Abstract
Fungemia is associated with a high mortality rate. We compared the performance of the Mycosis IC/F selective fungal medium and the Plus Aerobic/F standard bacteriological medium for the diagnosis of fungemia on the Bactec 9240 automatic system. We retrospectively analyzed 550 blood culture pairs composed of one Mycosis IC/F vial and one Plus Aerobic/F vial, drawn in 187 patients with fungemia. The positivity rate by vial was significantly higher on Mycosis IC/F medium than on Plus Aerobic/F medium (88.0% versus 74.9%, P < 0.0001). The positivity rate for fungus detection on Plus Aerobic/F medium fell to 26.9% when bacteria were present in the same vial. The positivity rate by patient was also significantly higher on Mycosis IC/F medium than on Plus Aerobic/F medium (92.5% versus 75.9%, P < 0.0001). A marked superiority of Mycosis IC/F medium was demonstrated for diagnosis of Candida glabrata fungemia (31 of 31, 100%, versus 18 of 31, 58.1%, P < 0.0001). The mean detection time was significantly shorter on Mycosis IC/F medium than on Plus Aerobic/F medium (28.9 ± 22.2 h versus 36.5 ± 24.6 h, P < 0.0001). The mean time saving was 8.8 h for Candida albicans and 43.7 h for C. glabrata. Mycosis IC/F medium enabled more sensitive and earlier diagnosis, particularly for the two strains most frequently responsible for fungemia, C. albicans and C. glabrata, and also in the event of the concomitant presence of both yeasts and bacteria. In patients with risk factors, it would thus appear to be sensible to draw a Mycosis IC/F vial in addition to the standard bacteriological vials.
Nosocomial systemic infections are a major cause of mortality and morbidity. Candida yeasts are currently the fourth most common pathogen isolated in nosocomial bloodstream infections (2, 15). Candida albicans is still the most common species, but there has been an increase in non-C. albicans species in the last decade, especially Candida glabrata (10). The mortality rate in fungemia cases is high (40 to 60%) due to difficulties diagnosing and treating it (17). It is therefore very important that microbiology laboratories can detect yeasts in the blood as quickly as possible and with a satisfactory level of sensitivity.
Blood cultures are the first-line test in the event of any suspected case of systemic mycosis. Current techniques use broth culture media processed using automated systems, which have considerably improved the sensitivity of blood cultures for the detection of microorganisms. However, the sensitivity of blood cultures for the diagnosis of fungemia is estimated to be only 50% (7). The Bactec system (BD Diagnostic Systems, Sparks, Md.) proposes media specifically formulated for the isolation of fungi in blood. The Fungal Blood Culture medium is used on Bactec NR first-generation noncontinuous monitoring systems and the Mycosis IC/F is used on Bactec 9000 second-generation continuous monitoring systems. The Bactec 9240 automatic system for blood cultures is based on the continuous-monitoring detection by fluorescence of CO2 produced during the growth of microorganisms.
Mycosis IC/F medium is a brain-heart broth enriched with sucrose, containing chloramphenicol and tobramycin to inhibit bacterial growth, and a lysis agent, saponin. The standard bacteriological blood culture vials are Plus Aerobic/F and Peds Plus/F media for the detection of aerobic bacteria and microaerophilic organisms and Plus Anaerobic/F medium for anaerobic bacteria. Plus Aerobic/F medium is a soybean-casein digest broth enriched with yeast extract, dextrose, sucrose, hemin, menadione, and vitamin B6. Peds Plus/F medium is the same formula as Plus Aerobic/F medium enriched with animal tissue digest and sodium pyruvate. Most yeasts are capable of growing on aerobic bacteriological blood culture media, but studies based on the inoculation of blood culture vials with standardized numbers of yeasts have shown that the positivity rate and the detection speed were better on Mycosis IC/F medium than on different standard bacteriological media in continuous monitoring (3, 14).
