Abstract
Background:
Recent research showed 82% of 233 retired National Football League players under age 50 had abnormal narrowing and blockages in arteries compared to the general population of the same age. It has been suggested that early screening and intervention in this at-risk population be a priority.
Hypothesis:
Omega-3 essential fatty acid has been shown to improve cardiovascular lipid risk factors and should improve lipid profiles in professional football players to help reduce their recently shown accelerated risk of developing cardiovascular disease.
Methods:
A total of 36 active national football players were randomly assigned to 2 groups: the first group (n = 20) was provided fish oil capsules (2200 mg of mixed docosahexaenoic acid and eicosapentaenoic acid and 360 mg of other omega-3s), and the second group (n = 16) served as controls during a 60-day trial. Vertical Auto Profile cholesterol tests directly measuring serum low-density lipoprotein, high-density lipoprotein, and other subfractions were performed. Compliance, side effects, and seafood consumption data were also collected. Baseline, midpoint, and poststudy blood work measured plasma docosahexaenoic acid and eicosapentaenoic acid.
Results:
Treatment increased high-density lipoprotein (average percent change: +25.96, control +14.16), decreased triglycerides treatment (–8.06, control +43.98), very low-density lipoprotein treatment (–13.98, control +23.18), intermediate density lipoprotein (–27.58, control +12.07), remnant lipoproteins (–23.86, control +8.33), and very low-density lipoprotein-3 (–17.10, control +7.77). An average increase of 106.67% for docosahexaenoic acid and 365.82% for eicosapentaenoic acid compared to control was also shown.
Conclusion:
Omega-3 supplementation significantly improved the lipid profile of active players randomized to treatment. These results suggest that fish oil supplementation is an effective way to increase eicosapentaenoic acid and docosahexaenoic acid levels in plasma and should be considered as a method to improve modifiable cardiovascular risk lipid factors in professional football players.
Clinical Relevance:
A prospective study examining the effects of 60 days of a highly purified fish oil supplementation in professional football players.
Keywords: cardiac risk factors, omega-3, professional football, vertical auto profile cholesterol test
Professional football players are some of the fittest athletes in sports. Despite rigorous physical conditioning, there is still a high risk for muscular, skeletal, and other high-velocity contact injuries that result in acute and chronic inflammation. The relationship between higher levels of circulating inflammatory eicosanoids and the associated health risk of vascular disease resulting in heart attack and strokes are rarely considered in this young man’s game.
The National Football League (NFL) has been the leader among professional sports teams with regard to player safety and health-related issues. From the introduction of protective pads and helmets to the modern use of computers for concussion testing and collision analysis, the NFL has sought out the most modern, state-of-the-art capabilities to protect and improve the players’ well-being. Most of these previous technological improvements have involved equipment and field improvements, which have resulted in longer NFL player careers. Many players remain on active NFL rosters into their early to mid-30s when the risk of cardiovascular disease (CVD) starts to become significant in men.19
Cardiovascular Risks in NFL Players
In all organized sports the most devastating event that can occur is the death of a player, on or off the field. Most sudden deaths in athletes older than 30 years are due to a heart attack from acute blockage of the coronary arteries.38,39 Athletes who are older than 30 are at increased risk for heart attack if they smoke, have high blood pressure, are obese or diabetic, have elevated abnormal blood lipids, or a strong family history of heart disease. In addition, low intakes or blood levels of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have been independently associated with increased risk of death from coronary heart disease22 (Table 1). In randomized secondary prevention trials, fish or fish oil have reduced total and CVD mortality.32,37
Table 1.
Summary of American Heart Association recommendations for omega-3 fatty acid intake relative to the incidence of coronary heart disease (CHD).a,1
| Patient Population | Recommendation |
|---|---|
| No documented history of CHD | Eat a variety of fish (preferably cold-water, oily fish) at least twice per week. Include oils and foods rich in alpha-linolenic acid (flaxseed, canola, and soybean oils; flaxseeds; walnuts) |
| Documented history of CHD | Consume approximately 1 g of EPA/DHA daily, preferably from oily fish. EPA/DHA capsule supplements may be used in consultation with a physician |
| Need to lower triglyceride level | Consume 2 to 4 grams of EPA/DHA daily in capsules, in consultation with a physician |
EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Hurst et al42 recently reported on 233 retired NFL football players, ages 35 to 65. More than 80% had evidence of vascular disease by the age of 50, which is higher than in the general population. These authors suggested early screening and intervention in this at-risk population.
Other research on retired NFL players of all ages has shown players are more prone to obesity, obstructive sleep apnea, and metabolic syndrome than the general population. Most disturbingly, there is a higher mortality rate to age-matched controls in some types of players.8,20,45
Short of requiring a preseason cardiac catheterization on all players, it has been difficult to determine an individual player’s exact cardiac risk. This study focused on the effect of highly purified fish oil supplementation on 2 modifiable risk markers of CVD. The first is serum lipid levels, as measured by low-density lipoproteins (LDL), high-density lipoproteins (HDL), and other lipid subfractions. These lipid subfractions include remnant lipoprotein (RLP) cholesterol, small/dense LDL, and lipoprotein a (Lp[a]). The second is plasma levels of EPA and DHA as a reflection of EPA/DHA intake and presumed attainment of documented cardioprotective benefits of omega-3 fatty acids.
