Abstract
Tendinosis is a troublesome clinical entity affecting many active people. Its treatment remains a challenge to sports medicine clinicians. The etiopathophysiology of tendinosis has not been well delineated. The known pathophysiology and the recent advances in the understanding of the etiologic process of tendinosis are discussed here, including new concepts in mechanotransduction and the biochemical alterations that occur during tendon overload. The optimal, nonoperative treatment of tendinosis is not clear. This article reviews recent evidence of the clinical efficacy of the following interventions: eccentric exercise, extracorporal shock wave treatment, corticosteroid and nonsteroidal anti-inflammatory medications, sclerosing injections, nitric oxide, platelet-rich plasma injections, and matrix metalloproteinase inhibitors. Eccentric exercise has strongest evidence of efficacy. Extracorporal shock wave treatment has mixed evidence and needs further study of energy and application protocols. Sclerosing agents show promising early results but require long-term studies. Corticosteroid and nonsteroidal anti-inflammatory medications have not been shown to be effective, and many basic science studies raise possible concerns with their use. Nitric oxide has been shown in several basic science studies to be promising, but clinical efficacy has not been well established. More clinical trials are needed to assess dosing, indications, and clinical efficacy of nitric oxide. Platelet-rich plasma injections have offered encouraging short-term results. Larger and longer-term clinical trials are needed to assess this promising modality. Matrix metalloproteinase inhibitors have had few clinical studies, and their role in the treatment of tendinosis is still in the early phase of investigation.
Keywords: tendinosis, tendinopathy, nonoperative treatment, pathology
Tendinosis is a problematic condition affecting many active people.5 Significant advances in understanding tendon response to mechanical loading have been made, but a complete picture of the pathophysiology of tendinosis is still elusive. The ability to prevent this condition and to restore normal structure and function after the establishment of a tendinosis lesion continues to be a significant challenge to sports medicine clinicians. This article reviews the pathophysiology of tendinosis and the current status of nonoperative treatments.
Background
Normal Tendon Anatomy and Function
Tendons function to transmit muscular force across joints, resulting in body movement and joint stabilization. Tendons are primarily composed of collagen, proteoglycans, water, and cells.39 The predominant constituent is collagen, which makes the tendon ideally suited to withstand and transfer tensile loads. Ninety-five percent of the collagen content is type I, with the remaining 5% being type III and IV. The predominant cell type is the tenocyte, which synthesizes and supports the tendon matrix.39 Vascularity within the tendon is relatively sparse and corresponds with the low metabolic/turnover rate of these tissues.17
Tendons display viscoelastic mechanical properties that confer time- and rate-dependent effects on the tissue. In particular, tendons are more elastic at low strain rates and stiffer at higher rates of tensile loading. Accordingly, the rate of tissue loading can influence the injury pattern of a tendon. Total tendon strains (percentage deformity) of 1% to 2% result in the straightening of the crimp pattern of unloaded tendon collagen. Strains of 2% to 6% are well tolerated by most healthy tendons. With a strain higher than 6%, incomplete tears start to occur within the tendon. Complete structural failure typically occurs in the range of 8% to 10%.38
The physical junction of tendon and bone is referred to as an enthesis.13 When 2 materials of different moduli of elasticity join, this interface is subject to stress concentration.96 Thus, the soft tissue–bone interface of an enthesis is vulnerable to acute and chronic injury.52 One role of the enthesis is to absorb and distribute this stress concentration over a broader area. The collagen, cartilage, periosteum, and bone tissues that constitute the tendon-bone interface have been collectively referred to as the enthesis organ.13 Overuse tendon injuries can occur in the midsubstance of the tendon but often occur near or at the enthesis. Lesions at or near the enthesis are typically referred to as tendinopathy, which, although appropriate, may be more precisely termed enthesopathy.
As is true of all connective tissue, tendons have a positive adaptive response to repeated physiologic mechanical loading. This has been best studied in bone, where Wolff’s law has been well established and widely accepted. In simple terms, this law states that healthy bone will respond to stress by becoming stronger. The reverse is also true, for bones subject to a decreased load.95 Tendons respond to cyclic physiologic tensile loads with biologic and mechanical changes. For example, when exposed to normal exercise, tenocytes produce several growth factors, such as transforming growth factor–beta 1 (TGF-β1) and insulin-like growth factor 1 (IGF-1), which promotes collagen synthesis and tenocyte replication.30 The resulting tendon changes produce increased tendon fiber diameter and greater mechanical strength.
Tendon Injury and Terminology
Tendon injury occurs through acute and chronic mechanisms. Acute injuries that disrupt vascular tissues within the tendon result in a well-studied healing process involving 3 overlapping phases: inflammation, repair, and remodeling.47 The first phase, inflammation, occurs as a hematoma forms with erythrocytes and activated platelets. This is followed by the infiltration of inflammatory cells, including neutrophils, monocytes, and macrophages that migrate to the injury site to remove debris. A short time later, chemotactic signals induce fibroblasts to start synthesizing collagen. The second phase, repair, is highly vascular and cellular and so involves the deposition of collagen and tendon matrix components.47 During the final phase, remodeling, the vascularity and cellularity of the injury site decrease, and the collagen becomes more structured and organized. The injured site never achieves the original histologic or mechanical features of a healthy uninjured tendon.47
Chronic or overuse injury of the tendon can be separated into superficial and intratendon pathologies. Paratenonitis is an inflammation of the outermost layer of the tendon, and it may be accompanied by synovitis of the tendon sheath, if present. Tendinosis, however, is an intratendinous degenerative lesion without an inflammatory component. Although these two pathologic processes can occur together, they are generally regarded as distinct independent conditions.
Terminology regarding tendon pathology has been somewhat confusing. The term tendonitis continues to be used clinically to describe any painful condition of the tendon. But accurate histologic and pathophysiologic terminology is needed. A generally accepted definition of terms has evolved. Table 1 summarizes Bonar’s modification of Clancy’s classification of tendinopathies.42 Tendinopathy is a broad, overarching term referring to any abnormal condition of the tendon. Tendinitis implies inflammation, and the term is now used to refer to situations where an incomplete structural disruption of the tendon has occurred, which results in vascular damage, bleeding, and the ensuing inflammation phase of healing. Paratenonitis refers to inflammation of the most peripheral layer of the tendon and, should the tendon be enclosed in a sheath, may include synovitis. Tendinosis refers to the intratendinous degeneration that is thought to be a result of chronic overuse and that does not have a significant inflammatory component. Both tendinitis and paratenonitis can develop within a short time frame. Tendinitis can result from a single traumatic episode, whereas tendinosis requires a more prolonged time frame to develop.5,39,43,47
Table 1.
