IFNγ to counterbalance tumor growth and tumor inhibition. Lately, NO has emerged as a potential anti-proliferative agent capable of overcoming tumor cell resistance to conventional therapeutic agents. Despite the relative beneficial effect of IFNγ-NO induction which inhibits RCC proliferation, a substantial percentage (15%) of the cells failed to respond to IFNγ treatment. For the first time, our results show that a certain subpopulation of RCC cells develop a mechanism of resistance to specific cytostatic stimuli that could largely explain the commonly observed lack of response to IFNγ in clinical trials. Mechanisms of resistance have been associated with IFNα defective Jak/Stat signaling pathways 50-52 that could be similar with IFNγ.
Interestingly, in our study, we showed that Renca cells which, upon stimulation with IFNγ express iNOS mRNA, did not express iNOS protein or NO production (Figure 2). Different mechanisms have been postulated to explain the lack of iNOS expression, but a novel finding has shown that loss of iNOS protein expression is exerted by translational inhibition specifically mediated by miR-146a 50. The goal of this study was not to understand the control of iNOS expression at the translational level, but to highlight that different RCC cell types can vary in their sensitivity to IFNγ and, therefore, to NO. These findings will have a high translational relevance because permitting us to identify RCC cells better and to determine their sensitivity to IFN treatment. It is possible that human RCC could present with similar characteristics to those seen in Renca cells. Therefore, these cells (or patients) will not benefit from IFNγ treatment not because IFNγ does not work but rather because these cells found a way to become resistant to this cytokine as a mechanism of survival. Therefore, in patients, the identification of resistance to INF treatment could be extremely important for a more personalized and effective treatment of RCC.
Production of either stimulatory or inhibitory cytokines by infiltrating or neoplastic cells may have more of an impact on the development of an effective anti-tumor response than in the promotion of tumor growth. When we tested cytokine production before IFNγ treatment, IL-1β, IL-6, TGFβ, and VEGF were the most predominant cytokines in all 3 cell lines. Our results showed a parallel effect of IL-1β and IL-6 reduction (CL-2 and CL-19) with the inhibitory effects of NO, an effect that could be associated with low ARG activity. Conversely, IFNγ did not have an effect on the production of VEGF and TGFβ, indicating that the inhibition of these cytokines can be mediated by other signaling pathways and that their presence can contribute to the maintenance of tumor growth and angiogenesis. A combination therapy of IFNγ with other drugs (e.g. sunitinib) which targets VGEF receptors could advance the treatment of RCC.
In summary, we provided new evidence that an improved understanding of IFNγ biology in the inhibition of tumor cell proliferation will provide new insights regarding the use of this cytokine, not only for the treatment of RCC, but for the treatment of other tumors where the use of interferons is indicated. Although the development of agents targeting VEGF and mTOR pathways has provided chemotherapeutic agents that are more active and less potentially toxic than interferons, regardless of the specific target, the end point of these therapies relies upon the induction of Th1 responses (mainly IFNγ expression). Our results indicate that IFNγ can play a key role in modulating L-arginine metabolism by inhibiting ARG activity and by enhancing the endogenous production of NO to inhibit the proliferation of RCC cells. Since numerous conflicting results about the role of iNOS and NO in cancer have been reported 53, understanding the multifaceted role of NO in cancer is imperative. The biochemistry, type of tumor, and the spatial and temporal effects of its production need to be more carefully studied in association with IFNγ. Our findings can have multiple implications in RCC treatment not only by eliminating tumor cells and inhibiting angiogenesis, but more importantly by identifying the sets of patients with RCC that will benefit from IFNγ treatment.