Better Cardiac Risk Assessment
We are now entering a new era of cardiac risk assessment where the concept of coronary artery plaque stabilization is realized by understanding the biochemistry of blood vessels becoming injured by vascular inflammation related to circulation proinflammatory eicosanoids53 and the oxidation of fats, as well as how this is a factor in the genesis of plaque formation.28 Abnormalities of blood lipids are now universally accepted as a significant risk factor for vascular disease due to their ability to both precipitate plaque formation and also as an inflammation-provoking substance upon entering the walls of blood vessels.10
Emerging evidence suggests that not all lipids carry the same risk for the development of CVD. Newer specialized blood tests can fractionate blood lipids into many component molecules in order to better assess which lipid subfraction may possess a greater risk for causing disease. Cardiotoxic LDL and very low-density lipids (vLDL) are well known for blood vessel damage, plaque formation, and ultimately vessel occlusion, while increases in HDL are associated with less cardiovascular risk.9,18,47
Omega-3 Fats EPA and DHA
The foundation of preventive medicine for cardiovascular disease is exercise and good nutrition. Possibly the most important heart-healthy nutrients are the omega-3 fats EPA and DHA from fish and fish oil. New and important findings are continually being reported about the benefits of fish oil for those with cardiovascular disease. These include evidence from randomized, controlled, clinical trials on how omega-3 fatty acids improve heart health by reducing triglyceride levels, decreasing the growth of atherosclerotic plaques, improving arterial endothelial function, lowering blood pressure, and reducing the risk of thrombosis.9,18,41,47
Fish oil is also a powerful supporter of the body’s natural anti-inflammatory response, which counteracts the progression of heart disease.12 Omega-3 fats within the endothelium reduce the inflammatory response that occurs from the oxidization of blood lipids that have invaded blood vessels. This invasion typically promotes the process of plaque development and initiates multiple atherogenic effects. Endothelial responses to oxidizing lipids within the intima cause a migration and activation of inflammatory cells, such as monocytes and macrophages, which release chemotactic chemicals, in turn accelerating the inflammatory response. An increased proportion of EPA and DHA within the cell membranes reduce the inflammatory response by inhibiting the production of the proinflammatory eicosanoid PGE2 while supporting the production of anti-inflammatory PGE3.22
Evidence from serum studies has shown that higher tissue levels of omega-3 fats significantly reduce the risk for heart attack. Red blood cell membrane omega-3 levels of 5% of total fatty acids is associated with a remarkable 70% reduction in the risk of heart attack.52
It is well established that fish oil directly improves heart health. As new research emerges, it is becoming increasingly evident that fish oil influences several additional parameters of cardiovascular and metabolic health. When overweight individuals (body mass index [BMI] > 25) combined fish oil (2 g total omega-3) and regular exercise, this combination lowered triglycerides, increased HDL cholesterol, and improved endothelium-dependent arterial vasodilatation when compared to sunflower oil placebo. Both fish oil and exercise independently reduced body fat and improved cardiovascular and metabolic health.25
Evidence from prevention studies suggests that taking EPA and DHA ranging from 500 mg to about 1500 mg per day significantly reduces deaths from heart disease (all causes). A significant and ever-growing body of scientific evidence that shows how important omega-3 fats are for cardiovascular health suggests that individuals with existing risk factors for cardiovascular disease should consume a minimum of 1 gram of EPA and DHA per day. Individuals with elevated serum lipids need 2 to 4 grams of EPA and DHA daily.32,40 For many, these recommendations cannot be easily achieved through diet alone, and a high-quality fish oil supplement is recommended. For this study the relatively short term of intervention combined with relatively large body mass of participants justified the selected omega-3 fatty acid dose of 2560 mg, which is on the high end of what is recommended for individuals with normal lipid levels.
Methods
Lipid Assessment
The Vertical Auto Profile or VAP Test (Atherotech Inc, Birmingham, Alabama) provides all the information found in a routine lipid panel plus measurements of all known cholesterol subclasses that play important roles in the development of CVD. The additional information provided by the VAP Test is a more specific lipid analysis to predict heart disease risk and was used in this study. Table 2 describes the components of the VAP test measures in a blood sample.43
Table 2.
Cholesterol subfractions, risk description, and normal values.a
| Cholesterol | |||
|---|---|---|---|
| Type | Major Components | Description | Desirable Score |
| Total cholesterol | Total cholesterol circulation in body. | All cholesterol | <200 mg/dL |
| LDL cholesterol | Considered to be the “bad” cholesterol because it is a primary cause of heart disease. | Lp(a), IDL, LDL-R | <130 mg/dL |
| HDL cholesterol | Considered the “good” cholesterol because low levels of this can lead to heart disease. | HDL2, HDL3 | ≥40 mg/dL |
| vLDL cholesterol | Carrier for triglycerides. If high it is a risk for heart disease. | vLDL3 | <30 mg/dL |
| Triglycerides | Molecules that provide energy to the entire body. If levels are high, triglycerides are a risk for heart disease. | Several | <150 mg/dL |
| Non-HDL cholesterol | Contains all bad cholesterol components and subclasses LDL and vLDL. | Several (see description) | <160 mg/dL |
| Cholesterol Subfractions | |||
| Type | Major Components | Description | Desirable Score |
| LDL cholesterol | Lp(a) | Very dangerous cholesterol that is harder than most to treat effectively with drugs. | <10 mg/dL |
| IDL | Dangerous if elevated. | <20 mg/dL | |
| Real LDL cholesterol | Component of LDL cholesterol, measures the real cholesterol circulation in the body. | <100 mg/dL | |
| LDL cholesterol pattern size | LDL cholesterol ranges from small and dense (pattern B) to large and buoyant (pattern A). The smaller the LDL size, the greater the risk for heart disease. | A | |
| HDL cholesterol | HDL2 | The most protective form of HDL, large and buoyant. | >10 mg/dL |
| HDL3 | Least protective form of HDL, small and dense. | >30 mg/dL | |
| vLDL cholesterol | vLDL3 | Most dense form of vLDL, greater risk factor than both vLDL1 and vLDL2. | <10 mg/dL |
LDL, low-density lipoprotein; Lp(a), lipoprotein a; IDL, intermediate density lipoprotein; LDL-R, low-density lipoprotein–remnant; HDL, high-density lipoprotein; vLDL, very low-density lipoprotein.
Plasma concentrations of the omega-3 fatty acids EPA and DHA (Metametrix Clinical Laboratory, Duluth, Georgia) are good biomarkers of dietary intake of these 2 essential fatty acids. Plasma EPA and DHA concentrations provide an objective measurement of compliance in fish oil feeding studies. In addition, plasma levels of EPA and DHA correlate with erythrocyte cell membrane levels of EPA and DHA. Erythrocyte cell membrane levels of EPA and DHA have been proposed as a physiologically relevant, easily modified, independent, and graded risk factor for death from CVD that could have significant clinical implication.22,52 Therefore, lower levels of omega-3 in the red blood cell membrane indicate a higher risk of CVD.
