Abstract
Cyclooxygenase-2 (COX-2) is an inducible form of COX and is overexpressed in diverse tumors, raising the possibility of a role for COX-2 in carcinogenesis. In addition, COX-2 contributes to angiogenesis. The Epstein–Barr virus (EBV) oncoprotein, latent membrane protein 1 (LMP1), is detected in at least 70% of nasopharyngeal carcinoma (NPC) and all EBV-infected preinvasive nasopharyngeal lesions. We found that in specimens of LMP1-positive NPC, COX-2 is frequently expressed, whereas LMP1-negative NPC rarely express the enzyme. We next found that expression of LMP1 in EBV-negative nasopharyngeal epithelial cells induced COX-2 expression. Coexpression of IκBα(S32A/S36A), which is not phosphorylated and prevents NF-κB activation, with LMP1 showed that NF-κB is essential for induction of COX-2 by LMP1. We also demonstrate that NF-κB is involved in LMP1-induced cox-2 promoter activity with the use of reporter assays. Two major regions of LMP1, designated CTAR1 and CTAR2, are signal-transducing domains of LMP1. Constructs expressing either CTAR1 or CTAR2 induce COX-2 but to a lesser extent than wild-type LMP1, consistent with the ability of both regions to activate NF-κB. Furthermore, we demonstrate that LMP1-induced COX-2 is functional because LMP1 increased production of prostaglandin E2 in a COX-2-dependent manner. Finally, we demonstrate that LMP1 increased production of vascular endothelial growth factor (VEGF). Treatment of LMP1-expressing cells with the COX-2-specific inhibitor (NS-398) dramatically decreased production of VEGF, suggesting that LMP1-induced VEGF production is mediated, at least in part, by COX-2. These results suggest that COX-2 induction by LMP1 may play a role in angiogenesis in NPC.
Cyclooxygenase (COX) is the key enzyme in the biosynthetic pathway of prostaglandins (PGs) and thromboxanes from arachidonic acid. There are two isoforms of COX, COX-1 and COX-2. COX-1 is considered a housekeeping protein because it is constitutively expressed. On the other hand, COX-2 is inducible by various stimuli such as cytokines, hormones, and mitogens. Overexpression of COX-2 has been reported recently in various tumors such as colon cancer (1), lung cancer (2), breast cancer (3), gastric cancer (4), esophageal cancer (5), and head and neck cancer (6), suggesting that COX-2 may be involved in carcinogenesis. In addition, a contribution of COX-2 to angiogenesis was recently reported. Overexpression of COX-2 in human colon cancer cells induces angiogenesis in vitro through induction of angiogenic factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (7). Furthermore, a selective COX-2 inhibitor inhibits angiogenesis in vitro (8) and in vivo (9).
Epstein–Barr virus (EBV), a ubiquitous human herpesvirus, is closely associated with several human malignancies such as Burkitt's lymphoma and nasopharyngeal carcinoma (NPC). In addition, EBV has been associated more recently with gastric carcinoma (10) and invasive breast cancer (11). In all of the tumors, EBV infection is predominantly latent. EBV genes expressed in latent infection are restricted to six EBV nuclear antigens (EBNAs; EBNA1,-2, -3A, -3B, -3C, -LP) and three latent membrane proteins (LMPs; LMP1, -2A, -2B). According to the pattern of expression of those genes, EBV latent infection is divided into three groups. In type I latency, only EBNA1 is always expressed as represented by Burkitt's lymphoma. In type III latency, all of the EBNAs and LMPs are expressed as in EBV lymphoproliferative diseases in immunocompromised hosts. In type II latency, EBNA1 and the three LMPs are expressed as in NPC and in nasal T/natural killer cell lymphoma. Among the EBV latency genes, LMP1 is considered an oncoprotein because it can transform rodent fibroblasts (12). The carboxyl-terminal portion of LMP1, which is in the cytoplasmic domain of the protein, contains two functional domains. The proximal domain, COOH-terminal activation region (CTAR) 1, interacts with tumor necrosis factor receptor-associated factors and activates NF-κB (13, 14). The distal domain, CTAR2, interacts with tumor necrosis factor receptor-associated death domain protein and also activates NF-κB (15). In addition to the activation of NF-κB, LMP1 activates c-Jun NH2-terminal kinase through CTAR2 (16) and also activates p38 mitogen-activated protein kinase through both CTAR1 and CTAR2 (17).
NPC is endemic in southern China and Taiwan, but it is rare in the United States, Europe, and Japan. EBV genomes are detected in virtually all NPC worldwide and are monoclonal (18), consistent with a causal role for NPC. LMP1 is detected in at least 70% of NPC and all EBV-infected preinvasive nasopharyngeal lesions (19), suggesting that LMP1 may play a role in NPC carcinogenesis. Here, we show that LMP1 induces expression of COX-2, which is mediated by NF-κB as an essential factor through both CTAR1 and CTAR2 in nasopharyngeal epithelial cells. We also show that induction of COX-2 is involved, at least in part, in the enhanced production of VEGF in LMP1-expressing cells. Because LMP1-positive NPCs tend to invade more frequently outside the nasopharynx and the lymph nodes than LMP1-negative NPCs (20), COX-2 induction by LMP1 may contribute to such invasive character through angiogenesis.