Acknowledgments
This work was supported by grant LEQSF(20-04-07)-RD-A-20 from the Louisiana Board of Regents and startup funds from the Stanley S. Scott Cancer Center at LSUHSC-NO. The authors greatly thank Dr. Robert H. Wiltrout who kindly provided us with the cell lines, Ms. Jonna Ellis for editing the manuscript, and Dr. Jeff Hobden for his critical review.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011 Jul;61(4):212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Staehler M, Rohrmann K, Bachmann A, Zaak D, Stief CG, Siebels M. Therapeutic approaches in metastatic renal cell carcinoma. BJU Int. 2005 Jun;95(8):1153–61. doi: 10.1111/j.1464-410X.2005.05537.x. [DOI] [PubMed] [Google Scholar]
- 3.Bleumer I, Oosterwijk E, De MP, Mulders PF. Immunotherapy for renal cell carcinoma. Eur Urol. 2003 Jul;44(1):65–75. doi: 10.1016/s0302-2838(03)00191-x. [DOI] [PubMed] [Google Scholar]
- 4.Atkins MB, Regan M, McDermott D. Update on the role of interleukin 2 and other cytokines in the treatment of patients with stage IV renal carcinoma. Clin Cancer Res. 2004 Sep 15;10(18 Pt 2):6342S–6S. doi: 10.1158/1078-0432.CCR-040029. [DOI] [PubMed] [Google Scholar]
- 5.McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, Kirkwood JM, Gordon MS, Sosman JA, Ernstoff MS, Tretter CP, Urba WJ. et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005 Jan 1;23(1):133–41. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 6.Atkins MB, Bukowski RM, Escudier BJ, Figlin RA, Hudes GH, Kaelin WG Jr, Linehan WM, McDermott DF, Mier JW, Pedrosa I, Rini BI, Signoretti S. et al. Innovations and challenges in renal cancer: summary statement from the Third Cambridge Conference. Cancer. 2009 May 15;115(10 Suppl):2247–51. doi: 10.1002/cncr.24229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R. et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009 Mar 15;15(6):2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 8.De Mulder PH. Targeted therapy in metastatic renal cell carcinoma. Ann Oncol. 2007 Jul;18(Suppl 9):ix98–102. doi: 10.1093/annonc/mdm303. [DOI] [PubMed] [Google Scholar]
- 9.Sosman JA, Puzanov I, Atkins MB. Opportunities and obstacles to combination targeted therapy in renal cell cancer. Clin Cancer Res. 2007 Jan 15;13(2 Pt 2):764s–9s. doi: 10.1158/1078-0432.CCR-06-1975. [DOI] [PubMed] [Google Scholar]
- 10.Morris SM Jr. Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol. 2009 Jul;157(6):922–30. doi: 10.1111/j.1476-5381.2009.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009 Oct;158(3):638–51. doi: 10.1111/j.1476-5381.2009.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005 Aug;5(8):641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 13.Viola A, Bronte V. Metabolic mechanisms of cancer-induced inhibition of immune responses. Semin Cancer Biol. 2007 Aug;17(4):309–16. doi: 10.1016/j.semcancer.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Peranzoni E, Marigo I, Dolcetti L, Ugel S, Sonda N, Taschin E, Mantelli B, Bronte V, Zanovello P. Role of arginine metabolism in immunity and immunopathology. Immunobiology. 2007;212(9-10):795–812. doi: 10.1016/j.imbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998 Nov 15;336( Pt 1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Meininger CJ, Kelly KA, Hawker JR Jr, Morris SM Jr, Wu G. Activities of arginase I and II are limiting for endothelial cell proliferation. Am J Physiol Regul Integr Comp Physiol. 2002 Jan;282(1):R64–R69. doi: 10.1152/ajpregu.2002.282.1.R64. [DOI] [PubMed] [Google Scholar]
- 17.Buga GM, Wei LH, Bauer PM, Fukuto JM, Ignarro LJ. NG-hydroxy-L-arginine and nitric oxide inhibit Caco-2 tumor cell proliferation by distinct mechanisms. Am J Physiol. 1998 Oct;275(4 Pt 2):R1256–R1264. doi: 10.1152/ajpregu.1998.275.4.R1256. [DOI] [PubMed] [Google Scholar]
- 18.Zea AH, Rodriguez PC, Culotta KS, Hernandez CP, DeSalvo J, Ochoa JB, Park HJ, Zabaleta J, Ochoa AC. L-Arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cell Immunol. 2004 Nov;232(1-2):21–31. doi: 10.1016/j.cellimm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999 Oct 1;163(7):3771–7. [PubMed] [Google Scholar]