The aim of our study was to compare, in a clinical situation and on a large population of patients, the efficacy of Mycosis IC/F fungal medium and Plus Aerobic/F standard bacteriological medium in terms of positivity rate and detection time for the diagnosis of fungemia.
MATERIALS AND METHODS
Patients.
We conducted a retrospective study of all fungemia cases that occurred at the Strasbourg University Hospital over a 46-month period, from January 1998 to November 2001. All patients who had at least one positive blood culture for fungi during this period were included in the analysis. Comparative analyses were then made exclusively on blood culture pairs, a pair being made up of one Mycosis IC/F vial and one Plus Aerobic/F vial, sampled from the patient at the same time and from the same site and processed simultaneously in the laboratory.
Blood culture processing.
All the vials were monitored at 35°C in the Bactec 9240 with automatic reading of fluorescence every 10 min. The protocol duration was 160 h for the Mycosis IC/F vials and 120 h for the Plus Aerobic/F vials, in accordance with the manufacturer's recommendations. Positive vials, along with their detection times, were signaled by the system. Each vial signaled as being positive by the system was the subject of direct examination to confirm the presence of fungi or bacteria. Blood cultures positive for fungi were then inoculated on Candiselect chromogenic medium (Bio-Rad, Marnes-la-Coquette, France) and incubated for 24 to 48 h at 35°C. C. albicans was identified by the blue color of the colonies. The other yeasts were identified on the basis of their morphology on potato-carrot-bile medium and according to their biochemical characters on the Auxacolor system (Bio-Rad). Blood cultures positive for bacteria were inoculated on standard bacteriological agars (blood agar, chocolate-polyvitex agar, Drigalski agar) for 24 to 48 h at 37°C, and the bacteria were identified using biochemical systems according to the initial guiding information.
Data analysis.
For each patient, we recorded the total number of blood cultures drawn on Mycosis IC/F medium and on Plus Aerobic/F medium, the number of blood cultures positive for fungi on each of the media, the fungal species involved, and the detection times of the positive vials. Since the processing protocol for the Plus Aerobic/F vials was only 120 h in comparison with 160 h for the Mycosis IC/F vials, we were unable to reconstitute pairs when the Mycosis IC/F vial was positive after 120 h. These Mycosis IC/F vials were therefore eliminated from the comparative analyses. The positivity rates were compared using Fisher's exact test. The detection times were compared using the Mann-Whitney test, since distribution of the values was not Gaussian. The cumulative positivity plots as a function of detection time were compared using the log-rank test. Values of P < 0.05 were considered to be statistically significant.
RESULTS
During the 46 months of the study, 68,150 Mycosis IC/F blood culture vials and 124,060 Plus Aerobic/F blood culture vials were drawn. In total, we recorded 1,148 blood cultures positive for fungi on Mycosis IC/F medium or Plus Aerobic/F medium, in 238 patients. For the comparative analysis of the media, we only retained Mycosis IC/F and Plus Aerobic/F blood culture couples that were strictly paired, as defined in the Materials and Methods section. Consequently, the final comparative analysis was conducted on 1,100 blood culture vials, forming 550 pairs, taken from 187 patients. We successively analyzed the positivity rates of the two media, the efficacy for diagnosis of fungemia by patient, the detection times of positive vials, and the influence of the concomitant presence of bacteria on the isolation of fungi.
Positivity rate by bottle.
The overall positivity rate of the blood cultures for isolation of yeasts was significantly higher for the Mycosis IC/F vials than for the Plus Aerobic/F vials (484 of 550, 88%, versus 412 of 550, 74.9%; P < 0.0001) (Table 1). The positivity rates varied depending on the fungal species isolated (Table 1). The Mycosis IC/F medium was revealed to be significantly better for C. parapsilosis (P = 0.0045) and, especially, for C. glabrata (P < 0.0001). For this species, 40 isolates (62.5%) were only detected on the Mycosis IC/F medium. In our study, one patient presented disseminated fusariosis. The Mycosis IC/F vial enabled isolation of Fusarium proliferatum, whereas the Plus Aerobic/F vial remained negative.