Materials and Methods
NPC Tissues.
Fourteen frozen NPC tissues were used. All samples were EBV-positive.
Cell Culture.
Ad-AH cells, kindly provided by Dr. E. Flemington (Tulane University, New Orleans), are an EBV-negative human nasopharyngeal epithelial cell line (21). An EBV-negative clone of NPC-KT cells, resulting from loss of EBV-DNA from EBV-positive NPC-KT cells, was isolated by serial dilution and designated as 422F6 cells. NPC-KT cells were originally derived by fusion of Ad-AH cells and NPC tissue (22). HeLa human cervical cancer cells and 293 human embryonal kidney cells were obtained from the American Type Culture Collection. All cells were cultured in DMEM supplemented with 10% FBS and penicillin and streptomycin.
Plasmids.
LMP1 expression plasmid was previously described (23). LMP1 mutants (LMP1 1–187, LMP1 1–231, and LMP1 Δ187–351) expression plasmids were described (24) and kindly provided by Dr. Nancy Raab-Traub (University of North Carolina). LMP1 1–187 contains neither CTAR1 nor CTAR2, LMP1 1–231 contains only CTAR1, and LMP1 Δ187–351 contains only CTAR2. IκBα(S32A/S36A) expression plasmid, in which both serines at position 32 and 36 were substituted by alanines, was kindly provided by Dr. Albert Baldwin (University of North Carolina). IκBα(S32A/S36A) is not phosphorylated because of these substitutions, resulting in prevention of both degradation of IκBα and subsequent translocation of NF-κB into nucleus. A series of cox-2 promoter reporter plasmids were described (25–28).
Transient and Stable Transfection.
The 422F6 cells, HeLa cells, and 293 cells were transfected with 1 μg of appropriate plasmid(s) with the use of an Effectene transfection kit (Qiagen) following the manufacturer's instructions. Ad-AH cells were transfected with 5 μg of appropriate plasmid(s) with Lipofectamine reagent (Life Technologies, Grand Island, NY) following the manufacturer's instructions. Stable cell lines were established by cultivating transfected Ad-AH cells in the presence of gentamicin (800 μg/ml Geneticin, Life Technologies) and designated as Ad-AH/transfected plasmid(s) here.
Western Blot Analysis.
Protein was extracted from cultured cells or NPC tissues, and concentration was calculated as described (29). One-hundred micrograms of cultured cell lysates were electrophoresed and transferred onto a nitrocellulose membrane. Equal loading of samples was confirmed with Ponceau-S staining of the membrane in all cases. Then, COX-2, LMP1, FLAG-tagged LMP1 mutants, or FLAG-tagged IκBα(S32A/S36A) and γ-tubulin were analyzed as reported with the use of rabbit anti-human COX-2 polyclonal antibody (Oxford Biomedical Research, Oxford, MI), mouse anti-LMP1 monoclonal antibody (Dako), mouse anti-FLAG M5 monoclonal antibody (Kodak), and mouse anti-human γ-tubulin monoclonal antibody (Sigma), respectively. Conditioned media collected as below were concentrated and electrophoresed under reducing conditions; VEGF production was then analyzed with mouse anti-VEGF monoclonal antibody (Santa Cruz Biotechnology).
Luciferase Reporter Assay.
Luciferase assays were performed after transient transfection of each construct as a reporter plasmid. Transfection efficiency was monitored by cotransfection with β-galactosidase reporter plasmid.
Electrophoretic Mobility Shift Assay.
Nuclear extracts were prepared as reported (29). Synthetic oligonucleotides used for probes were identical to either of the NF-κB binding sequences in the promoter region of COX-2. Five micrograms of nuclear extracts were analyzed as described (29).
Conditioned Media, Enzyme Immunoassay (EIA), and Gelatin Zymography.
After 48 h of transfection, cells were cultured for 24 h in DMEM with neither FBS nor antibiotics with or without treatment with NS-398 (20 μM, Cayman Chemicals, Ann Arbor, MI), phorbol 12-myristate 13-acetate (PMA) (100 ng/ml, Sigma) or in combination. Conditioned media were used for EIA for PGE2 (PGE2 EIA kit, Cayman Chemicals) with dilution. Gelatin zymography was performed as described (29).
Results
LMP1-Positive but Not LMP1-Negative NPC Frequently Express COX-2.
