Abstract
Osteochondritis dissecans of the knee is identified with increasing frequency in the young adult patient. Left untreated, osteochondritis dissecans can lead to the development of osteoarthritis at an early age, resulting in progressive pain and disability. Treatment of osteochondritis dissecans may include nonoperative or operative intervention. Surgical treatment is indicated mainly by lesion stability, physeal closure, and clinical symptoms. Reestablishing the joint surface, maximizing the osteochondral biologic environment, achieving rigid fixation, and ensuring early motion are paramount to fragment preservation. In cases where the fragment is not amenable to preservation, the treatment may include complex reconstruction procedures, such as marrow stimulation, osteochondral autograft, fresh osteochondral allograft, and autologous chondrocyte implantation. Treatment goals include pain relief, restoration of function, and the prevention of secondary osteoarthritis.
Keywords: osteochondritis dissecans, knee, cartilage, surgical treatment
Osteochondritis dissecans (OCD) is a pathological condition that results in destruction of subchondral bone with secondary damage to overlying articular cartilage.6 Factors such as inflammation, ossification, and repetitive trauma contribute to the pathogenesis of OCD. Left untreated, OCD can lead to the development of osteoarthritis at an early age, resulting in progressive pain and disability.12 OCD is classified as a juvenile or adult form based on the skeletal maturity of the patients.6 Juvenile OCD (JOCD) occurs in children and young adolescents who have open growth plates. Although adult OCD (AOCD) may arise de novo, it is more commonly the result of an incompletely healed, previously asymptomatic lesion from JOCD.20 JOCD has a much better prognosis than does AOCD, with higher rates of spontaneous healing with conservative treatment.5 AOCD lesions have a greater propensity for instability, and they typically follow a clinical course that is progressive and unremitting.30
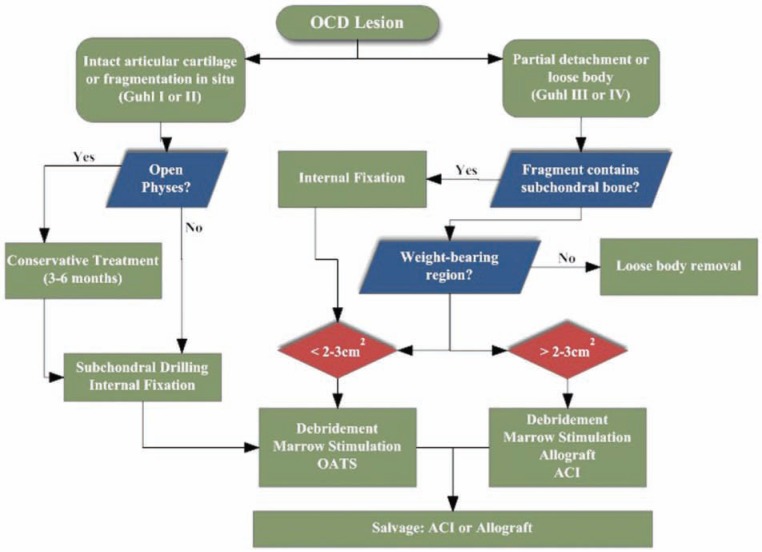
The highest rates of OCD appear among patients between 10 and 15 years old, ranking among the most common causes of knee pain and dysfunction in teenagers.5,30,35 The prevalence of OCD is estimated at 15 to 21 per 100 000.27 Lesions most frequently occur on the femoral condyles, but they are also found in the elbow, wrist, ankle, and femoral head.3,7,17,43,47 Treatments tend to fall into 1 of 2 categories: preservation or replacement. Nonoperative treatment, activity modification, drilling, and fragment fixation are used to preserve the native articular cartilage. Restorative biological therapies, such as marrow stimulation, autologous chondrocyte implantation, osteochondral autograft, and fresh osteochondral allograft, are indicated to replace the damaged cartilage with hyaline or hyaline-like tissue when preservation fails39 (Figure 1).
Figure 1.
Algorithm for surgical treatment of juvenile and adult osteochondritis dissecans.
Presentation and Imaging
The typical presentation of OCD includes knee pain and swelling related to activity. Instability is not usually reported, although mechanical symptoms (catching or locking) may occur in the presence of a loose body. Not uncommonly, patients may have an occult OCD and so initially present with mechanical symptoms. With fragment fixation or removal, many will do well clinically and have few symptoms related to the defect.
More than 70% of OCD lesions are found in the classic area (ie, posterolateral aspect of the medial femoral condyle), with inferior central lateral condylar lesions accounting for only 15% to 20% of cases and femoral trochlear lesions less than 1%. Patellar involvement is uncommon (5%-10%) and is typically located in the inferior medial area.30
On physical exam, tenderness may localize to the anterior medial part of the knee, in the case of the classic OCD lesion. The patient may walk with an antalgic gait or with the leg externally rotated (Wilson sign).38 Effusion, decreased range of motion, and quadriceps atrophy are variably present, depending on the severity and duration of the lesion.16 Alternatively, physical examination may reveal only a patient’s subjective discomfort with weightbearing in the region of the lesion.
Standard anteroposterior and lateral radiographs of the knee permit localization of the lesion and assessment of the patient’s physeal status. Additional images, such as a tunnel and sunrise views, are useful for suspected distal medial femoral condyle or patellar lesions, respectively. By convention, lesions may be anatomically localized using the Cahill classification6 (Figure 2). Magnetic resonance imaging is a mainstay for diagnosing OCD lesions. Lesion qualities, including bone edema, subchondral separation, and cartilage condition, may be evaluated before determining a treatment course (Figure 3). Intraoperatively, OCD lesions may be classified using the criteria suggested by Guhl24: grade 1, normal articular cartilage; grade 2, fragmentation in situ; grade 3, partial detachment; and grade 4, complete detachment, loose body present.
Figure 2.