Terminology of tendon pathologies.
| Pathology | Time of Onset | |
|---|---|---|
| Tendinopathy | Any abnormal condition of tendon | N/A |
| Tendinitis | Inflammation in partially torn tendon | Short |
| Paratenonitis | Inflammation of superficial structures of tendon | Short |
| Tendinosis | Intratendinous degeneration | Long |
Clinical Presentation
As applied today, the term tendinosis was first described by Puddu et al in 1976, who noted that such degeneration was troublesome in athletes.71 The lesion is typically well localized and tender to palpation. Any significant loading of the affected tendon causes pain. When the lesion is well established, the clinical problem is rather persistent and recalcitrant to traditional overuse interventions. Tendinosis occurs most commonly in the Achilles, patellar, supraspinatus, and lateral elbow common extensor tendons. Tendinosis appears to be the result of repeated heavy loads applied to a tendon, as exemplified by Achilles tendon lesions occurring in runners, patellar tendon lesions occurring in jumpers, and lateral epicondylar lesions occurring in patients who repeatedly and forcefully grasp an object. Clinical evaluation usually reveals that the patient has had some symptoms for a significant period of time before seeking medical attention. A clinical grading system for tendinopathy was first described by Blazina et al in 1973. Since then, several variations have been used.14 A grade 1 condition exists if there is no significant pain during athletic activity but the athlete has discomfort afterward. A grade 2 condition exists when the athlete has pain before and after activity but not enough during activity to alter his or her performance or schedule of activity. A grade 3 condition exists when the pain is severe enough that performance is affected and the athlete’s volume of activity has to be modified because of the painful condition.
Histology
The normal tendon has a characteristic histologic appearance. Collagen fibers are arrayed in an organized longitudinal parallel pattern with a periodic, slight waving pattern (Figure 1). These fibers are tightly packed with no other obvious ground substance.39,41,42,47 The tenocytes are long spindle-shaped cells and not readily apparent. Fibroblasts and myofibroblasts are not present, and vascularity is sparse within the collagen fibers. Superficial to the packed collagen fibers is the epitenon, which contains vascular, lymphatic, and neurologic supply to the tendon. Enveloping the entire tendon is the paratenon, which is loose areolar connective tissue with a thin layer of synovial cells.42
Figure 1.
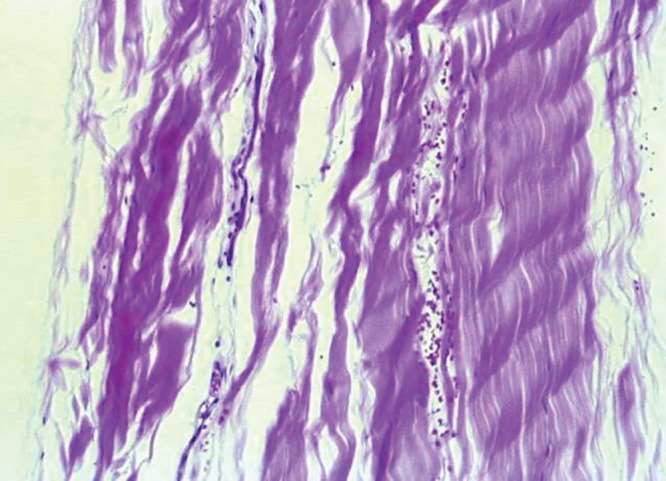
Histologic appearance of normal tendon with H&E staining.
Histologic evaluation of tendinosis lesions reveals striking changes to the above-described normal tendon structure (Figure 2).5 The collagen fibers are not tightly packed nor well structured in parallel alignment. The fibers are widely spread with mucoid ground substance interspersed between them, and they present a disorganized pattern.39,41,42,47 The tenocytes are more apparent and abnormal in shape.42 They lose their spindle shape and become more round and chondrocyte-like, amounting to a fibrocartilaginous metaplasia.41 There is increased cellularity, with the predominant cell population being mesenchymal-derived cells, such as fibroblasts, chondrocytes, endothelial cells, osteocytes, and lipocytes.47 Higher levels of apoptosis, or programmed cell death, have been demonstrated in the cells of overloaded supraspinatus and patella tendons.50,59,97 Neovascularization is obvious, resulting in a marked increase in vascularity within the lesion.41,42,47 In 1979, Nirschl coined the term angiofibroblastic hyperplasia when describing tendinosis of the lateral elbow.47 Studies of the histopathology of tendinosis confirm the lack of inflammatory cells.97 Accordingly, terms such as lateral epicondylitis and patellar tendonitis are not accurate nomenclature to describe these conditions.43
Figure 2.
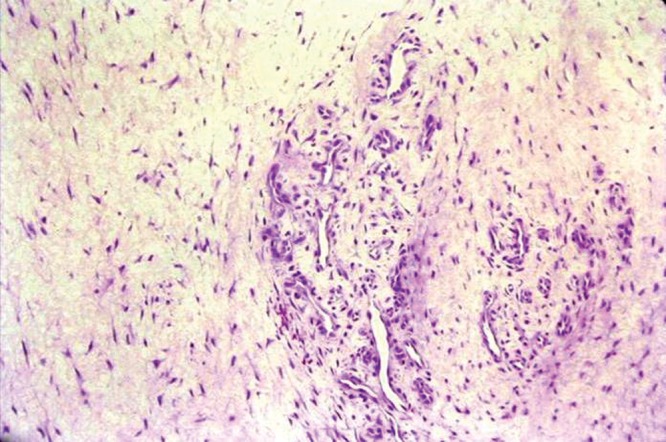
Histologic appearance of the disorganized structure of a tendinosis lesion.
The pattern of collagen disruption, mucoid ground substance, hypercellularity, hypervascularity, and lack of inflammatory cells summarizes what has been found consistently in histologic evaluation of lesions in the Achilles, patella, supraspinatus, and elbow tendons.5,39,41,42,47 Metaplastic changes resulting in chondroid, lipid, and bone deposition within the lesion have also been described.5,39,41,42,47 MRI findings of increased signal and ultrasonic findings of a hypoechoic area correlate with the histologic findings described above.26,69,98
Movin and Bonar scores are two grading systems that can be used to quantify the histologic changes associated with tendinosis.51 Maffulli et al showed that there is a high correlation between these 2 tools.51 The use of histologic grading will advance our ability to evaluate treatment interventions beyond clinical pain relief.