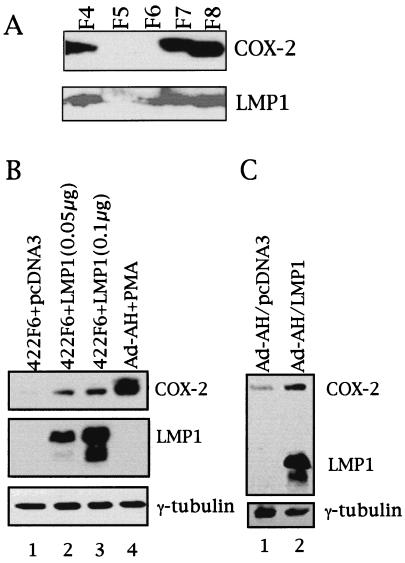
As summarized in Table 1, 7 of 10 LMP1-positive NPC clearly expressed COX-2 disclosed by Western blotting. On the other hand, only 1 of 4 LMP1-negative NPC expressed COX-2. Fig. 1A demonstrates representative images of COX-2 and LMP1 produced by Western blotting in NPC tissue extracts. LMP1-positive NPCs F4, F7, and F8 clearly expressed COX-2. COX-2 was not detected in the faintly LMP1-positive NPC F6, nor was COX-2, detected in the LMP1-negative NPC F5.
Table 1.
Expression of LMP1 and COX-2 in NPC
| Case | LMP1* | COX-2* |
| F3 | + | + |
| F4 | + | + |
| F7 | + | + |
| F8 | + | + |
| F10 | + | + |
| F13 | + | + |
| F14 | + | + |
| F9 | + | − |
| F11 | + | − |
| F6 | ± | − |
| F1 | − | + |
| F2 | − | − |
| F5 | − | − |
| F12 | − | − |
Detected by Western blotting.
Figure 1.
Expression of COX-2 and LMP1 in NPC (A) and induction of COX-2 by LMP1 in nasopharyngeal epithelial cells (B and C). (A) Lysates obtained from each tumor as indicated were analyzed for COX-2 and LMP1 expression by Western blotting. (B) 422F6 is an EBV-negative clone derived from the EBV-positive NPC-KT cell line. Two different amounts of LMP1 expression plasmid were transiently transfected into 422F6 cells and analyzed by Western blotting. Ad-AH cells treated with PMA (100 ng/ml) were used as a positive control for COX-2 induction. (C) Ad-AH cells stably expressing either pcDNA3 or LMP1 were analyzed.
LMP1 Induces COX-2 Expression in Nasopharyngeal Epithelial Cells.
Because NPC is a malignant tumor in which EBV is almost always detected, we first tested whether COX-2 expression is induced by LMP1 in epithelial cells including nasopharyngeal epithelial cells. As shown in Fig. 1B, transfection of an LMP1 expression plasmid clearly induced COX-2 in the EBV-negative 422F6 cells. Ad-AH cells treated with 100 ng/ml PMA were used as positive control for COX-2 expression. NPC-KT cells, in which LMP1 is faintly detected, disclosed a slight increase in expression of COX-2 (data not shown). Similarly, induction of COX-2 by LMP1 was observed in HeLa cells and 293 cells (data not shown). Furthermore, COX-2 was induced in Ad-AH/LMP1 cells compared with Ad-AH/pcDNA3 cells (Fig. 1C) Thus, LMP1 could induce COX-2 in diverse epithelial cell lines.
NF-κB Is Essential for Induction of COX-2 by LMP1.
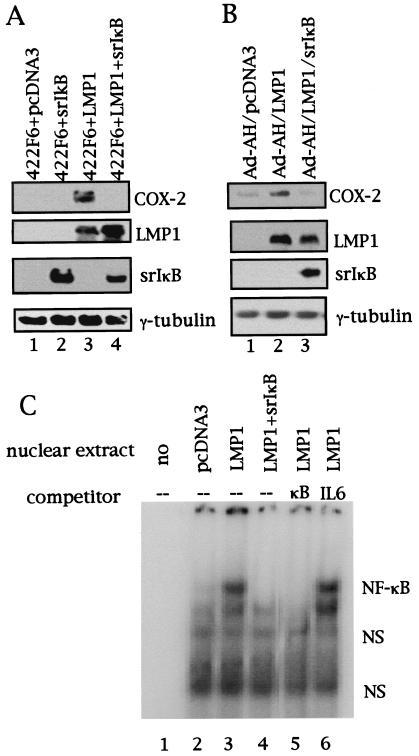
LMP1 has been shown to activate NF-κB. Therefore, we next tested whether NF-κB is involved or not in induction of COX-2 by LMP1 in epithelial cells including nasopharyngeal epithelial cells. We first confirmed that LMP1 activated NF-κB in 422F6, Ad-AH, HeLa, and 293 cells with the use of reporter assays (data not shown). Western blots showed that cotransfection of IκBα(S32A/S36A) with LMP1 completely abolished induction of COX-2 by LMP1 in 422F6 cells (Fig. 2A) and Ad-AH cells (Fig. 2B). Complete abolition of LMP1-induced COX-2 expression by cotransfection of IκBα(S32A/S36A) was also observed in both HeLa (data not shown) and 293 cells (data not shown). Correspondingly, increases in nuclear factor binding to NF-κB binding sequence in the cox-2 promoter in LMP1-expressing compared with vector-transfected cells were demonstrated (Fig. 2C). In addition, cotransfection of IκBα(S32A/S36A) abolished LMP1-induced increases of nuclear factor binding to the NF-κB binding sequence (Fig. 2C).