- 20.Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, Wood L, Elson P, Garcia J, Dreicer R, Bukowski R. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008 Oct 15;14(20):6674–82. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 21.Tatsumi T, Kierstead LS, Ranieri E, Gesualdo L, Schena FP, Finke JH, Bukowski RM, Mueller-Berghaus J, Kirkwood JM, Kwok WW, Storkus WJ. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002 Sep 2;196(5):619–28. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onishi T, Ohishi Y, Imagawa K, Ohmoto Y, Murata K. An assessment of the immunological environment based on intratumoral cytokine production in renal cell carcinoma. BJU Int. 1999 Mar;83(4):488–92. doi: 10.1046/j.1464-410x.1999.00925.x. [DOI] [PubMed] [Google Scholar]
- 23.Tate DJ Jr, Vonderhaar DJ, Caldas YA, Metoyer T, Patterson JR, Aviles DH, Zea AH. Effect of arginase II on L-arginine depletion and cell growth in murine cell lines of renal cell carcinoma. J Hematol Oncol. 2008;1:14. doi: 10.1186/1756-8722-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dutcher JP, Fine JP, Krigel RL, Murphy BA, Schaefer PL, Ernstoff MS, Loehrer PJ. Stratification by risk factors predicts survival on the active treatment arm in a randomized phase II study of interferon-gamma plus/minus interferon-alpha in advanced renal cell carcinoma (E6890) Med Oncol. 2003;20(3):271–81. doi: 10.1385/MO:20:3:271. [DOI] [PubMed] [Google Scholar]
- 25.Kirkwood JM, Bryant J, Schiller JH, Oken MM, Borden EC, Whiteside TL. Immunomodulatory function of interferon-gamma in patients with metastatic melanoma: results of a phase II-B trial in subjects with metastatic melanoma, ECOG study E 4987. Eastern Cooperative Oncology Group. J Immunother. 1997 Mar;20(2):146–57. doi: 10.1097/00002371-199703000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Berner V, Liu H, Zhou Q, Alderson KL, Sun K, Weiss JM, Back TC, Longo DL, Blazar BR, Wiltrout RH, Welniak LA, Redelman D. et al. IFN-gamma mediates CD4+ T-cell loss and impairs secondary antitumor responses after successful initial immunotherapy. Nat Med. 2007 Mar;13(3):354–60. doi: 10.1038/nm1554. [DOI] [PubMed] [Google Scholar]
- 27.Hillman GG, Younes E, Visscher D, Hamzavi F, Kim S, Lam JS, Montecillo EJ, Ali E, Pontes JE, Puri RK, Haas GP. Inhibition of murine renal carcinoma pulmonary metastases by systemic administration of interferon gamma: mechanism of action and potential for combination with interleukin 4. Clin Cancer Res. 1997 Oct;3(10):1799–806. [PubMed] [Google Scholar]
- 28.Gruys ME, Back TC, Subleski J, Wiltrout TA, Lee JK, Schmidt L, Watanabe M, Stanyon R, Ward JM, Wigginton JM, Wiltrout RH. Induction of transplantable mouse renal cell cancers by streptozotocin: in vivo growth, metastases, and angiogenic phenotype. Cancer Res. 2001 Aug 15;61(16):6255–63. [PubMed] [Google Scholar]
- 29.Zea AH, Culotta KS, Ali J, Mason C, Park HJ, Zabaleta J, Garcia LF, Ochoa AC. Decreased expression of CD3zeta and nuclear transcription factor kappa B in patients with pulmonary tuberculosis: potential mechanisms and reversibility with treatment. J Infect Dis. 2006 Nov 15;194(10):1385–93. doi: 10.1086/508200. [DOI] [PubMed] [Google Scholar]
- 30.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991 Jul;3(7):207–12. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 31.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O'Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005 Apr 15;65(8):3044–8. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 32.Haas GP, Hillman GG. Update on the Role of Immunotherapy in the Management of Kidney Cancer. Cancer Control. 1996 Nov;3(6):536–41. doi: 10.1177/107327489600300617. [DOI] [PubMed] [Google Scholar]
- 33.Hillman GG, Younes E, Visscher D, Hamzavi F, Kim S, Lam JS, Montecillo EJ, Ali E, Pontes JE, Puri RK, Haas GP. Inhibition of murine renal carcinoma pulmonary metastases by systemic administration of interferon gamma: mechanism of action and potential for combination with interleukin 4. Clin Cancer Res. 1997 Oct;3(10):1799–806. [PubMed] [Google Scholar]
- 34.Negrier S, Escudier B, Lasset C, Douillard JY, Savary J, Chevreau C, Ravaud A, Mercatello A, Peny J, Mousseau M, Philip T, Tursz T. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d'Immunotherapie. N Engl J Med. 1998 Apr 30;338(18):1272–8. doi: 10.1056/NEJM199804303381805. [DOI] [PubMed] [Google Scholar]
- 35.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002 Jan 1;20(1):289–96. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg SA, Lotze MT, Yang JC, Linehan WM, Seipp C, Calabro S, Karp SE, Sherry RM, Steinberg S, White DE. Combination therapy with interleukin-2 and alpha-interferon for the treatment of patients with advanced cancer. J Clin Oncol. 1989 Dec;7(12):1863–74. doi: 10.1200/JCO.1989.7.12.1863. [DOI] [PubMed] [Google Scholar]