Pathophysiology
Despite recent advances, the pathophysiologic trigger and process resulting in clinically significant tendinosis are poorly understood. Tendinosis has traditionally been thought to be the result of a failed healing response of the tendon to microdamage from chronic overuse.97 Some would use the terms failed reparative response or failed adaptive response because normal tendon turnover and remodeling does not involve the inflammation implied in the term healing. Tender or painful connective tissue is often assumed to be inflamed, but this is not the case in tendinosis lesions.43 The pain from these lesions may be due to local noxious stimuli and/or enhanced nocicep-tive fibers.3
It is not clear whether the initial event in the pathologic cascade occurs in the collagen matrix or the tenocyte.5,39 Overload of the tendon may result in collagen fiber disruption that may either trigger mechanoreceptors or directly damage the tenocytes causing them to replicate and differentiate into fibrocytes, endothelial cells, and other mesenchymal-derived cells.19 These cells then produce the hypercellularity, hypervascularity, collagen disorganization, and abnormal ground substance deposition seen in tendinosis lesions.76
To prevent and treat tendinosis, a better understanding of its pathogenesis is needed. Recent in vivo investigations of human tendons offer promise.10,12,47 Alfredson et al have used in situ microdialysis to study the presence of cell signal molecules in human Achilles tendons.1 They found no difference in prostaglandin E2 levels between normal and tendinosis lesions and concluded that chemical inflammation is not a significant contributor to the tendinosis process. They also noted increased levels of glutamate in the tendinosis lesions. Glutamate is a known excitatory neurotransmitter, and it has been shown to be a mediator of pain in the central nervous system.4 In subsequent studies, the researchers demonstrated the presence of N-metyol-D-aspartate in Achilles tendons. N-metyol-D-aspartate glutamate receptors are associated with nerve endings.2 The neuropeptides substance P and calcitonin gene-related peptide have also been identified in tendinosis lesions.24 This finding adds credence to the notion that neurogenic inflammation may be the cause of pain in tendinosis lesions.2
Higher levels of lactate have also been observed in tendinosis lesions, suggesting to some that anaerobic metabolism may be either a cause or a response to tendinosis.1 This finding may also relate to the increased apoptosis seen in tendinosis lesions.
Messner et al showed in a rat model that with tendon overload, the first histologic finding before collagen disruption was vascular and nerve filament ingrowth into the tendons.58 They also identified the presence of the neuropeptides substance P and calcitonin gene-related peptide in the overloaded tendons. Schubert et al found the sprouting of substance P positive nerve fibers in tendinosis lesions.82 Substance P has been associated with pain and nociceptive nerve endings. This finding adds evidence to the notion that the pain in tendinosis lesions is neurogenic rather than inflammatory.2 Several recent studies have well documented the hypervascularity of tendinosis lesions in the Achilles and patellar tendons.24 A correlation between the areas of maximal pain and maximal hypervascularity has also been established.2,20,35,36,41 This abnormal vascularity is not found in the asymptomatic contralateral limb.46 The presence of perivascular innervation in tendinosis lesions is being further studied.2,20 Danielson et al have shown that these innervations have a large sympathetic component.23,25
Scott et al recently showed that the density of mast cells in tendinosis lesions was 3 times that found in normal tendons.83 The density of mast cells correlated with the duration of symptoms and the amount of vascular hyperplasia present. These mast cells were positive for tryptase, a potent angiogenic factor.83 Mast cells are also known to be capable of producing a potent neurotrophin, or nerve growth factor.34 These finding support the findings of early neovascularization and neuro sprouting in tendinosis lesions.2,55
A glimpse into why established tendinosis lesions are so recalcitrant to treatment may be gleaned from the work by Rolf et al.76 Using immunohistochemistry, in situ zymography, and cell culture techniques, they showed that tendinosis cells were metabolically hyperactive and that their degenerative behavior persisted for many generations, even when removed from the tendinosis environment. It appears that once a normal spindle-shaped tenocyte differentiates into the more round cells found in tendinosis lesions, the process becomes difficult reverse.76
Clearly, tenocytes respond to mechanical stress stimuli. In fact, intermittent loading of tenocytes appears to be required to maintain tenocyte health.49 How fluid flow, strain, and shear are detected and transduced by cell membrane or cytoskeleton structures is unclear. Studies have demonstrated that one of the earliest mechanotransduction events in tenocytes is ion channel activity, specifically involving calcium.54,94 In the future, cellular mechanotransduction processes and intercellular communication mechanisms will be elucidated to expand not only our understanding of, but also the prevention and treatment of, tendinosis lesions.8
In efforts to better understand the response of tenocytes to mechanical loads, Arnoczky studied a rat tail tenocyte model.10,12 The tendon cell response to mechanical loading is dependent on both the frequency and the magnitude of the applied loads.10,12 Tenocytes appear to be programmed to sense a certain level of stress, and if they are not subjected to this level of stress, significant alterations occur in the tenocyte’s production of matrix metalloproteinases and tissue inhibitor of metalloproteinases.12 Thus, Arnoczky has postulated that the degenerative nature of tendinosis may be the result of the underloading of tenocytes as attributed to abnormal cell-matrix interaction, as opposed to the direct overload of the tenocyte.10-12 In an in vitro study, stress deprivation resulted in increased apoptosis in tendon cells.28 This concept is supported by work by Thornton et al and Gardner et al, who showed that stress deprivation of the tenocyte negatively affected the expression of matrix metalloproteinase and tissue inhibitor of metalloproteinase and, as such, may be a contributor to the development of tendinosis.32,88 Orchard et al have proposed the concept of stress shielding as a contributing factor in the development of tendinopathies.66
Tendinosis tends to occur in predictable and reproducible locations—for example, the patellar tendon, where the lesion almost invariably occurs in the posterior aspect of the proximal tendon. Both Lavagnino et al (in a computational model) and Dillon et al (in an in vivo study) have shown this to be the location of greatest tensile loading, hence contradicting the stress deprivation theory proposed above.27,48 An explanation may be that repeated high-stress episodes result in a disturbance of the normal tenocyte-matrix interaction, thus uncoupling the tenocyte from the mechanical signal of tendon loading.10,12 Once uncoupled, the tenocyte is no longer effective at supporting healthy and repairing damaged tendon matrix.10,12
An integrative model for tendinosis that incorporates the above observations has not been established. More research is needed into the interplay between mechanotransduction and local micro-environment factors such as hypoxia and the resulting failed healing response with the production of nociceptive signals, neovascularization, neuro sprouting, as well as the cell signaling that results in the differentiation of cells into nontenocyte mesenchymal cells. As these complex processes are better elucidated, new preventative and treatment options for tendinosis will become apparent.