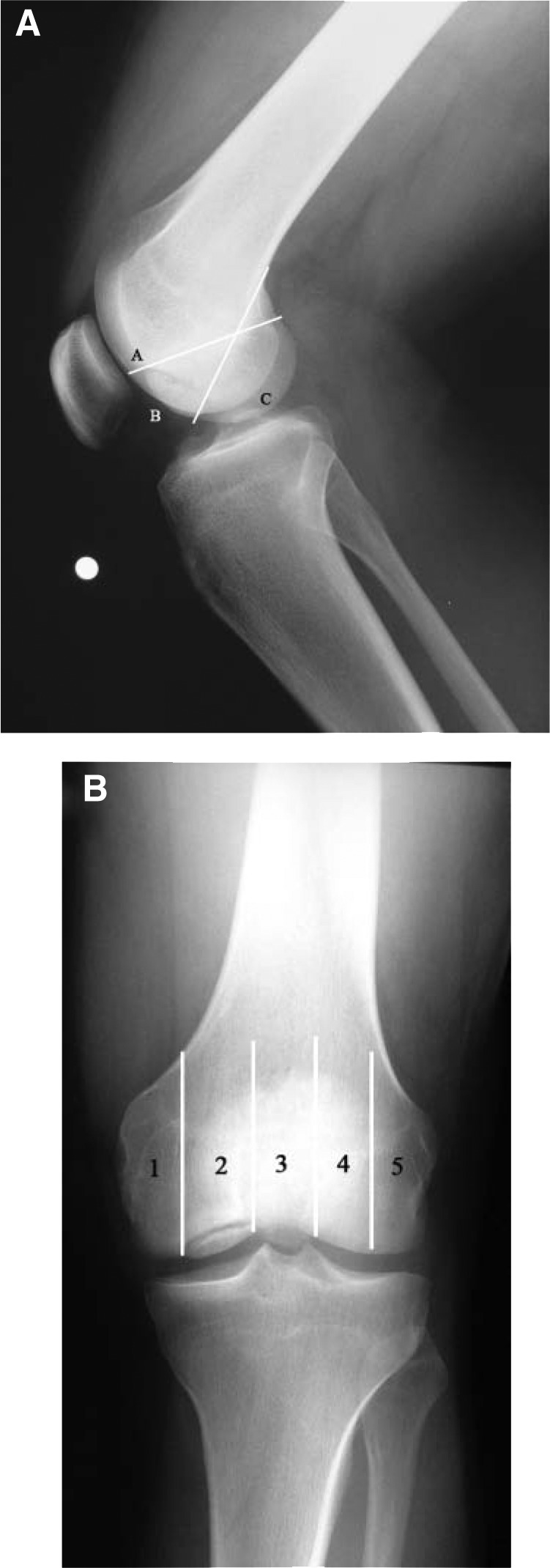
Anatomic locations of osteochondritis dissecans in the knee. A, lateral radiograph of a 21-year-old man with a BC lesion in the medial femoral condyle; B, anteroposterior radiograph of the same patient shows a grade 2 lesion occupying the weightbearing area of the femoral condyle. Numbering of the 5 anatomic areas begins in the middle side. The condyles are bisected, and area 3 is bounded by the walls of the intercondylar notch.
Figure 3.
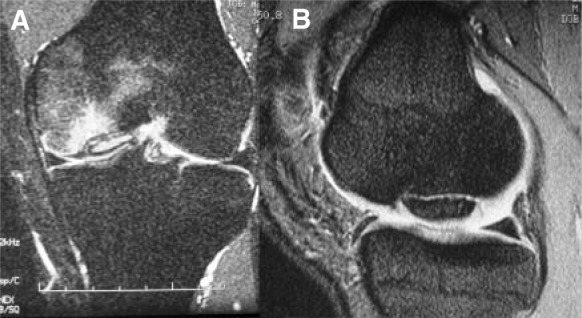
A, coronal magnetic resonance image of the knee of an osteochondritis dissecans lesion of the medial femoral condyle; note the amount of bone edema that is evident, as well as the high signal between the osteochondral fragment and subchondral bone. B, sagittal image through the medial femoral condyle—a high intensity interface between the osteochondritis dissecans lesion and underlying bone suggests instability.
Natural History and Prognosis
The natural history of untreated OCD lesions is poorly defined. Most AOCD cases arise from established untreated JOCD. Many younger AOCD patients have a history of knee symptoms, dating back to a time when their physes were open. These cases probably represent JOCD that did not heal and so evolved to AOCD. Spontaneous healing of JOCD lesions has been reported when the lesion is not in the classic location.10
Linden35 performed a long-term retrospective follow-up study (average, 33 years after diagnosis) of patients with OCD of the femoral condyles. The researcher concluded that patients with AOCD had an increasing incidence of gonarthrosis with age. In contrast, when JOCD was diagnosed, the patient had no increased risk of osteoarthritis later in life, when compared to the normal population. Linden also suggested that treatment complications do not usually develop with JOCD. In contrast, Twyman et al52 conducted a prospective follow-up study at middle age of 22 JOCD-afflicted knees. Fifty percent had some radiographic signs of osteoarthritis. The likelihood of osteoarthritis development was also found to be proportional to the size of the area involved.
Early studies evaluating treatment results of these lesions focused on fragment excision. Denoncourt et al11 treated 37 patients, with arthroscopic removal of the fragment and curettage of the lesion. Second-look arthroscopy confirmed complete healing in 10 cases. The researchers recommended this treatment for patients (adults and children) who failed initial attempts of nonoperative treatment. Similarly, Ewing and Voto15 excised the fragments and drilled the crater in 29 patients. They reported a satisfactory result in 72% of their patients with short-term follow-up (less than 1 year).
Further emphasis has been placed on fragment retention to minimize the chance for the long-term development of secondary arthritis. Recent reports suggest that pain relief due to fragment excision may be short-term, and they emphasize the importance of repairing the fragment, if possible. Anderson and Pagnani1 evaluated 19 patients (20 OCD lesions) who were treated with fragment excision. At follow-up (conducted between 2 and 20 years), only 5 patients could participate in strenuous activity without significant symptoms; 11 had pain with activities of daily living; and the remaining 3 had pain with light activities. Excision of the fragment produced short-term improvement, but symptoms worsened with time.