Subjects
Following Institutional Review Board (IRB) approval, 36 professional NFL football players from the Pittsburgh Steelers Football Club, ages 23 to 41 years (average, 28.03 years), volunteered to be randomly assigned to either the treatment (n = 20) or the control (c = 16) arms of the study. Each group contained similarly sized players, including BMI and position played. After informed consent was given, baseline questionnaires were completed to assess any allergies to fish or seafood, family and personal medical history of cardiac disease and risk factors, and dietary intake of fish and seafood. Vital signs and baseline VAP cholesterol tests were also assessed to directly measure the LDL, HDL, and other lipid subfractions, such as remnant lipoprotein cholesterol, small/dense LDL, Lp(a), and plasma fatty acid concentrations (EPA/DHA) (Table 2).
Study Design and Intervention
This study was designed to determine the effect of 2560 mg/day of mixed EPA/DHA omega-3 fatty acid supplements (650 mg EPA; 450 mg DHA; 180 mg other omega-3 fatty acids per 2 soft gels of ProOmega; Nordic Naturals, Watsonville, California) in the form of fish oil soft gels on healthy, professional football players. Testing took place during a 2-month period during the active 2006-2007 NFL season. Compliance, side effects, and seafood consumption data were collected using weekly questionnaires. Midpoint and poststudy VAP and plasma fatty acid blood work was obtained after the subjects fasted overnight.
The 20 players that were randomized to the treatment group were provided individual bottles of 28 fish oil soft gels and instructed to take 4 on a daily basis. These bottles were collected on a weekly basis to access compliance and new bottles were provided. The control subjects (n = 16) did not receive any additional supplements and were asked not to take any essential fatty acid supplements. All subjects were asked not to take any omega-3 fatty acid supplements for at least 1 month prior to the baseline blood work. A sample size estimate was not performed.
Results
Questionnaires revealed no significant difference in seafood and fish consumption between the treatment and control groups. In the treatment group there were no reported side effects, and compliance overall was 80% for taking at least 24 of the provided 28 capsules per week.