TABLE 1.
Positivity of Mycosis IC/F and Plus Aerobic/F vials on paired blood cultures according to species
| Species | No. of pairs | Positive on Mycosis IC/F
|
Positive on Plus Aerobic/F
|
P (Fisher's exact test) | ||
|---|---|---|---|---|---|---|
| No. of vials | % | No. of vials | % | |||
| C. albicans | 278 | 228 | 82.0 | 217 | 78.1 | 0.2887 |
| C. parapsilosis | 65 | 63 | 96.9 | 52 | 80.0 | 0.0045 |
| C. glabrata | 64 | 61 | 95.3 | 24 | 37.5 | <0.0001 |
| C. tropicalis | 56 | 51 | 91.1 | 47 | 83.9 | 0.3922 |
| C. krusei | 39 | 35 | 89.7 | 35 | 89.7 | 1.2885 |
| S. cerevisiae | 17 | 16 | 94.1 | 15 | 88.2 | 1.0000 |
| C. famata | 16 | 16 | 100 | 15 | 93.7 | 1.0000 |
| Othera | 15 | 14 | 93.3 | 7 | 46.7 | 0.0142 |
| Total | 550 | 484 | 88.0 | 412 | 74.9 | <0.0001 |
C. inconspicua, three pairs (three Mycosis IC/F vials positive, two Plus Aerobic/F vials positive); Cryptococcus neoformans, two pairs (one Mycosis IC/F vial positive, one Plus Aerobic/F vial positive); Rhodotorula mucilaginosus, two pairs (two Mycosis IC/F vials positive, zero Plus Aerobic/F vial positive); Malassezia furfur, one pair (one Mycosis IC/F vial positive, zero Plus Aerobic/F vial positive); C. guillermondii, one pair (one Mycosis IC/F vial positive, zero Plus Aerobic/F vial positive); Fusarium proliferatum, one pair (one Mycosis IC/F vial positive, zero Plus Aerobic/F vial positive); combination of two yeasts, five pairs (five Mycosis IC/F vials positive, four Plus Aerobic/F vials positive).
Positivity rate by patient.
Significantly more cases of fungemia were diagnosed on Mycosis IC/F medium than on Plus Aerobic/F medium (173 of 187, 92.5%, versus 142 of 187, 75.9%; P < 0.0001) (Table 2). Forty-five cases of fungemia (24.1%) were only detected on Mycosis IC/F medium. Analysis of the rates according to the fungal species did not demonstrate any significant difference between the two media for C. albicans, C. parapsilosis, C. tropicalis, and C. krusei. Conversely, a very marked superiority of the Mycosis IC/F medium was observed for C. glabrata (P < 0.0001). For this species, almost half of the cases of fungemia (13 of 31, 41.9%) were only diagnosed on Mycosis IC/F medium.
TABLE 2.
Fungemia cases diagnosed on Mycosis IC/F and Plus Aerobic/F media according to species
| Species | Total no. of episodes | Positive on Mycosis IC/F
|
Positive on Plus Aerobic/F
|
P (Fisher's exact test) | ||
|---|---|---|---|---|---|---|
| No. of episodes | % | No. of episodes | % | |||
| C. albicans | 98 | 87 | 88.8 | 80 | 81.6 | 0.2270 |
| C. glabrata | 31 | 31 | 100 | 18 | 58.1 | <0.0001 |
| C. parapsilosis | 18 | 17 | 94.4 | 14 | 77.8 | 0.3377 |
| C. tropicalis | 15 | 15 | 100 | 13 | 86.7 | 0.4828 |
| C. krusei | 10 | 8 | 80 | 8 | 80.0 | 1.4180 |
| S. cerevisiae | 5 | 5 | 100 | 4 | 80.0 | 1.0000 |
| Othera | 9 | 9 | 100 | 4 | 44.4 | 0.0294 |
| Total | 187 | 173 | 92.5 | 142 | 75.9 | <0.0001 |
C. inconspicua and Cryptococcus neoformans, one episode each, positive on both media. Rhodotorula mucilaginosus, Malassezia furfur, C. guillermondii, and Fusarium proliferatum, one episode each, positive on Mycosis IC/F only. Combination of two yeasts, three episodes, two positive on both media, one positive on Mycosis C/F only.