Patient age, skeletal maturity (physeal maturity), lesion appearance (size, location, and stability), and clinical symptoms influence surgical decisions. With initial nonoperative treatment, stable OCD lesions in young patients have a favorable prognosis. The goal of conservative treatment is to obtain lesion healing before physeal closure. Authors who focus on the biology of the fragment–subchondral bone interface argue that the knee should be protected in a knee immobilizer and treated similar to an intra-articular fracture.6 Alternatively, there are authors who place a premium on the health of the articular cartilage and cite the value of continuous motion.6 Hughston et al27 demonstrated the detrimental effects of prolonged immobilization, including stiffness, atrophy, and (potentially) chondropenia. Traditional nonoperative treatment for JOCD consists of an initial phase of knee immobilization with partial weightbearing (4-6 weeks). Once the patient is pain-free, weightbearing (as tolerated) is permitted, and a rehabilitation program ensues, emphasizing knee range of motion and low-impact strengthening exercises. During this time, competitive sports are restricted. If there are radiographic and clinical signs of healing at 3 or 4 months after the initial diagnosis, patients may participate in a gradual return to sports, with increasing intensity allowed in the absence of knee symptoms. The likelihood that the lesion will heal with this management is approximately 50% at 10 to 18 months.7
Operative treatment is indicated for young patients with detached or unstable lesions or for those approaching physeal closure whose lesions have been unresponsive to nonoperative management.6,15 In contrast, AOCD lesions have a greater propensity for instability and so typically follow a clinical course requiring early surgical intervention.20 A large multicenter review by the European Pediatric Orthopedic Society (509 knees, 318 juvenile and 191 adult, in 452 patients) suggests an improved prognosis with conservative treatment in young patients with a small lesion (less than 2 cm2) in the classic location with no signs of dissection or effusion. In cases of chondral separation, surgical results are better than those with nonsurgical treatments.26,49
Surgical Options
Reparative Treatments
The goal of reparative procedures is to restore the integrity of the native subchondral interface and preserve the overlying articular cartilage.38 Methodologies such as drilling and internal fixation are indicated for the symptomatic juvenile patient who has failed a course of nonoperative treatment (generally, 6 months). Depending on the symptom quality, when the presence of significant mechanical symptoms dominates the clinical presentation, a decision to operate might occur earlier. These procedures provide clinical relief in a majority of patients and leave many viable options for revision in case of inadequate symptomatic relief.
Drilling
The disruption of subchondral blood supply, whether from repeated microtrauma or rapid growth, is an important factor in the development of OCD.2 Fragment healing is enhanced by creating vascular channels to the devitalized region, also known as drilling. Drilling is generally limited to low-grade lesions that are intact or show minimal signs of separation (grades 1 and 2, respectively) in young patients with open physes. Typically, these lesions are not grossly unstable to palpation. Flap detachment and loose bodies must be addressed through removal, fixation, or replacement. Drilling may be an adjuvant to improve the blood supply to the repair; transchondral and retrograde approaches have been described.
Antegrade drilling—through the articular surface and into the femoral epiphysis—is done arthroscopically under direct visualization.8,31,36 If the lesion is not accessible via standard anterolateral and anteromedial portals, satellite portals are created to obtain an orthogonal drilling angle. A K-wire 2 cm longer than a small cannula facilitates the direction and depth of the channels.2 Return of blood and fat droplets through the articular surface confirms penetration of cancellous bone. Drilling can more commonly be performed by entering at a nonarticular location. For example, the classic OCD lesion, along the posterolateral aspect of the medial femoral condyle, can be entered at the anterior aspect of the posterior cruciate ligament origin, along the inner margin of the medial femoral condyle, with a K-wire introduced percutaneously or through the inferolateral portal.
Retrograde drilling is inherently more difficult when targeting the lesion base. C-arm visualization is needed and will help avoid joint penetration or dislodgement of the OCD fragment. Alternative methods have been proposed, including sonography4 and the use of an anterior cruciate ligament guide.32
Large-diameter drilling with iliac crest bone graft supplementation has also been described.33 Retrograde drilling is typically used for stable lesions not amenable to approaches through the intercondylar notch or gutter. In these instances, an anterior cruciate ligament guide is used for triangulation, which can avoid the need for cumbersome imaging during the procedure. By arthroscopic view, the defect is drilled without violating the articular cartilage.
Outcomes of OCD drilling are generally favorable, with patient age being the most prognostic factor. Patients with OCD who were diagnosed and treated with drilling as an adult have decreased radiographic healing and less favorable symptom outcomes,2 likely because these lesions are generally more unstable than those identified in patients with open physes and because the potential for spontaneous healing is low. Louisia et al36 noted 71% radiographic healing (12/17) and 2 poor results in JOCD, compared with 25% healing (2/8) and 4 poor results in AOCD patients. Overall, good to excellent results are observed in greater than 80% of adolescent patients, with 70% or more being able to return to sports.3,30,32,36
Internal Fixation
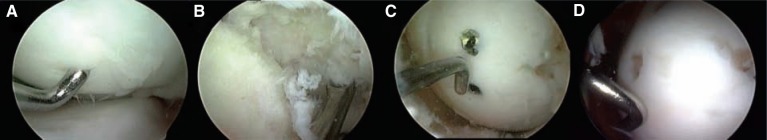
Higher-grade OCD lesions with articular cartilage flaps and loose bodies (grades 3 and 4, respectively) may cause the knee to catch or lock, and they are generally not amenable to conservative treatment, such as observation and activity modification. Reattachment of partially detached lesions and loose bodies is appropriate for large fragments containing sufficient subchondral bone, to provide union and support of the fixation system. Lower grade lesions (grade 1 or 2) may be fixed if conservative treatment has failed or there is clinical suspicion of instability. Fixation can be accomplished with metal or bioabsorbable devices.1,21,42 In vitro studies suggest that compression, resulting in friction between the fragment and base, improves stability and resistance to shear loading. Unstable “trap door” lesions that are partially elevated from the subchondral bed require fixation.44,54 If accessible, the base of the lesion and bony surface of the flap are debrided with a curette or rotary shaver. The fibrous tissue from the base (analogous to a fibrous nonunion) is completely debrided. Microfracture awls can be used to penetrate the base and so allow improved access to the subchondral blood supply. The fragment is reduced and temporarily fixed with K-wires to facilitate the final placement of the fixation device. In most cases, fixation is accomplished at 2 or more locations, to impart compression and rotational stability to the fragment. All devices should be recessed beneath the cartilage surface, with metal screws removed postoperatively when evidence of union is apparent (Figure 4). Postoperatively, patients may heel-touch weightbear and, when available, use a continuous passive motion machine for 4 to 6 hours per day. Bioabsorbable fixation is an option (Arthrex Bio-compression Screws, Arthrex Inc, Naples, Florida); however, the dual benefit of revisiting the lesion postoperatively is to verify defect healing and remove hardware that might become prominent should the fragment settle around the fixation device.