Nonoperative Treatment
Because knowledge of the etiology, pathologic cascade, and healing mechanisms of tendinosis is still incomplete, it is difficult to know the correct therapeutic intervention to recommend.7 As such, multiple approaches have been advocated, most with poor or only empirical evidence to support their use. Management of patients with tendinosis still revolves around the modulation of pain, despite the fact that the origin of this pain within the body of the tendon or the enthesis is presently unknown.
Although tendinosis has been poorly studied in a controlled fashion, clinicians widely accept that rest is clinically effective in early tendon overuse conditions, as is decreasing the magnitude and frequency of the loading episodes.42 Jelinsky et al demonstrated in a rat model that gene expression in the overloaded supraspinatus tendon was reversed with as little as 2 weeks of rest.37 Also widely accepted is the fact that rest is strikingly less effective in the treatment of established tendinosis lesions.†
Corticosteroid and Nonsteroidal Anti-inflammatory Medications
Both corticosteroid and nonsteroidal anti-inflammatory medications (NSAIDs) may provide pain relief in situations of mild, acute, or recent onset of tendon pain associated with true tendonitis injuries.8 Although steroids have been commonly used in the treatment of tendinosis, their benefit appears to be limited to short-term improvement in pain (ie, < 6 weeks). There is no evidence to suggest long-term improvement,8 nor is there significant evidence indicating that NSAIDs are effective in treating established tendinosis lesions in relieving long-term clinical symptoms or resolving the pathologic lesion.53,56 This lack of efficacy is likely explained by the absence of inflammation as a significant factor in tendinosis pathology. In fact, many basic science studies have documented the potential negative effects of NSAID use on the tendon-healing process.6,18,22,29,75,89-91 Accordingly, many authors suggest caution when prescribing NSAIDs for tendon injuries.
Sclerosing Treatments
Sclerosing treatments aim to address the neovascularization of tendinosis lesions.1,18,29,77 The hypervascular area is typically identified by ultrasound imaging, and a sclerosing agent such as polidocanol or hyperosmolar dextrose is injected into the desired area.57 Several studies have shown this approach to be promising in relieving clinical symptoms.36,65 No study, however, has demonstrated that sclerosing agents are effective in resolving the histopathology of tendinosis. Hoksrud et al demonstrated that, even when effective at reducing pain, the sclerosing treatment did not correlate with reduced vascularity in the long term.35 Work by Cook et al implied that the presence of increased vascular structures seemed to correlate with pain, more so than the actual blood flow through the vessels.20 They postulated the adjoining nerve fibers associated with the neovascularization may explain this finding. The denervation of the lesion may explain why sclerosing agents can so quickly relieve pain in an established tendinosis lesion. With these promising early results, further study needs to be pursued of the long-term affects and results of sclerosing techniques.53,72
Eccentric Exercise
Periodic eccentric exercise has been shown in many studies to be beneficial to patients with tendinosis lesions.3,64,85 Eccentric overload exercises were initially used to treat Achilles tendon lesions, but they have now been extended to other affected tendons. Nakamura et al demonstrated that eccentric exercises contributed to stable angiogenesis in early tendon injury whereas concentric exercises did not.64 Knobloch et al showed in a controlled study that in tendinosis lesions an eccentric loading program resulted in a significant decrease in the paratenon vascularity and pain while not changing the oxygen saturation of the paratenon tissues.44-46 Daily eccentric exercises were clinically beneficial and not harmful to tendon microcirculation.64 Shalabi et al demonstrated decreased tendon volume, decreased MRI signal in the tendinosis lesions, and improved clinical pain scores in patients with Achilles tendinosis who were treated with eccentric training regimens.84
In contrast to the findings of studies of eccentric exercises, concentric muscle activity does not appear to be beneficial.33 In a study comparing eccentric and concentric exercises, Rees et al found that there was no difference between the 2 exercises with respect to peak tendon force and tendon length change.74 They did find that the tendons that were subjected to eccentric loading all had high-frequency oscillations in tendon loads, whereas none of the concentric group exhibited these oscillations. The researchers speculate that this oscillation pattern may explain the different physiologic effects and clinical efficacies of the 2 exercise types. Stergioulas et al showed a clinical benefit to adding low-level laser therapy to an eccentric exercise program but that such therapy alone was not effective.86 A recent systematic review of nonoperative treatments of midportion Achilles tendinopathy concluded that eccentric exercise had the most evidence of efficacy.53 The optimal pattern and schedule of eccentric overloading programs have not yet been identified.8
Extracorporeal Shock Wave Treatment
Extracorporeal shock wave treatment (ESWT) has been used to treat tendinosis lesions.31,73,93 Chen et al demonstrated in a rat model that ESWT promoted tendon healing in a collagenase-induced tendinopathy and that it induced TGF-β1 and IGF-1 expression.16