Detection time.
The mean detection time of the positive vials was significantly shorter with the Mycosis IC/F medium than the Plus Aerobic/F medium (28.9 h versus 36.5 h, P < 0.0001) (Table 3). Analysis of the detection times by fungal species demonstrated a very significant time saving on Mycosis IC/F medium for the isolation of C. albicans, with a mean time saving of 8.8 h (P < 0.0001), and especially for C. glabrata, with a mean time saving of 43.7 h (P < 0.0001).
TABLE 3.
Mean detection time according to species
| Species | Mycosis IC/F
|
Plus Aerobic/F
|
P (Mann-Whitney) | ||
|---|---|---|---|---|---|
| No. of positive vials | Mean detection time (h) ± SD | No. of positive vials | Mean detection time (h) ± SD | ||
| C. albicans | 220 | 31.1 ± 23.3 | 194 | 39.9 ± 23.7 | <0.0001 |
| C. glabrata | 61 | 17.8 ± 9.1 | 19 | 61.5 ± 31.4 | <0.0001 |
| C. parapsilosis | 60 | 32.0 ± 26.1 | 51 | 24.4 ± 14.5 | 0.2990 |
| C. tropicalis | 48 | 18.5 ± 16.3 | 46 | 18.5 ± 11.7 | 0.2879 |
| C. krusei | 35 | 22.7 ± 6.4 | 28 | 26.7 ± 10.3 | 0.0984 |
| C. famata | 16 | 36.1 ± 22.4 | 15 | 31.9 ± 8.9 | 0.8125 |
| S. cerevisiae | 15 | 56.1 ± 21.5 | 15 | 74.0 ± 27.7 | 0.0649 |
| C. inconspicua | 3 | 34.8 ± 1.6 | 1 | 53.0 | NA |
| C. guillermondii | 1 | 33.0 | 0 | NAc | NA |
| Cryptococcus neoformans | 1 | 95.4 | 1 | 110.8 | NA |
| R. mucilaginosus | 2 | 48.0 ± 2.0 | 0 | NA | NA |
| M. furfur | 1 | 71.7 | 0 | NA | NA |
| Fusarium proliferatum | 1 | 58.0 | 0 | NA | NA |
| Total | 464a | 28.9 ± 22.2 | 370b | 36.5 ± 24.6 | <0.0001 |
Fifteen detection times were lost, and five combinations of two yeast species were not analyzed.
Thirty-eight detection times were lost, and four combinations of two yeast species were not analyzed.
NA, not applicable.
Overall efficacy of the two media.
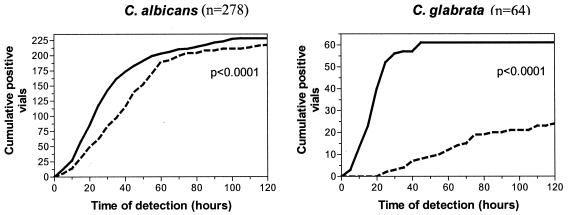
The overall efficacy is defined by the combination of positivity rate and time criteria of the blood cultures. We compared the cumulative positivity kinetic plots as a function of time for the two media for the most common strains. Mycosis IC/F medium was shown to be statistically more effective than Plus Aerobic/F medium for the isolation of the two species responsible for most cases of fungemia, C. albicans (P < 0.0001) and C. glabrata (P < 0.0001) (Fig. 1). The difference was not significant for C. parapsilosis (P = 0.0931), C. tropicalis (P = 0.2050), C. krusei (P = 0.2114), C. inconspicua (P = 0.8870), and Saccharomyces cerevisiae (P = 0.0704).