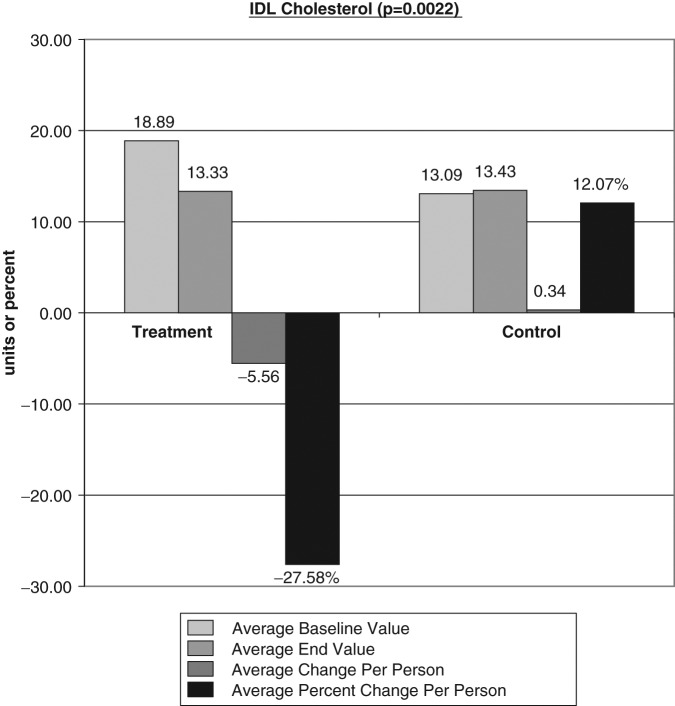
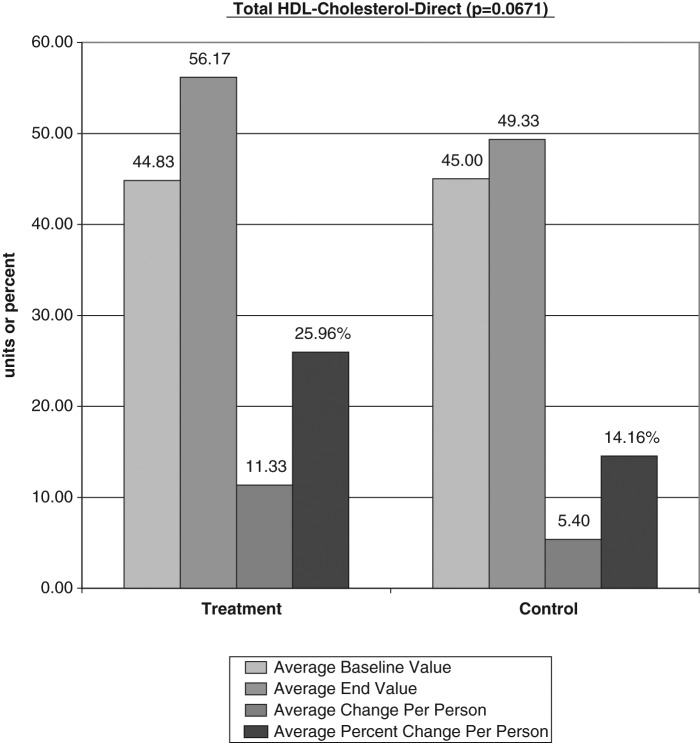
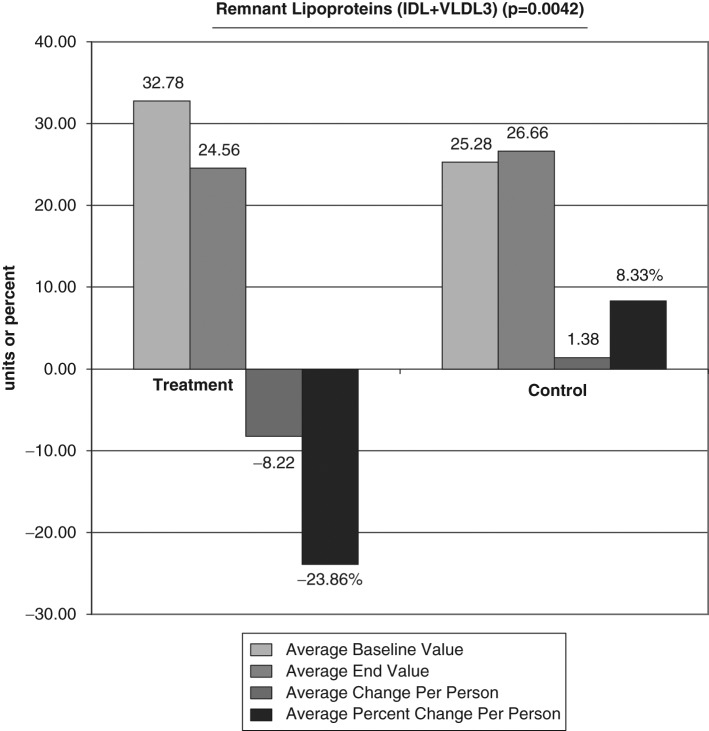
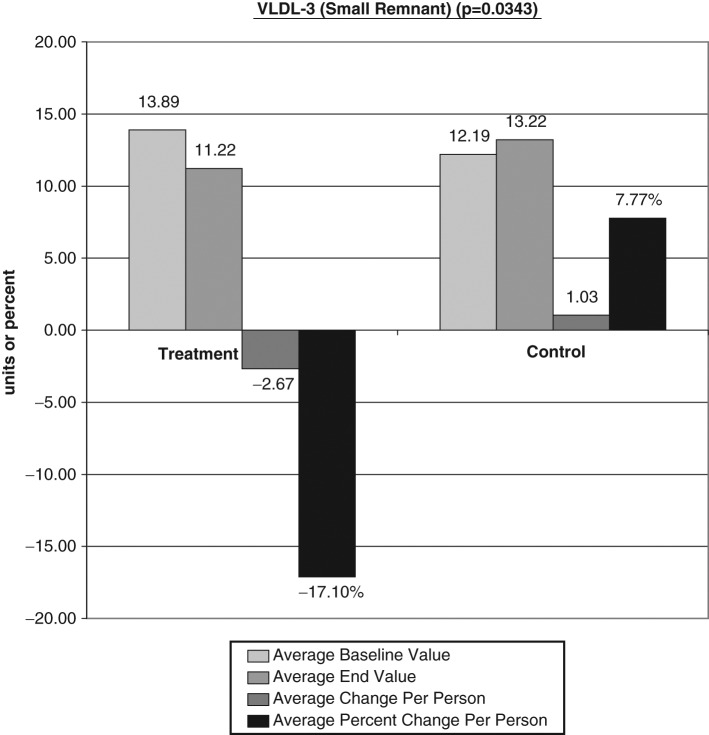
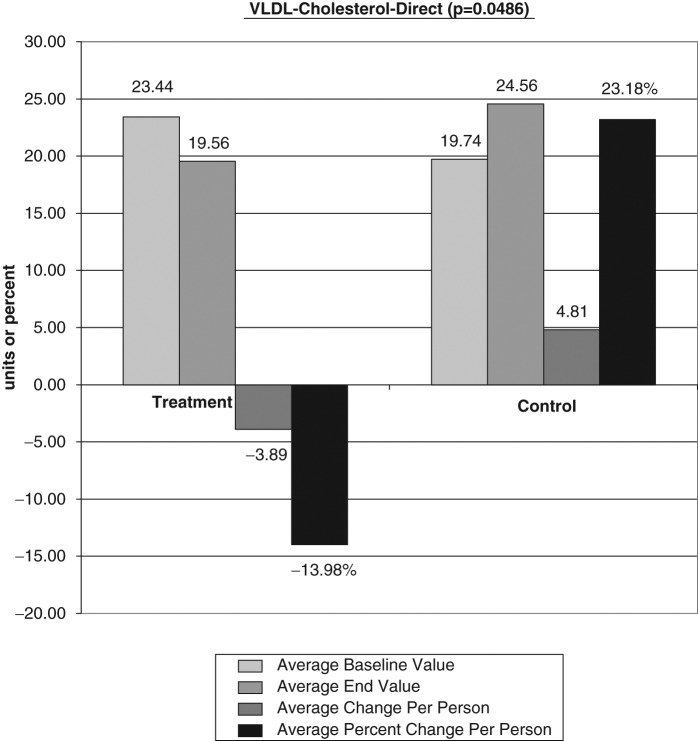
The average baseline value, final test value, average value change, and average percent changes of the VAP cholesterol panel were calculated (Figures 1-6). The treatment subjects (T) compared to the control subjects (C), using the unpaired t test, had significant decreases in IDL (average percent change [APC]: T = −27.58, C = 12.07), RLP (intermediate density lipoprotein [IDL] + vLDL3) (APC: T = −23.86, C = 8.33), triglycerides (APC: T = −8.06, C = 43.98), vLDL-3 (APC: T −17.10, C = 7.77), and vLDL (APC: T = −13.98, C = 23.18). Total HDL-cholesterol (HDL-C) direct (APC: T = 25.96, C = 14.16), HDL-2 (APC: T = 16.28, C = 1.164), and HDL-3 (APC: T = 35.52, C = 24.28) were greater in the treatment group but nonsignificant (P = .067). Additionally, the LDL-C and Lp(a) subfractions had nonsignificant improvement in the treatment group, and total cholesterol, LDL, and Apolipoprotein (Apo) B100 changes were similar in both groups.
Figure 1.
The average baseline value, final test value, average value change, and average percent changes of Vertical Auto Profile cholesterol panel were calculated comparing the treatment subjects (T) to the control subjects (C) using the unpaired t test. Figure 1 shows a significant decreases in intermediate density lipoprotein average percent change: T = −27.58, C = 12.07.
Figure 6.
Total HDL cholesterol (HDL-C) direct (average percent change: T = 25.96, C = 14.16) was improved but not significant (P = .067). HDL, high-density lipoprotein; T, treatment subjects; C, control subjects.
Figure 2.
Significant decreases in remnant lipoprotein (intermediate density lipoprotein [IDL] + vLDL3) average percent change: T = −23.86, C = 8.33. LDL, low-density lipoprotein; T, treatment subjects; C, control subjects.
Figure 3.
Significant decreases in triglycerides average percent change: T = −8.06, C = 43.98. T, treatment subjects; C, control subjects.
Figure 4.