Rompe et al showed in a randomized controlled trial that eccentric exercise, combined with ESWT, was more effective than eccentric exercise alone.80 In another randomized controlled trial comparing ESWT with eccentric exercises, Rompe et al showed ESWT to be more efficacious at 4 months.77 And in yet another randomized trial, Rompe et al found ESWT and eccentric exercises to be equally effective and better than controls.79 In a randomized placebo-controlled trial, Costa et al found no efficacy of ESWT.21 In a recent review of the literature regarding ESWT for patellar tendinopathy, van Leeuwen et al concluded that ESWT appeared to be a safe and promising treatment, with a positive effect on pain and function, but that the current evidence did not allow for a specific treatment protocol.92 In another review of the literature, Rompe and Maffulli found evidence in 10 randomized controlled trials that ESWT was effective for tennis elbow under well-defined and restrictive conditions.78 The ideal energy level and application schedule are not well defined for ESWT and so may explain the varied reports of efficacy.53
Platelet-Rich Plasma
Platelet-rich plasma (PRP) appears to be a promising intervention for tendinosis lesions. Schnabel et al showed that tendons cultured in PRP showed enhanced gene expression of anabolic agents without a corresponding increase in catabolic molecules.81 Arguelles et al showed a positive long-term result in 5 horses treated with PRP injections.9 Mishra and Pavelko showed significant benefit to PRP injections in a small pilot study of patients with chronic tennis elbow who had failed traditional measures and were considering surgery.60 Although theoretically attractive with promising early clinical results, a better understanding is needed of the soup of growth factors released in PRP. The clinical application of PRP to promote healing and adaptive responses clearly needs further basic science and controlled trials to ascertain its indications and efficacy.72
Nitric Oxide
Nitric oxide is an important cell signal molecule, and it appears to be involved in numerous tissue types’ response to mechanical loading. Nitric oxide is also involved in modulating tendon healing and collagen synthesis.61 Several basic science studies are encouraging regarding nitric oxide’s potential role in enhancing tendon healing. Szomor et al demonstrated that nitric oxide synthases are upregulated in tendon overuse.61,87,96 Molloy et al showed that nitric oxide influences tenocyte gene expression during tendon healing.61 Xia and colleagues’ study demonstrated that exogenous nitric oxide can enhance collagen synthesis in cultured tenocytes.96 Murrell also demonstrated that nitric oxide enhances tendon healing and that systematic inhibition of nitric oxide synthesis resulted in a smaller cross-sectional area of, and the lower mechanical strength of, healing Achilles tendons in rats.62 Topical nitrate has been reported to be clinically effective in the treatment of chronic tendinopathies62,68,69; however, a recent randomized controlled clinical trial by Kane et al failed to show clinical benefit to topical glyceryl trinitrate patches in noninsertional Achilles tendinopathy.40 More clinical trials are needed to assess dosing, indications, and efficacy of nitric oxide as a therapeutic intervention for tendinosis.8,53
Matrix Metalloproteinase
Matrix metalloproteinase inhibitors are another recent intervention for tendinosis. Matrix metalloproteinases are endopeptidases involved in the normal remodeling of the extracellular matrix of connective tissues. An increase in their activity in causing degradation of the extracellular matrix is thought to contribute to the development of tendinosis.11 Matrix metalloproteinase inhibitors aim to decrease the catabolic enzymatic activity in the lesions.11 Although some initial success has been reported, clinical efficacy has not been well established at this time.15,67
Conclusion
Recent advances in our understanding of the pathophysiology of tendinosis are promising. As we further define the processes of mechanotransduction and biochemical signaling of tendon overload, our efforts to prevent and treat tendinosis lesions should be greatly advanced. With respect to evidence of clinical efficacy, eccentric exercises have the strongest level of evidence. ESWT, PRP, sclerosing injections, and nitric oxide show early promise but require further clinical studies. Matrix metalloproteinase inhibitors are in the early phase of clinical study. Corticosteroids and NSAIDs have not been shown to be effective in treating tendinosis.
Footnotes
References
- 1. Alfredson H, Bjur D, Thorsen K, et al. High intratendinous lactate levels in painful chronic Achilles tendinosis: an investigation using microdialysis technique. J Orthop Res. 2002;20:934-938 [DOI] [PubMed] [Google Scholar]
- 2. Alfredson H, Ohberg L, Forsgren S. Is vasculo-neural ingrowth the cause of pain in chronic Achilles tendinosis? An investigation using ultrasonography and colour Doppler, immunohistochemistry, and diagnostic injections. Knee Surg Sports Traumatol Arthrosc. 2003;11:334-338 [DOI] [PubMed] [Google Scholar]
- 3. Alfredson H, Pietila T, Jonsson P, et al. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26:360-366 [DOI] [PubMed] [Google Scholar]
- 4. Alfredson H, Thorsen K, Lorentson R. In situ microdialysis in tendon tissue: high levels of glutamate, but not prostaglandin E2 in chronic Achilles tendon pain. Knee Surg Sports Traumatol Arthrosc. 1999;7:378-381 [DOI] [PubMed] [Google Scholar]
- 5. Almekinders LC. Tendinitis and other chronic tendinopathies. J Am Acad Orthop Surg. 1998;6:157-164 [DOI] [PubMed] [Google Scholar]
- 6. Almekinders LC, Deol G. The effects of aging, antiinflammatory drugs, and ultrasound on the in vitro response of tendon tissue. Am J Sports Med. 1999;27:417-421 [DOI] [PubMed] [Google Scholar]
- 7. Andres BM, Murrell GA. Molecular and clinical developments in tendinopathy: editorial comment. Clin Orthop Rel Res. 2008;466:1519-1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Rel Res. 2008;466:1539-1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arguelles D, Carmona JU, Climent F, et al. Autologous platelet concentrates as a treatment for musculoskeletal lesions in five horses. Vet Rec. 2008;162:208-211 [DOI] [PubMed] [Google Scholar]