Figure 4.
A, intraoperative arthroscopic view of an unstable osteochondral fragment (25 × 25 mm); B, the base was completely denuded of fibrocartilage; C, anatomic reduction of the osteochondral fragment was performed, and 2 standard screws were used for fixation; D, removal of the screws 2 months after surgery, with complete healing.
Favorable outcomes after internal fixation have been reported for metallic and bioabsorbable devices. Kivistö et al28 noted good to excellent results in 86% of young patients treated with staple fixation (53% radiographic healing). A study of Herbert compression screw fixation yielded the following outcomes: 87% (13/15) of the knees healed to normal per IKDC grading and 93% (14/15) per radiographic assessment.37 Gomoll et al21 evaluated 12 adolescent patients with unstable Cahill type 2C lesions treated with compression screw fixation with average 6-year follow-up. All lesions healed without clinical or radiographic evidence of degenerative disease. Fixation with self-reinforced PLLA (poly-L-lactic acid) nails and pins permit radiographic healing in 60% to 100% of cases.13,45 A cohort study by Weckström et al53 suggests that implant geometry (ie, presence of barbs or a flared head) is a factor in successful outcomes. Complications associated with OCD fixation include damage to opposing cartilage surfaces from proud hardware, broken hardware, loose bodies, and synovitis.18,28,49
Restorative Treatments
Restorative procedures attempt to replace damaged cartilage with hyaline or hyaline-like tissue.8,34 As such, the surgeon must consider the “next step” option if initial surgical management fails and the patient has classic symptoms related to the defect. The treatment algorithm proceeds from the least destructive and invasive methodologies, to avoid “burning bridges” (ie, precluding future options).11 All restoration procedures should be performed in the setting where comorbidities are considered, including malalignment, ligament insufficiency, and a meniscectomized state.
Every attempt to repair the osteochondral fragment should be made, even if supplemental bone grafting is required. In the event of subchondral bone loss, cancellous bone can be used, as harvested arthroscopically via osteochondral autograft harvesting tubes (Arthrex Inc) to extract 7-mm cylinders of bone from the intercondylar notch edge. In the event that the fragment cannot be initially stabilized (and thus requires excision) or that it fails to heal after initial fixation, the clinical relevance of the remaining defect needs to be determined. Many patients have coexisted with OCD only to become symptomatic when the osteochondral fragment becomes unstable. Fragment removal can lead to symptom relief. Because the natural history of OCD is poorly understood, performing a restorative procedure immediately is debatable.
Carefully questioning the patient before fragment fixation or following fragment removal can help determine if the defect bed is clinically relevant. Complaints of achy discomfort, effusions unrelated to mechanical symptoms, or weightbearing pain over the lesion may indicate that symptoms are due to the defect rather than the unstable or displaced fragment. When the defects are relatively small or from areas withless contact pressure50 (ie, classic OCD) and are associated with the acute onset of mechanical symptoms in a skeletally mature adult, fragment removal and observation may be all that is required.
Appropriately educating patients about defect-related symptoms is critical to assessing further need for intervention and cartilage restoration at follow-up. Lesions that are large or weightbearing (such as OCD of the lateral femoral condyle) are likely to be associated with the worst natural history and may therefore require, depending on the depth of subchondral bone loss, high-level restorative intervention.
Marrow Stimulation Techniques
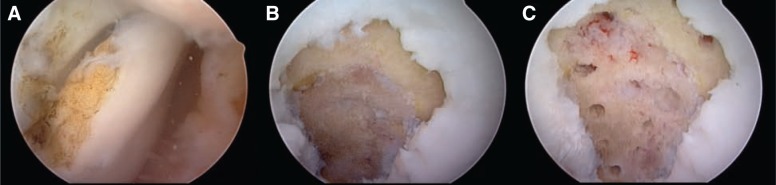
Abrasion, subchondral drilling, and microfracture involve breaching the subchondral bone to allow the influx of pluripotent stem cells from the marrow into the osteochondral defect, resulting in fibrocartilage formation.51 Microfracture is indicated in patients with a localized cartilage defect (less than 2 to 3 cm2). This technique can also be used for patients with a bigger lesion and low demand. The calcified cartilage layer is carefully debrided, and surgical awls are used to penetrate the subchondral bone to enhance the defect fill19 (Figure 5). Postoperative rehabilitation requires up to 6 weeks of nonweightbearing with the use of a continuous passive motion machine for 6 hours per day.
Figure 5.
A, intraoperative arthroscopic view of a loose body secondary to osteochondritis dissecans disease; B, the calcified layer was completely removed; C, the microfracture holes normally began at the periphery of the lesion adjacent to the stable cartilage rim and continued to the center.