- 37.Spencer WF, Linehan WM, Walther MM, Haas GP, Lotze MT, Topalian SL, Yang JC, Merino MJ, Lange JR, Pockaj BA. Immunotherapy with interleukin-2 and alpha-interferon in patients with metastatic renal cell cancer with in situ primary cancers: a pilot study. J Urol. 1992 Jan;147(1):24–30. doi: 10.1016/s0022-5347(17)37124-0. [DOI] [PubMed] [Google Scholar]
- 38.Ellerhorst JA, Kilbourn RG, Amato RJ, Zukiwski AA, Jones E, Logothetis CJ. Phase II trial of low dose gamma-interferon in metastatic renal cell carcinoma. J Urol. 1994 Sep;152(3):841–5. doi: 10.1016/s0022-5347(17)32587-9. [DOI] [PubMed] [Google Scholar]
- 39.Ernstoff MS, Nair S, Bahnson RR, Miketic LM, Banner B, Gooding W, Day R, Whiteside T, Hakala T, Kirkwood JM. A phase IA trial of sequential administration recombinant DNA-produced interferons: combination recombinant interferon gamma and recombinant interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 1990 Oct;8(10):1637–49. doi: 10.1200/JCO.1990.8.10.1637. [DOI] [PubMed] [Google Scholar]
- 40.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002 Apr;13(2):95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 41.Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, Kleinman HK, Reaman GH, Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995 Jul 1;182(1):155–62. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ignarro LJ. The unique role of nitric oxide as a signaling molecule in the cardiovascular system. Ital Heart J. 2000 Sep;1(Suppl 3):S28–S29. [PubMed] [Google Scholar]
- 43.Li H, Meininger CJ, Hawker JR Jr, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM Jr, Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab. 2001 Jan;280(1):E75–E82. doi: 10.1152/ajpendo.2001.280.1.E75. [DOI] [PubMed] [Google Scholar]
- 44.Wang WW, Jenkinson CP, Griscavage JM, Kern RM, Arabolos NS, Byrns RE, Cederbaum SD, Ignarro LJ. Co-induction of arginase and nitric oxide synthase in murine macrophages activated by lipopolysaccharide. Biochem Biophys Res Commun. 1995 May 25;210(3):1009–16. doi: 10.1006/bbrc.1995.1757. [DOI] [PubMed] [Google Scholar]
- 45.Gotoh T, Mori M. Arginase II downregulates nitric oxide (NO) production and prevents NO-mediated apoptosis in murine macrophage-derived RAW 264.7 cells. J Cell Biol. 1999 Feb 8;144(3):427–34. doi: 10.1083/jcb.144.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sousa MS, Latini FR, Monteiro HP, Cerutti JM. Arginase 2 and nitric oxide synthase: Pathways associated with the pathogenesis of thyroid tumors. Free Radic Biol Med. 2010 Sep 15;49(6):997–1007. doi: 10.1016/j.freeradbiomed.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Hussain SP, Trivers GE, Hofseth LJ, He P, Shaikh I, Mechanic LE, Doja S, Jiang W, Subleski J, Shorts L, Haines D, Laubach VE. et al. Nitric oxide, a mediator of inflammation, suppresses tumorigenesis. Cancer Res. 2004 Oct 1;64(19):6849–53. doi: 10.1158/0008-5472.CAN-04-2201. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007 Feb 15;109(4):1568–73. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh R, Pervin S, Karimi A, Cederbaum S, Chaudhuri G. Arginase activity in human breast cancer cell lines: N(omega)-hydroxy-L-arginine selectively inhibits cell proliferation and induces apoptosis in MDA-MB-468 cells. Cancer Res. 2000 Jun 15;60(12):3305–12. [PubMed] [Google Scholar]
- 50.Korkola JE, Blaveri E, DeVries S, Moore DH, Hwang ES, Chen YY, Estep AL, Chew KL, Jensen RH, Waldman FM. Identification of a robust gene signature that predicts breast cancer outcome in independent data sets. BMC Cancer. 2007;7:61. doi: 10.1186/1471-2407-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brinckmann A, Axer S, Jakschies D, Dallmann I, Grosse J, Patzelt T, Bernier T, Emmendoerffer A, Atzpodien J. Interferon-alpha resistance in renal carcinoma cells is associated with defective induction of signal transducer and activator of transcription 1 which can be restored by a supernatant of phorbol 12-myristate 13-acetate stimulated peripheral blood mononuclear cells. Br J Cancer. 2002 Feb 1;86(3):449–55. doi: 10.1038/sj.bjc.6600066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shang D, Liu Y, Ito N, Kamoto T, Ogawa O. Defective Jak-Stat activation in renal cell carcinoma is associated with interferon-alpha resistance. Cancer Sci. 2007 Aug;98(8):1259–64. doi: 10.1111/j.1349-7006.2007.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006 Jul;6(7):521–34. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]