FIG. 1.
Cumulative positive curve according to the time of detection of C. albicans and C. glabrata in Mycosis IC/F (solid line) and Plus Aerobic/F (dashed line) vials. Statistical analysis by the log-rank test.
Concomitant presence of fungi and bacteria.
To evaluate the influence of the presence of bacteria on detection of fungi in blood cultures containing both microorganisms, we analyzed 52 blood culture pairs in which a bacterium was isolated in the Plus Aerobic/F vial corresponding to a Mycosis IC/F vial positive for fungus. The rate for the isolation of fungus on Plus Aerobic/F medium was statistically lower when bacteria were present in the same sample (14 of 52, 26.9%) than in the absence of bacteria (398 of 498, 79.9%) (P < 0.0001). These 52 blood culture pairs corresponded to 39 patients. Thus, 20.9% of the patients in our study had at least one concomitant isolation of yeasts and bacteria. Fungemia was diagnosed in the Mycosis IC/F vial for all 39 patients and in the Plus Aerobic/F vial for only 23 patients (P < 0.0001).
DISCUSSION
Advances in the field of blood culture media and detection technologies have considerably improved the sensitivity of diagnosis of bloodstream infections (12). The diagnosis of fungemia is still difficult, however, while the number of patients with a high risk of fungal infection is steadily rising. Standard bacteriological media allow the growth of most fungi and several studies have compared the efficacy of various bacteriological media and different detection systems for the isolation of fungi in the blood. Among the latest-generation continuous monitoring systems, comparable performance levels have been found for BacT/Alert FAN Aerobic bacteriological medium monitored on the BacT/Alert system and Plus Aerobic/F medium monitored on the Bactec 9240 system for the diagnosis of fungemia (8, 11).
Only the Bactec system proposes media specifically formulated for fungi. McDonald et al. compared the BacT/Alert FAN medium on a continuous system and the Fungal Blood Culture medium monitored on the Bactec NR660 noncontinuous system and found comparable performances for the diagnosis of fungemia (9). However, Vigano et al. compared the continuous-monitoring detection times of BacT/Alert Fan Aerobic, Plus Aerobic/F, and Mycosis IC/F media inoculated with standard quantities of yeasts (14). The detection times were always shorter on Mycosis IC/F medium than on the bacteriological media, with a mean time saving of 5 h over the Plus Aerobic/F medium and 9 h over the BacT/Alert Fan Aerobic medium, suggesting a possible increased sensitivity with Mycosis IC/F medium for the diagnosis of fungemia in clinical practice.
The aim of our study was to compare, in a clinical situation and on a large population of patients, the performance of Mycosis IC/F and Plus Aerobic/F media processed in parallel in the Bactec 9240 system by continuous monitoring. The results show that the Mycosis IC/F medium clearly has several advantages over the Plus Aerobic/F medium for the isolation of yeasts in the blood. The positivity rates are statistically better on the Mycosis IC/F medium, when analyzed both by sample and by patient. In particular, analysis by species demonstrates the very marked superiority of Mycosis IC/F medium for the isolation of C. glabrata. The detection time was significantly shorter on Mycosis IC/F than on Plus Aerobic/F medium. All species combined, yeasts were detected, on average, 7.6 h earlier with the Mycosis IC/F medium, confirming the data obtained with media spiked with yeasts (3, 14).