Figure 2.
NF-κB is involved and essential for COX-2 induction by LMP1. Suppression of LMP1-induced COX-2 expression by IκBα(S32A/S36A) is shown. COX-2 expression was analyzed with or without coexpression of IκBα(S32A/S36A) (indicated as srIκB) with LMP1 in 422F6 cells (A) or in Ad-AH cells (B) by Western blotting. (C) Induction of nuclear factor binding to NF-κB binding sequence in the cox-2 promoter by LMP1 and its suppression by IκBα. Nuclear extracts of 293 cells transfected as indicated were mixed with 32P-labeled NF-κB probe and analyzed by electrophoretic mobility shift assay. An excess of nonlabeled NF-κB (indicated as κB) or NF-IL6 (indicated as IL6) probe (100×) was used as competitor (NF-κB, 5′-CAGGAGAGTGGGGACTACCCCCTCTGCT-3′; NF-IL6, 5′-CACCGGGCTTACGCAATTTTTTTAA-3′). Underlined letters indicate binding sequences in the promoter of the cox-2 gene. srIκB, IκBα(S32A/S36A); NS, nonspecific binding.
Both LMP1 CTAR1 and CTAR2 Induce COX-2 in Nasopharyngeal Epithelial Cells.
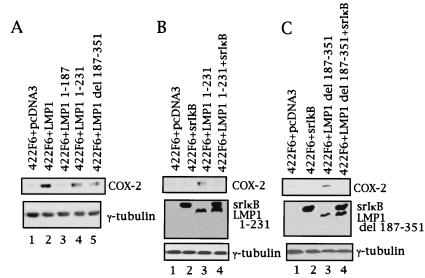
Two major regions of LMP1 are well known in the signal transduction induced by the oncoprotein: CTAR1 and CTAR2. Therefore, we next investigated which LMP1 CTAR plays a role in induction of COX-2 in nasopharyngeal epithelial cells. As shown in Fig. 3A, COX-2 was not induced in 422F6 cells by LMP1 1–187, which lacks both CTAR1 and CTAR2. On the other hand, COX-2 was induced both by LMP1 1–231 and LMP1 Δ187–351, but to a lesser extent than by LMP1 wild type in 422F6 cells (Fig. 3A); the first construct contains only CTAR1 in the C terminus; the second contains only CTAR2. Similarly, Ad-AH/LMP1 1–231 and Ad-AH/LMP1 Δ187–351 cells showed COX-2 induction, but to a lesser extent than in Ad-AH/LMP1 cells; however, induction was not observed in Ad-AH/LMP1 1–187 cells (data not shown). Therefore, both CTAR1 and CTAR2 seem to be needed for induction of COX-2.
Figure 3.
Both CTAR1 and CTAR2 of LMP1 partially induce COX-2 through NF-κB. (A) COX-2 expression in each LMP1 mutant-expressing 422F6 cells as indicated was analyzed by Western blotting. Involvement of NF-κB in COX-2 induction by each CTAR was analyzed by coexpression of IκBα(S32A/S36A) (indicated as srIκB) with LMP1 1–231 (B) or LMP1 Δ187–351 (C) in 422F6 cells.
NF-κB Is Essential for Induction of COX-2 by Both CTAR1 and CTAR2 of LMP1.
LMP1 has been shown to activate NF-κB through both CTAR1 and CTAR2. Therefore, we tested involvement of NF-κB in induction of COX-2 by both domains of LMP1 in nasopharyngeal epithelial cells. We first confirmed that LMP1 1–231 or LMP1 Δ187–351 but not LMP1 1–187 activated NF-κB in both 422F6 and Ad-AH cells with the use of reporter assays (data not shown). Then, cotransfection of IκBα(S32A/S36A) with LMP1 mutants was carried out. Cotransfection of IκBα(S32A/S36A) with LMP1 1–187 had no effect in 422F6 and Ad-AH cells (data not shown). On the other hand, cotransfection of IκBα(S32A/S36A) abolished COX-2 induction by LMP1 1–231 in 422F6 cells (Fig. 3B) and Ad-AH cells (data not shown). Similarly, cotransfection of IκBα(S32A/S36A) essentially abolished COX-2 induction by LMP1 Δ187–351 in 422F6 cells (Fig. 3C) and Ad-AH cells (data not shown).