- 10. Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int J Exp Pathol. 2007;88:217-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnoczky SP, Lavagnino M, Egerbacher M, et al. Matrix metalloproteinase inhibitors prevent a decrease in the mechanical properties of stress-deprived tendons: an in vitro experimental study. Am J Sports Med. 2007;35:763-769 [DOI] [PubMed] [Google Scholar]
- 12. Arnoczky SP, Lavagnino M, Egerbacher M, et al. Loss of homeostatic strain alters mechanostat “set point” of tendon cells in vitro. Clin Orthop Relat Res. 2008;466:1583-1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benjamin M, Toumi H, Ralphs JR, et al. Where tendons and ligaments meet bone: attachment sites (“entheses”) in relation to exercise and/or mechanical load. J Anat. 2006;208:471-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blazina ME, Kerlan RK, Jobe FW, et al. Jumper’s knee. Orthop Clin North Am. 1973;4:665-678 [PubMed] [Google Scholar]
- 15. Brown R, Orchard J, Kinchington M, et al. Aprotinin in the management of Achilles tendinopathy: a randomised controlled trial. Br J Sports Med. 2006;40:275-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen YJ, Wang CJ, Yang KD, et al. Extracorporeal shock waves promote healing of collagenase-induced Achilles tendinitis and increase TGF-beta1 and IGF-I expression. J Orthop Res. 2004;22:854-861 [DOI] [PubMed] [Google Scholar]
- 17. Clancy WGJ. Tendon trauma and overuse injuries. In: Leadbetter WB, Buckwalter JA, Gordon SL, eds. Sports-Induced Inflammation: Clinical and Basic Science Concepts. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1990:609-618 [Google Scholar]
- 18. Cohen DB, Kawamura S, Ehteshami JR, et al. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34:362-369 [DOI] [PubMed] [Google Scholar]
- 19. Cook JL, Feller JA, Bonar SF, et al. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes’ patellar tendons. J Orthop Res. 2004;22:334-338 [DOI] [PubMed] [Google Scholar]
- 20. Cook JL, Malliaras P, De Luca J, et al. Vascularity and pain in the patellar tendon of adult jumping athletes: a 5 month longitudinal study. Br J Sports Med. 2005;39:458-461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Costa ML, Shepstone L, Donell ST, et al. Shock wave therapy for chronic Achilles tendon pain: a randomized placebo-controlled trial. Clin Orthop Rel Res. 2005;440:199-204 [DOI] [PubMed] [Google Scholar]
- 22. Dahners LE, Mullis BH. Effects of nonsteroidal anti-inflammatory drugs on bone formation and soft-tissue healing. J Am Acad Orthop Surg. 2004;12:139-143 [DOI] [PubMed] [Google Scholar]
- 23. Danielson P, Alfredson H, Forsgren S. Distribution of general (PGP 9.5) and sensory (substance P/CGRP) innervations in the human patellar tendon. Knee Surg Sports Traumatol Arthrosc. 2006;14:125-132 [DOI] [PubMed] [Google Scholar]
- 24. Danielson P, Alfredson H, Forsgren S. Studies on the importance of sympathetic innervation, adrenergic receptors, and a possible local catecholamine production in the development of patellar tendinopathy (tendinosis) in man. Microsc Res Tech. 2007;70:310-324 [DOI] [PubMed] [Google Scholar]
- 25. Danielson P, Andersson G, Alfredson H, et al. Marked sympathetic component in the perivascular innervation of the dorsal paratendinous tissue of the patellar tendon in arthroscopically treated tendinosis patients. Knee Surg Sports Traumatol Arthrosc. 2008;16:621-626 [DOI] [PubMed] [Google Scholar]
- 26. Davies SG, Baudouin CJ, King JB, et al. Ultrasound, computed tomography and magnetic resonance imaging in patellar tendinitis. Clin Radiol. 1991;43:52-56 [DOI] [PubMed] [Google Scholar]
- 27. Dillon EM, Erasmus PJ, Muller JH, et al. Differential forces within the proximal patellar tendon as an explanation for the characteristic lesion of patellar tendinopathy: an in vivo descriptive experimental study. Am J Sports Med. 2008;36:2119-2127 [DOI] [PubMed] [Google Scholar]
- 28. Egerbacher M, Arnoczky SP, Caballero O, et al. Loss of homeostatic tension induces apoptosis in tendon cells: an in vitro study. Clin Orthop Rel Res. 2008;466:1562-1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferry ST, Dahners LE, Afshari HM, et al. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am J Sports Med. 2007;35:1326-1333 [DOI] [PubMed] [Google Scholar]
- 30. Fu SC, Wang W, Pau HM, et al. Increased expression of transforming growth factor-beta1 in patellar tendinosis. Clin Orthop Rel Res. 2002;400:174-183 [DOI] [PubMed] [Google Scholar]
- 31. Furia JP. High-energy extracorporeal shock wave therapy as a treatment for chronic noninsertional Achilles tendinopathy. Am J Sports Med. 2008;36:502-508 [DOI] [PubMed] [Google Scholar]
- 32. Gardner K, Arnoczky SP, Caballero O, et al. The effect of stress-deprivation and cyclic loading on the TIMP/MMP ratio in tendon cells: an in vitro experimental study. Disabil Rehabil. 2008;30:1523-1529 [DOI] [PubMed] [Google Scholar]
- 33. Grigg NL, Wearing SC, Smeathers JE. Eccentric calf muscle exercise produces a greater acute reduction in Achilles tendon thickness than concentric exercise [published online ahead of print November 19, 2008]. Br J Sports Med. [DOI] [PubMed] [Google Scholar]
- 34. Hart DA, Frank CB, Kydd A, Ivie T, Sciore P, Reno C. Neurogenic mast cell and gender variables in tendon biology: potential role in chronic tendinopathy. In: Maffulli N, Renstrom P, Leadbetter WB. (eds). Tendon Injuries: Basic Science and Clinical Medicine. London, UK: Springer; 2005:40-48 [Google Scholar]
- 35. Hoksrud A, Ohberg L, Alfredson H, et al. Color Doppler ultrasound findings in patellar tendinopathy (jumper’s knee). Am J Sports Med. 2008;36:1813-1820 [DOI] [PubMed] [Google Scholar]
- 36. Hoksrud A, Ohberg L, Alfredson H, et al. Ultrasound-guided sclerosis of neovessels in painful chronic patellar tendinopathy: a randomized controlled trial. Am J Sports Med. 2006;34:1738-1746 [DOI] [PubMed] [Google Scholar]
- 37. Jelinsky SA, Lake SP, Archambault JM, et al. Gene expression in rat supraspinatus tendon recovers from overuse with rest. Clin Orthop Rel Res. 2008;466:1612-1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jozsa L, Kannus P. Functional and mechanical behavior of tendons. In: Human Tendons. Champaign, IL: Human Kinetics; 1997:98-102 [Google Scholar]