Significant decreases in vLDL-3 average percent change: T = −17.10, C = 7.77. LDL, low-density lipoprotein; T, treatment subjects; C, control subjects.
Figure 5.
Significant decreases in vLDL average percent change: T = −13.98,C = 23.18. LDL, low-density lipoprotein; T, treatment subjects; C, control subjects.
There was an average increase of 106.67% for DHA and 365.82% for EPA in the treatment group. There was a 58.29% increase in the EPA and no change in the DHA of the control group. Alpha-linolenic acid changes were similar in both groups. There were no significant complications or side effects in either group. (Figures 1-8; Table 2).
Figure 8.
Average increase of 106.67% for DHA in the treatment group indicating compliance. DHA, docosahexaenoic acid.
Figure 7.
Average increase of 365.82% for EPA in the treatment group indicating compliance. EPA, eicosapentaenoic acid.
Discussion
Coronary artery disease (CAD) is a leading cause of morbidity and mortality in the United States.1 Professional football players, although often at the peak of physical conditioning, still may possess significant CVD risk factors. Gender, family history—including race—dietary excess, obesity, and other disease conditions such as diabetes, hypertension, and abnormal blood lipid profile are all risk factors for CVD in this select population. Some of these risks are fixed, but the CVD risk factor of abnormal blood lipids is one of the most discriminating factors for early detection of CVD.6
The size of blood lipids is very important with regard to whether abnormal lipid values can increase CVD risk.2 Low-density lipoproteins vary in particle size and density, which are factors related to their ability to induce vascular oxidative damage and cause blood vessel plaque formation. Lipid subtype research now confirms that the predominance of small LDL is a much more significant cardiovascular risk factor than other subtypes. Larger LDL subfractions are less frequently associated with CVD, while HDLs are often considered protective to the development of CVD due to their large size.2,31
The therapeutic modulation of distinct LDL subspecies is therefore of great benefit in reducing the risk of cardiovascular events. Low-density lipoprotein size correlates positively with plasma HDL levels and negatively with plasma triglyceride concentrations.3,17 The combination of small dense LDL, decreased HDL cholesterol, and increased triglycerides has been coined by Austin et al3 in 1990 as the “atherogenic lipoprotein phenotype.”
By using an expanded cholesterol subfraction test for this study (VAP) we demonstrated the possible effects of omega-3 essential fatty acid (EFA) dietary supplements as an intervention for CVD.
Our evaluation using 2560 mg/day of mixed EPA/DHA omega-3 fatty acid supplements for a 60-day period revealed the most significant blood lipid changes in the IDL and RLP. The IDL showed a significant decrease of 27.58% in the treatment group compared to a 12.07% increase in the control group (P = .0022). Increased plasma IDL levels have been related to the presence and severity of angiographically determined CVD and to the progression of arterial lesions.58
In addition, RLP had a significant decrease of 23.86% in the treatment group compared to an 8.33% increase in the control group (P = .0042). Remnant lipoproteins are formed intravascularly when chylomicrons of intestinal origin and vLDL of hepatic origin are converted by lipoprotein lipase into smaller and denser particles. Remnant lipoproteins are depleted of triglycerides, enriched with cholesteryl esters and apolipoprotein E (a major component of vLDL) and believed to be more atherogenic than the larger size lipids. Evidence have implicated RLPs in premature and accelerated atherosclerosis.29
Omega-3 fatty acids have been shown to reduce RLP levels. In 2007, Satoh et al51 evaluated 44 obese, type-2 diabetic Japanese patients with metabolic syndrome and reported significant reductions in RLP cholesterol (P = .035) following 3 months of 1.8 g/day EPA supplementation.
Very low lipoprotein is the major carrier of plasma triglycerides. They are a diverse group of lipoprotein particles that vary in triglyceride and cholesterol content, apolipoprotein C and apolipoprotein E, and in their metabolism. The vLDL3, which is the smallest of the vLDL subfractions, showed a significant decrease of 17.10% in the treatment group over time compared to a 7.77% increase in the control group (P = .0343). Since this entire lipoprotein particle can enter the arterial intima, the vLDL concentration and particle size are most important variables. The vLDL3 subfraction is the dense, cholesterol-laden portion with less triglyceride that comprises the greatest risk for cardiovascular disease. Lower vLDL3 levels correlate to decreased cardiovascular risk.43 Additionally, the treatment group’s total vLDL demonstrated a significant decrease of 13.98% compared to increases of 23.18% in the control group (P = .0486). Tornvall et al57 in 1993 confirmed that vLDL3 was the smallest of the vLDL particles and demonstrated a correlation between the number of cholesteryl ester molecules in vLDL3 and global coronary atherosclerosis in hypertriglyceridemic patients. They concluded that it was the small cholesterylester-rich vLDL3 particles that predicted the CVD severity.
The treatment group’s triglyceride lipid portion demonstrated a significant decrease of 8.06% compared to increases of 43.98% in the control group (P = .0298). Plasma triglyceride concentration is a significant predictor of coronary heart disease. A meta-analysis by Hokanson and Austin26 of observational studies suggested that an 89-mg/dL elevation in triglycerides was associated with a 14% to 37% higher incidence of CVD independent of other risk factors. Bucher et al,6 in a meta-analysis of randomized, controlled trials of omega-3 diets or supplementation effects on CVD, found an average triglyceride reduction of 20% with no significant effect on LDL-C or HDL-C. Hamazaki et al21 performed a study evaluating omega-3 supplementation on serum levels of triglycerides and found a significant decrease in triglyceride levels in the omega-3 treated group compared to the control group after 4, 8, and 12 weeks (P < .05). Many studies of omega-3 fatty acids have found marked lowering of vLDL and triglycerides of 15% to 40%, depending on the dose. Our results are consistent with the observations from these studies. However, total cholesterol and LDL-C results have been inconsistent among other studies. In general, decreases in LDL have been found if dietary saturated fat intake was decreased.13,23,27,50,54
The use of omega-3 EFA for the treatment of hypertriglyceridemia has recently been approved by the Food and Drug Administration (FDA) using a prescription form of EPA/DHA (Omacor; LOVAZA; GlaxoSmithKline, Middlesex, United Kingdom). Calabresi et al7 evaluated patients who received 4 capsules daily of Omacor (providing 3.4 g EPA and DHA per day) or placebo for 8 weeks in a randomized, double-blind, crossover study. They found that Omacor significantly lowered plasma triglycerides and vLDL-C levels by 27% and 18%, respectively.