LMP1 Induces the cox-2 Promoter in an NF-κB-Dependent Manner.
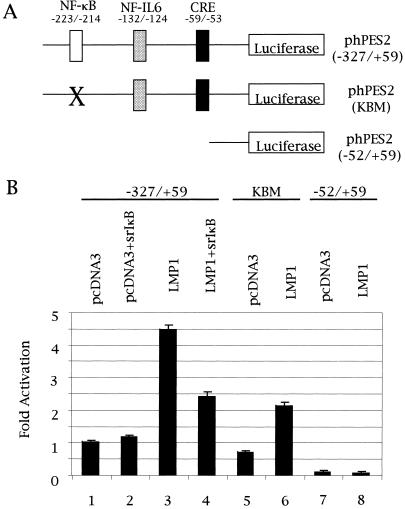
Cox-2 promoter activity was analyzed by luciferase assays with the constructs shown in Fig. 4A. As shown in Fig. 4B, LMP1 induced cox-2 promoter activity. Coexpression of IκBα(S32A/S36A) markedly, but not completely, repressed cox-2 promoter activity induced by LMP1 (Fig. 4B). Similarly, mutation in the NF-κB binding site repressed LMP1-induced cox-2 promoter activation (Fig. 4B).
Figure 4.
LMP1 induces cox-2 promoter activity. (A) Schematic diagram of cox-2 promoter reporter constructs. X indicates the mutation in the NF-κB binding site in the cox-2 promoter region. (B) Relative luciferase activity with each construct from three independent duplicate experiments is shown with the standard deviation.
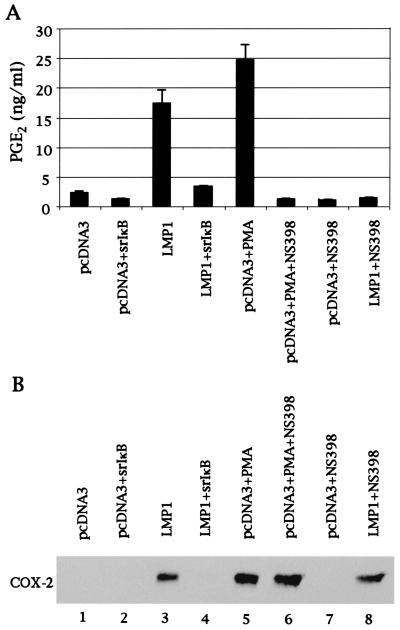
LMP1 Increases Prostaglandin E2 Production in Nasopharyngeal Epithelial Cells Through Induction of COX-2.
We next tested whether LMP1-induced COX-2 is functional. To this end, we measured production of PGE2 with EIA in 422F6 cells because PGE2 is one of the COX-2-mediated metabolic products of arachidonic acid. Fig. 5A shows that LMP1 markedly increased production of PGE2. To make certain that increased production of PGE2 is through COX-2, we treated cells with the COX-2-specific inhibitor, NS-398, and showed that the inhibitor clearly decreased LMP1-induced PGE2 as well as PMA-induced PGE2 production (Fig. 5A). Because COX-2 expression induced by LMP1 and PMA was not affected by NS-398 as shown in Fig. 5B, the inhibitory effect of NS-398 on PGE2 production was not because of the down-regulation of COX-2 expression. Coexpression of IκBα(S32A/S36A) with LMP1 dramatically decreased production of PGE2 (Fig. 5A) because LMP1-induced COX-2 expression was inhibited by IκBα(S32A/S36A) (Fig. 5B).
Figure 5.
LMP1 increases production of PGE2 through induction of COX-2. (A) Conditioned medium was diluted, and production of PGE2 was analyzed with EIA. PMA, 100 ng/ml PMA; NS-398, 20 μM NS-398. (B) One-hundred micrograms of lysates from matched samples used in A were analyzed for expression of COX-2 and LMP1 by Western blotting.
LMP1 Induces VEGF Production, at Least in Part, in a COX-2-Dependent Manner.
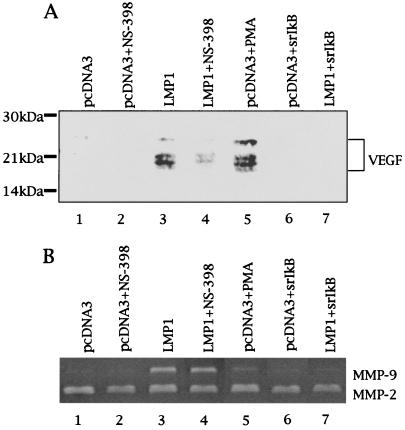
Finally, VEGF production in the conditioned medium used in the experiment described in Fig. 5A was analyzed under reducing conditions to dissociate dimer formation. VEGF production in medium of LMP1-expressing 422F6 cells was increased as shown in Fig. 6A. Because conditioned media were analyzed, detected bands must be components of VEGF121 and VEGF165, which are isoforms of secretory-type VEGF. Treatment of LMP1-expressing 422F6 cells with NS-398 clearly decreased VEGF production (Fig. 6A), although COX-2 expression was unaffected as shown in Fig. 5B. Expression of VEGF induced by LMP1 was also clearly suppressed by IκBα(S32A/S36A) (Fig. 6A). Enzymatic activity of matrix metalloproteinase-2 (MMP-2) in each conditioned medium was unchanged in contrast to induction of MMP-9 in LMP1-expressing cells (Fig. 6B) as shown before (23, 29), which indicates that each conditioned medium was obtained from an equal amount of cells.