In a study by Gudas et al,23 normally large lesions (more than 2 cm2) treated with microfracture demonstrated deterioration with time because of decreased fibrocartilage resilience and stiffness40; however, specific outcomes of OCD lesions were not assessed in this study.
Knutsen et al29 compared 80 patients at 2- and 5-year follow-up who had a single chronic symptomatic cartilage defect on the femoral condyle of the knee. Patients were randomized to either microfracture or autologous chondrocyte implantation. In sum, 28% of the lesions were due to OCD, and 77% of patients in both groups showed satisfactory results at 5 years. No significant difference between the 2 treatment groups was evident, as based on clinical, histological, and radiographic results. The researchers proposed that microfracture should be preferred as the first-line treatment option for defects located on the medial or lateral femoral condyle of the knee. Microfracture should be considered as the first-line treatment in small lesions (< 2 cm2) with subchondral bone integrity and in patients with lower physical demands and slightly larger lesions (2-4 cm2). Microfracture can only resurface defects; it cannot reconstitute subchondral bone. If reconstruction is needed, osteochondral autograft or allograft are better options.
Osteochondral Autograft Transplantation
The indications and optimal patient population for transplanting osteochondral tissue from a nonweightbearing region of the knee to restore a damaged articular surface remain narrow.25 A single-plug autograft is preferred for defects smaller than 2 cm. However, some authors have performed mosaicplasty with multiple smaller plugs for defects as large as 4 cm2, with encouraging results.25 Protected weightbearing is encouraged for up to 6 weeks after surgery. The advantages of the osteochondral autograft transplantation technique include absence of disease transmission risk and the lower cost of a single-stage procedure. Disadvantages include donor site morbidity and limited available graft volume. In addition, it is technically difficult to position the plugs to re-create the contour of curved surfaces. Despite these limitations, results from isolated small- to medium-sized lesions of the femoral condyle have been good: at greater than 3 years, 91% of cases had good to excellent results.25
Miniaci and Tytherleigh-Strong42 suggested using the osteochondral autograft transplantation technique for the fixation of unstable OCD lesions of the knee. Twenty patients with OCD lesions were fixed in situ with multiple 4.5-mm osteochondral dowel grafts harvested from the edges of the trochlea. At 6 months postoperatively, all knees were radiographically healed; at 18 months postoperatively, all knees were scored as normal. One advantage of this technique is that a considerable volume of the original lesion is replaced by autologous bone graft, providing stable biological fixation. Outerbridge et al46 reported favorable short-term results in 10 patients with large femoral OCD lesions, using autografts harvested from the lateral facet of the patella. Yoshizumi et al55 reported successful osteochondral graft treatment of OCD lesions in 3 skeletally mature patients 18 years old and younger. Overall, experimental and clinical data demonstrate that transplanted autograft hyaline cartilage has a good rate of survival.25
Osteochondral Allografting
Larger OCD lesions (> 2 cm2) may be treated with osteochondral allograft transplantation,22 which provides subjective improvement in 75% to 85% of patients and has the longest follow-up in the literature22 (Figures 6-8). In a cohort of 64 patients treated with fresh osteochondral allograft transplantation, 72% had good to excellent clinical outcomes at 7.7 years after surgery.14 Garret20 reported on a series of 17 patients treated with a osteochondral allografts, with 94% clinical success at a mean follow-up of 3 years. McCulloch et al40 studied the clinical outcomes of 25 patients who underwent delayed fresh osteochondral allografting (these grafts are harvested and typically maintained in dimethyl sulfoxide at 4°C, up to 42 days), 6 of whom were diagnosed with OCD. The researchers reported 84% patient satisfaction and 88% radiographic incorporation of delayed fresh allografts to the femoral condyle.
Figure 6.
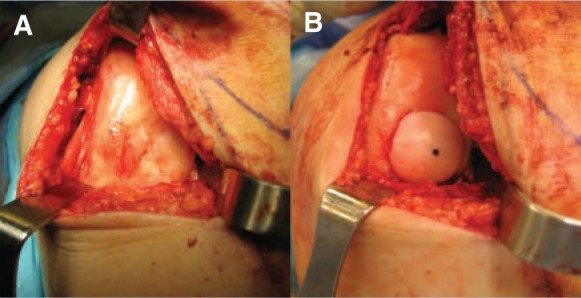
A, intraoperative photo demonstrating a large osteochondritis dissecans lesion located in the medial femoral condyle; B, the lesion was treated with a 20-mm fresh osteochondral allograft.
Figure 8.
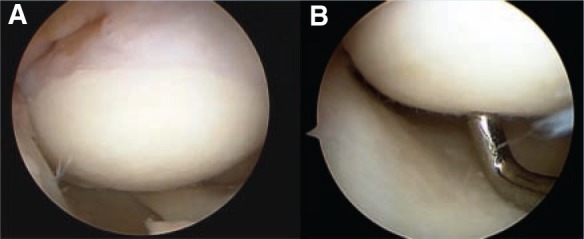
A, intraoperative arthroscopic view, 24 months postoperatively (osteochondral allografting); the graft appeared completely firm with hyaline-like cartilage. B, indentation evident (a stable, firm allograft).
Figure 7.
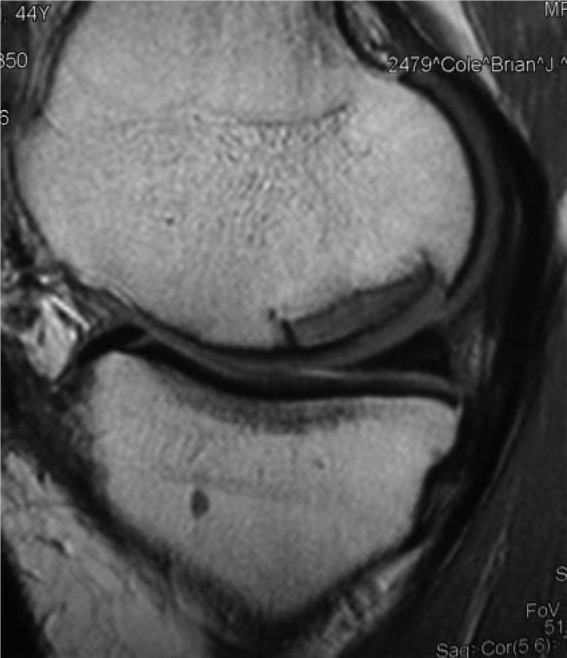
Sagittal T1-weighted image demonstrating good graft incorporation at 6 months postoperatively (fresh osteochondral allograft to femoral condyle).