Piolot et al46 reported significant reductions in vLDL triglyceride and vLDL-C concentrations following daily ingestion of 6 grams of omega-3 EFA (EPA/DHA) during a 2-month period (P < .05). Rivellese et al evaluated the effects of omega-3 EFA on lipid metabolism in healthy individuals undergoing either a diet rich in monounsaturated fats or one rich in saturated fatty acids.49 They, too, discovered that the addition of omega-3 EFA supplements significantly reduces triglyceride as well as vLDL cholesterol, regardless of the type of diet. Other authors have shown that the benefits of omega-3 EFAs on coronary mortality are due to their effects on cardiac arrhythmia,48 platelet aggregation,32,37,55 hemostatic variables,32,37,55 and vascular reactivity37 in addition to how they affect lipid metabolism.35,44
High-density lipoprotein is considered the “good” cholesterol because it forages cholesterol from the tissues and delivers it to the liver for degradation.59 It may be for this reason that HDL and its subfractions are acknowledged as protective factors against coronary heart disease.36,54
The average total HDL cholesterol (direct) showed a nearly significant increase of 25.96% in the treatment group compared to a 14.16% increase the control group (P = .0671). The subfraction of HDL, HDL2, and HDL3 also increased in the treatment group compared to the control, although not statistically significant. This suggests that a larger number of subjects may have provided significant results.
Thomas et al56 also observed significant increases in HDL and HDL2 concentrations following omega-3 EFA supplementation of 4 g/day for 4 weeks in recreationally active men. These increases might be a means by which omega-3 EFA supplementation improves the CVD risk profile in patients.
Our results demonstrated no significant difference in HDL and subfractions HDL2 and HDL3, which may confirm that the previously observed increases in HDL are associated with exercise, for which both groups of players are regular participants. Durstine and Haskell14 reported elevations in HDL and HDL3 concentrations following a session of exercise independent of omega-3 EFA supplements. Thomas et al, while also investigating the use of omega-3 EFA supplementation and exercise, concluded that both omega-3 EFA and exercise could raise HDL concentrations; the omega-3 EFA was believed to be responsible for a greater HDL2 increase, while HDL3-C was elevated by exercise.56
Finally, both EPA and DHA plasma concentrations showed significant elevations of (EPA 365.8% [P = .0207], DHA 106.67% [P = .0555]) in the treatment group. These increases confirm compliance among the treatment group and show that a treatment period of 60 days is long enough to allow for a plasma increase in the proportion of omega-3. Plasma concentrations of EPA and DHA reflect current omega-3 status and are predictors of future tissue status. Tissue status of omega-3 fatty acids are currently being investigated as a powerful independent risk factor for death from coronary heart disease.22,33 The plasma elevations of EPA and DHA found in the treatment group could be interpreted as therapeutic endpoints independent of changes in blood lipids found among the treatment group.
The control group also experienced a 58.29% increase in EPA. Although less than the treatment group, and despite dietary diaries (suggest, recorded dietary intake) showing similar seafood use, this result is difficult to explain. The most likely explanation is the metabolic conversion of the vegetable or nut sources of omega-3 fatty acids, such as walnuts or almonds, or alpha-linolenic acid converted to EPA. It should be noted that the majority of this average increase in EPA was found in only 1 control participant.
Omega-3 EFA and Lipids
In addition to omega-3 EFA, there are other supplements that have been suggested as possible adjuncts to the treatment of abnormal blood lipids: antioxidant vitamins E and C, garlic, soy products, plant stanols/sterols, and fiber.30
Of this group omega-3 EFAs have had the most success for improving blood lipid profiles. In fact Brown et al5 showed that some antioxidants may inhibit HDL improvements found with the combination therapy of niacin and statin medication.
The omega-3 EFAs are believed to lower lipids by inhibiting the synthesis of vLDL in the liver. This results in smaller, less-dense vLDL and LDL particles and, therefore, a reduced plaque-producing lipid profile.34
The dose used in this study was 2.2 g/day of mixed EPA and DHA. Higher dosing of 3 to 4 grams a day have resulted in greater blood lipid improvements in many clinical trials, although current AHA guidelines recommend only 1 gram a day of omega-3 EFA for CVD patients (Table 2). Omega-3 fatty acid supplementation in doses of up to 3 grams a day is considered safe. Reported side effects have included gastrointestinal upset and loose stool. At higher doses there is a possible but low risk of bleeding.32
In 2001, the Adult Treatment Panel (ATP III) of the National Cholesterol Education Program recommended that omega-3 fatty acids be used as an adjunct to pharmacological therapy for lowering triglycerides. Additionally they concluded that the most practical way to achieve this quantity of omega-3 fatty acids is through the use of fish oil supplements.15,16
Finally, because of the lack of significant side effects and complications associated with fish oil supplements, chronic or lifelong use is very possible, as both a prevention and treatment alternative in select cases to potentially toxic medications. Statins remain an excellent means to reduce total cholesterol, but subfraction lipid testing reveals that they are not effective at reducing some harmful cholesterol subfractions. The proportion of the smaller, potentially more dangerous vLDL lipids may not be adequately reduced and the HDL fractions not improved to the levels many believe are required to lessen cardiac risk factors.24 The side effect profiles of statins are also not insignificant and include potentially damaging myopathy and increases in hepatic transaminases. Serious drug interactions with statins are also a concern.4
Conclusion
This evaluation is limited by the relatively small number of participants (N = 36). Despite this fact, rather dramatic improvements in blood lipid profiles of professional football players were achieved using a moderately high dose of omega-3 EFA fish oil supplementation. Our results along with those cited in the literature suggest that omega-3 EFA supplementation can have a significant effect on improving blood lipid–related CVD risk factors in professional football players.
Based on the results of our study population, the professional NFL player should consider continued use of omega-3 EFA supplementation throughout his active years as well as during retirement, when additional risk factors for CVD become much more prevalent as a function of aging.
Acknowledgments
The authors would like to thank NFL Charities for their generous grant for this study and also the copyediting work provided by Stephanie Bost.