Figure 6.
LMP1 increases VEGF production, at least in part, in a COX-2-dependent manner. (A) The same conditioned media used in Fig. 5 were analyzed for VEGF production by Western blotting. PMA, 100 ng/ml PMA; NS-398, 20 μM NS-398. (B) Enzymatic activity of MMP-2 and MMP-9 in conditioned media was analyzed by gelatin zymography. PMA, 100 ng/ml; NS-398, 20 μM.
Discussion
Recent studies have revealed that COX-2 is overexpressed in diverse tumors (1–6), suggesting that COX-2 may be involved in carcinogenesis. In fact, a selective COX-2 inhibitor reduced tumorigenesis or tumor cell growth (30–32). NPC frequently expresses the EBV oncoprotein LMP1, prompting us to investigate whether LMP1 induces COX-2 in nasopharyngeal epithelial cells. First, we showed that, with the use of Western blotting, LMP1-positive NPC frequently (but LMP1-negative LMP1 rarely) expresses COX-2. Then, we showed that transient or stable transfections clearly revealed that LMP1 induces COX-2 in nasopharyngeal epithelial cells. A role for overexpression of COX-2 in various cancers is still unclear; however, the angiogenic role of COX-2 has been focused on recently. Angiogenesis is one of the factors contributing to metastatic potential as well as growth of cancers (33); therefore, this result raises the possibility that LMP1 may contribute to angiogenesis through induction of COX-2 in proliferating NPC.
Several consensus sequences for nuclear factor binding are found in the 5′-flanking region of the cox-2 gene. Among them, NF-κB, nuclear factor for interleukin-6 expression (NF-IL6), and cAMP response element sites have been identified as the regulatory sequences involved in COX-2 induction in response to various stimuli in different species and cell types (26, 34, 35). Western blots demonstrated that NF-κB is involved and essential for COX-2 induction by LMP1. However, reporter assays showed residual promoter activity even with a mutation in the NF-κB binding site, suggesting that factor(s) other than NF-κB may be involved in the cox-2 promoter activation. It is reported that activation of epidermal growth factor receptor (EGFR) induced COX-2 (36). Because LMP1 is known to constitutively activate EGFR (37), the LMP1-induced EGFR signaling pathway may be central to the mechanism of COX-2 induction.
LMP1 mimics CD40, a member of the tumor necrosis factor receptor family, in its signal transduction pathway (38, 39). LMP1 activates NF-κB through both its CTAR1 and CTAR2 regions and also activates AP-1 through CTAR2 and p38 mitogen-activated protein kinase through CTAR1 and CTAR2. Our data demonstrate that both CTAR1 and CTAR2 partially induce COX-2. Furthermore, NF-κB activation is involved and essential in induction of COX-2 by each CTAR. These results suggest that activation of NF-κB from both CTAR1 and CTAR2 is required for full induction of COX-2 by LMP1.
VEGF is a potent angiogenic factor; therefore, a contribution of LMP1 to angiogenesis is suggested, as this study demonstrates that VEGF production is enhanced by LMP1. Furthermore, NS-398, a specific COX-2 inhibitor, clearly decreased production of VEGF in LMP1-expressing cells, which suggests that induction of COX-2 by LMP1 is involved, at least in part, in production of VEGF. Residual VEGF production even after treatment of LMP1-transfected cells with NS-398 was observed. This result suggests that factor(s) other than COX-2 may be involved in VEGF production by LMP1. EGFR is a candidate because it is reported that EGFR up-regulated VEGF in glioblastoma cells (40). Coexpression of IκBα(S32A/S36A) with LMP1 abolished VEGF production, suggesting that residual VEGF production after NS-398 treatment may depend on NF-κB. LMP1-induced COX-2 is functional in our system because production of PGE2, one of the COX-2-mediated metabolites of arachidonic acid, is greatly increased. It has been reported that PGE2 stimulates IL-8 gene expression in human colonic epithelial cells (41). Because IL-8 is another potent angiogenic factor (42), LMP1-induced COX-2 may contribute to angiogenesis through IL-8.