Osteochondral allograft transplantation can resurface larger and deeper defects with mature hyaline cartilage while addressing the underlying bone deficit. Potential disadvantages include limited graft availability, decreased cell viability, immunogenicity, and disease transmission.
Commercially available instrumentation systems (Arthrex Inc) permit sizing and matching of a cylindrical allograft plug to the defect. It is usually possible to press-fit the graft. If necessary, the allograft can be fixed with bioabsorbable compression screws (Arthrex Inc) or headless differentially pitched titanium screws. Postoperatively, restricted weightbearing is recommended for at least 8 weeks.
Autologous Chondrocyte Implantation
The goal of autologous chondrocyte implantation is to produce a repair tissue that resembles type II hyaline cartilage, thus restoring the durability and natural function of the knee joint. Autologous chondrocyte implantation is ideal for symptomatic, unipolar, well-contained chondral osteochondral defects between 2 and 10 cm2 with bone loss less than 6 to 8 mm deep. Healthy chondrocytes are biopsied from a nonweightbearing region and expanded in vitro over 4 to 6 weeks. Alternatively, cells may be cryopreserved for up to 5 years. At implantation, defect preparation involves debriding the calcified cartilage base and creating vertical walls of healthy cartilage to shoulder the lesion. A periosteal patch from the proximal tibia or a synthetic collagen membrane is attached to the perimeter using interrupted 5-0 or 6-0 Vicryl sutures. The edges of the patch are sealed with fibrin glue, and the cells are injected into the chamber. Postoperatively, nonweightbearing and continuous passive motion is indicated. Defects deeper than 8 to 10 mm can be approached by concomitant or staged bone grafting. Bone grafting should be performed up to the level of the subchondral bone.41 Before bone grafting but following debridement, drilling through the bed allows appropriate blood flow into the defect, thereby ensuring subsequent bone graft incorporation.
Peterson et al48 evaluated 58 patients (JOCD, 35; AOCD, 23; mean age, 26.4) who underwent autologous chondrocyte implantation after a mean follow-up of 2 to 10 years. Integrated nonarticular cartilage repair tissue had formed,28 and successful clinical results were noted in more than 90% of patients. Thirty percent of the 27 patients with preoperative and postoperative radiographs showed joint space narrowing. Results evaluated at a minimum 2-year follow-up are similar to the 76% successful outcomes at 4-year follow-up reported in the literature9 (Figures 9 and 10).
Figure 9.
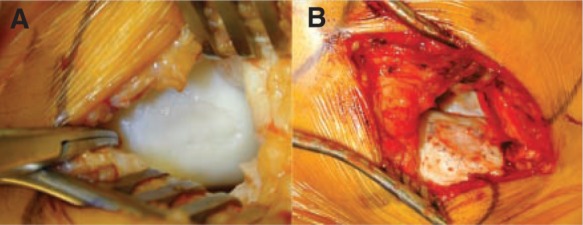
A, intraoperative photo demonstrating a large osteochondritis dissecans lesion located in the medial femoral condyle; exposure was obtained through a medial parapatellar arthrotomy, and the extent of the lesion is determined by direct visualization. B, osteochondritis dissecans lesion following debridement, injection of cultured chondrocytes, and suturing of periosteal graft in place.
Figure 10.
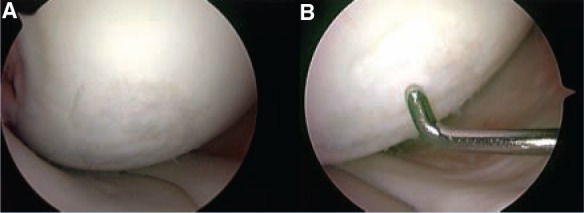
A, intraoperative arthroscopic view, 10 months post–autologous chondrocyte implantation of the previous lesion; B, indentation of a stable graft, with some irregularities of the surface (probably due to short follow-up after the procedure).
Summary
OCD of the knee requires a timely diagnosis to prevent compromise of the articular cartilage and to maximize the opportunity to perform a restorative procedure. Indications for surgical treatment are based on lesion stability, physeal closure, and clinical symptoms. Reestablishment of the joint surface, improvement of the fragment’s blood supply, rigid fixation, and early motion are primary goals for osteochondral fragment preservation. When the fragment is not suitable for preservation, careful consideration of defect location and the patient’s clinical presentation will determine when cartilage restoration procedures should be performed. Successful restorative options should relieve pain, restore function, and prevent the development of secondary osteoarthritis.
Footnotes
No potential conflict of interest declared.