Footnotes
One or more authors has declared a potential conflict of interest. Dr Maroon is on the scientific advisory board of Nordic Naturals.
References
- 1. American Heart Association Cardiovascular disease statistics. 2006. http://www.americanheart.org/presenter.jhtml?identifier=4478 Accessed August 8, 2007
- 2. Austin MA, Hokanson JE, Brunzell JD. Characterization of low-density lipoprotein subclasses: methodologic approaches and clinical relevance. Curr Opin Lipidol. 1994;5:395-403 [DOI] [PubMed] [Google Scholar]
- 3. Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990;82:495-506 [DOI] [PubMed] [Google Scholar]
- 4. Bellosta S, Paoletti R, Corsini A. Safety of statins focus on clinical pharmacokinetics and drug interactions. Circulation. 2004;109:III-50-III-57 [DOI] [PubMed] [Google Scholar]
- 5. Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583-1592 [DOI] [PubMed] [Google Scholar]
- 6. Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2002;112:298-304 [DOI] [PubMed] [Google Scholar]
- 7. Calabresi L, Donati D, Pazzucconi F, Sirtori CR, Franceschini G. Omacor in familial combined hyperlipidemia: effects on lipids and low subclasses. Atherosclerosis. 2000;148(2):387-396 [DOI] [PubMed] [Google Scholar]
- 8. Callahan LF, Currey SS, Jonas BL, et al. Osteoarthritis in retired national football league (NFL) players: the role of injuries and playing position. Arthritis Rheum. 2002;46:S415 [Google Scholar]
- 9. Campos H, Genest JJ, Blijlevens E, et al. Low-density lipoprotein particle size and coronary artery disease. Arterioscler Thromb.1992;12:187-195 [DOI] [PubMed] [Google Scholar]
- 10. Castelli WP, Anderson K, Wilson PW, Levy D. Lipids and risk of coronary heart disease. The Framingham Study. Ann Epidemiol. 1992;2(1-2):23-28 [DOI] [PubMed] [Google Scholar]
- 11. Castelli WP. Lipids, risk factors, and ischaemic heart disease. Atherosclerosis. 1996;124(Suppl l):S1-S9 [DOI] [PubMed] [Google Scholar]
- 12. Colussi G, Catena C, Baroselli S, et al. Omega-3 fatty acids: from biochemistry to their clinical use in the prevention of cardiovascular disease. Recent Patents Cardiovasc Drug Discov. 2007;2:13-21 [DOI] [PubMed] [Google Scholar]
- 13. Connor WE, Conner SL. Diet, atherosclerosis, and fish oil. Adv Intern Med. 1990;35:139-171 [PubMed] [Google Scholar]
- 14. Durstine JL, Haskell WL. Effects of exercise training on plasma lipids and lipoproteins. Exerc Sport Sci Rev. 1994;22:477-521 [PubMed] [Google Scholar]
- 15. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497 [DOI] [PubMed] [Google Scholar]
- 16. Fletcher B, Berra K, Ades P, et al. Managing abnormal blood lipids: a collaborative approach. Circulation. 2005;112:3184-3209 [DOI] [PubMed] [Google Scholar]
- 17. Frost RJA, Otto C, Geiss HC, Schwandt P, Parhofer KG. Effects of Atorvastatin versus Fenofibrate on lipoprotein profiles, low-density lipoprotein subfraction distribution, and hemorheologic parameters in type 2 diabetes mellitus with mixed hyperlipoproteinemia. Am J Cardiol.2001;87:44-48 [DOI] [PubMed] [Google Scholar]
- 18. Griffin BA, Freeman DJ, Tait GW, et al. Role of plasma triglyceride in the regulation of plasma low-density lipoprotein (LDL) subfractions: relative contribution of small, dense LDL to coronary heart disease risk. Atherosclerosis. 1994;106:241-253 [DOI] [PubMed] [Google Scholar]
- 19. Grundy SM, Pasternak R, Greenland P, Smith S, Jr, Fuster V. Assessment of cardiovascular risk by use of multiple risk–factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation.1999;100:1481-1492 [DOI] [PubMed] [Google Scholar]
- 20. Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903-909 [DOI] [PubMed] [Google Scholar]
- 21. Hamazaki K, Itomura M, Huan M, et al. n-3 long-chain FA decrease serum levels of TG and remnant-like particle-cholesterol in humans. Lipids. 2003;38(4):353-358 [DOI] [PubMed] [Google Scholar]
- 22. Harris WS, Von Schacky C. The omega-3 index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212-220 [DOI] [PubMed] [Google Scholar]
- 23. Haynes WG. Triglyceride-rich lipoproteins and vascular function. Arterioscler Thromb Vasc Biol. 2003;23;153-155 [DOI] [PubMed] [Google Scholar]
- 24. Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomized, placebo-controlled trial. Lancet. 2002;360:7-2212114036 [Google Scholar]
- 25. Hill AM, Buckley JD, Murphy KJ, Howe PR. Combining fish-oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors. Am J Clin Nutr. 2007;85:1267-1274 [DOI] [PubMed] [Google Scholar]
- 26. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213-219 [PubMed] [Google Scholar]
- 27. Jeppesen J, Hein H, Suadicani P, et al. Triglyceride concentration and ischemic heart disease: an 8-year follow-up in the Copenhagen Male Study. Circulation. 1998;97:1029-1036 [DOI] [PubMed] [Google Scholar]
- 28. Jialal I, Devaraj S. The role of oxidized low-density lipoprotein in atherogenesis. J Nutr. 1996;126(4 Suppl):1053S-1057S [DOI] [PubMed] [Google Scholar]
- 29. Jialal I, Devaraj S. Remnant lipoproteins: measurement and clinical significance. Clin Chem. 2002;48:217-219 [PubMed] [Google Scholar]
- 30. Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R; Stresa Workshop Participants Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc. 2003;78(8):965-978 [DOI] [PubMed] [Google Scholar]
- 31. Krauss RM. Low-density lipoprotein subclasses and risk of coronary artery disease. Curr Opin Lipidol.1991;2:248-252 [Google Scholar]