There is a report stating that EBV suppresses PGE2 production through inhibition of COX-2 expression in human monocytes (43). This result seems to conflict with the present findings. However, experimental procedures in that study were completely different, and the data also indicate that viral replication and/or neosynthesized viral proteins were involved. In contrast, EBV infection in NPC is predominantly latent, and only a restricted set of latency genes and no replicative genes are expressed.
Taken together with our previous reports showing that LMP1 increased tumor cell invasiveness through up-regulation of MMP-9, a type IV collagenase (23, 29), it is becoming clear that LMP1, the principal oncoprotein of EBV, not only has cell-transforming activity, it is also able to induce a constellation of elements activated in the process of invasion and metastasis. Moreover, an association of LMP1 and MMP-9 with tumor metastasis in NPC has been reported recently (44), which is compatible with our previous observations in cell culture (23, 29). The association of LMP1, COX-2, and VEGF with angiogenesis in NPC is currently being investigated. Although EBV is the only tumor virus thus far shown to induce factors needed for tumor invasion, other viruses associated with tumors with invasive phenotype may have a similar capability.
Acknowledgments
We thank Dr. Luwen Zhang for helpful discussions. We thank Drs. Nancy Raab-Traub and Albert Baldwin for providing LMP1 mutant expression plasmids and IκBα(S32A/S36A) expression plasmid, respectively, and for reviewing the manuscript. We also thank Dr. E. Flemington for providing Ad-AH cells. This work was supported by National Institutes of Health Grant P01CA19014. S.M. was supported in part by the Sumitomo Life Enterprise Group for Social Welfare, Osaka, Japan.
Abbreviations
- COX
cyclooxygenase
- CTAR
COOH-terminal activation region
- EGFR
epidermal growth factor receptor
- EBNA
Epstein–Barr virus nuclear antigen
- EBV
Epstein–Barr virus
- EIA
enzyme immunoassay
- LMP
latent membrane protein
- MMP
matrix metalloproteinase
- NPC
nasopharyngeal carcinoma
- PG
prostaglandin
- PMA
phorbol 12-myristate 13-acetate
- VEGF
vascular endothelial growth factor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Sano H, Kawahito Y, Wilder R L, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 2.Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A. Cancer Res. 1998;58:4997–5001. [PubMed] [Google Scholar]
- 3.Hwang D, Scollard D, Byrne J, Levine E. J Natl Cancer Inst. 1998;90:455–460. doi: 10.1093/jnci/90.6.455. [DOI] [PubMed] [Google Scholar]
- 4.Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M. Cancer Res. 1997;57:1276–1280. [PubMed] [Google Scholar]
- 5.Zimmermann K C, Sarbia M, Weber A A, Borchard F, Gabbert H E, Schror K. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]
- 6.Chan G, Boyle J O, Yang E K, Zhang F, Sacks P G, Shah J P, Edelstein D, Soslow R A, Koki A T, Woerner B M, et al. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- 7.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois R N. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 8.Jones M K, Wang H, Peskar B M, Levin E, Itani R M, Sarfeh I J, Tarnawski A S. Nat Med. 1999;5:1418–1423. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- 9.Sawaoka H, Tsuji S, Tsujii M, Gunawan E S, Sasaki Y, Kawano S, Hori M. Lab Invest. 1999;79:1469–1477. [PubMed] [Google Scholar]
- 10.Shibata D, Weiss L M. Am J Pathol. 1992;140:769–774. [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnet M, Guinebretiere J M, Kremmer E, Grunewald V, Benhamou E, Contesso G, Joab I. J Natl Cancer Inst. 1999;91:1376–1381. doi: 10.1093/jnci/91.16.1376. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Liebowitz D, Kieff E. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- 13.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 14.Devergne O, Hatzivassiliou E, Izumi K M, Kaye K M, Kleijnen M F, Kieff E, Mosialos G. Mol Cell Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izumi K M, Kieff E D. Proc Natl Acad Sci USA. 1997;94:12592–12597. doi: 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kieser A, Kilger E, Gires O, Ueffing M, Kolch W, Hammerschmidt W. EMBO J. 1997;16:6478–6485. doi: 10.1093/emboj/16.21.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eliopoulos A G, Gallagher N J, Blake S M, Dawson C W, Young L S. J Biol Chem. 1999;274:16085–16096. doi: 10.1074/jbc.274.23.16085. [DOI] [PubMed] [Google Scholar]