References
- 1. Anderson AF, Pagnani MJ. Osteochondritis dissecans of the femoral condyles: long-term results of excision of the fragment. Am J Sports Med. 1997;25(6):830-834 [DOI] [PubMed] [Google Scholar]
- 2. Anderson AF, Richards DB, Pagnani MJ, Hovis WD. Antegrade drilling for osteochondritis dissecans of the knee. Arthroscopy. 1997;13(3):319-324 [DOI] [PubMed] [Google Scholar]
- 3. Bauer M, Jonsson K, Linden B. Osteochondritis dissecans of the ankle: a 20-year follow-up study. J Bone Joint Surg Br. 1987;69(1):93-96 [DOI] [PubMed] [Google Scholar]
- 4. Berna-Serna JD, Martinez F, Reus M, Berna-Mestre JD. Osteochondritis dissecans of the knee: sonographically guided percutaneous drilling. J Ultrasound Med. 2008;27(2):255-259 [DOI] [PubMed] [Google Scholar]
- 5. Bradley J, Dandy DJ. Osteochondritis dissecans and other lesions of the femoral condyles. J Bone Joint Surg Br. 1989;71(3):518-522 [DOI] [PubMed] [Google Scholar]
- 6. Cahill BR. Osteochondritis dissecans of the knee: treatment of juvenile and adult forms. J Am Acad Orthop Surg. 1995;3(4):237-247 [DOI] [PubMed] [Google Scholar]
- 7. Cahill BR. Current concepts review: osteochondritis dissecans. J Bone Joint Surg Am. 1997;79(3):471-472 [PubMed] [Google Scholar]
- 8. Cain EL, Clancy WG. Treatment algorithm for osteochondral injuries of the knee. Clin Sports Med. 2001;20(2):321-342 [DOI] [PubMed] [Google Scholar]
- 9. Cole BJ, Lee SJ. Complex knee reconstruction: articular cartilage treatment options. Arthroscopy. 2003;19(suppl 1):1-10 [DOI] [PubMed] [Google Scholar]
- 10. Crawfurd EJ, Emery RJ, Aichroth PM. Stable osteochondritis dissecans: does the lesion unite? J Bone Joint Surg Br. 1990;72(2):320. [DOI] [PubMed] [Google Scholar]
- 11. Denoncourt PM, Patel D, Dimakopoulos P. Arthroscopy update #1: treatment of osteochondrosis dissecans of the knee by arthroscopic curettage, follow-up study. Orthop Rev. 1986;15(10):652-657 [PubMed] [Google Scholar]
- 12. Detterline AJ, Goldstein JL, Rue JP, Bach BR., Jr. Evaluation and treatment of osteochondritis dissecans lesions of the knee. J Knee Surg. 2008;21(2):106-115 [DOI] [PubMed] [Google Scholar]
- 13. Dines JS, Fealy S, Potter HG, Warren RF. Outcomes of osteochondral lesions of the knee repaired with a bioabsorbable device. Arthroscopy. 2008;24(1):62-68 [DOI] [PubMed] [Google Scholar]
- 14. Emmerson BC, Gortz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907-914 [DOI] [PubMed] [Google Scholar]
- 15. Ewing JW, Voto SJ. Arthroscopic surgical management of osteochondritis dissecans of the knee. Arthroscopy. 1988;4(1):37-40 [DOI] [PubMed] [Google Scholar]
- 16. Flynn JM, Kocher MS, Ganley TJ. Osteochondritis dissecans of the knee. J Pediatr Orthop. 2004;24(4):434-443 [DOI] [PubMed] [Google Scholar]
- 17. Fowler JL, Wicks MH. Osteochondritis dissecans of the lunate. J Hand Surg [Am]. 1990;15(4):571-572 [DOI] [PubMed] [Google Scholar]
- 18. Friederichs MG, Greis PE, Burks RT. Pitfalls associated with fixation of osteochondritis dissecans fragments using bioabsorbable screws. Arthroscopy. 2001;17(5):542-545 [DOI] [PubMed] [Google Scholar]
- 19. Frisbie DD, Trotter GW, Powers BE, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses. Vet Surg. 1999;28(4):242-255 [DOI] [PubMed] [Google Scholar]
- 20. Garrett JC. Fresh osteochondral allografts for treatment of articular defects in osteochondritis dissecans of the lateral femoral condyle in adults. Clin Orthop Relat Res. 1994(303):33-37 [PubMed] [Google Scholar]
- 21. Gomoll AH, Flik KR, Hayden JK, Cole BJ, Bush-Joseph CA, Bach BR., Jr. Internal fixation of unstable Cahill Type-2C osteochondritis dissecans lesions of the knee in adolescent patients. Orthopedics. 2007;30(6):487-490 [DOI] [PubMed] [Google Scholar]
- 22. Gross AE. Repair of cartilage defects in the knee. J Knee Surg. 2002;15(3):167-169 [PubMed] [Google Scholar]
- 23. Gudas R, Stankevicius E, Monastyreckiene E, Pranys D, Kalesinskas RJ. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):834-842 [DOI] [PubMed] [Google Scholar]
- 24. Guhl JF. Arthroscopic treatment of osteochondritis dissecans. Clin Orthop Relat Res. 1982(167):65-74 [PubMed] [Google Scholar]
- 25. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25-32 [DOI] [PubMed] [Google Scholar]
- 26. Hefti F, Beguiristain J, Krauspe R, et al. Osteochondritis dissecans: a multicenter study of the European Pediatric Orthopedic Society. J Pediatr Orthop B. 1999;8(4):231-245 [PubMed] [Google Scholar]
- 27. Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg Am. 1984;66(9):1340-1348 [PubMed] [Google Scholar]
- 28. Kivistö R, Pasanen L, Leppilahti J, Jalovaara P. Arthroscopic repair of osteochondritis dissecans of the femoral condyles with metal staple fixation: a report of 28 cases. Knee Surg Sports Traumatol Arthrosc. 2002;10(5):305-309 [DOI] [PubMed] [Google Scholar]
- 29. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture: findings at five years. J Bone Joint Surg Am. 2007;89(10):2105-2112 [DOI] [PubMed] [Google Scholar]