We found a high level of disparity between species. The mean time saving provided by the Mycosis IC/F medium was 8.8 h for C. albicans and 43.7 h for C. glabrata. Fricker-Hidalgo et al. had also observed a marked time saving, of 9.5 h for C. albicans and 105.2 h for C. glabrata, on Mycosis IC/F medium in comparison with the results seen with Plus Aerobic/F medium on the Bactec 9240 system (3). In our study, analysis of the global efficacy by combining the criteria for positivity rate and speed, clearly demonstrates the advantage of the Mycosis IC/F medium for C. albicans and C. glabrata. This increased sensitivity and earlier diagnosis are crucial for C. albicans and C. glabrata, which alone represent 70% of all fungemia cases (2). C. glabrata is currently the second commonest agent responsible for fungemia behind C. albicans and is associated with low sensitivity to fluconazole and with particularly high mortality rates (1). The value of Mycosis IC/F medium for the diagnosis of C. glabrata fungemia is therefore obvious.
Mycosis IC/F medium provides a considerable advantage when yeasts are present concomitantly with bacteria. These combinations are common. They represented 21% of the fungemia cases in our study and 18% in the study by Grillot et al. (R. Grillot, H. Fricker-Hidalgo, B. Lebeau, H. Pelloux, J. Croize, C. Recule, and P. Ambroise-Thomas, 39th Intersci. Conf. Antimicrob. Agents Chemother., 1999, abstract J975). In this situation, the absence of detection of yeasts on bacteriological media could be due to inhibition of fungal growth by more rapid bacterial development or by the production of antifungal substances by the bacteria. In the study by Vigano et al. with simulated blood cultures, the mean detection time for Candida spp. was 29.2 h, whereas the majority of bacterial strains tested presented a shorter detection time (14). It was also shown that bacterial flora inhibited detection of yeasts in 50% of polymicrobial samples inoculated on media not spiked with antibiotics (13) and that Pseudomonas aeruginosa was capable of inhibiting the growth in vitro of several yeast species (4). An in vivo model of coinfection with C. albicans and P. aeruginosa has confirmed these data (6).
Mycosis IC/F medium also appears to be more effective than a standard bacteriological medium for the isolation of Fusarium sp. and certain low growing or exigent yeasts, such as Malassezia spp., C. guillermondii, or Cryptococcus neoformans (3, 5). In our study, the low number of these particular species did not allow statistical comparisons. In addition, we could not evaluate the ability of the Mycosis IC/F medium in recovering Histoplasma capsulatum or other dimorphic fungi from blood since these pathogens are very rare in Europe. It would be interesting to evaluate the Mycosis IC/F medium, especially in comparison with the reference Isolator system, for the detection of dimorphic fungal pathogens.
In conclusion, the Mycosis IC/F medium was shown to be more effective than the Plus Aerobic/F medium for the diagnosis of fungemia in terms of positivity rate and detection speed. The advantage was particularly obvious for C. albicans and C. glabrata as well as in cases of blood cultures combining both bacteria and fungi. The risk factors for systemic Candida infection are currently clearly identified (16, 17), and it would therefore seem sensible to draw a Mycosis IC/F vial in addition to the standard bacteriological vials to improve the sensitivity and speed of diagnosis of fungemia in high-risk patients.