- 39. Jozsa L, Kannus P. Overuse injuries of tendons. In: Human Tendons: Anatomy, Physiology, and Pathology. Champaign, IL: Human Kinetics; 1997:164-253 [Google Scholar]
- 40. Kane TP, Ismail M, Calder JD. Topical glyceryl trinitrate and noninsertional Achilles tendinopathy: a clinical and cellular investigation. Am J Sports Med. 2008;36:1160-1163 [DOI] [PubMed] [Google Scholar]
- 41. Khan KM, Bonar F, Desmond PM, et al. : Victorian Institute of Sport Tendon Study Group Patellar tendinosis (jumper’s knee): findings at histopathologic examination, US, and MR imaging. Radiology. 1996;200:821-827 [DOI] [PubMed] [Google Scholar]
- 42. Khan KM, Cook JL, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408 [DOI] [PubMed] [Google Scholar]
- 43. Khan KM, Cook JL, Kannus P, et al. Time to abandon the “tendinitis” myth. Br Med J. 2002;324:626-627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knobloch K. Eccentric training in Achilles tendinopathy: is it harmful to tendon microcirculation? Br J Sports Med. 2007;41:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Knobloch K, Kraemer R, Jagodzinski M, et al. Eccentric training decreases paratendon capillary blood flow and preserves paratendon oxygen saturation in chronic achilles tendinopathy. J Orthop Sports Phys Ther. 2007;37:269-276 [DOI] [PubMed] [Google Scholar]
- 46. Knobloch K, Kraemer R, Lichtenberg A, et al. Achilles tendon and paratendon microcirculation in midportion and insertional tendinopathy in athletes. Am J Sports Med. 2006;34:92-97 [DOI] [PubMed] [Google Scholar]
- 47. Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow): clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999;81:259-278 [PubMed] [Google Scholar]
- 48. Lavagnino M, Arnoczky SP, Elvin N, et al. Patellar tendon strain is increased at the site of the jumper’s knee lesion during knee flexion and tendon loading: results and cadaveric testing of a computational model. Am J Sports Med. 2008;36:2110-2118 [DOI] [PubMed] [Google Scholar]
- 49. Lavagnino M, Arnoczky SP, Kepich E, et al. A finite element model predicts the mechanotransduction response of tendon cells to cyclic tensile loading. Biomech Model Mechanobiol. 2008;7:405-416 [DOI] [PubMed] [Google Scholar]
- 50. Lian O, Scott A, Engebretsen L, et al. Excessive apoptosis in patellar tendinopathy in athletes. Am J Sports Med. 2007;35:605-611 [DOI] [PubMed] [Google Scholar]
- 51. Maffulli N, Longo UG, Franceschi F, et al. Movin and Bonar scores assess the same characteristics of tendon histology. Clin Orthop Rel Res. 2008;466:1605-1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries: ideas on insertional tendinopathy. Sports Med. 2004;34:1005-1017 [DOI] [PubMed] [Google Scholar]
- 53. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64 [DOI] [PubMed] [Google Scholar]
- 54. Magra M, Hughes S, El Haj AJ, et al. VOCCs and TREK-1 ion channel expression in human tenocytes. Am J Physiol Cell Physiol. 2007;292:1053-1060 [DOI] [PubMed] [Google Scholar]
- 55. Marinova T, Philipov S, Aloe L. Nerve growth factor immunoreactivity of mast cells in acute involuted human thymus. Inflammation. 2007;30:38-43 [DOI] [PubMed] [Google Scholar]
- 56. Marsolais D, Cote CH, Frenette J. Nonsteroidal anti-inflammatory drug reduces neutrophil and macrophage accumulation but does not improve tendon regeneration. Lab Invest. 2003;83:991-999 [DOI] [PubMed] [Google Scholar]
- 57. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. AJR Am J Roentgenol. 2007;189:W215-W220 [DOI] [PubMed] [Google Scholar]
- 58. Messner K, Wei Y, Andersson B, Gillquist J, Rasanen R. Rat model of Achilles tendon disorder: a pilot study. Cells Tissues Organs. 1999;165:30-39 [DOI] [PubMed] [Google Scholar]
- 59. Millar NL, Wei AQ, Molloy TJ, et al. Heat shock protein and apoptosis in supraspinatus tendinopathy. Clin Orthop Rel Res. 2008;466:1569-1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34:1774-1778 [DOI] [PubMed] [Google Scholar]
- 61. Molloy TJ, de Bock CE, Wang Y, et al. Gene expression changes in SNAP-stimulated and iNOS-transfected tenocytes: expression of extracellular matrix genes and its implications for tendon-healing. J Orthop Res. 2006;24:1869-1882 [DOI] [PubMed] [Google Scholar]
- 62. Murrell GA. Oxygen free radicals and tendon healing. J Shoulder Elbow Surg. 2007;16:S208-S214 [DOI] [PubMed] [Google Scholar]
- 63. Murrell GA, Tang G, Appleyard RC, et al. Addition of nitric oxide through nitric oxide-paracetamol enhances healing rat achilles tendon. Clin Orthop Rel Res. 2008;466:1618-1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nakamura K, Kitaoka K, Tomita K. Effect of eccentric exercise on the healing process of injured patellar tendon in rats. J Orthop Sci. 2008;13:371-378 [DOI] [PubMed] [Google Scholar]
- 65. Ohberg L, Alfredson H. Sclerosing therapy in chronic Achilles tendon insertional pain-results of a pilot study. Knee Surg Sports Traumatol Arthrosc. 2003;11:339-343 [DOI] [PubMed] [Google Scholar]
- 66. Orchard JW, Cook JL, Halpin N. Stress-shielding as a cause of insertional tendinopathy: the operative technique of limited adductor tenotomy supports this theory. J Sci Med Sport. 2004;7:424-428 [DOI] [PubMed] [Google Scholar]
- 67. Orchard J, Massey A, Brown R, et al. Successful management of tendinopathy with injections of the MMP-inhibitor aprotinin. Clin Orthop Rel Res. 2008;466:1625-1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Paoloni JA, Appleyard RC, Nelson J, et al. Topical glyceryl trinitrate treatment of chronic noninsertional achilles tendinopathy: a randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am. 2004;86:916-922 [DOI] [PubMed] [Google Scholar]
- 69. Paoloni JA, Appleyard RC, Nelson J, et al. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2003;31:915-920 [DOI] [PubMed] [Google Scholar]