- 30. Kocher MS, Micheli LJ, Yaniv M, Zurakowski D, Ames A, Adrignolo AA. Functional and radiographic outcome of juvenile osteochondritis dissecans of the knee treated with transarticular arthroscopic drilling. Am J Sports Med. 2001;29(5):562-566 [DOI] [PubMed] [Google Scholar]
- 31. Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current concepts review. Am J Sports Med. 2006;34(7):1181-1191 [DOI] [PubMed] [Google Scholar]
- 32. Kouzelis A, Plessas S, Papadopoulos AX, Gliatis I, Lambiris E. Herbert screw fixation and reverse guided drillings, for treatment of types III and IV osteochondritis dissecans. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):70-75 [DOI] [PubMed] [Google Scholar]
- 33. Lebolt JR, Wall EJ. Retroarticular drilling and bone grafting of juvenile osteochondritis dissecans of the knee. Arthroscopy. 2007;23(7):794, e791-e794 [DOI] [PubMed] [Google Scholar]
- 34. Lewis PB, McCarty LP, III, Kang RW, Cole BJ. Basic science and treatment options for articular cartilage injuries. J Orthop Sports Phys Ther. 2006;36(10):717-727 [DOI] [PubMed] [Google Scholar]
- 35. Linden B. Osteochondritis dissecans of the femoral condyles: a long-term follow-up study. J Bone Joint Surg Am. 1977;59(6):769-776 [PubMed] [Google Scholar]
- 36. Louisia S, Beaufils P, Katabi M, Robert H. Transchondral drilling for osteochondritis dissecans of the medial condyle of the knee. Knee Surg Sports Traumatol Arthrosc. 2003;11(1):33-39 [DOI] [PubMed] [Google Scholar]
- 37. Makino A, Muscolo DL, Puigdevall M, Costa-Paz M, Ayerza M. Arthroscopic fixation of osteochondritis dissecans of the knee: clinical, magnetic resonance imaging, and arthroscopic follow-up. Am J Sports Med. 2005;33(10):1499-1504 [DOI] [PubMed] [Google Scholar]
- 38. Mandelbaum BR, Browne JE, Fu F, et al. Articular cartilage lesions of the knee. Am J Sports Med. 1998;26(6):853-861 [DOI] [PubMed] [Google Scholar]
- 39. McCarty LP., III Primary repair of osteochondritis dissecans in the knee. In: Cole BJ, Sekiya JK. eds. Surgical Techniques of the Shoulder, Elbow, and Knee in Sports Medicine. Philadelphia, PA: WB Saunders; 2008:chap 53 [Google Scholar]
- 40. McCulloch PC, Kang RW, Sobhy MH, Hayden JK, Cole BJ. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: minimum 2-year follow-up. Am J Sports Med. 2007;35(3):411-420 [DOI] [PubMed] [Google Scholar]
- 41. Minas T, Peterson L. Advanced techniques in autologous chondrocyte transplantation. Clin Sports Med. 1999;18(1):13-44, v-vi [DOI] [PubMed] [Google Scholar]
- 42. Miniaci A, Tytherleigh-Strong G. Fixation of unstable osteochondritis dissecans lesions of the knee using arthroscopic autogenous osteochondral grafting (mosaicplasty). Arthroscopy. 2007;23(8):845-851 [DOI] [PubMed] [Google Scholar]
- 43. Mitsunaga MM, Adishian DA, Bianco AJ., Jr. Osteochondritis dissecans of the capitellum. J Trauma. 1982;22(1):53-55 [DOI] [PubMed] [Google Scholar]
- 44. Morelli M, Poitras P, Grimes V, Backman D, Dervin G. Comparison of the stability of various internal fixators used in the treatment of osteochondritis dissecans: a mechanical model. J Orthop Res. 2007;25(4):495-500 [DOI] [PubMed] [Google Scholar]
- 45. Nakagawa T, Kurosawa H, Ikeda H, Nozawa M, Kawakami A. Internal fixation for osteochondritis dissecans of the knee. Knee Surg Sports Traumatol Arthrosc. 2005;13(4):317-322 [DOI] [PubMed] [Google Scholar]
- 46. Outerbridge HK, Outerbridge AR, Outerbridge RE. The use of a lateral patellar autologous graft for the repair of a large osteochondral defect in the knee. J Bone Joint Surg Am. 1995;77(1):65-72 [DOI] [PubMed] [Google Scholar]
- 47. Pappas AM. Osteochondrosis dissecans. Clin Orthop Relat Res. 1981(158):59-69 [PubMed] [Google Scholar]
- 48. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85(suppl 2):17-24 [DOI] [PubMed] [Google Scholar]
- 49. Scioscia TN, Giffin JR, Allen CR, Harner CD. Potential complication of bioabsorbable screw fixation for osteochondritis dissecans of the knee. Arthroscopy. 2001;17(2):E7. [DOI] [PubMed] [Google Scholar]
- 50. Simonian PT, Sussmann PS, Wickiewicz TL, Paletta GA, Warren RF. Contact pressures at osteochondral donor sites in the knee. Am J Sports Med. 1998;26(4):491-494 [DOI] [PubMed] [Google Scholar]
- 51. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484 [DOI] [PubMed] [Google Scholar]
- 52. Twyman RS, Desai K, Aichroth PM. Osteochondritis dissecans of the knee: a long-term study. J Bone Joint Surg Br. 1991;73(3):461-464 [DOI] [PubMed] [Google Scholar]
- 53. Weckström M, Parviainen M, Kiuru MJ, Mattila VM, Pihlajamaki HK. Comparison of bioabsorbable pins and nails in the fixation of adult osteochondritis dissecans fragments of the knee: an outcome of 30 knees. Am J Sports Med. 2007;35(9):1467-1476 [DOI] [PubMed] [Google Scholar]
- 54. Wouters DB, Bos RR, Mouton LJ, van Horn JR. The meniscus Arrow or metal screw for treatment of osteochondritis dissecans? In vitro comparison of their effectiveness. Knee Surg Sports Traumatol Arthrosc. 2004;12(1):52-57 [DOI] [PubMed] [Google Scholar]
- 55. Yoshizumi Y, Sugita T, Kawamata T, Ohnuma M, Maeda S. Cylindrical osteochondral graft for osteochondritis dissecans of the knee: a report of three cases. Am J Sports Med. 2002;30(3):441-445 [DOI] [PubMed] [Google Scholar]