- 32. Kris-Etherton PM, Harris WS, Appel LJ; American Heart Association Nutrition Committee Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747-2757 [DOI] [PubMed] [Google Scholar]
- 33. Kuriki K, Nagaya T, Tokudome Y, et al. Plasma concentrations of (n-3) highly unsaturated fatty acids are good biomarkers of relative dietary fatty acid intakes: a cross-sectional study. J Nutr. 2003;133:3643-3650 [DOI] [PubMed] [Google Scholar]
- 34. Leaf A, Weber PC. Cardiovascular effects of n-3 fatty acids. N Engl J Med. 1988;318:549-557 [DOI] [PubMed] [Google Scholar]
- 35. Luo J, Rizkalla SW, Vidal H, et al. Moderate intake of n-3 fatty acids for 2 months has no detrimental effect on glucose metabolism and could ameliorate the lipid profile in type 2 diabetic men. Results of a controlled study. Diabetes Care. 1998;21:717-724 [DOI] [PubMed] [Google Scholar]
- 36. Mack WJ, Krauss RM, Hodis HN. Lipoprotein subclasses in the Monitored Atherosclerosis Regression Study (MARS): treatment effects and relation to coronary angiographic progression. Arterioscler Thromb Vasc Biol. 1996;16:697-704 [DOI] [PubMed] [Google Scholar]
- 37. Marchioli R. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet.1999;354:447-455 [PubMed] [Google Scholar]
- 38. Maron BJ, Chaitman BE, Ackerman MJ, et al. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular disease. Circulation.2004;109:2807-2816 [DOI] [PubMed] [Google Scholar]
- 39. Maron BJ. Sudden death in young athletes. N Engl J Med.2003;349:1064-1075 [DOI] [PubMed] [Google Scholar]
- 40. Maroon JC, Bost J. Fish Oil: The Natural Anti-Inflammatory. Laguna, CA: Basic Health Books; 2006 [Google Scholar]
- 41. Massaro M, Habib A, Lubrano L, et al. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKCeqinhibition. Proc Natl Acad Sci U S A. 2006;103(41):15184-15189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mayo Clinic Press Release Retired National Football League players at increased risk for heart problems. Medical News Today Web site. http://www.medicalnewstoday.com/articles/101971.php. Published March 29, 2008; accessed October 23, 2008
- 43. Metamatrix Company Brochure Basic description of lipids and lipoproteins. http://www.cholesterol-tests.com/Description_Lipids.html Accessed August 8, 2007
- 44. Minihane AM, Khan S, Leigh-Firbank EC, et al. ApoE polymorphism and fish oil supplementation in subjects with an atherogenic lipoprotein phenotype. Arterioscler Thromb Vasc Biol. 2000;20:1990-1997 [DOI] [PubMed] [Google Scholar]
- 45. US Department of Health & Human Services National Institute for Occupational Safety and Health (NIOSH) NFL Mortality Study. January 1994. http://www.cdc.gov/niosh/pdfs/nflfactsheet.pdf Accessed October 23, 2008
- 46. Piolot A, Blache D, Boulet L, et al. Effect of fish oil on LDL oxidation and plasma homocysteine concentrations in health. J Lab Clin Med. 2003;141(1):41-49 [DOI] [PubMed] [Google Scholar]
- 47. Rajman I, Kendall MJ, Cramb R, et al. Investigation of low-density lipoprotein subfractions as a coronary risk factor in normotriglyceridemic men. Atherosclerosis.1996;125:231-242 [DOI] [PubMed] [Google Scholar]
- 48. Reiffel WS, McDonald A. Antiarrhythmic effects of omega-3 fatty acids. Am J Cardiol. 2006;98(suppl):50i-60i [DOI] [PubMed] [Google Scholar]
- 49. Rivellese AA, De Natale C, Lilli S. Type of dietary fat and insulin resistance. Ann N Y Acad Sci. 2002;967:329-335 [DOI] [PubMed] [Google Scholar]
- 50. Sacks FM, Alaupovic P, Moye LA, et al. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the cholesterol and recurrent events (CARE) trial. Circulation. 2000;102:1886. [DOI] [PubMed] [Google Scholar]
- 51. Satoh N, Shimatsu A, Kotani K, et al. Purified eicosapentaenoic acid reduces small dense LDL, remnant lipoprotein particles, and C-Reactive protein in metabolic syndrome. Diabetes Care.2007;30:144-146 [DOI] [PubMed] [Google Scholar]
- 52. Siscovick DS, Raghunathan TE, King I, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363-1367 [DOI] [PubMed] [Google Scholar]
- 53. Spagnoli LG, Bonanno E, Sangiorgi G, Mauriello A. Role of inflammation in atherosclerosis. J Nucl Med. 2007;48(11):1800-1815 [DOI] [PubMed] [Google Scholar]
- 54. Stampfer MJ, Krauss RM, Ma J, et al. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276:882-888 [PubMed] [Google Scholar]
- 55. Stone NJ. Fish consumption, fish oil, lipids, and coronary heart disease. Circulation. 1996;94:2337-2340 [DOI] [PubMed] [Google Scholar]
- 56. Thomas TR, Smith BK, Donahue OM, Altena TS, James-Kracke M, Sun GY. Effects of omega-3 fatty acid supplementation and exercise on low-density lipoprotein and high-density lipoprotein subfractions. Metabolism. 2004;53(6):749-754 [DOI] [PubMed] [Google Scholar]
- 57. Tornvall P, Bavenholm P, Landou C, de Faire U, Hamsten A. Relation of plasma levels and composition of apolipoprotein B-containing lipoproteins to angiographically defined coronary artery disease in young patients with myocardial infarction. Circulation. 1993;88(5 Pt 1):2180-2189 [DOI] [PubMed] [Google Scholar]
- 58. Sutherland WH, Restieaux NJ, Nye ER, et al. IDL composition and angiographically determined progression of atherosclerotic lesions during Simvastatin therapy. Arterioscler Thromb Vasc Biol. 1998;18:577-583 [DOI] [PubMed] [Google Scholar]
- 59. Yamashita S, Sakai N, Hirano K, Ishigami M, Maruyama T, Nakajima N, Matsuzawa Y. Roles of plasma lipid transfer proteins in reverse cholesterol transport. Front Biosci. 2001;6:D366-D387 [DOI] [PubMed] [Google Scholar]