- 70. Popp JE, Yu JS, Kaeding CC. Recalcitrant patellar tendinitis: magnetic resonance imaging, histologic evaluation, and surgical treatment. Am J Sports Med. 1997;25:218-222 [DOI] [PubMed] [Google Scholar]
- 71. Puddu G, Ippolito E, Postacchini F. A classification of Achilles tendon disease. Am J Sports Med. 1976;4:145-150 [DOI] [PubMed] [Google Scholar]
- 72. Rabago D, Best TM, Zgierska A, Zeisig E, Ryan M, Crane D. A systematic review of four injection therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood and platelet rich plasma [published online ahead of print January 21, 2009]. Br J Sports Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rasmussen S, Christensen M, Mathiesen I, et al. Shockwave therapy for chronic Achilles tendinopathy: a double-blind, randomized clinical trial of efficacy. Acta Orthop. 2008;79:249-256 [DOI] [PubMed] [Google Scholar]
- 74. Rees JD, Lichtwark GA, Wolman RL, et al. The mechanism for efficacy of eccentric loading in Achilles tendon injury; an in vivo study in humans. Rheumatology. 2008;47:1493-1497 [DOI] [PubMed] [Google Scholar]
- 75. Riley GP, Cox M, Harrall RL, et al. Inhibition of tendon cell proliferation and matrix glycosaminoglycan synthesis by non-steroidal anti-inflammatory drugs in vitro. J Hand Surg [Br]. 2001;26:224-228 [DOI] [PubMed] [Google Scholar]
- 76. Rolf CG, Fu BS, Pau A, et al. Increased cell proliferation and associated expression of PDGFRbeta causing hypercellularity in patellar tendinosis. Rheumatology. 2001;40:256-261 [DOI] [PubMed] [Google Scholar]
- 77. Rompe JD, Furia J, Maffulli N. Eccentric loading compared with shock wave treatment for chronic insertional achilles tendinopathy: a randomized, controlled trial. J Bone Joint Surg Am. 2008;90:52-61 [DOI] [PubMed] [Google Scholar]
- 78. Rompe JD, Maffulli N. Repetitive shock wave therapy for lateral elbow tendinopathy (tennis elbow): a systematic and qualitative analysis. Br Med Bull. 2007;83:355-378 [DOI] [PubMed] [Google Scholar]
- 79. Rompe JD, Nafe B, Furia JP, et al. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007;35:374-383 [DOI] [PubMed] [Google Scholar]
- 80. Rompe JD, Nafe B, Furia JP, Maffulli N. Eccentric loading versus eccentric loading plus shock-wave treatment for midportion achilles tendinopathy: a randomized controlled trial [published online ahead of print December 15, 2008]. Am J Sports Med. [DOI] [PubMed] [Google Scholar]
- 81. Schnabel LV, Mohammed HO, Miller BJ, et al. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res. 2007;25:230-240 [DOI] [PubMed] [Google Scholar]
- 82. Schubert TE, Weidler C, Lerch K, et al. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis. 2005;64:1083-1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Scott A, Lian O, Bahr R, et al. Increased mast cell numbers in human patellar tendinosis: correlation with symptom duration and vascular hyperplasia. Br J Sports Med. 2008;42:753-757 [DOI] [PubMed] [Google Scholar]
- 84. Shalabi A, Kristoffersen-Wilberg M, Svensson L, et al. Eccentric training of the gastrocnemius-soleus complex in chronic Achilles tendinopathy results in decreased tendon volume and intratendinous signal as evaluated by MRI. Am J Sports Med. 2004;32:1286-1296 [DOI] [PubMed] [Google Scholar]
- 85. Stanish WD, Rubinovich RM, Curwin S. Eccentric exercise in chronic tendinitis. Clin Orthop Rel Res. 1986;208:65-68 [PubMed] [Google Scholar]
- 86. Stergioulas A, Stergioula M, Aarskog R, et al. Effects of low-level laser therapy and eccentric exercises in the treatment of recreational athletes with chronic achilles tendinopathy. Am J Sports Med. 2008;36:881-887 [DOI] [PubMed] [Google Scholar]
- 87. Szomor ZL, Appleyard RC, Murrell GA. Overexpression of nitric oxide synthases in tendon overuse. J Orthop Res. 2006;24:80-86 [DOI] [PubMed] [Google Scholar]
- 88. Thornton GM, Shao X, Chung M, Sciore P, Boorman RS, Hart DA, Lo IK. Changes in mechanical loading lead to tendon-specific alterations in MMP and TIMP expression: influence of stress-deprivation and intermittent cyclic hydrostatic compression on rat supraspinatus and achilles tendons [published online ahead of print September 18, 2008]. Br J Sports Med. [DOI] [PubMed] [Google Scholar]
- 89. Tsai WC, Hsu CC, Chen CP, et al. Ibuprofen inhibition of tendon cell migration and down-regulation of paxillin expression. J Orthop Res. 2006;24:551-558 [DOI] [PubMed] [Google Scholar]
- 90. Tsai WC, Hsu CC, Chou SW, et al. Effects of celecoxib on migration, proliferation and collagen expression of tendon cells. Connect Tissue Res. 2007;48:46-51 [DOI] [PubMed] [Google Scholar]
- 91. Tsai WC, Tang FT, Hsu CC, et al. Ibuprofen inhibition of tendon cell proliferation and upregulation of the cyclin kinase inhibitor p21CIP1. J Orthop Res. 2004;22:586-591 [DOI] [PubMed] [Google Scholar]
- 92. van Leeuwen MT, Zwerver J, van den Akker-Scheek I. Extracorporeal shockwave therapy for patellar tendinopathy: a review of the literature [published online ahead of print August 21, 2008]. Br J Sports Med. [DOI] [PubMed] [Google Scholar]
- 93. Vulpiani MC, Vetrano M, Savoia V, et al. Jumper’s knee treatment with extracorporeal shock wave therapy: a long-term follow-up observational study. J Sports Med Phys Fitness. 2007;47:323-328 [PubMed] [Google Scholar]
- 94. Wall ME, Banes AJ. Early responses to mechanical load in tendon: role for calcium signaling, gap junctions and intercellular communication. J Musculoskelet Neuronal Interact. 2005;5:70-84 [PubMed] [Google Scholar]
- 95. Wolff J. The Law of Bone Remodeling [translation of the German 1892 edition]. New York, NY: Springer; 1986 [Google Scholar]
- 96. Xia W, Szomor Z, Wang Y, et al. Nitric oxide enhances collagen synthesis in cultured human tendon cells. J Orthop Res. 2006;24:159-172 [DOI] [PubMed] [Google Scholar]
- 97. Xu Y, Murrell GA. The basic science of tendinopathy. Clin Orthop Related Res. 2008;466:1528-1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yu J, Popp J, Kaeding C, et al. Correlation of MR imaging and pathologic findings in athletes undergoing surgery for chronic patellar tendinitis. Am J Roentgenol. 1995;165:115-118 [DOI] [PubMed] [Google Scholar]