- 18.Raab-Traub N, Flynn K. Cell. 1986;47:883–889. doi: 10.1016/0092-8674(86)90803-2. [DOI] [PubMed] [Google Scholar]
- 19.Pathmanathan R, Prasad U, Sadler R, Flynn K, Raab-Traub N. N Engl J Med. 1995;333:693–698. doi: 10.1056/NEJM199509143331103. [DOI] [PubMed] [Google Scholar]
- 20.Hu L F, Chen F, Zhen Q F, Zhang Y W, Luo Y, Zheng X, Winberg G, Ernberg I, Klein G. Eur J Cancer. 1995;5:658–660. doi: 10.1016/0959-8049(94)00468-k. [DOI] [PubMed] [Google Scholar]
- 21.Takimoto T, Furukawa M, Hatano M, Umeda R. Ann Otol Rhinol Laryngol. 1984;93:166–169. doi: 10.1177/000348948409300213. [DOI] [PubMed] [Google Scholar]
- 22.Takimoto T, Kamide M, Umeda R. Arch Otorhinolaryngol. 1984;239:87–92. doi: 10.1007/BF00454266. [DOI] [PubMed] [Google Scholar]
- 23.Yoshizaki T, Sato H, Furukawa M, Pagano J S. Proc Natl Acad Sci USA. 1998;95:3621–3626. doi: 10.1073/pnas.95.7.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller W E, Mosialos G, Kieff E, Raab-Traub N. J Virol. 1997;71:586–594. doi: 10.1128/jvi.71.1.586-594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue H, Nanayama T, Hara S, Yokoyama C, Tanabe T. FEBS Lett. 1994;350:51–54. doi: 10.1016/0014-5793(94)00731-4. [DOI] [PubMed] [Google Scholar]
- 26.Inoue H, Yokoyama C, Hara S, Tone Y, Tanabe T. J Biol Chem. 1995;270:24965–24971. doi: 10.1074/jbc.270.42.24965. [DOI] [PubMed] [Google Scholar]
- 27.Inoue H, Tanabe T. Biochem Biophys Res Commun. 1998;244:143–148. doi: 10.1006/bbrc.1998.8222. [DOI] [PubMed] [Google Scholar]
- 28.Inoue H, Umesono K, Nishimori T, Hirata Y, Tanabe T. Biochem Biophys Res Commun. 1999;254:292–298. doi: 10.1006/bbrc.1998.9939. [DOI] [PubMed] [Google Scholar]
- 29.Murono S, Yoshizaki T, Sato H, Takeshita H, Furukawa M, Pagano J S. Cancer Res. 2000;60:2555–2561. [PubMed] [Google Scholar]
- 30.Sheng H, Shao J, Kirkland S C, Isakson P, Coffey R J, Morrow J, Beauchamp R D, DuBois R N. J Clin Invest. 1997;99:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddy B S, Hirose Y, Lubet R, Steele V, Kelloff G, Paulson S, Seibert K, Rao C V. Cancer Res. 2000;60:293–297. [PubMed] [Google Scholar]
- 32.Rioux N, Castonguay A. Cancer Res. 1998;58:5354–5360. [PubMed] [Google Scholar]
- 33.Folkman J. Semin Cancer Biol. 1992;3:65–71. [PubMed] [Google Scholar]
- 34.Kim Y, Fischer S M. J Biol Chem. 1998;273:27686–27694. doi: 10.1074/jbc.273.42.27686. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto K, Arakawa T, Ueda N, Yamamoto S. J Biol Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 36.Coffey R J, Hawkey C J, Damstrup L, Graves-Deal R, Daniel V C, Dempsey P J, Chinery R, Kirkland S C, DuBois R N, Jetton T L, Morrow J D. Proc Natl Acad Sci USA. 1997;94:657–662. doi: 10.1073/pnas.94.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller W E, Earp H S, Raab-Traub N. J Virol. 1995;69:4390–4398. doi: 10.1128/jvi.69.7.4390-4398.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gires O, Zimber-Strobl U, Gonnella R, Ueffing M, Marschall G, Zeidler R, Pich D, Hammerschmidt W. EMBO J. 1997;16:6131–6140. doi: 10.1093/emboj/16.20.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uchida J, Yasui T, Takaoka-Shichijo Y, Muraoka M, Kulwichit W, Raab-Traub N, Kikutani H. Science. 1999;286:300–303. doi: 10.1126/science.286.5438.300. [DOI] [PubMed] [Google Scholar]
- 40.Maity A, Pore N, Lee J, Solomon D, O'Rourke D M. Cancer Res. 2000;60:5879–5886. [PubMed] [Google Scholar]
- 41.Yu Y, Chadee K. J Immunol. 1998;161:3746–3752. [PubMed] [Google Scholar]
- 42.Yoshida S, Ono M, Shono T, Izumi H, Ishibashi T, Suzuki H, Kuwano M. Mol Cell Biol. 1997;17:4015–4023. doi: 10.1128/mcb.17.7.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savard M, Belanger C, Tremblay M J, Dumais N, Flamand L, Borgeat P, Gosselin J. J Immunol. 2000;164:6467–6473. doi: 10.4049/jimmunol.164.12.6467. [DOI] [PubMed] [Google Scholar]
- 44.Horikawa T, Yoshizaki T, Sheen T S, Lee S Y, Furukawa M. Cancer. 2000;89:715–723. doi: 10.1002/1097-0142(20000815)89:4<715::aid-cncr1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]