REFERENCES
- 1.Bodey, G. P., M. Mardani, H. A. Hanna, M. Boktour, J. Abbas, E. Girgawy, R. Y. Hachem, D. P. Kontoyiannis, and X. Raad II. 2002. The epidemiology of Candida glabrata and Candida albicans fungemia in immunocompromised patients with cancer. Am. J. Med. 112:380-385. [DOI] [PubMed] [Google Scholar]
- 2.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 3.Fricker-Hidalgo, H., F. Chazot, B. Lebeau, H. Pelloux, P. Ambroise-Thomas, and R. Grillot. 1998. Use of simulated blood cultures to compare a specific fungal medium with a standard microorganism medium for yeast detection. Eur. J. Clin. Microbiol. Infect. Dis. 17:113-116. [DOI] [PubMed] [Google Scholar]
- 4.Grillot, R., V. Portmann-Coffin, and P. Ambroise-Thomas. 1994. Growth inhibition of pathogenic yeasts by Pseudomonas aeruginosa in vitro: clinical implications in blood cultures. Mycoses 37:343-347. [DOI] [PubMed] [Google Scholar]
- 5.Hennequin, C., C. Ranaivoarimalala, T. Chouaki, M. Tazerout, T. Ancelle, J. J. Cabaud, and C. P. Raccurt. 2002. Comparison of aerobic standard medium with specific fungal medium for detecting Fusarium spp in blood cultures. Eur. J. Clin. Microbiol. Infect. Dis. 21:748-750. [DOI] [PubMed] [Google Scholar]
- 6.Hockey, L. J., N. K. Fujita, T. R. Gibson, D. Rotrosen, J. Z. Montgomerie, and J. E. Edwards. 1982. Detection of fungemia obscured by concomitant bacteremia: in vitro and in vivo studies. J. Clin. Microbiol. 16:1080-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones, J. M. 1990. Laboratory diagnosis of invasive candidiasis. Clin. Microbiol. Rev. 3:32-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jorgensen, J. H., S. Mirrett, L. C. McDonald, P. R. Murray, M. P. Weinstein, J. Fune, C. W. Trippy, M. Masterson, and L. B. Reller. 1997. Controlled clinical laboratory comparison of BACTEC plus aerobic/F resin medium with BacT/Alert aerobic FAN medium for detection of bacteremia and fungemia. J. Clin. Microbiol. 35:53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald, L. C., M. P. Weinstein, J. Fune, S. Mirrett, L. G. Reimer, and L. B. Reller. 2001. Controlled comparison of BacT/ALERT FAN aerobic medium and BACTEC fungal blood culture medium for detection of fungemia. J. Clin. Microbiol. 39:622-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfaller, M. A., R. N. Jones, S. A. Messer, M. B. Edmond, and R. P. Wenzel. 1998. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. SCOPE Participant Group. Surveillance and Control of Pathogens of Epidemiologic. Diagn. Microbiol. Infect. Dis. 30:121-129. [DOI] [PubMed] [Google Scholar]
- 11.Pohlman, J. K., B. A. Kirkley, K. A. Easley, B. A. Basille, and J. A. Washington. 1995. Controlled clinical evaluation of BACTEC Plus Aerobic/F and BacT/Alert Aerobic FAN bottles for detection of bloodstream infections. J. Clin. Microbiol. 33:2856-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reimer, L. G., M. L. Wilson, and M. P. Weinstein. 1997. Update on detection of bacteremia and fungemia. Clin. Microbiol. Rev. 10:444-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandven, P., and J. Lassen. 1999. Importance of selective media for recovery of yeasts from clinical specimens. J. Clin. Microbiol. 37:3731-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vigano, E. F., E. Vasconi, C. Agrappi, and P. Clerici. 2002. Use of simulated blood cultures for time to detection comparison between BacT/ALERT and BACTEC 9240 blood culture systems. Diagn. Microbiol. Infect. Dis. 44:235-240. [DOI] [PubMed] [Google Scholar]
- 15.Vincent, J. L., D. J. Bihari, P. M. Suter, H. A. Bruining, J. White, M. H. Nicolas-Chanoin, M. Wolff, R. C. Spencer, and M. Hemmer. 1995. The prevalence of nosocomial infection in intensve care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) study. EPIC International Advisory Committee. JAMA 274:639-644. [PubMed] [Google Scholar]
- 16.Wenzel, R. P. 1995. Nosocomial candidemia: risk factors and attributable mortality. Clin. Infect. Dis. 20:1531-1534. [DOI] [PubMed] [Google Scholar]
- 17.Wey, S. B., M. Mori, M. A. Pfaller, R. F. Woolson, and R. P. Wenzel. 1988. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch. Intern. Med. 148:2642-2645. [DOI] [PubMed] [Google Scholar]